Abstract
Objective(s):
Dendritic cells (DCs) play a critical role in activation of T cell responses. Induction of type1 T helper (Th1) immune response is essential to generate protective immunity against cutaneous leishmaniasis. The intrinsic tendency of liposomes to have interaction with antigen-presenting cells is the main rationale to utilize liposomes as antigen carriers. In the present study, the effect of lipid phase transition temperature on DCs maturation and liposome uptake by murine bone marrow derived dendritic cells and human monocyte derived dendritic cells was investigated.
Materials and Methods:
Two cationic liposomal formulations consisting of DOTAP and DSPC/DOTAP were prepared and contained soluble leishmania antigen. Liposomes were incubated with immature or mature DCs derived from bone marrow (BMDCs) of C57BL/6 (which are resistant to cutaneous leishmaniasis), BALB/c mice (susceptible to cutaneous leishmaniasis) or DCs derived from human monocytes (MoDCs). The expression of DCs co-stimulatory markers and liposomal uptake were evaluated by flow cytometry method.
Results:
DCs which were encountered to liposomes consisting of DSPC showed significantly more expression of co-stimulatory molecules in cells from both human and C57BL/6 mice but not in cells from BALB/c mice.
Conclusion:
It is concluded that cationic liposomes consisting of DSPC are an effective adjuvant for antigen delivery in case of MoDCs and BMDCs from C57BL/6 mice. Moreover, DCs from different origins act differently in uptake of liposomes.
Keywords: Cationic liposomes, Dendritic cells, Immune response, Leishmaniasis, Phase transition - temperature, Th1
Introduction
Dendritic cells (DCs) are a group of professional antigen-presenting cells (APCs) and have an essential role in activating T lymphocytes (1, 2). DCs are present in two different forms of immature and mature state and have special capabilities in each state (3). Immature DCs do not present co-stimulatory signals that are essential to activate T cells but are capable of antigen uptake by means of phagocytosis, endocytosis and/or micropinocytosis. This process together with exposure to cytokines can result in maturation of DCs, which is characterized by antigen uptake and processing down-regulation. Mature DCs migrate to the local draining lymphoid tissue and simultaneously their antigen-presenting function is upregulated. This maturation process can be specified by overexpression of cell surface markers such as co-stimulatory molecules like CD80, CD86 and major histocompatibility complex class II (MHC-II) molecules. In human DCs, CD83 is also overexpressed and is the marker of cell activation (4). In Leishmaniasis, the inducing of Th1type of immune response results in resistance to the infection. In vitro maturation of DCs by some stimuli such as LPS will develop a Th1 immune response rather than Th2 which is partly related to IL-12 release by DCs (3).
DCs intrinsic capacity in antigen take up and processing can be used for the antigen’s targeted delivery to these APCs. It has been demonstrated that antigens encapsulated within particles are better presented by DCs rather than in solution (4). Among lipid-based nanoparticles, liposomes are used widely because of their low immunogenicity and toxicity. Liposomes are lipid-bilayer vesicles that can be used as important delivery systems to carry different types of antigens due to their high versatility (5-7). Some studies indicated that liposomes have a striking tendency to interact with APCs and this is one of the main reasons for utilizing them as carriers of antigens to induce antibody and T cell responses (5, 6, 8). Physical or chemical properties of the liposomes influence their function as carriers of antigens. Surface charge is of high importance in their adjuvant mechanism and electrostatic interaction with DCs (9-11). As the cellular membrane has a negative net charge, cationic liposomes are well suited to enhance antigen uptake by APCs (10). As well as surface charge, fluidity of lipid bilayer, the other physical characteristic of liposomes, can affect maturation of DCs (12). Current studies demonstrate that the more rigid (higher gel-liquid crystal transition temperature (Tm)) the liposomes are, the more potent antibody and cell-mediated responses are induced (7).
Infection with Leishmania parasites continues to be a major problem in public health of some countries and causes significant mortality and morbidity especially in the poorest people. In leishmaniasis, induction of Th1 response, CD8+ T cell activation and production of high levels of IFN-γ accompanies with treatment. In contrast, a Th2 immune response is associated with the susceptibility to the disease (13, 14). First generation vaccines which are killed parasites have shown limited efficacy in trials and furthermore, distinct molecules have not yet reached phase 3 trials (15). Leishmania vaccine’s limited efficacy is partly because of lack of a suitable adjuvant. Soluble Leishmania antigen (SLA) as a first generation Leishmania vaccine was used in the current study as a model antigen. SLA which is a cocktail of soluble antigens is more potent in stimulating T- cells and producing IFNγ than each of its fractions alone (16). A proper cationic SLA liposomal formulation can induce a Th1 immune response and cause protection against Leishmania infection (8, 17). Incorporation of SLA into cationic liposomes might be the main reason for effective targeting to and stimulating DCs and results in protection against Leishmania infection. To assess this idea, we studied the extent of liposomal SLA uptake by DCs and their maturation in vitro.
Several murine models are used for leishmania major infection and are functional for inducing Th1 or Th2 responses (18). A Th1 response in mice can result in self-healing of wounds whereas Th2 activation is associated with disease progression (1, 3). Most inbred mice like C57BL/6, are genetically resistant to L. major infection and resolve it. This self-healing phenotype is because of generated Th1 immune response (19). Mouse strains such as BALB/c are susceptible in contrast and induce Th2 immune response which lead to progression of disease and finally death (20). It has become obvious that production of Th1 associated cytokines, IL-12 and IFNγ, are essential for protection against intracellular pathogens including Leishmania parasites, while Th2 activation produces IL-4, IL-5, IL-6, IL-10 and IL-13 is accompanied with humoral immunity (21).
In the present study, two cationic liposomal formulations with low and high Tm which had shown Th1 response results in previous studies (7, 12, 17, 22, 23) were selected in order to investigate their effects on DCs maturation and uptake by murine bone marrow derived dendritic cells (BMDCs) as well as human monocyte derived dendritic cells (MoDCs) in vitro. Moreover, as our knowledge, the uptake study and maturation markers of DCs in susceptible/resistant murine and human models of leishmaniasis are studied together, for the first time.
