Abstract
Hygienic behaviour is a group defence in which dead or diseased individuals are excluded. In the honeybee, Apis mellifera, hygienic behaviour refers to uncapping and removing dead and diseased larvae and pupae from sealed brood cells. We quantified removal of freeze-killed and chalkbrood-infected larvae from open cells in 20 colonies. We also measured removal of freeze-killed brood from sealed cells. Study colonies ranged from non-hygienic to fully hygienic (52–100% removal within 2 days). All larvae killed in open cells were removed. This shows that all colonies, including those with low hygienic behaviour against dead brood in sealed cells, are highly hygienic against dead brood in open cells and suggests that low hygienic behaviour against dead brood in sealed cells is a trait in its own right. This may also contribute to understanding why hygienic behaviour is uncommon in A. mellifera, which is puzzling as it reduces several diseases without detrimental effects. In particular, the result provides indirect support for the hypothesis that there are two adaptive peaks conferring disease resistance: (i) high hygienic behaviour: diseased brood are removed quickly, in some cases before becoming infective; (ii) low hygienic behaviour: diseased brood remain isolated within sealed cells.
This article is part of the Theo Murphy meeting issue ‘Evolution of pathogen and parasite avoidance behaviours'.
Keywords: hygienic behaviour, Ascosphaera apis, group-level defence, Apis mellifera, freeze-killed brood, twin adaptive peaks
1. Introduction
Diseases are a challenge to all organisms, but especially to group-living species due to increased opportunities for pathogen transmission (e.g. humans [1], lizards [2], Lepidoptera [3] and birds [4]). However, group-living organisms also have group-level defences against disease (humans [5], primates [6] and birds [4]). Eusocial insects display a wide variety of group defences [7], including allogrooming behaviour [8–10], the use of antimicrobial resins [11–13], social fever [14], antimicrobial secretions [15,16] and hygienic behaviour [7].
Hygienic behaviour is a group defence against diseases in which workers remove dead, dying and diseased individuals from the nest [7]. In the honeybee, Apis mellifera, the term hygienic behaviour has a well-established meaning that specifically refers to the uncapping of sealed cells and the removal of brood (larvae and pupae) from those cells [17–21]. This helps colony defence against pests and diseases such as American foulbrood [22], chalkbrood [23], Varroa mites [21,24,25] and deformed wing virus [24,26]. Hygienic behaviour is a heritable trait not a learned behaviour [19,20,27–29], and seems to have no negative effect on colony performance as it does not increase the removal of healthy brood [30] and increases honey production [31].
Most colonies of honeybees are less than fully hygienic [32,33], defined as removing 95% or more sealed brood within 2 days after freeze-killing with liquid nitrogen [34]. It is a puzzle why hygienic behaviour is uncommon given that it seems to have no negative effects on the colony. One hypothesis is that in honeybees there are two alternative and disjunct adaptive peaks [35–38]: (i) a high level of hygienic behaviour, so that diseased brood are removed swiftly, in some cases before the pathogen becomes infective [39,40]; (ii) a low level of hygienic behaviour, so that infected brood remain isolated within sealed brood cells as reported in Apis cerana for Varroa mites, European foulbrood and Thai sacbrood virus [38].
One prediction of the two adaptive peaks hypothesis is that honeybee colonies will remove dead and diseased brood from open cells, whether or not the colony has a high level of hygienic behaviour against dead and diseased brood in sealed cells. This prediction is made because it would not be advantageous to allow dead larvae to remain in open cells, as such cells will not have a capping to isolate the contents. We tested this hypothesis by quantifying the removal of dead (freeze-killed) and infected (with spores of Ascosphaera apis, the causative agent of chalkbrood disease) larvae from open cells in 20 colonies for which we also measured the level of hygienic behaviour against freeze-killed brood (FKB) in sealed cells. Our results support the hypothesis. All colonies, including those with low levels of hygienic behaviour against FKB in sealed cells, removed 100% dead brood from open cells.
2. Material and methods
(a). Study colonies
We studied 20 honeybee colonies, Apis mellifera, located in a single apiary in Sussex, UK, in summer, July–September 2013. Each colony was in a hive consisting of a single ‘commercial’ 56-l brood chamber, containing 11 frames (43.8 × 25.4 cm), bottom board, inner cover and telescopic cover. From May 2013, no frames of brood were transferred into any study colony so that the young worker bees in each colony were the progeny of the colony's queen at the time of the experiment. As part of normal hive management, all colonies were inspected regularly for visible symptoms of brood diseases. Dead brood showing symptoms of sac brood, American foulbrood and European foulbrood were never seen. Chalkbrood mummies were observed in brood cells in 8 of the 20 colonies, but always at low levels (less than 12 cells per colony per inspection).
