Abstract
Objective(s):
Weight gain and metabolic disturbances such as dyslipidemia, are frequent side effects of second-generation antipsychotics, including olanzapine. This study examined the metabolic effects of chronic olanzapine exposure. In addition, we investigated the hepatic fatty acid effects of olanzapine in female C57BL/6J mice fed a normal diet.
Materials and Methods:
Female C57BL/6J mice orally received olanzapine or normal saline for 7 weeks. The effects of long-term olanzapine exposure on body weight changes, food efficiency, blood glucose, triglyceride (TG), insulin, and leptin levels were observed. Hepatic TG and abdominal fat mass were investigated, and fat cell morphology was analyzed through histopathological methods. The levels of protein markers of fatty acid regulation in the liver, namely fatty acid synthase (FAS) and stearoyl-CoA desaturase-1 (SCD-1), were measured.
Results:
Olanzapine treatment increased the food intake of the mice as well as their body weight. Biochemical analyses showed that olanzapine increased blood TG, insulin, leptin, and hepatic TG. The olanzapine group exhibited increased abdominal fat mass and fat cell enlargement in abdominal fat tissue. Western blotting of the mouse liver revealed significantly higher (1.6-fold) levels of SCD-1 in the olanzapine group relative to the control group; by contrast, FAS levels in the two groups did not differ significantly.
Conclusion:
Enhanced lipogenesis triggered by increased hepatic SCD-1 activity might be a probable peripheral mechanism of olanzapine-induced dyslipidemia. Some adverse metabolic effects of olanzapine may be related to the disturbance of lipid homeostasis in the liver.
Keywords: Adiposity, Insulin, Olanzapine, Stearoyl-CoA desaturase-1- (SCD-1), Triglyceride
Introduction
Antipsychotic drugs are extensively employed to treat mental disorders, including bipolar disorder, delusional disorder, and schizophrenia. Second-generation antipsychotics (SGAs) carry a lower risk of extrapyramidal syndrome development than do first-generation antipsychotics. However, SGA use may result in weight gain, impaired glucose tolerance, hyperlipidemia, and increased adiposity (1-3), which in turn may lead to higher morbidity because of their association with hypertension, type II diabetes, stroke, and cardiovascular disease (4-8). The side effects of SGAs may limit their use and have negative effects on patient compliance (9, 10). Among all SGAs, olanzapine and clozapine have the most significant effects on weight gain and metabolic changes (4). The mechanisms underlying metabolic disturbance induced by SGAs, which have yet to be clarified, are probably multifactorial, involving the central and peripheral nervous systems (11).
Enhanced lipogenesis triggered by increased enzymatic activity is one probable peripheral mechanism for SGA-induced adverse metabolic effects such as hyperlipidemia. The major enzymes involved in biosynthesizing fatty acid are stearoyl-CoA desaturase-1 (SCD-1; predominantly expressed in the liver) and fatty acid synthase (FAS). Furthermore, some clinical and rodent studies have evidenced that SCD-1 is crucial for regulating triglyceride (TG) biosynthesis and lipid homeostasis. Moreover, animal studies have demonstrated that SCD-1 mutation is related to impaired SCD-1 activity and TG biosynthesis (12, 13). In a rodent disease model, the pharmacological inhibition of SCD-1 reduced glucose and TG levels (14). Few studies have reported on the activity and hepatic expression of SCD-1 in humans, but some have positively correlated elevation in SCD-1 activity to the level of insulin resistance and plasma TG (15, 16). The foregoing observations suggest that SCD-1 is strongly involved in TG synthesis and lipid homeostasis.
Antipsychotic medications have been reported to induce lipid accumulation and upregulate lipogenic gene expression; this is attributed to the effects of sterol regulatory element–binding proteins (SREBPs), including SCD-1 (17-19). Moreover, in patients treated with olanzapine, peripheral blood cells had higher FAS and SCD-1 mRNA expression than did those of drug-free patients (20). The aforementioned results indicate that antipsychotics may affect lipogenic genes and may impair lipid metabolism. Although the underlying mechanism remains unclear, an increasing body of evidence suggests that the lipogenic activity of SGAs is attributable, at least partially, to direct effects on peripheral tissues (21).
Most research on the metabolic side effects of olanzapine has concentrated on the central nervous system, and little is understood about the effects of olanzapine on peripheral organs, such as the liver and adipose tissue, which are essential organs for lipid metabolic homeostasis. The relationships between the metabolic effects of antipsychotics, adipose tissue morphology, and enzymatic activity involved in lipid metabolism need to be investigated. Accordingly, in this rodent model study, we examined the outcomes of chronic exposure to olanzapine in terms of body weight, biochemical changes, abdominal fat mass, and the levels of hepatic FAS and SCD-1 proteins.