Materials and Methods
Materials
All lipids including 1,2-distearoyl-sn-glycero-3-phos-phocholine (DSPC; 20 mM), cholesterol (CHOL; 10 mM) and N-[1-(2,3-Dioleoyloxy) propyl]-N,N,N- trimethyl ammonium methyl-sulfate (DOTAP; 20 mM) were purchased from Avanti Polar Lipid (Alabaster, USA). The fluorescent lipophilic dyes, 1,1_-dioctadecyl-3,3,3_,3_-tetramethylindocarbocyanine perchlorate (DiI) and 1,1_-dioctadecyl-3,3,3_,3_ tetramethylindodicarbocyanine (DiD) were purchased from Invitrogen (USA). Bovine serum albumin (BSA) was from Fluka (USA). 4-(2-hyd roxyethyl)-1 piperazineethanesulfonic acid (HEPES), Iscove’s Modified Dulbecco’s Medium (IMDM), Roswell Park Memorial Institute (RPMI) 1640 medium and phosphate-buffered saline (PBS; 155 mM NaCl, 1.5 mM potassium phosphate monobasic, 2.7 mM sodium phosphate dibasic, pH 7.2) were from Sigma (USA), human granulocyte macrophage colony-stimulating factor (GM-CSF), murine GM-CSF, human IL-4 and murine IL-4 were from R&D (USA). L-Glutamine was from Biosera. Anti-murine FITC conjugated CD11c and PE conjugated CD40, CD80, CD86, MHC-II and anti-human FITC conjugated CD14 and PE conjugated CD83, CD86 and HLA-DR antibodies were from BD Biosciences (USA). Lipopolysaccharide (LPS) was from Sigma (USA). Fetal calf serum (FCS) were purchased from Invitrogen (USA). CD14 human microbeads (Cat. No.#130-050-201) were purchased from Miltenyi Biotec (Germany).
Preparation of soluble leishmania antigen (SLA)
SLA preparation was carried out using the protocol developed by Scott et al. with minor modifications (24). Briefly, the parasites were harvested at stationary phase and washed 4 times using HEPES-sucrose buffer (10 mM, sucrose 10% w/v, pH7.4). Then, the number of promastigotes was adjusted to 1.2 × 109/ml in buffer solution containing enzyme inhibitor cocktail, 50 μl/ml (Sigma, USA). Then, the parasites were lysed using freeze-thaw method followed by probe sonication in an ice bath. The supernatant of centrifuged lysate parasites was collected, dialyzed against buffer solution, sterilized using a 0.22 μm membrane and stored at -70 °C until use. The protein concentration of the preparation was determined using BCA protein assay method (Thermo Scientific, USA).
Preparation of liposomes
Liposomes containing SLA were prepared using lipid film hydration method. Briefly, two liposomal formulations consisting of (DOTAP/cholesterol) (1:1 molar ratio) as a low Tm formulation and (DSPC/DOTAP/cholesterol) (3:1:1 molar ratio) as a high Tm formulation with DiI or DiD (0.2 mol% of phospholipid as a florescent label) were dissolved in chloroform: methanol (2:1, v/v) in a sterile tube. The solvent was then removed by rotary evaporation (Heidolph, Germany) resulting in deposition of a thin lipid film on the tube’s wall. The lipid film was then freeze-dried (TAITEC, Japan) overnight to ensure complete removal of the solvent. The film was hydrated and dispersed in sterile buffer (10 mM HEPES, 10% sucrose, pH 7.4) containing SLA (1 mg/ml) at 65 °C. The resultant multilamellar dispersions were vortexed and reduced in size and lamellarity by soniaction using bath sonicator (Bandelin, Germany), at 60 °C. Liposomes were then extruded 13 times through 400 and 200 nm polycarbonate filters, respectively, using the Mini Extruder (Avestin, Canada) to make liposomes with the size of around 200 nm. Dialysis against buffer was done using dialysis bags (cut off 300 kD) in order to separate the un-entrapped SLA from liposomal one.
Characterization of liposomes
Liposome size and poly dispersity index (PDI) were determined by dynamic light scattering (DLS) using a particle size analyzer (Malvern Instruments, UK). The zeta potential of liposomes was determined after a 20-fold dilution in HEPES buffer.
The concentration of SLA entrapped in liposomes was determined using BCA protein assay kit (Thermo Scientific, USA) using bovine serum albumin as a standard. The phospholipid content of the liposomes was also determined according to Bartlett method (25).
Culture of murine BMDCs
Femurs and tibiae of 6–8 weeks old female C57BL/6 and BALB/c mice were removed and completely purified from the surrounding muscle tissues. Thereafter, intact bones were left in 70% ethanol for 2–5 min for disinfection and washed with IMDM media. Then both ends were cut with scissors and the marrow flushed with IMDM using a syringe with 26- gauge needle. The bone marrow cells were centrifuged 5 min at 234 ×g at room temperature. The top part containing most of the platelets were carefully removed. The cells (3×106 cells per well) were seeded into a 6-well tissue culture plate containing 3.5 ml of complete culture medium consisting IMDM (ISCOVE) supplemented with 10% FCS, 100 U/ml penicillin and 100 g/ml streptomycin, L-Glutamine 2 mM, GM-CSF (25 ng/ml) and IL-4 (5 ng/ml) per well and incubated 3 days at 37 °C.
At day 3, culture supernatant containing stimulating factors was collected and added to fresh complete culture medium (21 ml). The cells was seeded into two 6-well tissue culture plates and incubated 2 days at 37 °C.
At day 5, cells were collected in a sterile test tube. Using complete fresh medium, cells were reseeded in two 12 –well plate tissue culture plates (250000 cells/500µl/well). LPS (1 µg/ml) was added to one of the plates and incubated at 37 °C for a further 24 hr to generate mature BMDCs. Liposomal formulations (100 µM) were added to the other plate containing immature BMDCs and incubated 1 day at 37 °C, 5% CO2. Buffer (HEPES 10 mM, 10% sucrose, pH 7.4) was used as negative control. At day 6, mature BMDCs were incubated with liposomal formulations (50 µl) for another 24 hr at 37 °C, 5% CO2.
