Abstract
Objective(s):
To explore whether endoplasmic reticulum (ER) stress regulates inflammation in adipose tissue of obese rats via TLR4 signaling.
Materials and Methods:
Sprague Dawley rats were randomly divided into four groups, and body weight, food intake, and free fatty acids (FFA) were measured. Real-time PCR and Western blot were used to determine mRNA or protein expression of TLR4, TRAF6, IKKβ, TNF-α, IL-6, and GRP78. Immunohistochemistry was used to detect GRP78 protein expression.
Results:
The FFA levels in HFD, HFD+PBA, and HFD+VIPER groups were higher than that in the control group (P<0.05). Compared with the control group, HFD induced GRP78 expression significantly (P<0.05), which could be decreased by ER stress inhibitor but not by TLR4 blocker. The mRNA expression of TLR4, TRAF6, TNF-α, and IL-6, and protein levels of TLR4, TNF-α, and IKKβ in the HFD group increased significantly compared with the control group (P<0.05), while these changes could be suppressed by PBA or VIPER (P<0.05). The immunohistochemistry staining indicated GRP78 expression in the HFD group was higher than that of the control group, which could be inhibited by PBA or VIPER.
Conclusion:
HFD could induce inflammation in adipose tissue via ER stress and its downstream TLR4 signaling.
Keywords: Adipocyte, Endoplasmic reticulum stress, Inflammation, Obesity, Toll-like receptor 4
Introduction
Obesity, as a serious threat to human health and quality of life, can lead to insulin resistance, diabetes, and cardiovascular diseases. Accumulating evidence indicates that obesity is a chronic low-grade inflammatory disease (1, 2). It is reported that excessive ingestion of fat without concomitant ingestion of antioxidant-rich foods/beverages may contribute to inflammation attributed to obesity, and study on the interaction of microbiota with food and obesity suggested a new hypothesis for the obesity/fat diet relationship with inflammation (3). Adipose tissue is not only an energy storage, but an important endocrine organ with multiple metabolic roles in regulating whole-body physiology and plays an important role in the regulation of energy metabolism (4, 5). Small adipocytes of lean individuals promote metabolic homeostasis, while the enlarged adipocytes in obese individuals recruit macrophages and promote inflammation and the release of a series of factors that predispose to insulin resistance (6). Research showed that one-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice (7). Yida et al. also demonstrated that High fat diet-induced inflammation and oxidative stress (8). Researchers reported that high-fat diet-induced obesity primes inflammation in adipose tissue prior to the liver in C57BL/6j mice (9).
Recent studies have pointed out that the endoplasmic reticulum (ER) stress is a core signaling to connect with obesity and inflammation (10, 11).
ER is notable for its vital roles in lipid biosynthesis, calcium ion storage, and protein sorting and processing, and it can also regulate diverse cellular processes including nutrient metabolism, inflammatory and insulin signaling, and cell proliferation and death via a signaling pathway called the unfolded protein response (UPR) (12).
Under some circumstances, such as increased fatty acids and stimulation of pathogenic microorganisms destructing the ER homeostasis and leading to the ER stress, the UPR in the cell will be activated in response to these stimuli (13, 14). Chronic UPR activation has been found in adipose tissue and/or liver of dietary and genetic murine models of obesity, and in human obesity and non-alcoholic fatty liver disease (NAFLD) (12).
These stimuli, such as fatty acids frequently occur in the obese body. UPR can also activate the inflammatory response pathway via regulation of gene transcription and protein synthesis (15-17). Therefore, more attenu-ation has been paid to the role of ER stress in obesity and related metabolic disorders, and obesity-induced ER stress may cause chronic inflammation in adipose tissue (18).
Toll-like receptor 4 (TLR4) is important for the development of chronic inflammation (19). It has been indicated that the occurrence and development of obesity, diabetes, atherosclerosis, myocardial infarction, and other non-bacterial inflammatory diseases, are closely related to TLR4 (20, 21). In addition, researchers (22) observed that TLR4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia.