(b). Quantifying hygienic behaviour in sealed cells using the freeze-killed brood bioassay
We determined the level of hygienic behaviour towards dead brood in sealed worker cells in the 20 study colonies using the FKB bioassay [22,24,30,34,39,40,41]. Colonies showing high levels of FKB removal have resistance against brood diseases (e.g. [21,22,24]). Each colony was tested four times from late July to late August at 7–10 day intervals. At this time, all colonies were strong with approximately equal populations (8–9 frames of brood and 10–11 frames of bees), several frames of honey, a marked egg-laying queen and frames with empty cells for egg laying or food storage. For each FKB bioassay, we followed standard methods [24,30,41]. We removed a test frame with a large area of sealed brood from each colony. We pressed two metal cylinders (6.5 cm diameter × 8 cm height) into the sealed brood to the mid rib of the comb. Approximately 150 ml of liquid nitrogen was poured into each cylinder to kill the circle of sealed brood inside. After a few minutes, when the liquid nitrogen had evaporated, we removed the cylinders. The frame was then photographed and returned to the hive, and photographed again 48 ± 2 h later. From the photographs, we determined the proportion of FKB-sealed brood cells cleaned out by worker hygienic behaviour.
(c). Preparation of chalkbrood (Ascosphaera apis) spore suspension
Black chalkbrood mummies (dead larvae with visible symptoms of chalkbrood disease, in which the fungus is sporulating [42]), n = 20, were collected from University of Sussex honeybee colonies. The mummies were placed in pairs onto Sabouraud dextrose agar (SDA) media plates and incubated at 28°C. Hyphae grew from each mummy. Spores formed where two hyphal mats of different mating types met [42,43]. Spores were allowed to mature before being harvested from above the SDA media. A spore suspension was made by gently grinding a small amount of spore material in a glass tissue homogenizer with 200 µl sterile deionized water. The supernatant was made up to 1 ml with sterile deionized water and left to stand for 20 min to ensure any spore clumps had settled to the bottom. A 0.5 ml aliquot was taken from the central column of the spore suspension and placed in an Eppendorf tube. The concentration of spores in the aliquot was determined using a FastRead disposable haemocytometer (Immune Systems, UK) and the viability determined [44]. An appropriate final dilution gave a concentration of 1 × 106 spores per millilitre, which is enough to infect and kill honeybee larvae [45].
(d). Quantifying removal of larvae treated with chalkbrood spores
In September, soon after the FKB tests were completed, two frames of brood with larvae of different ages, including both sealed and unsealed brood cells, were chosen from each colony and taken into a warm room (25°C), to reduce the chance of harming the brood by chilling, in the laboratory. A clear acetate sheet was attached to each test frame with drawing pins. On the first frame, nine groups of 30 uncapped cells, each containing a worker larva, were traced onto the acetate so that the position of each cell was precisely recorded, thereby allowing the cell to be relocated. Three groups contained small larvae (less than 1 day old; figure 1), three older larvae that half-filled the base of the cell (around 2–3 days old; figure 1), and three large larvae that filled the base of the cell and which would normally be sealed with a wax capping within one day (around 4–5 days old; figure 1). In each of these three larval-size categories, one group of cells acted as a control with no treatment, one acted as a control treated with 5 µl sterile deionized water, and the third was treated with 5 µl suspension of around 5000 spores.