For flow cytometry analysis, immature and mature DCs were incubated with different formulations, harvested 24 hr later, i.e. at day 6 for immature and at day 7 for mature ones.
Culture of human MoDCs
Peripheral blood was obtained from the local blood bank as standard buffy coat preparations from normal donors. MoDCs were generated from magnetic cell-sorted CD14+ monocytes (>95% purity) purified from human peripheral blood mononuclear cells (PBMCs) (14). Briefly, PBMCs were isolated by density gradient centrifugation and subjected to magnetic cell sorting using CD14 microbeads, an MS column and a miniMACS™ seperator Immature MoDCs were prepared by culturing CD14+ monocytes (1×106 cells/well in 6-well plates) in RPMI 1640 supplemented with 10% (v/v) fetal calf serum (FCS), 100 U/ml penicillin and 100 g/ml streptomycin in the presence of 800 U/ml GM-CSF and 1000 U/ml IL-4 and cultured for 5 days in a CO2 incubator; refreshment of the culture was done on day 3. Mature MoDCs were generated by the addition of LPS (100 ng/ml) on day 5 following incubation for a further 24 hr. Liposomes (100 µM) were added directly to the wells (250000 cells/500 µl/well) and the mixtures were incubated one day at 37 °C. flowcytometric analysis was done at day 7.
Flow cytometry analysis
Harvested DCs from cultures were centrifuged and resuspended in cell-staining buffer (sterile PBS, 2% FCS). Cells, 105 cells per each tube, were incubated directly with florescent labeled mAbs for 30 min at 4 °C and then washed to remove unbounded mAbs. The live population was gated based on size and granularity and then only CD11c+ BMDCs were considered for the evaluation of the expression of CD40, CD80, CD86, and MHC-II. Moreover, in human DCs, CD14+ MoDCs were considered for the evaluation of the expression of CD86, CD83 and HLA-DR.
Cells were analyzed by flow cytometry using a FACS Calibur flow cytometer and Cell Quest software (BD Biosciences) that can be used to assess cell-associated fluorescence and examine interaction of DiI or DiD-labelled liposomes with DCs. The Flowjo V.10 software was used for further analysis of FACS-data.
Statistical analysis
Data were analyzed using PRISM software. One-way ANOVA statistical test was used to assess the significance of differences among various groups. In case of significant P-value, multiple comparison Tukey test was used to compare the means of different groups. Results with P<0.05 were considered to be statistically significant.
Results
Characterization of liposomes
The composition and characterization of liposomal formulations are summarized in Table 1. DOTAP liposomes had a mean particle size of 248 nm and DOTAP/DSPC formulation showed 237 nm in size. The polydispersity index (PDI) of liposome formulations was 0.1 and thus both formulations were homogenous. The zeta potential of DOTAP formulation was 47 mV compared to DSPC formulation which was 55 mV. The entrapment efficacy for SLA was 51.73% in DOTAP liposomes and 58.56% in DSPC liposomes. The phosphate content for DSPC liposomes was 8.79 mM. We couldn’t do phosphate assay for DOTAP formulation because of phosphorous absence in formulation.
Table 1.
Characteristics of Liposomes
| Name | Composition (lipid molar ratio) | Z average (nm) | Pdi | Zeta potentiala (mv) | SLA entrapment efficacy (%) | Phosphate content mM |
|---|---|---|---|---|---|---|
| DOTAP | DOTAP/CHOL/SLA (4:4) | 248.20±16.43 | 0.17±0.43 | 47.23±7.10 | 51.73 | - |
| DOTAP/DSPC | DSPC/DOTAP/CHOL/SLA (12:4:4) | 237.43±17.51 | 0.14±0.56 | 55.76±5.48 | 58.56 | 8.79 |
Flow cytometric analysis of liposome uptake by DCs
The uptake of DiD or DiI labeled liposomes by DCs was assessed as shown in Figure 1 and Figure 2, respectively. In C57BL/6 mice, uptake of DSPC liposomes was significantly higher than DOTAP liposomes (P < 0.0001) in both immature and mature states. In contrast, in BALB/c mice, uptake of DOTAP liposomes was higher than DSPC (P<0.0001) In both immature and mature states. Interestingly, human MODCs showed different uptake behavior. There was no significant difference between DOTAP formulation and DSPC ones in immature state. In mature state, uptake of DSPC ones was significantly higher than DOTAP ones. It seems that uptake process by DCs was dependent on the DCs origin. However, there was no significant difference between immature and mature DCs in their uptake behavior among different DCs origins in this study.
Figure 1.
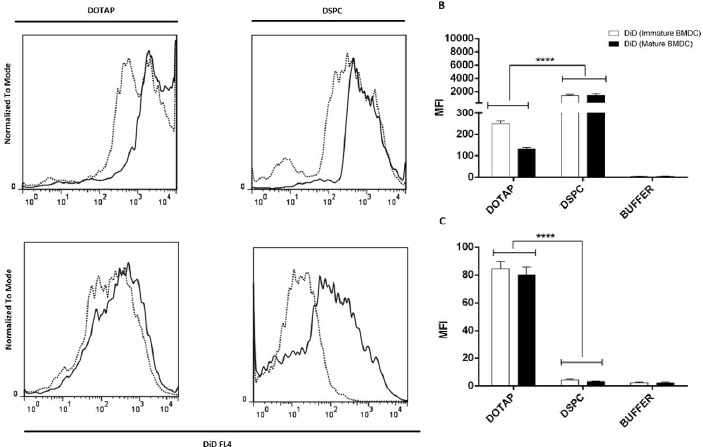
Flow cytometric analysis of DiD uptake in immature and mature murine DCs. (A) Histograms comparing DiD uptake in immature and mature states; Solid line is indicative of immature state, dotted line is indicative of mature ones. (B) DiD uptake in BMDCs from C57BL/6 mice presented by mean flourcent intensity value (MFI). (C) DiD uptake in BMDCs from BALB/C mice presented by MFI value
Figure 2.