However, how obesity-induced ER stress regulates TLR4 signaling and the role of this regulation in inflammatory responses of adipose tissue, are still unclear. The present study aimed to feed the rats with a high-fat diet and to observe the changes of ER stress regulating TLR4 signaling pathway in rat adipose tissue.
Materials and Methods
Animals and experimental protocol
Animal studies were in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health. One hundred Sprague Dawley rats (body weight 110–120 g) were purchased from Shanghai SIPPR-BK Experimental Animal Co. Ltd. All animals were housed in individual cages in a room with temperature (22±5 °C) and humidity control (50±10%), and allowed access to standard rat rodent chow and distilled water ad libitum. After 1 week of accommodation to environmental conditions, the animals were divided into four groups with 25 rats per group: 1) control group, fed with normal diet; 2) high-fat diet (HFD) group, fed with HFD purchased from Shanghai SIPPR-BK Experimental Animal Co. Ltd.; 3) HFD+PBA (an ER stress inhibitor, IP, 10 mg/kg) group; and 4) HFD+VIPER (a TLR4 blocker, IV, 0.1 mg/kg).
Sample acquirement
After 10 weeks of treatment, the rats were fasted for 14 hr, weighted, and anesthetized with 10% chloral hydrate (IP, 0.3 mg/kg). Blood was taken from the apex, centrifuged immediately at 4 °C, and stored in -80 °C refrigerator. Thereafter, the rats were sacrificed and abdominal adipose tissue was quickly removed without connective tissue and sliced into two parts. One part was immersed in 10% neutral-buffered formalin for histopathological examinations and another part was snap frozen in liquid nitrogen and kept at -80 °C for RNA and protein extraction.
Changes in rat body physiological and metabolic parameters
Food intake and body weight were examined, and free fatty acids (FFA) were measured enzymatically (Hitachi 7600-020 automatic chemical analyzer) as previously described (23).
Real-time PCR
Quantitative real-time PCR was used to measure mRNA expression of inflammatory TLR4, TNF receptor-associated factor 6 (TRAF6), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) in adipose tissue. Fat tissue (100 mg) was cut into pieces and fully homogenized, and total RNA was isolated by using Trizol reagent (Invitrogen, USA). RNA purity was determined using Tecan infinite M200 multifunctional microplate spectrophotometer. Thereafter, RNA (200 ng) was reverse transcribed to cDNA using High Capacity cDNA Reverse Transcription Kits (Invitrogen, USA). The primers for target genes and referenced gene (GAPDH) were designed and synthesized by Shanghai Sangon Biotech (Table 1). According to the instruction of SYBRR Select Master Mix (Life technology, USA), real-time quantitative PCR reaction was performed by using the Light Cycler 480 fluorescence quantitative PCR instrument. The parameters for PCR reaction were as follows: reaction system 20 μl; pre-degeneration with 50 °C for 2 min; degeneration with 95 °C for 2 min; and then 45 cycles of 95 °C for 15 sec and 60 °C for 1 min. The results were analyzed with Light Cycler480 1.5 software and the ratio of target gene to reference gene GAPDH was calculated.
Table 1.