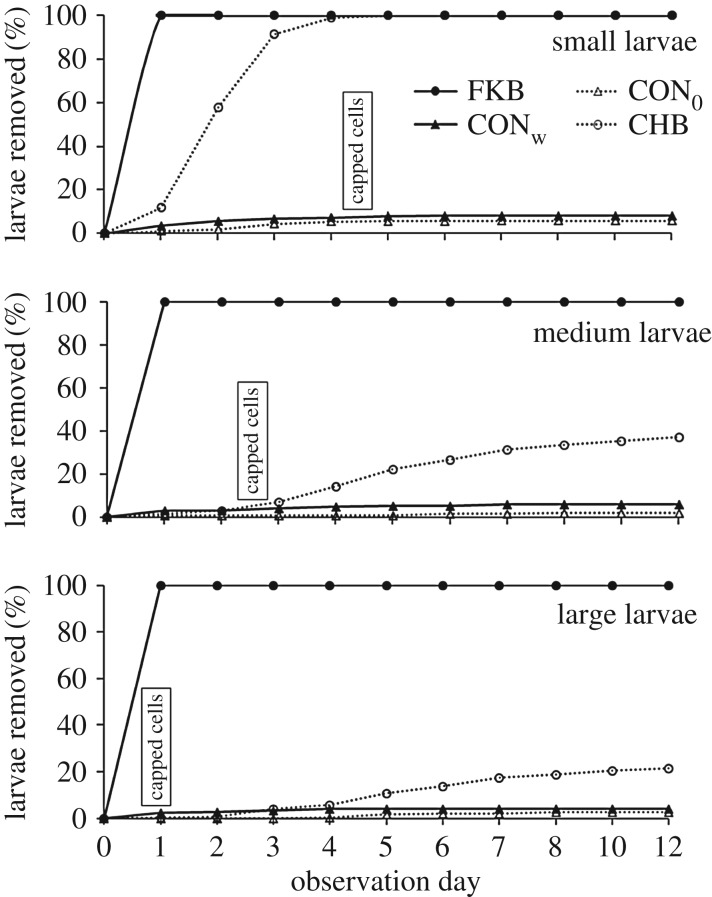
Figure 1.
Examples of small, medium and old larvae in open cells. Blue (small larvae, aged less than 1 day post hatching), yellow (medium, around 2–3 days post hatching) and white (large, around 4–5 days post hatching and within 1 day of being capped (solid line)). Larvae already being capped (white dashed circle) and eggs (red circle) are also shown. Larvae in unmarked cells are of intermediate sizes and were not used. Sealed brood cells with brown wax cappings and empty cells can also be seen. (Online version in colour.)
The water or spore suspension was placed in the larval food at the base of the cell using a Gilson pipette. Care was taken not to touch the larva with the pipette to avoid physical damage. On the second frame, a further 90 cells in three patches, small, medium and large, were traced following the same procedure. All 90 larvae were then killed using liquid nitrogen placed into the study cells using a 10 ml stainless steel spoon, rather than by treating a circle of cells as in the standard FKB bioassay.
Whether or not each larva had been removed from its cell was determined 1, 2, 3, 4, 5, 6, 7, 8, 10 and 12 days after treatment. Cells from which larvae had not been removed after 12 days were uncapped to determine whether they contained a healthy pupa or a chalkbrood mummy.
3. Results
Our 20 study colonies showed considerable variation in freeze-killed-brood removal, 53–100% within 2 days. Importantly, this range included colonies with both low and high levels of hygienic behaviour.
All fixed effects (treatment type, larval size and days since treatment) and each of their 2-way interactions, as well as FKB removal from sealed cells, had highly significant effects on the removal of larvae treated in open cells (ANOVA results shown in table 1).
Table 1.
Significance levels in the statistical analysis of fixed effects on the removal of larvae treated in open cells. Data were analysed by ANOVA using IBM SPSS version 20.
| fixed effects | F-value | p-value |
|---|---|---|
| 1. Larval-size when treated in open cell (small, medium and large) | 303.10 | <0.001 |
| 2. Days since larval treatment | 182.84 | <0.001 |
| 3. Treatment (control, water control, freezing and chalk brood spores) | 1999.54 | <0.001 |
| 4. FKB removal (%) from sealed cells in the same colony | 29.17 | <0.001 |
| 1 × 2 interaction | 22.05 | <0.001 |
| 1 × 3 interaction | 486.55 | <0.001 |
| 2 × 3 interaction | 173.86 | <0.001 |
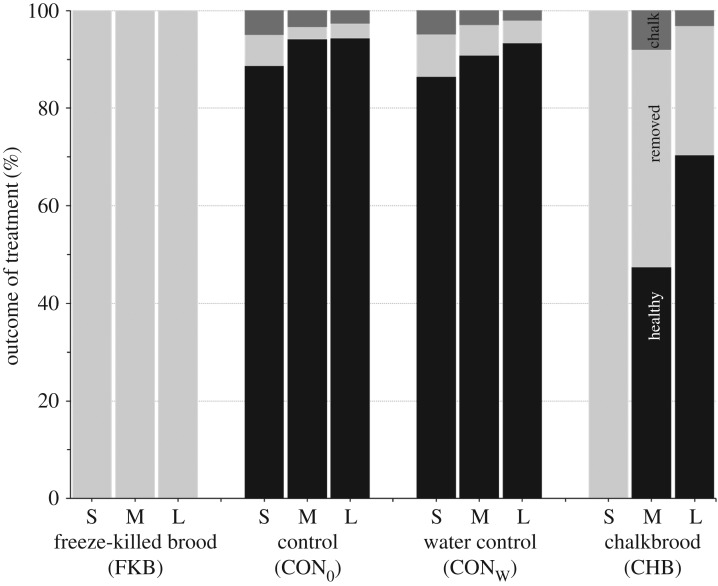
The key results (figures 2 and 3) are that all larvae in open cells killed with liquid nitrogen (n = 1800) were removed within 1 day and all small larvae treated with chalkbrood spores (n = 600) were removed within 5 days. Removal was low for both controls for small, medium and large larvae in the 12 days after treatment. Medium and large larvae treated with chalkbrood spores were not removed before cell capping in greater numbers than for the water only control larvae (figure 2). Large proportions of the medium and large larvae treated with chalkbrood spores survived to form healthy pupae (medium: mean 49%, range 37–63%; large: mean 73%, range 60–80%; figure 3).
