Abstract
背景与目的
小细胞肺癌(small cell lung cancer, SCLC)是恶性程度极高的神经内分泌肿瘤,对放化疗敏感。目前,广泛期SCLC的一线标准化疗方案为铂类联合依托泊苷方案,但大多数接受一线化疗的患者在1年-2年内复发。一旦疾病复发,预后不良。本研究旨在研究广泛期SCLC总体和一线化疗的生存情况及其影响因素。
方法
收集2001年2月-2011年12月经病理学或细胞学确诊为广泛期的SCLC患者394例,采用Kaplan-Meier法计算总生存时间(overall survival, OS)和无进展生存时间(progression-free survival, PFS)并绘制生存曲线,单因素及Cox回归多因素分析各种因素对生存期的影响。
结果
全组中位OS为14.8个月,1年、2年、5年生存率分别为58.9%、27.2%、7.8%。全组OS与年龄(P=0.006)、ECOG评分(P=0.021)、肝转移(P < 0.001)、骨转移(P < 0.001)、是否化疗(P < 0.001)密切相关。一线化疗广泛期SCLC患者的中位OS为15.1个月,中位PFS为7.5个月。多因素分析结果显示一线化疗广泛期SCLC的OS与吸烟(P=0.041)、肝转移(P < 0.001)、骨转移(P < 0.001)、化疗疗程数(P < 0.001)相关;一线化疗PFS与吸烟(P=0.003)、肝转移(P=0.001)、骨转移(P < 0.001)、化疗疗程数(P < 0.001)相关。胸部放疗并非广泛期SCLC OS和PFS的独立影响因素。
结论
年龄 < 60岁、体能状况好、无肝、骨转移的广泛期SCLC患者预后更好。广泛期SCLC患者应积极进行化疗,一线化疗的化疗疗效达到部分缓解-完全缓解有益于生存;适合的化疗疗程数目是4-6疗程。胸部放疗在广泛期SCLC治疗中的作用需要进一步研究。
Keywords: 肺肿瘤, 广泛期, 生存, 影响因素
Abstract
Background and objective
Small cell lung cancer (SCLC) is the most malignant neuroendocrine tumor but highly sensitive to chemotherapy and radiotherapy. At present, the standard first-line chemotherapy regimen of extensive-stage SCLC is platinum combined etoposide regimen. However, most patients who receive first-line chemotherapy will relapse within one to two years. Once recurrent, it indicates poor prognosis. In this study, we analyzed the survival among all extensive-stage SCLC and patients who received first-line chemotherapy and determined prognostic factors.
Methods
Total of 394 patients who were diagnosed as extensive-stage small cell lung cancer from February 2001 to December 2011 hospitalized in Peking Union Medical College Hospital were collected. Kaplan-Meier method was used to calculate the overall survival (OS) and progression-free survival (PFS). Univariate analysis and Cox regression analysis were used to detect the influence factors of survival.
Results
The median OS of all extensive-stage small cell lung cancer was 14.8 months; 1-year, 2-year and 5-year survival rates were 58.9%, 27.2% and 7.8%, respectively. According to the results of univariate and Cox multivariate analysis, OS of extensive-stage SCLC was closely associated with age (P=0.006), ECOG PS (P=0.021), liver metastasis (P < 0.001), bone metastasis (P < 0.001) and chemotherapy (P < 0.001). The mortality risk of patients who didn't receive chemotherapy was 4.919 times higher than that who received; the mortality risk of patients without liver, bone metastasis was reduced by approximately 50 percent. The first-line chemotherapy was mainly EP (DDP+VP-16) or CE (CBP+VP-16) regimens (accounting for 82.8%) with 4-6 cycles. The median OS and PFS in first-line chemotherapy were 15.1 months and 7.5 months, respectively. The result of Cox regression analysis indicated that OS in first-line chemotherapy was remarkably related to smoking history (P=0.041), liver metastasis (P < 0.001), bone metastasis (P < 0.001), chemotherapy cycle number (P < 0.001); PFS was relevant with smoking history (P=0.003), liver metastasis (P=0.001), bone metastasis (P < 0.001), chemotherapy cycle number (P < 0.001). Thoracic radiotherapy was not an independent influence factor of OS and PFS in extensive-stage small cell lung cancer.