Primers used for quantitative real-time PCR
| Genes | Forward primer | Reverse primer |
|---|---|---|
| TLR4 | 5’-CCGCTCTGGCATCATCTTCA-3’ | 5’-CCCACTCGAGGTAGGTGTTTCTG-3’ |
| TRAF6 | 5’-TCTCCCCTGCCTTCATTGTT-3’ | 5’-AGGCTGGCGATTTTGTGTTT-3’ |
| TNF-α | 5’-GGCGTGTTCATCCGTTCTC-3’ | 5’-CTTCAGCGTCTCGTGTGTTTCT-3’ |
| IL-6 | 5’-ATTGTATGAACAGCGATGATGCAC-3’ | 5’-CCAGGTAGAAACGGAACTCCAGA-3’ |
| GAPDH | 5’-GCAAGTTCAACGGCACAG-3’ | 5’-GCCAGTAGACTCCACGACAT-3’ |
Western blot
Western blot was used to measure the protein expression of inflammatory TLR4 (Sigma Aldrich, USA,1:200), inhibitor kappa B kinaseβ(IKKβ) (Sigma Aldrich, USA,1:200), TNF-α (Santa, USA,1:200), mitochondrial stress-related protein, and Glucose Regulated Protein-78 (GRP78) (Santa, USA,1:200). Epididymal adipose tissue (100 mg) was taken and followed by adding the appropriate amount of lysate, phosphatase inhibitors, protease inhibitors, and Phenylmethanesulfonyl fluoride (PMSF) (KGP 250 Kit). After homogenization (8 min, twice), the protein extracts were placed in an ice bath for 30 min and then centrifuged to abstain the supernatant. Bicinchoninic Acid (BCA) method was used to measure protein concentration. An equal amount of protein was mixed with the SDS-PAGE sample buffer and boiled at 100 °C for 5 min, and then separated by 10% SDS polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membrane. After blocking with 5% BSA for 1 hr, the membrane was incubated with primary antibodies at 4 °C overnight. After three washes with PBST buffer, the membranes were incubated with HRP-labeled IgG for 1 hr at room temperature. The protein bands were detected using ECL reagents. Chemiluminescent signals were detected with Alphaview 1.3 and analyzed using the Image-pro Plus 6.0 Imaging System. Beta-actin (Sigma Aldrich, USA,1:300) was used as reference and the ratios of the target protein to β-actin were calculated.
Immunohistochemistry
To detect the protein expression of GRP78 (Santa, USA) in adipose tissue, the adipose tissue slices were placed into an oven for 30 min at 60 °C to fix slices. Thereafter, the slices were immersed in xylene for 10 min, washed with PBS twice, blocked with 10% serum, and incubated with primary antibody for 2 hr at room temperature. The second antibody was added for 20 min at 37 °C followed by washing with PBS for 2 min three times. Stained with DAB and hematoxylin, resin was used to seal the glass slides for microscopic study to assess the histological change. Image-pro Plus 6.0 was used for image analysis.
Statistical analysis
All data were statistically analyzed using SPSS 17 software. All data were expressed as the mean± standard deviation (x±s), n refers to the number of rats. Differences between groups were compared using independent t-test analysis. The differences were considered statistically significant when P<0.05.
Results
Changes in rat body physiological and metabolic parameters
No significant difference in food intake among the four groups was observed. The body weight and FFAs levels of HFD, HFD+PBA, and HFD+VIPER groups were higher obviously than those of the control group (Table 2).
Table 2.
The effects of high-fat diet on rat physiological and metabolic parameters
| Group | n | Food intake (g/d) | Body weight (g) | FFAs (mmol/l) |
|---|---|---|---|---|
| Control | 25 | 152.76±5.86 | 476.12±9.36* | 0.36±0.02* |
| HFD | 25 | 139.77±12.61 | 526.26±11.35 | 0.82±0.32 |
| HFD+PBA | 25 | 142.26±11.26 | 522.51±10.65 | 0.79±0.11 |
| HFD+VIPER | 25 | 136.65±13.87 | 515.02±8.97 | 0.80±0.51 |
Note:
P<0.05 vs. others groups
Changes in mitochondrial stress-related protein GRP78 in adipose tissue
As shown in Figure 1, GRP78 protein level in the HFD group was higher than that in the control group (P<0.05), while GRP78 protein expression in the HFD+PBA group was lower significantly than that in the HFD group (P<0.05), indicating HFD induced GRP78 expression, which could be decreased by ER stress inhibitor but not by TLR4 blocker (Figure 1).
Figure 1.