Figure 2.
Mean proportions of small (0–1 day post hatching; see figure 1), medium (2–3 days) and large (4–5 days) larvae removed from cells in 20 A. mellifera colonies. All larvae were in unsealed cells when treated (day 0). Cell sealing, shown as ‘capped cells’, occurs when a larva is around 6 days old, which would be around 5, 3 and 1 days after treatment for small, medium and large larvae, respectively. Treatments were: CON0, cell monitored but not treated; CONw, 5 µl distilled water placed in base of a cell in larval food at day 0; CHB, 5 µl water suspension of around 5000 chalkbrood, Ascosphaera apis, spores placed in base of cell in larval food at day 0; FKB, larva killed with liquid nitrogen introduced into the cell on day 0.
Figure 3.
Mean proportions of cells 12 days after treatment that contained either a healthy pupa (black), were empty as the larva or pupa or mummy had been removed (light grey), or contained a chalkbrood mummy (dark grey). Each bar shows the mean of 20 colonies × 30 cells per colony = 600 cells. Larvae were all treated in open cells when small (0–1 day post hatching), medium (2–3 days) or large (4–5 days) in size (see figure 1).
4. Discussion
The removal of all larvae (n = 1800) killed with liquid nitrogen from open cells shows that all honeybee colonies, even colonies with low levels of hygienic behaviour for removal of FKB from sealed cells, have a maximal level of hygienic behaviour with respect to dead brood in open cells. The same picture is seen in young larvae, 0–1 day post hatching, treated with Ascosphaera apis spores with all 600 removed before cell capping. The fact that removal took up to 5 days indicates that the larvae were not killed right away, as would have occurred with liquid nitrogen. Approximately half and two-thirds of the medium and large larvae treated with spores in open cells, respectively, resulted in healthy pupae (figure 3). Previous research has shown that older honeybee larvae are less likely to die when infected by disease spores [45,46,47] and that it takes several days or more for a treated larva to die.
Fully hygienic honeybee colonies are not common. For example, of 31 colonies in Derbyshire, England, screened using the FKB bioassay only 1 was fully hygienic, defined as removing greater than 95% of the dead brood within 2 days [32], with an average of only 46% removal [30]. In a Canadian study, only 1 of 30 colonies had greater than 95% removal after 1 day [33].
Hygienic behaviour in the honeybee is known to be a heritable trait [39,40,41] under the control of multiple genetic loci [19,28,48]. However, it is puzzling that so much variation exists, with both hygienic and non-hygienic colonies in the same populations and with enough genetic variation available to carry out programmes of selective breeding which in a few generations can result in colonies with greater than 95% removal of FKB from sealed cells [39,40].
One obvious general hypothesis for the uncommonness of hygienic behaviour is that it is costly to the colony. However, hygienic colonies have similar or better performance (honey production) in comparison to non-hygienic colonies [31]. In addition, there is no correlation between the removal of healthy brood with the removal of FKB indicating that hygienic colonies do not mistakenly remove healthy brood [30]. Overall, the benefits of hygienic behaviour are thought to outweigh the costs [49].
As mentioned in the Introduction, another hypothesis for the uncommonness of hygienic behaviour is that there are two alternative adaptive peaks for disease control, high and low hygienic behaviour, with the former removing diseased brood from sealed cells swiftly and the latter leaving diseased brood sealed in [35–38]. High levels of hygienic behaviour would more likely be selected when disease is abundant, and low levels when disease is rare. This could also lead to periods when selection is changing, resulting in an intermediate situation with variation in observed levels of hygienic behaviour. It is possible that this is the current situation in A. mellifera, especially with managed colonies. Beekeeping has likely increased the prevalence and transmission of diseases in A. mellifera [50]. In addition, disease control carried out by beekeepers will likely have reduced natural selection for resistance mechanisms including hygienic behaviour [51,52].