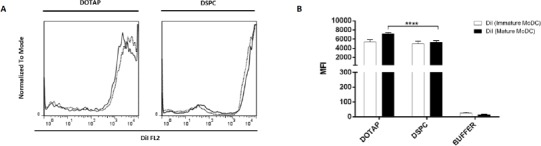
Flow cytometric analysis of DiI uptake in immature and mature human DCs. (A) Histograms comparing DiI uptake in immature and mature states; solid line is indicative of immature state, dotted line is indicative of mature ones. (B) DiI uptake in MoDCs presented by MFI value
Flow cytometric analysis of cell surface marker’s expression on DCs
As shown in Figure 3 and Figure 4, differentiation of cells to DCs and their maturation process was done successfully.
Figure 3.
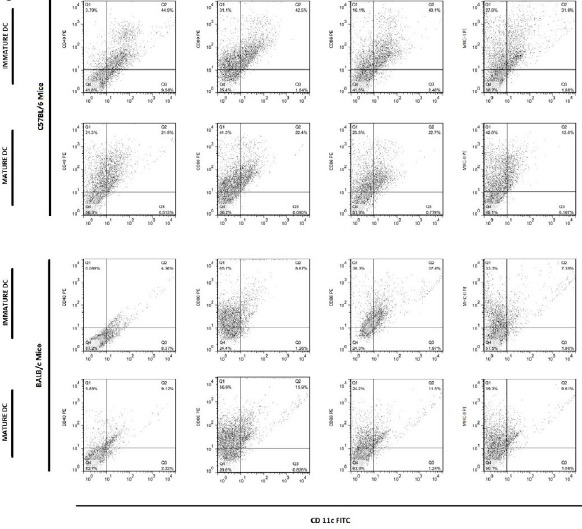
Flow cytometric analysis comparing murine DCs in immature and mature states. Immature DCs have been matured by LPS
Figure 4.
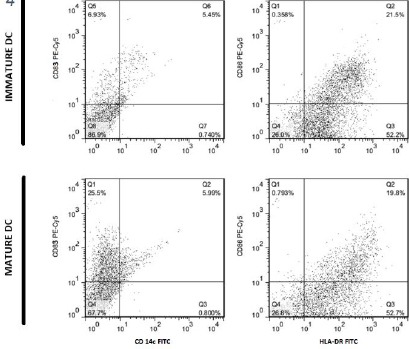
Flow cytometric analysis comparing human DCs in immature and mature states, before encountering with liposomal formulations
Dot plots obtained from murine (C57BL/6 and BALB/c mice) and human DCs are presented in Figure 5, 6 and 7, respectively. Expression of cell surface markers was upregulated upon maturation in DCs originated from C57BL/6 mice and human MoDCs. In BALB/c mice a different behavior was observed.
Figure 5.
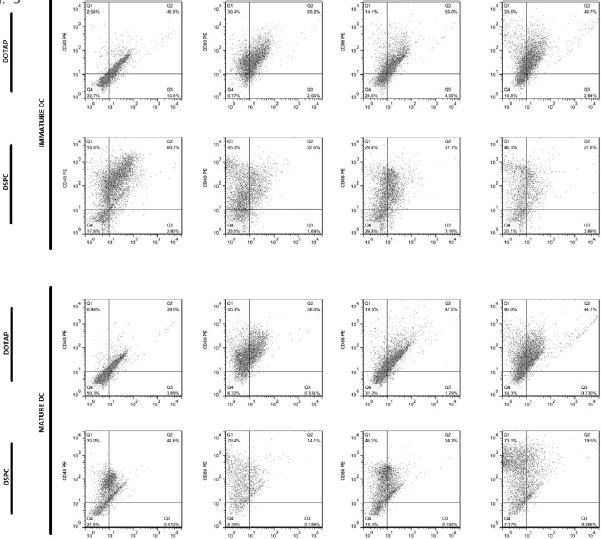
Dot plots showing flow cytometric analysis of cell surface marker’s expression on immature and mature BMDCs from C57BL/6 mice, after overnight incubation with liposomal forulations at 37 °C
Figure 6.
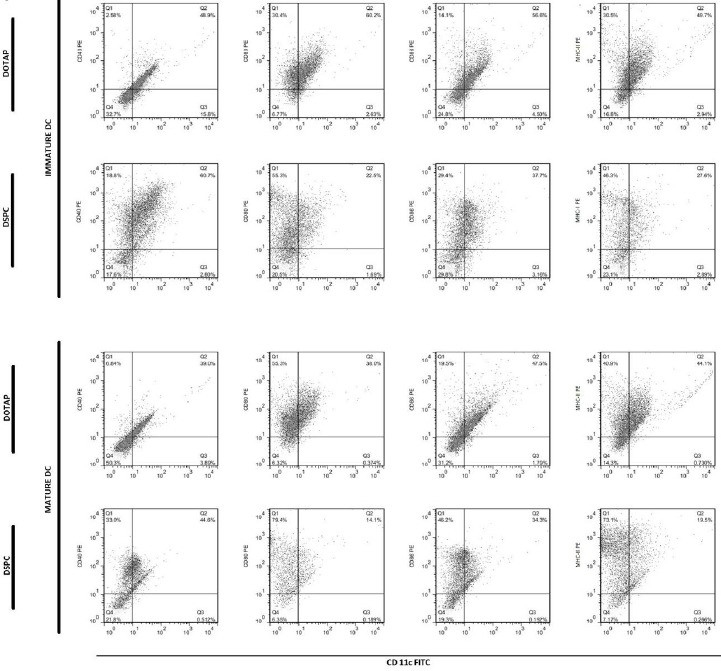
Dot plots showing flow cytometric analysis of cell surface marker’s expression on immature and mature BMDCs from BALB/c mice, after overnight incubation with liposomal forulations at 37 °C
Fig. 7.