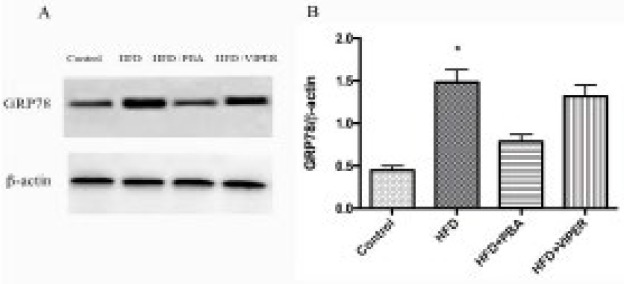
Changes in mitochondrial stress-related protein GRP78 in adipose tissue. (A) Protein expression of GRP78 in the presence of TLR4 blocker and ER stress inhibitor. (B) Statistical analysis of protein expression. *P<0.05 vs others groups; n=10
Changes in TLR4-related inflammatory signaling in rat adipose tissue
Real-time PCR and Western blot were used to measure the mRNA and protein levels of key factors of the TLR4 signaling pathway. As shown in Figures 2 and 3, compared with the control group, the mRNA expression of TLR4, TRAF6, TNF-α, and IL-6, and protein levels of TLR4, TNF-α, and IKKβ, in the HFD group, increased significantly. In addition, these changes could be suppressed by PBA or VIPER treatment (Figures 2 and 3).
Figure 2.
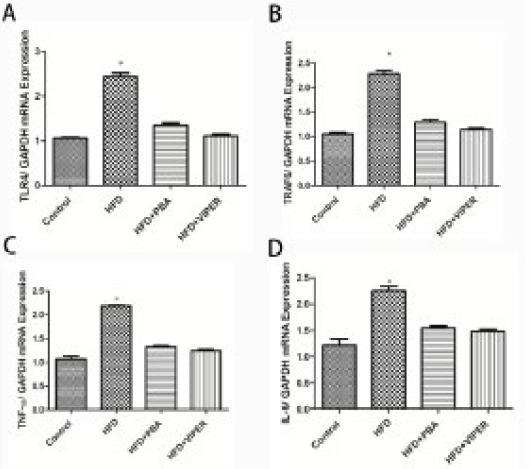
The mRNA expression of the inflammatory gene in rat adipose tissue.
The mRNA expression of TLR4 (A), TRAF6 (B), TNF-α (C), and IL-6 (D) is higher than that in the control group, while the increase could be suppressed by PBA or VIPER treatment. *P<0.05 vs others groups; n=10
Figure 3.
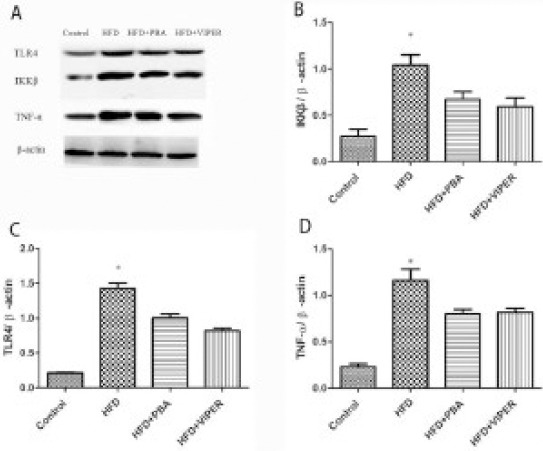
The protein expression of the inflammatory gene in rat adipose tissue
(A) Compared with the control group, the protein expression TLR4, IKKβ, TNF-α is higher in the HFD group, and it is blocked by TLR4 blocker or ER stress inhibitor. (B, C, D) Statistical analysis of protein expression. *P<0.05 vs others groups; n=10
Changes in ER stress in rat adipose tissue
Immunohistochemistry was used to observe GRP78 expression in different groups. The GRP78 protein expression in the HFD group was higher than that of the control group, which could be inhibited by PBA or VIPER treatment (Figure 4).
Figure 4.