The key result of the present study, that all colonies are highly hygienic in the removal of dead brood from open cells, gives an additional perspective to hygienic behaviour in A. mellifera. In particular, it suggests that low hygienic behaviour should probably be considered a trait in its own right and not simply the absence of a trait. The result is also indirect support for the twin adaptive peaks hypothesis. This is because for larvae in open cells there is only one adaptive peak: a high level of hygienic behaviour. There would not be any advantage in terms of disease control to allow dead larvae to remain in open cells, as these cells lack a capping to isolate the dead larva from the colony.
A single adaptive peak may also occur in the stingless bees, Meliponini, although for a different reason. Stingless bees appear to have higher levels of hygienic behaviour than A. mellifera. A total of five species have been studied using brood in sealed cells freeze-killed with liquid nitrogen. Two days after freezing, removal was 65% in Melipona beecheii and 98% in Scaptotrigona pectoralis (n = 8 colonies per species, Mexico [53]), and 99% in M. scutellaris, 80% in S. depilis and 62% in Tetragonisca angustula (n = 8 colonies per species, Brazil [54]). Across the five stinglesss bee species the average FKB removal was 81% and all colonies removed 100% in under 3 to 7 days depending on species.
Like honeybees, stingless bees rear brood in sealed cells but, unlike honeybees, stingless bees never re-use brood cells which are always torn down after one use [55–57]. New cells are constructed in the vacated space when needed. Therefore, leaving cells containing dead brood is not an option and so there is only one adaptive peak.
Another possibly important difference between honeybees and stingless bees is colony lifespan. Wild A. mellifera colonies survive, on average, only a few years [58–60], whereas stingless bee colonies can live for decades [37]. If a honeybee colony leaves cells containing disease-killed brood sealed, these cells cannot later be used for food storage or brood rearing. If a colony is very long lived, but not if the colony is short lived, the number of such cells might increase to a level where much of the nest space was wasted so that leaving dead brood sealed in cells is not a viable option. However, this argument also depends on the number of cells affected. If disease is low, then few cells would be affected. Colony and nest lifespan also vary among honeybee species, as some species and subspecies relocate their nests frequently due to absconding or migration. However, these differences do not result in a clear correlation with levels of hygienic behaviour [54].
When Charles Darwin wrote On the Origin of the Species he knew nothing about genetics and based his theory of natural selection on the idea that heritable traits that helped an organism in the struggle for existence would increase [61,62]. In the case of hygienic behaviour in the honeybee, we know that the trait is heritable and we also know something about its genetics. However, we struggle to understand the benefits of hygienic behaviour. In particular, we struggle to understand why a trait that has been shown to decrease levels of four serious brood diseases in colonies is not more common.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Thomas AJ. 2009. The Lambeth cholera outbreak of 1848–1849: the setting, causes, course and aftermath of an epidemic in London. Jefferson, NC: McFarland & Co. [Google Scholar]
- 2.Godfrey SS, Bull CM, Murray K, Gardner MG. 2005. Transmission mode and distribution of parasites among groups of the social lizard Egernia stokesii. Parasitol. Res. 99, 223–230. ( 10.1007/s00436-005-0120-9) [DOI] [PubMed] [Google Scholar]
- 3.Hochberg ME. 1991. Viruses as costs to gregarious feeding behaviour in the Lepidoptera. Oikos 61, 291–296. ( 10.2307/3545236) [DOI] [Google Scholar]