Conclusion
The patients who were younger than 60-year old, with good KPS, absence of liver and bone metastasis had better prognosis. Patients should receive chemotherapy with first-line standard regimen (CE/EP regimen). It was beneficial to survival if the effect of first-line chemotherapy was SD or PR-CR and the proper chemotherapy cycle number was 4-6 cycles. The role of thoracic radiotherapy in extensive-stage small cell lung cancer needed to be investigated further.
Keywords: Lung neoplasms, Extensive-stage, Survival, Prognostic factors
小细胞肺癌(small cell lung cancer, SCLC)是恶性程度极高的神经内分泌肿瘤,属于肺癌的未分化型,约占所有肺癌的15%[1]。初诊时约60%-70%的SCLC患者属于广泛期,30%-40%属于局限期。广泛期SCLC中位生存时间约10个月-14个月,5年生存率为6.5%[2, 3]。近30年虽然有新的化疗药物出现,但广泛期SCLC患者生存情况的改善不明显。1981至2008年所有广泛期SCLC的Ⅲ期临床数据显示,生存时间每年只提高了0.63天[4]。本文回顾性地分析了在北京协和医院住院治疗并于2001年2月-2011年12月经病理学或细胞学确诊的394例广泛期SCLC患者的临床资料,研究广泛期SCLC的总体和一线化疗的生存情况及其影响因素,旨在为临床治疗广泛期SCLC和判断预后提供一定的参考依据。
1. 资料与方法
1.1. 研究对象
收集于2001年1月-2011年12月在北京协和医院住院治疗的SCLC患者的临床资料。所有患者需符合以下条件:①年龄 > 18周岁;②经细胞学或组织病理学检查确诊为SCLC;③经胸腹部增强CT、全身骨扫描、头颅增强MRI检查,根据美国退伍军人肺癌研究组(Veterans Administration Lung Study Group, VALG)制定的分期方法判定为广泛期SCLC;④病历资料完整。
1.2. 研究方法
收集患者完整的临床资料,包括:患者的年龄、性别、吸烟情况和ECOG(Eastern Cooperative Oncology Group)评分;病理获取时间、分期、转移部位;化疗方案、最佳疗效、疾病进展时间、是否行胸部放疗;末次随访时间及死亡时间。采用电话形式进行随访,随访时间至患者死亡时间或2013年4月15日为止,存活时间以月为单位。
1.3. 评定标准
根据实体瘤疗效评价标准(Response Evaluation Criteria in Solid Tumor, RECIST)评估疗效,分为完全缓解(complete response, CR)、部分缓解(partial response, PR)、稳定(stable disease, SD)和进展(progressive disease, PD)。有效率(RR)=(CR+PR)/全部病例数×100%。敏感复发是指初始治疗有效且初始治疗结束到疾病进展的时间 > 90天;耐药复发是指初始治疗结束到疾病进展的时间≤90天。总生存时间(overall survival, OS)指从病理获取时间至患者死亡或末次随访时间(月)。无进展生存时间(progression-free survival, PFS)指从病理获取时间至疾病进展或患者死亡的时间或末次随访时间(月)。
1.4. 统计学处理
采用SPSS 18.0软件进行数据统计分析。采用Kaplan-Meier法计算患者的OS和PFS,并绘制生存曲线。采用单因素以及Cox回归多因素分析各种因素对生存期的影响。P < 0.05为差异有统计学意义。
2. 结果
2.1. 全组一般情况
查阅于2001年1月-2011年12月在北京协和医院住院治疗的SCLC患者的临床资料802例,根据实验对象入选标准,符合条件的广泛期SCLC患者共394例。全组患者年龄20岁-93岁,中位年龄为62岁。病理组织学上,经典SCLC 389例,混合癌5例。最常见的转移部位包括骨、胸腔积液、肝、脑和肾上腺。患者一般情况见表 1。
1.