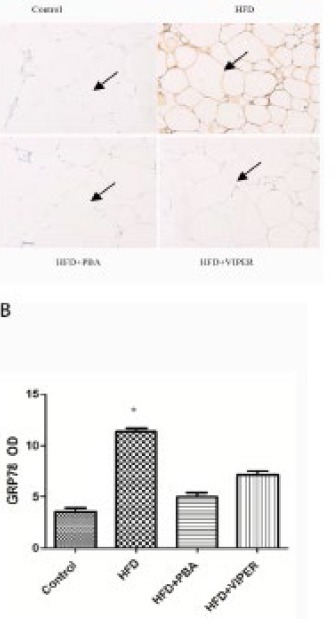
GRP78 protein expression in rat adipose tissue was determined by immunohistochemistry. (A) GRP78 protein expression in adipose tissue in the presence of TLR4 blocker and ER stress inhibitor. The arrow indicates GRP78 protein expression (200 x). (B) Statistical analysis *P<0.05 vs others groups; n=15
Discussion
ER stress has drawn much attention recently because of its potential roles in inflammation (24-26). It is reported that inflammation is associated with diseases caused by primary misfolding mutations and ER stress, since ER stress and activation of the unfolded protein response is a feature of some autoimmune and inflammatory diseases, and ER stress may be both a trigger and consequence of inflammation (27). Liu et al. reported ER stress is involved in inflammation and ER stress-regulated immunity via activation of the NOD-like receptor (NLRP) 3/caspase1 inflammasome and in diabetes pathogenesis via the release of cytokines (28). A study also reported that ER stress is the crossroads of inflammation, autophagy, and apoptosis signaling pathways and participates in liver fibrosis (29).
Recent studies have shown that TLR4 signaling is crucial to the development of ER stress (22). TLR4 signaling induces tissue pro-inflammatory cytokine release and ER stress (33). TLR4 signaling is a major source of pro-inflammatory cytokines (30) such as TNF-α (31). TLR4 plays a crucial role in synovial inflammation and may contribute to the pathogenesis of rheumatoid arthritis (32). TLR4 signaling promotes autonomic dysfunction, inflammation, and microglia activation, through neuronal ER stress, in the hypothalamic paraventricular nucleus (33). It is reported TLR4 enhanced inflammatory responses and osteoclastogenesis, and then promoted cholesteatoma-induced bone destruction and deafness (34). Researchers demonstrated that peripheral TLR4 stimulation acts as a transient counter-regulatory mechanism for inflammatory pain in vivo, and increases the release of opioid peptides from monocytes in vitro (35). It is reported that TLR4-mediated ER stress in intestinal crypts induces necrotizing enterocolitis (36). However, how obesity-induced ER stress regulates TLR4 signaling and the role of this regulation in inflammatory responses of adipose tissue remains less reported.
In the present study, we showed that ER stress regulates inflammation in adipocytes of obese rats via TLR4 signaling. GRP78 is a marker protein of ER stress that is an ER stress chaperone whose decreased expression is associated with Type II diabetes by inhibiting AKT activation in the GLUT signaling pathway to facilitate insulin resistance. In this study, compared with the control group, HFD induced GRP78 expression, which could be decreased by ER stress inhibitor but not by TLR4 blocker. These findings indicate that inflammation and ER stress set a positive feedback mechanism, thereby contributing to autonomic dys-function. It is reported that GRP78 plays an essential role in adipogenesis and postnatal growth in mice, and GRP78 is required for adipocyte differentiation, glucose homeostasis, and balanced secretion of adipokines (37).
ER stress can drive a pro-inflammatory program, and it regulates inflammation and immunity through multiple mechanisms and facilitates the processing and secretion of cytokines and chemokines (38). ER stress inhibitor and TLR4 blocker decreased the expression of TLR4, its downstream factors such as TRAF6, IKKβ, and inflammatory factors TNF-α and IL-6, indicating that TLR4 is the key signaling element of ER stress regulating inflammation in adipose tissue. This is supported by our findings that ER stress inhibition attenuated the increase of TNF-α, IL-6, TLR4, and GRP78. After suppression by PBA or VIPER treatment in the experiment group, the mRNA expression inflammatory genes (TLR4, TRAF6, TNF-α, and IL-6) in rat adipose tissue decreased significantly, suggesting that PBA or VIPER inhibited the inflammatory response. Masson et al. also demonstrated that inflammation induced by TLR4 activation is suppressed by ER Stress blocker or TLR4 blocker (33).