- 4.Møller AP, Merino S, Brown CR, Robertson RJ. 2001. Immune defense and host sociality: a comparative study of swallows and martins . Am. Nat. 158, 136–145. ( 10.1086/321308) [DOI] [PubMed] [Google Scholar]
- 5.Curtis V, Cairncross S. 2003. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet 3, 275–281. ( 10.1016/S1473-3099(03)00606-6) [DOI] [PubMed] [Google Scholar]
- 6.Freeland WJ. 1979. Primate social groups as biological islands. Ecology 60, 719–728. ( 10.2307/1936609) [DOI] [Google Scholar]
- 7.Wilson-Rich N, Spivak M, Fefferman NH, Starks PT. 2007. Genetic, individual, and group facilitation of disease resistance in insect societies. Annu. Rev. Entomol. 54, 405–423. ( 10.1146/annurev.ento.53.103106.093301) [DOI] [PubMed] [Google Scholar]
- 8.Hughes WO, Eilenberg J, Boomsma JJ. 2002. Trade-offs in group living: transmission and disease resistance in leaf-cutting ants. Proc. R. Soc. Lond. B 269, 1811–1819. ( 10.1098/rspb.2002.2113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunn CL, Altizer S. 2006. Infectious disease in primates. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Schmid-Hempel P. 1998. Parasites in social insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 11.Christe P, Oppliger A, Bancala F, Castella G, Chapuisat M. 2003. Evidence for collective medication in ants. Ecol. Lett. 6, 19–22. ( 10.1046/j.1461-0248.2003.00395.x) [DOI] [Google Scholar]
- 12.Simone M, Evans JD, Spivak M. 2009. Resin collection and social immunity in honey bees. Evolution 63, 3016–3022. ( 10.1111/j.1558-5646.2009.00772.x) [DOI] [PubMed] [Google Scholar]
- 13.Stow A, Briscoe D, Gillings M, Holley M, Smith S, Leys R, Silberbauer T, Turnbull C, Beattie A. 2007. Antimicrobial defences increase with sociality in bees. Biol. Lett. 3, 422–424. ( 10.1098/rsbl.2007.0178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starks PT, Blackie CA, Seeley TD. 2000. Fever in honey bee colonies. Naturwissenschaften 87, 229–231. ( 10.1007/s001140050709) [DOI] [PubMed] [Google Scholar]
- 15.Brown WL. 1968. An hypothesis concerning the function of the metapleural glands in ants. Am. Nat. 102, 188–191. ( 10.1086/282536) [DOI] [Google Scholar]
- 16.Tranter C, Hughes WOH. 2015. Acid, silk and grooming: alternative strategies in social immunity in ants? Behav. Ecol. Sociobiol. 69, 1687–1699. ( 10.1007/s00265-015-1980-3) [DOI] [Google Scholar]
- 17.Park OW. 1937. Testing for resistance to American foulbrood in honeybees. J. Econ. Entomol. 30, 504–512. ( 10.1093/jee/30.3.504) [DOI] [Google Scholar]
- 18.Rinderer TE, Harris JW, Hunt GJ, De Guzman LI. 2010. Breeding for resistance to Varroa destructor in North America. Apidologie 41, 409–424. ( 10.1051/apido/2010015) [DOI] [Google Scholar]
- 19.Rothenbuhler WC. 1964. Behaviour genetics of nest cleaning in honey bees. IV. Responses of F 1 and backcross generations to disease-killed brood. Am. Zool. 4, 111–123. [DOI] [PubMed] [Google Scholar]
- 20.Rothenbuhler WC. 1964. Behaviour genetics of nest cleaning in honey bees. I. Responses of four inbred lines to disease-killed brood. Anim. Behav. 12, 578–583. ( 10.1016/0003-3472(64)90082-X) [DOI] [PubMed] [Google Scholar]
- 21.Spivak M. 1996. Honeybee hygienic behaviour and defense against Varroa jacobsoni. Apidologie 27, 245–260. ( 10.1051/apido:19960407) [DOI] [Google Scholar]
- 22.Spivak M, Reuter G. 2001. Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behaviour. Apidologie 32, 555–565. ( 10.1051/apido:2001103) [DOI] [Google Scholar]
- 23.Oldroyd BP. 1996. Evaluation of Australian commercial honey bees for hygienic behaviour, a critical character for tolerance to chalkbrood. Aust. J. Exp. Ag. Anim. Husb. 36, 625–629. ( 10.1071/EA9960625) [DOI] [Google Scholar]
- 24.Al Toufailia HM, Amiri E, Scandian L, Kryger P, Ratnieks FLW. 2014. Towards integrated control of varroa: effect of variation in hygienic behaviour among honey bee colonies on mite population increase and deformed wing virus incidence. J. Apic. Res. 53, 555–562. ( 10.3896/IBRA.1.53.5.10) [DOI] [Google Scholar]