全组广泛期小细胞肺癌患者的一般情况和总生存期的单因素分析
Clinical characteristics and univariate analysis of overall survival (OS) of all extensive-stage samll cell lung
| Factor | n (%) | OS (month) | 95%CI (month) | χ2 | P | |
| ECOG PS: Eastern Cooperative Oncology Group performance status | ||||||
| Sex | 0.918 | 0.338 | ||||
| Male | 300 (76.1) | 13.8 | 12.21-15.33 | |||
| Female | 94 (23.9) | 16.5 | 14.36-18.64 | |||
| Age (yr) | 6.936 | 0.008 | ||||
| ≥60 | 232 (58.9) | 13.2 | 10.45-16.02 | |||
| < 60 | 162 (41.1) | 15.4 | 13.66-17.14 | |||
| Smoking history | 2.269 | 0.132 | ||||
| Yes | 297 (75.4) | 13.4 | 11.30-15.45 | |||
| No | 97 (24.6) | 15.6 | 13.63-17.51 | |||
| ECOG PS | 18.963 | < 0.001 | ||||
| 0-1 | 304 (77.2) | 15.6 | 14.00-17.14 | |||
| 2-4 | 90 (22.8) | 9.9 | 5.90-13.96 | |||
| Brain metastasis | 0.435 | 0.510 | ||||
| Yes | 71 (18.0) | 11.8 | 5.92-17.68 | |||
| No | 323 (82.0) | 14.9 | 13.30-16.50 | |||
| Liver metastasis | 28.153 | < 0.001 | ||||
| Yes | 78 (20.0) | 9.3 | 7.60-11.00 | |||
| No | 316 (80.0) | 16.4 | 15.16-17.64 | |||
| Bone metastasis | 20.270 | < 0.001 | ||||
| Yes | 128 (32.5) | 10.0 | 8.26-11.80 | |||
| No | 266 (67.5) | 16.6 | 15.32-17.82 | |||
| Adrenal metastasis | 0.237 | 0.626 | ||||
| Yes | 62 (15.7) | 14.5 | 11.82-17.20 | |||
| No | 332 (84.3) | 14.8 | 13.12-16.54 | |||
| Malignant pleural fluid | 0.045 | 0.832 | ||||
| Yes | 104 (26.4) | 15.5 | 12.83-18.23 | |||
| No | 290 (73.6) | 14.5 | 12.79-16.28 | |||
| Chemotherapy | 41.372 | < 0.001 | ||||
| Yes | 369 (93.7) | 15.1 | 13.43-16.84 | |||
| No | 25 (6.3) | 1.6 | 0.62-2.64 | |||
| Thoracic radiotherapy | 14.017 | < 0.001 | ||||
| Yes | 164 (41.6) | 16.8 | 14.36-19.25 | |||
| No | 230 (58.4) | 12.3 | 9.93-14.73 | |||
2.2. 全组治疗情况
394例患者中25例未行化疗,369例接受化疗;164例患者进行了胸部放疗,91例患者在出现脑转移后进行了脑部放疗,8例行预防性全脑放射治疗。369例进行化疗患者的化疗方案包括EP或CE[顺铂(DDP)或卡铂(CBP)+依托泊苷(VP-16)]方案各75例和230例,其他化疗方案64例包括拓扑替康(TPT)+DDP方案12例、CODE[环磷酰胺(CTX)+阿霉素(ADM)/表阿霉素(EPI)+DDP+VP-16]方案10例、氨柔比星+DDP方案10例、紫杉醇(TAX)+DDP/CBP方案8例、替尼泊苷(VM26)+卡莫司汀(BCNU)方案4例、力比泰+DDP方案4例、口服VP-16方案4例、异环磷酰胺(IFO)+VP-16方案3例、吉西他滨(GEM)+DDP方案2例、伊立替康(CPT-11)+DDP方案2例、IFO+CBP方案1例、丝裂霉素(MMC)+DDP方案1例、VIP(VP-16+IFO+DDP/CBP)方案1例、CPE(CBP+TAX+VP-16)方案1例、COME[CTX+长春新碱(VCR)+甲氨蝶呤(MTX)+VP-16]方案1例。化疗1个-3个疗程者102例,4个-6个疗程者221例,7个疗程以上者46例;疗效PD者28例,SD者79例,PR/CR者197例,疗效不详者65例;化疗后耐药复发者158例,3个月-6个月复发者52例,6个月以上复发者31例,复发情况不详者128例;接受化疗的患者中126例接受了放射治疗。
2.3. 全组生存情况及其影响因素
全组的中位总生存时间为14.8个月。1年、2年、5年生存率分别为58.9%、27.2%、7.8%。分析性别、年龄、吸烟情况、ECOG评分、转移部位、是否进行化疗、是否进行胸部放疗对全组394例广泛期SCLC OS的影响。单因素分析的结果表明广泛期SCLC的OS与年龄、ECOG评分、肝转移、骨转移、是否化疗、是否胸部放疗密切相关,其中未进行化疗患者的死亡风险是进行化疗的患者的4.919倍,若无肝、骨转移则死亡风险下降约50%;与其他因素如性别、是否吸烟、脑转移、肾上腺转移、有无胸腔积液的关系无统计学意义(表 1)。多因素分析的结果表明年龄、ECOG评分、肝转移、骨转移和是否化疗是广泛期SCLC OS的独立影响因素(表 2)。单因素分析结果显示广泛期小细胞肺癌OS与是否进行胸部放疗有关,但多因素分析结果显示OS与是否进行胸部放疗无关。
2.