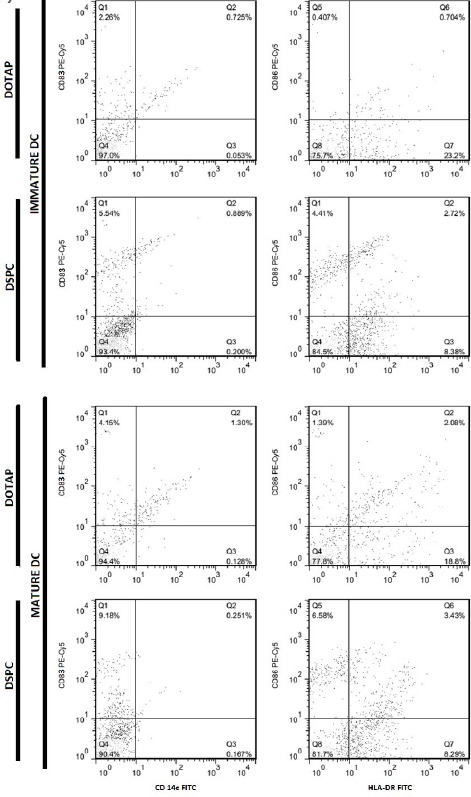
Dot plots showing flow cytometric analysis of cell surface marker’s expression on immature and mature MoDCs, after overnight incubation with liposomal formulations at 37°C.
In cells from C57BL/6 mice BMDCs and human MoDCs, the highest expression of markers was seen with DSPC formulation. In cells from C57BL/6 mice, the expression of MHC-II was significantly higher in case of DSPC formulation than buffer. In human MoDCs, expression of all markers was significantly (P<0.0001) higher when DSPC was used. On the contrary, in BALB/c mice, the highest expression belonged to DOTAP formulation, but only the expression of CD80 and MHC-II was significantly (P<0.05 and P<0.01, respectively) higher than buffer (Figure 8).
Figure 8.
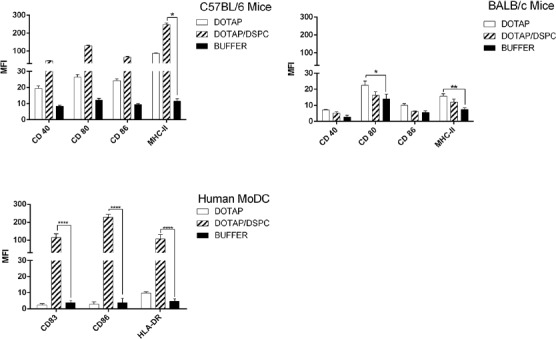
MFI values of markers determined by flow cytometric analysis of immature DCs. (A) MFI values in immature BMDCs from C57BL/6 mice. (B) MFI values in immature BMDCs from BALB/C mice. (C) MFI values in immature MoDCs
The results related to mature DCs are shown in Figure 9. In all DCs models, DSPC formulation induced higher level of markers’ expression compared to DOTAP one. The biggest difference was seen in human MoDCs model (> 10 fold increase in markers MFI level when DSPC formulation was used). In C57BL/6 mice, the highest MFI level was due to HLA marker when DSPC formulation was used and induced a significant (P< 0.05) expression compared to other markers. However, in BALB/c mice, the highest MFI level was seen in CD80 marker when DSPC formulation was used and it was significant (P<0.05) when compared to buffer group. In cells from human MoDCs, the highest MFI level was seen in CD83 when DSPC formulation was used (P<0.0001), although there was no significant difference when compared to CD86.
Figure 9.
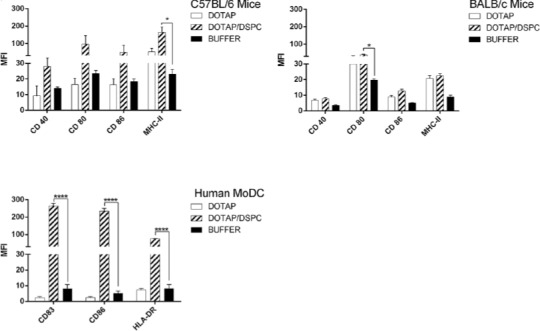
MFI values of markers determined by flow cytometric analysis of mature DCs. (A) MFI values in mature BMDCs from C57BL/6 mice. (B) MFI values in mature BMDCs from BALB/C mice. (C) MFI values in mature MoDCs
Discussion
In lipid formulations, lipid bilayer has a significant role on interaction of nanoparticle with APCs, especially DCs (26, 27). Owing to interaction with negative surface charge of APCs, cationic liposomes can affect efficiently maturation of DCs and uptake of antigens (28). It has been also proved that particular cationic lipids such as DOTAP with an ammonium ion head group, because of their innate positive charge, have a potent immunostimulatory effect. In case of vaccine development, capacity of cationic liposomes to form bilayer structures makes them a good choice as a delivery system and this is because they can offer efficient encapsulation of negatively charged proteins (29). Current evidence suggests that DOTAP liposomes raise the proficiently immunogenic profiles of peptide or protein antigens and induce cytotoxic T lymphocyte (CTL) and Th1 responses (30). DOTAP, which is a cationic lipid, powerfully interface with the anionic parts on the surface of APCs and help antigens to undergo efficient endocytosis (30, 31). Those were the reasons to make our liposomes with DOTAP and also we have previously shown that DOTAP liposomes containing SLA induce a Th1 response against leishmaniasis in murine models (17).