In the current study, the protein expression of TLR4, IKKβ, and TNF-α is higher in the HFD group that is blocked by TLR4 blocker or ER stress inhibitor. This fact suggests that TLR4/IKKbeta Signaling Pathways plays an essential role in inflammation. Zhang et al. reported that berberine could reduce the level of ET of obese rats, down-regulate the TLR4/IKKbeta/NF-kappaB inflammation signal pathway in skeletal muscle and improve insulin resistance of skeletal muscle through inhibiting the TLR4/IKKbeta/NF-kappaB signaling pathway (39). In our study, we also found that GRP78 protein expression decreased in adipose tissue in the presence of TLR4 blocker and ER stress inhibitor, indicating that TLR4 signaling plays an important role in the process of ER stress regulating inflammation.
Conclusion
The experimental data showed that HFD could induce ER stress, and further enhance inflammation in adipose tissue. However, these changes could be suppressed by ER stress inhibitor or TLR4 blocker, these results indicate that ER stress plays important pathophysiological roles in obesity-induced adipose tissue dysfunction, and TLR4 was the key signaling for ER Stress regulating inflammation in adipose tissue. The present study may suggest a new strategy for the treatment of chronic inflammation induced by obesity.
Acknowledgment
The study was supported by Shanghai Municipal Commission of Health and Family Planning(201540126), and Talent-development Project of Pudong Heath and Family planning Commission of Shanghai (PWRd2014-16).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. The J ClinI Inves. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010:1–10. doi: 10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 7.Waise TM, Toshinai K, Naznin F, NamKoong C, Md Moin AS, Sakoda H, et al. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem Biophys Res Commun. 2015;464:1157–1162. doi: 10.1016/j.bbrc.2015.07.097. [DOI] [PubMed] [Google Scholar]
- 8.Yida Z, Imam MU, Ismail M, Ismail N, Ideris A, Abdullah MA. High fat diet-induced inflammation and oxidative stress are attenuated by N-acetylneuraminic acid in rats. J Biomed Sci. 2015;22:96–106. doi: 10.1186/s12929-015-0211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Heijden RA, Sheedfar F, Morrison MC, Hommelberg PP, Kor D, Kloosterhuis NJ, et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging (Albany NY) 2015;7:256–268. doi: 10.18632/aging.100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 11.Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms:role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 12.Pagliassotti MJ, Kim PY, Estrada AL, Stewart CM, Gentile CL. Endoplasmic reticulum stress in obesity and obesity-related disorders:An expanded view. Metabolism. 2016;65:1238–1246. doi: 10.1016/j.metabol.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- 14.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 15.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 17.Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D. Stress-induced gene expression requires programmed recovery from translational repression. The EMBO J. 2003;22:1180–1187. doi: 10.1093/emboj/cdg112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2:799. doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szatmary Z. Molecular biology of toll-like receptors. Gen Physiol Biophys. 2012;31:357–366. doi: 10.4149/gpb_2012_048. [DOI] [PubMed] [Google Scholar]
- 20.Erridge C. Diet, commensals and the intestine as sources of pathogen-associated molecular patterns in atherosclerosis, type 2 diabetes and non-alcoholic fatty liver disease. Atherosclerosis. 2011;216:1–6. doi: 10.1016/j.atherosclerosis.2011.02.043. [DOI] [PubMed] [Google Scholar]
- 21.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 22.Yao L, Kan EM, Lu J, Hao A, Dheen ST, Kaur C, et al. Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia:role of TLR4 in hypoxic microglia. J Neuroinflammation. 2013;10:23–43. doi: 10.1186/1742-2094-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Liu X, Wang W, Song W, Chen L, Fang Q, et al. The expression of inflammatory cytokines on the aorta endothelia are up-regulated in pinealectomized rats. Inflammation. 2013;36:1363–1373. doi: 10.1007/s10753-013-9676-1. [DOI] [PubMed] [Google Scholar]