全组广泛期小细胞肺癌OS的多因素分析
Multivariate analysis of OS in all extensive-stage SCLC patients
| Factor | HR | 95%CI | Wald | P | |
| Compare with patients who were more than 60 years old, whose ECOG performance status 2-4, who were present with liver metastasis and bone metastasis and who received chemotherapy, respectively. | |||||
| Age (yr) | < 60 | 0.68 | 0.52-0.90 | 7.565 | 0.006 |
| ECOG PS | 0-1 | 0.71 | 0.52-0.96 | 5.295 | 0.021 |
| Liver metastasis | No | 0.49 | 0.36-0.66 | 21.247 | < 0.001 |
| Bone metastasis | No | 0.55 | 0.41-0.74 | 15.970 | < 0.001 |
| Chemotherapy | No | 4.92 | 2.77-8.75 | 29.375 | < 0.001 |
2.4. 一线化疗生存情况及其影响因素
接受一线化疗广泛期SCLC患者的中位OS为15.1个月。分析年龄、性别、ECOG评分、吸烟情况、不同转移部位、化疗方案、化疗疗效、化疗疗程数、是否进行胸部放疗、复发时间对一线化疗OS的影响。单因素分析的结果显示一线化疗广泛期小细胞肺癌的OS与年龄、ECOG评分、肝转移、骨转移、化疗疗效、化疗疗程数、胸部放疗、复发时间相关(表 3)。多因素分析的结果显示一线化疗广泛期SCLC的OS只与吸烟、骨转移、肝转移、化疗疗程数相关(表 4)。一线化疗的中位PFS为7.5个月。同样分析各因素对一线化疗PFS的影响。单因素和多因素分析的结果均显示一线化疗PFS与吸烟、肝转移、骨转移、化疗疗程数相关,单因素分析的结果显示一线化疗PFS与胸部放疗相关,但多因素分析的结果显示PFS与胸部放疗无关(表 3,表 5)。
3.
广泛期小细胞肺癌一线化疗OS和PFS的单因素分析
Univariate analysis of OS and PFS in extensive-stage SCLC patients who received first-line chemotherapy
| Factor | n | OS (95%CI) | χ2 | P | PFS (95%CI) | χ2 | P | ||
| EP: etoposide+cisplatin; CE: carboplatin+etoposide; PD: progressive disease; SD: stable disease; PR: partial response; CR: complete response. | |||||||||
| Sex | 2.520 | 0.112 | 4.058 | 0.044 | |||||
| Male | 285 | 14.3 (12.66-15.94) | 7.2 (6.70-7.70) | ||||||
| Female | 84 | 17.0 (13.19-20.75) | 7.8 (5.27-10.33) | ||||||
| Age (yr) | 5.406 | 0.020 | 0.268 | 0.605 | |||||
| ≥60 | 213 | 13.4 (10.81-15.93) | 7.3 (6.78-7.82) | ||||||
| <60 | 156 | 15.6 (13.71-17.43) | 7.7 (7.08-8.38) | ||||||
| Smoking history | 3.736 | 0.053 | 7.335 | 0.007 | |||||
| Yes | 280 | 14.1 (12.08-16.13) | 7.2 (6.62-7.78) | ||||||
| No | 89 | 16.8 (14.36-19.24) | 8.4 (5.87-10.99) | ||||||
| ECOG PS | 7.664 | 0.006 | 2.112 | 0.146 | |||||
| 0-1 | 297 | 15.6 (14.05-17.10) | 7.6 (6.89-8.31) | ||||||
| 2-4 | 72 | 13.0 (9.01-16.99) | 7.2 (6.42-7.92) | ||||||
| Brain metastasis | 1.369 | 0.242 | 0.013 | 0.909 | |||||
| Yes | 67 | 11.8 (5.92-17.68) | 7.6 (6.92-8.22) | ||||||
| No | 302 | 15.4 (13.71-17.09) | 7.4 (6.89-7.80) | ||||||
| Liver metastasis | 22.794 | < 0.001 | 7.879 | 0.005 | |||||
| Yes | 71 | 9.4 (7.61-11.19) | 6.9 (5.10-8.64) | ||||||
| No | 298 | 16.5 (15.23-17.77) | 7.7 (7.14-8.32) | ||||||
| Bone metastasis | 23.212 | < 0.001 | 20.231 | < 0.001 | |||||