- 25.Locke B, Forsgren E, de Miranda JR. 2014. Increased tolerance and resistance to virus infections: a possible factor in the survival of Varroa destructor-resistant honey bees (Apis mellifera). PLoS ONE 9, e99998 ( 10.1371/journal.pone.0099998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schöning C, Gisder S, Geiselhardt S, Kretschmann I, Bienefeld K, Hilker M, Genersch E. 2012. Evidence for damage-dependent hygienic behaviour towards Varroa destructor-parasitised brood in the western honey bee, Apis mellifera. J. Exp. Biol. 215, 264–271. ( 10.1242/jeb.062562) [DOI] [PubMed] [Google Scholar]
- 27.Momot JP, Rothenbuhler WC. 1971. Behaviour genetics of nest cleaning in honey bees. V. Interactions of age and genotype of bees, and nectar flow. J. Apic. Res. 10, 11–21. ( 10.1080/00218839.1971.11099665) [DOI] [Google Scholar]
- 28.Oxley PR, Spivak M, Oldroyd BP. 2010. Six quantitative trait loci influence task thresholds for hygienic behaviour in honeybees (Apis mellifera). Mol. Ecol. 19, 1452–1461. ( 10.1111/j.1365-294X.2010.04569.x) [DOI] [PubMed] [Google Scholar]
- 29.Boutin S, Alburaki M, Mercier P-L, Giovenazzo P, Derome N. 2015. Differential gene expression between hygienic and non-hygienic honeybee (Apis mellifera L.) hives. BMC Genomics 16, 500 ( 10.1186/s12864-015-1714-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bigio G, Al Toufailia H, Ratnieks FLW. 2014. Honey bee hygienic behaviour does not incur a cost via removal of healthy brood. J. Evol. Biol. 27, 226–230. ( 10.1111/jeb.12288) [DOI] [PubMed] [Google Scholar]
- 31.Spivak M, Reuter GS. 1998. Performance of hygienic honey bee colonies in a commercial apiary. Apidologie 29, 291–302. ( 10.1051/apido:19980308) [DOI] [Google Scholar]
- 32.Pérez-Sato JA, Châline N, Martin SJ, Hughes WOH, Ratnieks FLW. 2009. Multi-level selection for hygienic behaviour in honeybees. Heredity 102, 609–615. ( 10.1038/hdy.2009.20) [DOI] [PubMed] [Google Scholar]
- 33.Harpur BA, Chernyshova C, Soltani A, Tsvetkov N, Mahjoorighasrodashti M, Xu Z, Zayed A. 2014. No genetic tradeoffs between hygienic behaviour and individual innate immunity in the honey bee, Apis mellifera. PLoS ONE 9, e104214 ( 10.1371/journal.pone.0104214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spivak M, Downey DL. 1998. Field assays for hygienic behaviour in honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 91, 64–70. ( 10.1093/jee/91.1.64) [DOI] [Google Scholar]
- 35.Boecking O, Spivak M. 1999. Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 30, 141–158. ( 10.1051/apido:19990205) [DOI] [Google Scholar]
- 36.Spivak M, Gilliam M. 1991. New ideas on the role of hygienic behaviour in disease resistance in honey bees. Am. Bee J. 131, 782. [Google Scholar]
- 37.Spivak M, Gilliam M. 1993. Facultative expression of hygienic behaviour in honey bees in relation to disease resistance. J. Apic. Res. 32, 147–157. ( 10.1080/00218839.1993.11101300) [DOI] [Google Scholar]
- 38.Boecking O. 1999. Sealing up and non-removal of diseased and Varroa jacobsoni infested drone brood cells is part of the hygienic behaviour in Apis cerana. J. Apicult. Res. 38, 159–168. ( 10.1080/00218839.1999.11101006) [DOI] [Google Scholar]
- 39.Ratnieks FLW. 1992. American foulbrood: a review of an important honey bee disease. Bee World 73, 177–191. ( 10.1080/0005772X.1992.11099136) [DOI] [Google Scholar]
- 40.Bigio G, Al Toufailia H, Hughes WOH, Ratnieks FLW. 2014. The effect of one generation of controlled mating on the expression of hygienic behaviour in honey bees. J. Apic. Res. 53, 563–568. ( 10.3896/IBRA.1.53.5.07) [DOI] [Google Scholar]
- 41.Spivak M, Reuter GS. 1998. Honey bee hygienic behaviour. Am. Bee J. 138, 283. [Google Scholar]
- 42.Spiltoir CR. 1955. Life cycle of Ascosphaera apis . Am. J. Bot. 42, 501–518. ( 10.1002/j.1537-2197.1955.tb11154.x) [DOI] [Google Scholar]