Other factors capable of influencing DCs maturation and liposome uptake are fluidity of lipid bilayer and phase transition temperature of lipids (11). In this study, we investigated the effect of using a high Tm lipid, i.e. DSPC, on the ability of cationic liposomes to induce uptake and maturation of DCs. Our results showed that the presence of DSPC in DOTAP formulation had no significant effect on liposomes size; because we optimized the DSPC molar ratio to make liposomes with the same size of DOTAP formulation. In fact, different liposome sizes induce significant differences in their uptake by APCs and that was why we had to prepare the same size liposomes. Surface charge was also just a bit higher in DSPC formulation than DOTAP ones and there was no significant differences between them. The reason might be due to same DOTAP concentration which we used in liposomal formulations. Since SLA has negative charge in our formulation pH (pH 7.4), a decrease in zeta potential compared to empty DOTAP liposome (> 60 mV) was expected which was in agreement with our previous results (32). There was also no significant difference in SLA entrapment amount in liposomes that means the presence of high Tm lipid didn’t influence antigen content of liposomes compared to low Tm lipid. The main force to entrap SLA in our liposomal formulations is electrostatic interaction between DOTAP and SLA during preparation method. Since DOTAP concentration in both formulation are the same, the same entrapment efficacy will be expected.
Liposomes which consist of DSPC are more rigid and resistant to particle adsorption because of higher melting point leading to reduction of the plasma promoted bilayer destabilization (33, 34). Generally, high Tm phospholipids induce higher immune responses than low Tm phospholipids (35). Previous studies also revealed that liposomes with higher Tm induce greater antibody and cell-mediated responses to antigens (7). The increased in vivo stability of DSPC liposomes appeared to influence the effect of these vesicles in triggering both humoral and cell-mediated immune responses compared with those composed of more fluid PC (36).
In this study, we used three different sources of DCs which have different outcomes when engage in Leishmania infection in vivo. Similar to human DCs, C57BL/6 mice are genetically resistant to L. major infection and resolve infection spontaneously (19). On the other hand, BALB/c mice induce Th2 response which results in exaggeration of infection (20). Production of bone BMDCs, or MoDCs, In vitro, can be done through different protocols by culturing precursors in the presence of appropriate cytokines for multiple days (37). Murine bone marrow DCs were generated from two different mouse models, C57BL/6 and BALB/c which differ in their response to Leishmania infection. CD4+ Th1 cells induction in resistant phenotype to L. major such as C57BL/6 results in production of cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). Nitric oxide production stimulated by above mentioned cytokines, results in clearance or inhibition of Leishmania parasites. In contrast, BALB/c mice develop Th2 responses and some cytokines such as IL-4, IL-10 and transforming growth factor-β (TGF-β) are produced which results in deactivation of macrophages (14, 38, 39).
Our results showed that DCs from different origins act differently in uptake of SLA-containing liposomes. Interestingly, those originated from C57BL/6 mice were similar to human MoDCs. They could uptake cationic liposomal formulations in both mature and immature states with no significant difference and independent to their lipid’s transition temperature.
C57BL/6 mice could significantly uptake DSPC formulations more than DOTAP ones. In contrast, BALB/c mice, which represent the susceptible murine model, could uptake DOTAP formulations to a greater extent than DSPC ones. We don’t have any explanations for these results now and more studies are suggested to be done to find out the reason. Although the role of surface charge density and bilayer composition of liposomes on uptake and maturation of DCs have been studied previouly (11, 37, 40), the role of DCs origins during those studies for a specific disease such as leishmaniasis have not been studied so far. We studied the maturation markers of DCs and liposome uptake in susceptible and resistant murine models of leishmaniasis and our results showed that DCs origins might have a significant effect on liposomal uptake by these cells.
Immature DCs can express low levels of surface co-stimulatory molecules, MHC class I and II. They are able to take up antigens and target them to MHC class II-positive lysosomes but they are not able to present antigens to T cells efficiently. After detecting antigen as a second danger signal, immature DCs convert to mature DCs which are immunogenic. In contrast, mature DCs have low antigen uptake capacity but they are able to stimulate T cells. (41-44). Upon maturation, surface co-stimulatory molecules, MHC class I, and T cell adhesion molecules are all upregulated. Existence or lack of various molecules on the cell surface and morphological features are the main factors for identification of DCs. CD83 functions as a specific marker for human blood DCs and has been known as one of the best markers for mature dendritic cells. Upon activation of human DCs, CD 86 expression is upregulated on the surface (45). On the other hand, in mice, co-stimulatory molecules including CD40, CD80, and CD86 are upregulated during DCs activation. CD40 ligation will result in the secretion of several proteins which allows a turning of T-cell maturation to the Th1 response. CD86 tends to be a marker of DCs maturation in the early phase, while CD80 only exists in mature DCs (46, 47). Fully activated DCs express high levels of cell-surface major histocompatibility complex (MHC) molecules (48). According to Singh-Jasuja and colleagues, CD11c expression is down-regulated upon activation of DCs in C57BL/6 mice and this was observed in our model, too (49).
High level of CD40, CD80, CD86, and MHC-class II was expressed in immature BMDCs of C57BL/6 mice, when cationic liposomes used as antigen delivery systems compared to buffer. It seems that our formulations, particularly DSPC one results in maturation state of BMDCs because there was a significant difference between liposomal formulations and buffer group. The similar results were seen in human MoDCs but not in BALB/c mice. Although there was a very significant difference between DSPC liposomes and DOTAP one, regarding the expression of surface markers including CD83, CD86, and HLA-DR, there was no significant difference between these two liposomal formulations in BALB/c murine model. Interestingly, human MoDCs showed very different results in response to high or low Tm phospholipids present in liposomal formulation, but there was no or less difference between these two formulations when murine BMDCs were used in this study. The reason for this might be due to the different origin of human MoDCs and thus different cell characteristics of them compared to murine BMDCs. Therefore, further studies with more details are needed to be done to explain this effect. The high expression level of maturation markers after DCs loading with cationic liposome has been reported previously. Recent data showed that cationic lipids can activate specific cellular signaling pathways and a number of kinases from the MAPK pathways. Activation of these kinases results in chemokine production and induction of cell surface expression of CD80/CD86 (50). Extracellular-signal-regulated kinase (ERK) phosphorylation induction for DOTAP and diC14-amidine liposomes was also shown (51).