| Yes | 118 | 10.2 (8.33-12.01) | 5.4 (4.21-6.53) | ||||||
| No | 251 | 16.8 (15.03-18.57) | 8.1 (7.33-8.81) | ||||||
| Adrenal metastasis | 0.109 | 0.741 | 0.694 | 0.405 | |||||
| Yes | 61 | 14.9 (11.35-18.45) | 7.9 (6.96-8.90) | ||||||
| No | 308 | 15.4 (13.44-17.30) | 7.5 (6.98-7.96) | ||||||
| Malignant pleural fluid | 0.827 | 0.363 | 0.800 | 0.371 | |||||
| Yes | 95 | 15.6 (13.01-18.13) | 7.6 (6.81-8.39) | ||||||
| No | 274 | 14.8 (12.99-16.67) | 7.5 (6.93-8.01) | ||||||
| Chemotherapy regimen | 4.956 | 0.084 | 0.716 | 0.699 | |||||
| CE regimen | 230 | 14.9 (13.12-16.68) | 7.7 (6.95-8.45) | ||||||
| EP regimen | 75 | 17.4 (13.23-21.51) | 7.2 (6.14-8.26) | ||||||
| Other regimens | 64 | 11.5 (9.20-13.80) | 7.4 (6.40-8.34) | ||||||
| Curative effect | 62.003 | < 0.001 | 145.099 | < 0.001 | |||||
| PD | 28 | 5.9 (3.48-8.32) | 2.0 (1.39-2.61) | ||||||
| SD | 79 | 13.4 (9.82-16.92) | 5.9 (4.28-7.52) | ||||||
| PR/CR | 197 | 16.6 (15.21-17.93) | 8.2 (7.51-8.96) | ||||||
| Unknown | 65 | 10.0 (7.20-12.80) | 8.4 (5.23-11.63) | ||||||
| Cycle number | 67.903 | < 0.001 | 84.932 | < 0.001 | |||||
| 1-3 | 102 | 7.7 (5.62-9.78) | 3.2 (2.53-3.93) | ||||||
| 4-6 | 221 | 16.4 (14.93-17.87) | 7.6 (7.08-8.06) | ||||||
| ≥7 | 46 | 16.6 (14.50-18.64) | 10.6 (8.90-12.36) | ||||||
| Thoracic radiotherapy | 14.557 | < 0.001 | 10.744 | 0.001 | |||||
| Yes | 164 | 18.6 (14.51-22.75) | 8.9 (7.80-9.95) | ||||||
| No | 13.1 (10.94-15.20) | 6.9 (6.00-7.74) | |||||||
| Recurrence status | 37.415 | < 0.001 | 170.031 | < 0.001 | |||||
| Resistant | 158 | 11.6 (9.96-13.31) | 5.1 (4.71-5.54) | ||||||
| 3-6 months | 52 | 18.5 (14.38-22.68) | 12.8 (11.52-14.14) | ||||||
| ≥6 months | 31 | 24.9 (11.30-38.56) | 13.2 (8.96-17.51) | ||||||
| Unknown | 128 | 13.2 (8.96-17.51) | 8.4 (7.61-9.25) | ||||||
4.
广泛期小细胞肺癌一线化疗OS的多因素分析
Multivariate analysis of OS in extensive-stage SCLC patients who received first-line chemotherapy
| Factor | HR | 95%CI | Wald | P | |
| Compare with patients who did not smoke, who were absence of bone metastasis and liver metastasis, whose cycle number was 1-3, respectively. | |||||
| Smoking | Yes | 1.39 | 1.01-1.91 | 4.156 | 0.041 |
| Liver metastasis | Yes | 2.20 | 1.57-3.07 | 20.952 | < 0.001 |
| Bone metastasis | Yes | 1.70 | 1.26-2.28 | 12.27 | < 0.001 |
| Cycle number | 43.771 | < 0.001 | |||
| ≥7 | 0.22 | 0.14-0.36 | 37.592 | < 0.001 | |
| 4-6 | 0.39 | 0.28-0.55 | 29.696 | < 0.001 | |
5.