- 43.Jensen AB, Pedersen BV, Eilenberg J. 2009. Differential susceptibility across honey bee colonies in larval to chalkbrood resistance. Apidologie 40, 524–534. ( 10.1051/apido/2009029) [DOI] [Google Scholar]
- 44.Vojvodic S, Jensen AB, James RR, Boomsma JJ, Eilenberg J. 2011. Temperature dependent virulence of obligate and facultative fungal pathogens of honeybee brood. Vet. Microbiol. 149, 200–205. ( 10.1016/j.vetmic.2010.10.001) [DOI] [PubMed] [Google Scholar]
- 45.Aronstein KA, Murray KD, Saldivar E. 2010. Transcriptional responses in honey bee larvae infected with chalkbrood fungus. BMC Genomics 11, 391 ( 10.1186/1471-2164-11-391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bambrick JF, Rothenbuhler WC. 1961. Resistance to American foulbrood in honey bees. IV. The relationship between larval age at inoculation and mortality in a resistant and in a susceptible line. J. Invertebr. Pathol. 3, 381–390. [Google Scholar]
- 47.Brødsgaard CJ, Ritter W, Hansen H. 1998. Response of in vitro reared honey bee larvae to various doses of Paenibacillus larvae larvae spores. Apidologie 29, 569–578. ( 10.1051/apido:19980609) [DOI] [Google Scholar]
- 48.Lapidge KL, Oldroyd BP, Spivak M. 2002. Seven suggestive quantitative trait loci influence hygienic behaviour of honey bees. Naturwissenschaften 89, 565–568. [DOI] [PubMed] [Google Scholar]
- 49.Leclercq G, Pannebakker B, Gengler N, Nguyen BK, Francis F. 2017. Drawbacks and benefits of hygienic behavior in honey bees (Apis mellifera L.): a review. J. Apicult. Res. 56, 366–375. ( 10.1080/00218839.2017.1327938) [DOI] [Google Scholar]
- 50.Seeley TD, Smith ML. 2015. Crowding honeybee colonies in apiaries can increase their vulnerability to the deadly ectoparasite Varroa destructor. Apidologie 46, 716–727. ( 10.1007/s13592-015-0361-2) [DOI] [Google Scholar]
- 51.Neumann P, Blacquière T. 2016. The Darwin cure for apiculture? Natural selection and managed honeybee health. Evol. Appl. 10, 226–230. ( 10.1111/eva.12448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seeley TD. 2017. Darwinian beekeeping: an evolutionary approach to apiculture. Bees Dev. J. 122, 3–8. [Google Scholar]
- 53.Medina LM, Hart AG, Ratnieks FLW. 2009. Hygienic behaviour in the stingless bees Melipona beecheii and Scaptotrigona pectoralis (Hymenoptera: Meliponini). Genet. Mol. Res. 8, 571–576. ( 10.4238/vol8-2kerr010) [DOI] [PubMed] [Google Scholar]
- 54.Toufailia HM A, Alves D, Bento JMS, Marchini LC, Ratnieks FLW. 2016. Hygienic behaviour in Brazilian stingless bees. Biol. Open 5, 1712–1718. ( 10.1242/bio.018549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winston ML. 1991. The biology of the honey bee. Cambridge, MA: Harvard University Press. [Google Scholar]
- 56.Michener CD. 1974. The social behaviour of the bees. Cambridge, MA: Harvard University Press. [Google Scholar]
- 57.Nogueira-Neto P. 1997. Vida e criação de abelhas indígenas sem ferrão. São Paulo, Brazil: Editora Nogueirapis. [Google Scholar]
- 58.Seeley TD. 1978. Life history strategy of the honey bee, Apis mellifera. Oecologia 32, 109–118. ( 10.1007/BF00344695) [DOI] [PubMed] [Google Scholar]
- 59.Seeley TD. 2017. Life-history traits of wild honey bee colonies living in forests around Ithaca, NY, USA. Apidologie 48, 743–754. ( 10.1007/s13592-017-0519-1) [DOI] [Google Scholar]
- 60.Oldroyd BP, Thexton EG, Lawler SH, Crozier RH. 1997. Population demography of Australian feral bees (Apis mellifera). Oecologia 111, 381–387. ( 10.1007/s004420050249) [DOI] [PubMed] [Google Scholar]
- 61.Darwin C. 1859. On the origin of species by means of natural selection. London, UK: John Murray. [Google Scholar]
- 62.Ratnieks FLW, Foster KR, Wenseleers T. 2010. Darwin's special difficulty: the evolution of ‘neuter insects’ and current theory. Behav. Ecol. Sociobiol. 65, 481–492. ( 10.1007/s00265-010-1124-8) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.