Conclusion
Overall, in both immature and mature states, the total expression of markers is higher in C57BL/6 mice and human MoDCs, especially in case of DSPC liposomes. This can be related to the lower uptake of formulations by BALB/c derived BMDCs. We concluded that DSPC which has a higher transition temperature than DOTAP is more successful in stimulation of dendritic cells and their maturation in our model, but further mechanistic and immunologic studies are needed to be done to explain these results.
Acknowledgment
We would like to thank the Iran Natinal Science Foundation (INSF) for their financial support. The results described in this paper were part of student thesis.
Conflicts of Interest
Authors have no conflict of interest to report.
References
- 1.Labeur MS, Roters B, Pers B, Mehling A, Luger TA, Schwarz T, et al. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J Immunol. 1999;162:168–175. [PubMed] [Google Scholar]
- 2.Sozzani S, Allavena P, D'Amico G, Luini W, Bianchi G, Kataura M, et al. Cutting edge:differential regulation of chemokine receptors during dendritic cell maturation:a model for their trafficking properties. J Immunol. 1998;161:1083–1086. [PubMed] [Google Scholar]
- 3.Mellman I, Steinman RM. Dendritic cells:specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 4.Copland MJ, Baird MA, Rades T, McKenzie JL, Becker B, Reck F, et al. Liposomal delivery of antigen to human dendritic cells. Vaccine. 2003;21:883–890. doi: 10.1016/s0264-410x(02)00536-4. [DOI] [PubMed] [Google Scholar]
- 5.Alving CR. Liposomes as carriers of antigens and adjuvants. J Immunol Methods. 1991;140:1–13. doi: 10.1016/0022-1759(91)90120-5. [DOI] [PubMed] [Google Scholar]
- 6.Schwendener RA. Liposomes as vaccine delivery systems:a review of the recent advances. Ther Adv Vaccines. 2014;2:159–182. doi: 10.1177/2051013614541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines:influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012;30:2256–2272. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravindran R, Maji M, Ali N. Vaccination with liposomal leishmanial antigens adjuvanted with monophosphoryl lipid–trehalose dicorynomycolate (MPL-TDM) confers long-term protection against visceral leishmaniasis through a human administrable route. Mol Pharm. 2011;9:59–70. doi: 10.1021/mp2002494. [DOI] [PubMed] [Google Scholar]
- 9.Brewer JM, Tetley L, Richmond J, Liew FY, Alexander J. Lipid vesicle size determines the Th1 or Th2 response to entrapped antigen. J Immunol. 1998;161:4000–4007. [PubMed] [Google Scholar]
- 10.Christensen D, Korsholm KS, Rosenkrands I, Lindenstrøm T, Andersen P, Agger EM. Cationic liposomes as vaccine adjuvants. Expert Rev Vaccines. 2007;6:785–796. doi: 10.1586/14760584.6.5.785. [DOI] [PubMed] [Google Scholar]
- 11.Soema PC, Willems G-J, Jiskoot W, Amorij J-P, Kersten GF. Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach. Eur J Pharm Biopharm. 2015;94:427–435. doi: 10.1016/j.ejpb.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Vangasseri DP, Cui Z, Chen W, Hokey DA, Falo LD, Jr, Huang L. Immunostimulation of dendritic cells by cationic liposomes. Mol Membr Biol. 2006;23:385–395. doi: 10.1080/09687860600790537. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham AC. Parasitic adaptive mechanisms in infection by Leishmania. Exp Mol Pathol. 2002;72:132–141. doi: 10.1006/exmp.2002.2418. [DOI] [PubMed] [Google Scholar]
- 14.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 15.Silvestre R, Cordeiro-da-Silva A, Ouaissi A. Live attenuated Leishmania vaccines:a potential strategic alternative. Arch Immunol Ther Exp (Warsz) 2008;56:123–126. doi: 10.1007/s00005-008-0010-9. [DOI] [PubMed] [Google Scholar]
- 16.Rafati seyedi yazdi s, Couty-jouve s, Alimohamadian mh, Dowlati y 1997. Evaluation of cellular immune responses to amastigote soluble leishmania major antigens in recovered cases of cutaneous leishmaniasis. Med J Islam Repub Iran. 11:33–38. [Google Scholar]
- 17.Firouzmand H, Badiee A, Khamesipour A, Shargh VH, Alavizadeh SH, Abbasi A, et al. Induction of protection against leishmaniasis in susceptible BALB/c mice using simple DOTAP cationic nanoliposomes containing soluble Leishmania antigen (SLA) Acta Trop. 2013;128:528–535. doi: 10.1016/j.actatropica.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Barbi J, Brombacher F, Satoskar AR. T cells from Leishmania major-susceptible BALB/c mice have a defect in efficiently up-regulating CXCR3 upon activation. J Immunol. 2008;181:4613–4620. doi: 10.4049/jimmunol.181.7.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 20.Lohoff M, Sommer F, Solbach W, Röllinghoff M. Coexistence of Antigen-Specific T H 1 and T H 2 Cells in Genetically Susceptible BALB/c Mice Infected with Leishmania major. Immunobiology. 1989;179:412–421. doi: 10.1016/S0171-2985(89)80045-2. [DOI] [PubMed] [Google Scholar]
- 21.Scott P, Novais FO. Cutaneous leishmaniasis:immune responses in protection and pathogenesis. Nat Rev Immunol. 2016;16:581–592. doi: 10.1038/nri.2016.72. [DOI] [PubMed] [Google Scholar]
- 22.Stamatatos L, Leventis R, Zuckermann MJ, Silvius JR. Interactions of cationic lipid vesicles with negatively charged phospholipid vesicles and biological membranes. Biochemistry. 1988;27:3917–3925. doi: 10.1021/bi00411a005. [DOI] [PubMed] [Google Scholar]
- 23.Maji M, Mazumder S, Bhattacharya S, Choudhury ST, Sabur A, Shadab M, et al. A lipid based antigen delivery system efficiently facilitates MHC class-I antigen presentation in dendritic cells to stimulate CD8+T cells. Sci Rep. 2016;6:27206. doi: 10.1038/srep27206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott P, Pearce E, Natovitz P, Sher A. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987;139:221–227. [PubMed] [Google Scholar]