广泛期小细胞肺癌一线化疗PFS的多因素分析
Multivariate analysis of PFS in extensive-stage SCLC patients who received first-line chemotherapy
| Factor | HR | 95%CI | Wald | P | |
| Compare with patients who did not smoke, who were absence of liver metastasis and bone metastasis, whose cycle number was 1-3, respectively. | |||||
| Smoking history | Yes | 1.52 | 1.16-2.01 | 8.878 | 0.003 |
| Liver metastasis | Yes | 1.63 | 1.22-2.20 | 10.549 | 0.001 |
| Bone metastasis | Yes | 1.64 | 1.28-2.11 | 14.988 | < 0.001 |
| Cycle number | 47.159 | < 0.001 | |||
| ≥7 | 0.25 | 0.17-0.38 | 44.261 | < 0.001 | |
| 4-6 | 0.48 | 0.36-0.64 | 10.262 | 0.001 | |
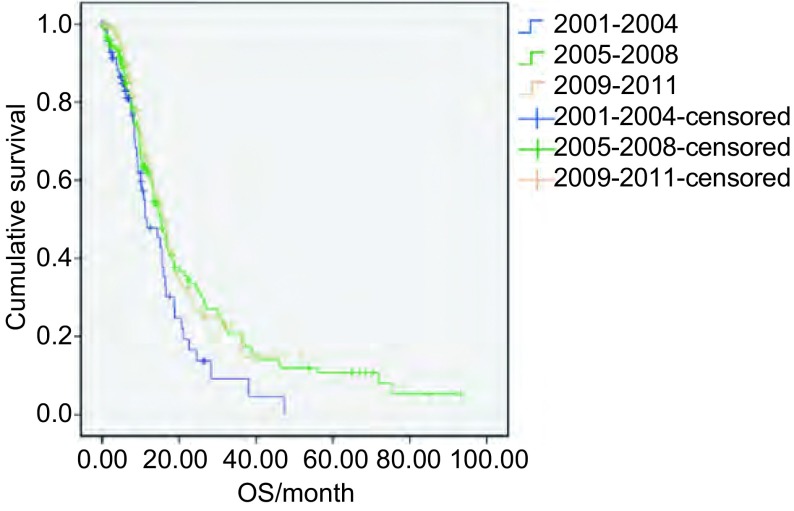
2.5. 不同年份一线化疗OS的比较
将369例进行化疗的患者按诊断时间的不同分为3组,即2001年-2004年组80例、2005年-2008年组152例、2009年-2011年组137例,3组的OS和95%可信区间分别为11.6(6.55-16.72)、15.0(12.56-17.44)和15.6(13.04-18.10)个月,无明显统计学差异(χ2=4.338, P=0.144)。3组的生存曲线见图 1。
1.
不同年份广泛期小细胞肺癌一线化疗OS的比较
Comparison of OS in extensive-stage SCLC patients who received first-line chemotherapy of different years. OS: overall survival; SCLC: small cell lung cancer.