- 24.Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meares GP, Liu Y, Rajbhandari R, Qin H, Nozell SE, Mobley JA, et al. PERK-dependent activation of JAK1 and STAT3 contributes to endoplasmic reticulum stress-induced inflammation. Mol Cell Biol. 2014;34:3911–3925. doi: 10.1128/MCB.00980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leonard A, Paton AW, El-Quadi M, Paton JC, Fazal F. Preconditioning with endoplasmic reticulum stress ameliorates endothelial cell inflammation. PLoS One. 2014;9:e110949. doi: 10.1371/journal.pone.0110949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasnain SZ, Lourie R, Das I, Chen AC, McGuckin MA. The interplay between endoplasmic reticulum stress and inflammation. Immunol Cell Biol. 2012;90:260–270. doi: 10.1038/icb.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Cao MM, Wang Y, Li LC, Zhu LB, Xie GY, et al. Endoplasmic reticulum stress is involved in the connection between inflammation and autophagy in type 2 diabetes. Gen Comp Endocrinol. 2015;210:124–129. doi: 10.1016/j.ygcen.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Wang Y, Wang H, Huang C, Huang Y, Li J. Endoplasmic reticulum stress is the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in liver fibrosis. Inflamm Res. 2015;64:1–7. doi: 10.1007/s00011-014-0772-y. [DOI] [PubMed] [Google Scholar]
- 30.Nair AR, Elks CM, Vila J, Del Piero F, Paulsen DB, Francis J. A blueberry-enriched diet improves renal function and reduces oxidative stress in metabolic syndrome animals:potential mechanism of TLR4-MAPK signaling pathway. PLoS One. 2014;9:e111976. doi: 10.1371/journal.pone.0111976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salomao R, Brunialti MK, Rapozo MM, Baggio-Zappia GL, Galanos C, Freudenberg M. Bacterial sensing, cell signaling, and modulation of the immune response during sepsis. Shock. 2012;38:227–242. doi: 10.1097/SHK.0b013e318262c4b0. [DOI] [PubMed] [Google Scholar]
- 32.Xu D, Yan S, Wang H, Gu B, Sun K, Yang X, et al. IL-29 Enhances LPS/TLR4-Mediated Inflammation in Rheumatoid Arthritis. Cellular physiology and biochemistry:international journal of experimental Cell Physiol Biochem. 2015;37:27–34. doi: 10.1159/000430330. [DOI] [PubMed] [Google Scholar]
- 33.Masson GS, Nair AR, Dange RB, Silva-Soares PP, Michelini LC, Francis J. Toll-like receptor 4 promotes autonomic dysfunction, inflammation and microglia activation in the hypothalamic paraventricular nucleus:role of endoplasmic reticulum stress. PLoS One. 2015;10:e0122850. doi: 10.1371/journal.pone.0122850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Si Y, Chen YB, Chen SJ, Zheng YQ, Liu X, Liu Y, et al. TLR4 drives the pathogenesis of acquired cholesteatoma by promoting local inflammation and bone destruction. Sci Rep. 2015;5:16683–16695. doi: 10.1038/srep16683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sauer RS, Hackel D, Morschel L, Sahlbach H, Wang Y, Mousa SA, et al. Toll like receptor (TLR)-4 as a regulator of peripheral endogenous opioid-mediated analgesia in inflammation. Mol Pain. 2014;10:10–24. doi: 10.1186/1744-8069-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Afrazi A, Branca MF, Sodhi CP, Good M, Yamaguchi Y, Egan CE, et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J Biol Chem. 2014;289:9584–9599. doi: 10.1074/jbc.M113.526517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu G, Ye R, Jung DY, Barron E, Friedline RH, Benoit VM, et al. GRP78 plays an essential role in adipogenesis and postnatal growth in mice. FASEB J. 2013;27:955–964. doi: 10.1096/fj.12-213330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee AS. Glucose-regulated proteins in cancer:molecular mechanisms and therapeutic potential. Nat Rev Cancer. 2014;14:263–276. doi: 10.1038/nrc3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang DS, Bai XH, Yao YJ, Mu DZ, Chen J. [Effect of berberine on the insulin resistance and TLR4/IKKbeta/NF-kappaB signaling pathways in skeletal muscle of obese rats with insulin resistance] Sichuan Da Xue Xue Bao Yi Xue Ban. 2015;46:827–831. [PubMed] [Google Scholar]