- 25.Torchilin V, Weissig V. Liposomes:a practical approached. Oxford University Press; 2003. p. 33. Chapter 2. [Google Scholar]
- 26.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 27.Nobs L, Buchegger F, Gurny R, Allémann E. Current methods for attaching targeting ligands to liposomes and nanoparticles. J Pharm Sci. 2004;93:1980–1992. doi: 10.1002/jps.20098. [DOI] [PubMed] [Google Scholar]
- 28.Quer CB, Elsharkawy A, Romeijn S, Kros A, Jiskoot W. Cationic liposomes as adjuvants for influenza hemagglutinin:more than charge alone. Eur J Pharm Biopharm. 2012;81:294–302. doi: 10.1016/j.ejpb.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Henriksen-Lacey M, Bramwell VW, Christensen D, Agger E-M, Andersen P, Perrie Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J Control Release. 2010;142:180–186. doi: 10.1016/j.jconrel.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 30.Brgles M, Habjanec L, Halassy B, Tomašić J. Liposome fusogenicity and entrapment efficiency of antigen determine the Th1/Th2 bias of antigen-specific immune response. Vaccine. 2009;27:5435–5442. doi: 10.1016/j.vaccine.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Copland MJ, Rades T, Davies NM, Baird MA. Lipid based particulate formulations for the delivery of antigen. Immunol Cell Biol. 2005;83:97–105. doi: 10.1111/j.1440-1711.2005.01315.x. [DOI] [PubMed] [Google Scholar]
- 32.Heravi Shargh V, Jaafari MR, Khamesipour A, Jaafari I, Jalali SA, Abbasi A, et al. Liposomal SLA co-incorporated with PO CpG ODNs or PS CpG ODNs induce the same protection against the murine model of leishmaniasis. Vaccine. 2012;30:3957–3964. doi: 10.1016/j.vaccine.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 33.Antimisiaris SG, Jayasekera P, Gregoriadis G. Liposomes as vaccine carriers:incorporation of soluble and particulate antigens in giant vesicles. J Immunol Methods. 1993;166:271–280. doi: 10.1016/0022-1759(93)90368-h. [DOI] [PubMed] [Google Scholar]
- 34.Moghimi SM, Patel HM. Tissue specific opsonins for phagocytic cells and their different affinity for cholesterol-rich liposomes. FEBS lett. 1988;233:143–147. doi: 10.1016/0014-5793(88)81372-3. [DOI] [PubMed] [Google Scholar]
- 35.Kersten GF, Crommelin DJ. Liposomes and ISCOMS as vaccine formulations. Biochim Biophys Acta. 1995;1241:117–138. doi: 10.1016/0304-4157(95)00002-9. [DOI] [PubMed] [Google Scholar]
- 36.Mazumdar T, Anam K, Ali N. Influence of phospholipid composition on the adjuvanticity and protective efficacy of liposome-encapsulated Leishmania donovani antigens. J Parasitol. 2005;91:269–274. doi: 10.1645/GE-356R1. [DOI] [PubMed] [Google Scholar]
- 37.Foged C, Arigita C, Sundblad A, Jiskoot W, Storm G, Frokjaer S. Interaction of dendritic cells with antigen-containing liposomes:effect of bilayer composition. Vaccine. 2004;22:1903–1913. doi: 10.1016/j.vaccine.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Noben-Trauth N, Kropf P, Muller I. Susceptibility to Leishmania major infection in interleukin-4-deficient mice. Science. 1996;271:987. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- 39.Scharton-Kersten T, Scott P. The role of the innate immune response in Th1 cell development following Leishmania major infection. J Leukoc Biol. 1995;57:515–522. doi: 10.1002/jlb.57.4.515. [DOI] [PubMed] [Google Scholar]
- 40.Ma Y, Zhuang Y, Xie X, Wang C, Wang F, Zhou D, et al. The role of surface charge density in cationic liposome-promoted dendritic cell maturation and vaccine-induced immune responses. Nanoscale. 2011;3:2307–2314. doi: 10.1039/c1nr10166h. [DOI] [PubMed] [Google Scholar]
- 41.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 42.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells:which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 43.Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+/CD25+regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101:4862–4869. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]
- 44.Dieu M-C, Vanbervliet B, Vicari A, Bridon J-M, Oldham E, Aït-Yahia S, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLellan AD, Starling GC, Williams LA, Hock BD, Hart DN. Activation of human peripheral blood dendritic cells induces the CD86 co-stimulatory molecule. Eur J Immunol. 1995;25:2064–2068. doi: 10.1002/eji.1830250739. [DOI] [PubMed] [Google Scholar]
- 46.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, et al. BDCA-2, BDCA-3, and BDCA-4:three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 47.Tsuji S, Matsumoto M, Takeuchi O, Akira S, Azuma I, Hayashi A, et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin:involvement of toll-like receptors. Infect Immun. 2000;68:6883–6890. doi: 10.1128/iai.68.12.6883-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shortman K, Liu Y-J. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 49.Singh-Jasuja H, Thiolat A, Ribon M, Boissier M-C, Bessis N, Rammensee H-G, et al. The mouse dendritic cell marker CD11c is down-regulated upon cell activation through Toll-like receptor triggering. Immunobiology. 2013;218:28–39. doi: 10.1016/j.imbio.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Lonez C, Vandenbranden M, Ruysschaert J-M. Cationic liposomal lipids:from gene carriers to cell signaling. Prog Lipid Res. 2008;47:340–347. doi: 10.1016/j.plipres.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka T, Legat A, Adam E, Steuve J, Gatot JS, Vandenbranden M, et al. DiC14-amidine cationic liposomes stimulate myeloid dendritic cells through toll-like receptor 4. Eur J Immunol. 2008;38:1351–1357. doi: 10.1002/eji.200737998. [DOI] [PubMed] [Google Scholar]