3. 讨论
本研究全组中位OS为14.8个月,1年、2年、5年生存率分别为58.9%、27.2%、7.8%,一线化疗中位OS为15.1个月,中位PFS为7.5个月,与文献报道基本一致[2, 3],另外,3个时间段OS的比较提示广泛期SCLC的生存情况未得到明显改善。全组OS与年龄、ECOG评分、肝转移、骨转移、是否化疗密切相关。一般SCLC多于中老年发病,该研究394例患者的年龄跨度为20岁-93岁,中位年龄为62岁。年龄 < 60岁死亡风险较年长者下降32%,这可能与年龄较轻的患者一般状况较好、合并的全身性疾病少,更能耐受化疗有关。多项研究显示ECOG评分明显与SCLC的预后相关[5],该研究同样证实了该观点。广泛期SCLC的治疗核心是化疗,未接受化疗的广泛期SCLC的生存期只有1个月-3个月,化疗比最佳支持治疗(best supportive care, BSC)能延长生存期[6]。本研究中进行化疗的患者的中位总生存时间为15.1个月,未进行化疗的中位总生存时间为1.6个月,未进行化疗患者的死亡风险是进行化疗的患者的4.919倍,故广泛期SCLC患者应积极接受化疗。另一个影响预后的因素是转移部位,有研究称肝转移与预后明显相关[3],而且转移部位越多预后越差[7],本研究证实若无肝、骨转移则死亡风险下降约50%。
若SCLC患者接受一线化疗,则OS、PFS都与化疗疗效、化疗疗程数相关,与化疗方案无关。首先,化疗疗效是预测广泛期SCLC预后的重要指标。本研究达到PR/CR患者的中位OS和PFS分别为16.6个月和8.2个月,PD的中位OS和PFS分别为5.9个月和2.0个月,即PR/CR患者较PD患者的OS延长1年、PFS延长半年,死亡风险下降62%,疾病进展风险下降85%,故如果化疗疗效能达到PR将有益于生存。这与临床经验相一致,因为如果疗效达到PR说明患者对化疗敏感,而如果是PD则提示耐药。而达到SD的患者的OS为13.4个月,与PR/CR患者的OS比较无统计学意义(χ2=5.512, P=0.019 > α’=0.05/6=0.008),即达到SD患者的生存时间并没有明显差于PR/CR的患者。其次,化疗疗程数同样可以影响OS和PFS。单因素结果显示化疗1个-3个疗程者的OS和PFS只有7.7个月和3.2个月,因为一般化疗1个-3个疗程者一般状况较差或者对化疗药物不敏感;化疗4-6疗程和≥7疗程的中位OS差别不大(χ2=2.706, P=0.100),而两者的中位PFS有明显差异(χ2=14.580, P < 0.001);Bozcuk[8]的meta分析也提示增加化疗周期(即维持治疗)只能增加中位进展时间,却没能明显改善生存,故适合的化疗疗程数是4个-6个疗程。值得注意的是胸部放疗在全组OS、一线化疗的OS和PFS的单因素和多因素分析结果是不一致的,有关胸部放疗在广泛期SCLC治疗中的作用有待进一步研究。
综上所述,年龄 < 60岁、体能状况好、无肝、骨转移的广泛期SCLC患者预后更好。广泛期SCLC患者应积极进行化疗,一线化疗的化疗疗效达到SD、PR-CR有益于生存;适合的化疗疗程数目是4个-6个疗程。
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Navada S, Lai P, Schwartz AG, et al. Temporal trends in small cell lung cancer: Analysis of the national Surveillance, Epidemiology, and End-Results (SEER) database. J Clin Oncol. 2006;24(18):384s–384s. [Google Scholar]
- 3.Wu C, Li F, Jiao SC. Prognostic factors for survival of patients with extensive stage small cell lung cancer--a retrospective single institution analysis. Asian Pac J Cancer Prev. 2012;13(10):4959–4962. doi: 10.7314/APJCP.2012.13.10.4959. [DOI] [PubMed] [Google Scholar]
- 4.Oze I, Hotta K, Kiura K, et al. Twenty-seven years of phase Ⅲ trials for patients with extensive disease small-cell lung cancer: disappointing results. PLoS One. 2009;4(11):e7835. doi: 10.1371/journal.pone.0007835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster NR, Mandrekar SJ, Schild SE, et al. Prognostic factors differ by tumor stage for small cell lung cancer: a pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2009;115(12):2721–2731. doi: 10.1002/cncr.v115:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelayo Alvarez M, Gallego Rubio O, Bonfill Cosp X, et al. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst Rev. 2009;(4):CD001990. doi: 10.1002/14651858.CD001990. [DOI] [PubMed] [Google Scholar]
- 7.Bremnes RM, Sundstrom S, Aasebo U, et al. The value of prognostic factors in small cell lung cancer: results from a randomised multicenter study with minimum 5 year follow-up. Lung Cancer. 2003;39(3):303–313. doi: 10.1016/S0169-5002(02)00508-1. [DOI] [PubMed] [Google Scholar]
- 8.Bozcuk H, Artac M, Ozdogan M, et al. Does maintenance/consolidation chemotherapy have a role in the management of small cell lung cancer (SCLC)? A metaanalysis of the published randomized controlled trials. Cancer. 2005;104(12):2650–2657. doi: 10.1002/cncr.21540. [DOI] [PubMed] [Google Scholar]