Materials and Methods
Animals and diets
The Institutional Animal Care and Use Committee at National Chung Hsing University approved the experimental procedures and the animal conditions (approval No.: NCHU IACUC 100-28). Four-week-old female C57BL/6J mice were sourced from the Education Research Resource Department at National Laboratory Animal Center, Taiwan. For 8 weeks preceding the experiment, the mice were fed a standard diet (Laboratory Rodent Diet 5001, PMI Nutrition International Inc, MO, USA), with metabolizable energy of 3.02 kcal/g. The mice, placed in standard rodent cages stored in quarters controlled at 22 °C, were subjected to a 12-hr light–dark cycle. The animals were given ad libitum access to water and pelleted mouse chow. All procedures were performed per appropriate guidelines recommended by the Taiwanese government (i.e., Guidelines for the Care and Use of Laboratory Animals).
Drug treatment
The mice were randomly categorized into two groups: The first group received 6 mg/kg olanzapine (Zyprexa, Eli Lilly Company, USA) per day through gavage for 7 weeks, and the second group (i.e., the control group) was administered 0.01 ml/g normal saline once per day through gavage. The two groups did not differ significantly in body weight, and the olanzapine dosage was set on the basis of earlier studies on olanzapine-induced metabolic changes (22-25).
Determination of food intake, body weight, and biochemical analyses
Food intake and body weight were assessed weekly for 7 weeks. To measure blood glucose levels, blood samples were drawn from the tail vein of overnight-fasted animals and analyzed using a OneTouch Ultra 2 glucose meter (LifeScan Inc., CA, USA). Following the 7-week experimental period, all mice were anesthetized, and tissues and sera were harvested for analysis. Serum TG levels were detected using Accutrend GCT (Roche Diagnostic, Mannheim, Germany), a TG-specific point-of-care testing device, and the levels of serum insulin and leptin were detected using ELISA kits from Crystal Chem Inc. (Downers Grove, IL, USA).
Determination of liver lipids
Lipids were harvested from the liver by following the methods reported by Spisni et. al. (26) and Chang et. al. (27) and subjected to TG analysis on commercial kits (BioVision), as follows: The harvested tissues underwent cold homogenization (2 min suffices for complete homogenization of liver or similar tissues) in a 2:1 (v/v) high-performance liquid chromatography–grade mixture of chloroform and methanol (Carlo Erba, Italy) to a dilution of twentyfold the tissue volume. The resulting homogenate was passed through filter paper containing anhydrous sodium sulfate into a rotavapor-glass tube and dried in a rotavapor at 56 °C for 15 min. The dried sample was dissolved again in the chloroform–methanol mixture, filtered into a glass tube containing 20 ml of 0.1 M KCl, and incubated overnight at 4 °C. Subsequently, the upper phase (i.e., the aqueous fraction) was discarded, and the lower phase was dried as explained earlier for 15 min. Next, the resulting sample was dissolved again in the aforementioned mixture and dried under a 56 °C nitrogen jet. Then, the sample was transferred to a screw-cap glass tube, to which was added 5 ml of a methanol–5% sulfuric acid mixture as the methylation mixture. This tube was left overnight at 58 °C in an oven. The methylated sample was decanted into a 100-ml glass tube, to which was added 5 ml of petroleum benzene (GR, Merck, Germany) and 90 ml of double-distilled water. Finally, the upper layer of the sample was extracted and frozen at –20 °C for TG analysis.
Histological examination
The liver and adipose tissue specimens extracted from the mice were weighed. The excised specimens were fixed in 10% formalin, embedded in paraffin, and subjected to hematoxylin and eosin (H&E) staining per standard procedures (28, 29). Staining images were captured and analyzed using a digital microscope (Olympus BX51) and ImageJ 1.33, respectively.
Western blot analysis
The gastrocnemius muscles of the mice were removed rapidly, minced coarsely, homogenized immediately, and subjected to Western blotting as explained in a prior report (30). Proteins were extracted from the lysed samples by using a protein extraction reagent (Pierce, Rockford, IL, USA) as well as protease and phosphatase inhibitors. The protein concentration was measured with a BCA protein assay kit (Pierce) per manufacturer instructions. The protein samples were denatured in an SDS sample buffer containing 8% SDS (pH 6.8) and 250 mM Tris-HCL, 40% glycerol, 20% β-mercaptoethanol, and 0.016% bromophenol blue. The denatured samples were analyzed using SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The blotted membranes were blocked using 5% fat-free milk in Tris-buffered saline containing tween 20 (TBS-T) at room temperature for 1 hr. Subsequently, the membranes were incubated overnight at 4 °C with primary antibodies against SCD-1 and FAS. Then, the membranes were washed thrice in TBS-T and incubated at room temperature for 1 hr with horseradish peroxidase-linked antibodies and antimouse or antirabbit IgG antibodies. Enhanced chemiluminescence reagents (Pierce) were used to detect the immunoreactive signals. Finally, the membranes were exposed on X-ray films (Konica, Tokyo, Japan), and protein phosphorylation and expression on the films were quantified using Scion Image (Scion Corporation, Frederick, MD, USA).
Statistical analysis
Data are presented as the mean±standard error of the mean (SEM). Differences in the data of the two mouse groups were evaluated using a t-test with equal variances, with P<0.05 considered significant.
Results
Effects of olanzapine on food intake, body weight, body weight gain, and food efficiency
The body weight and body weight gain of the mice in the two groups differed because of the differences in the dietary intakes of the groups (Figure 1). At the end of the study period, the body weight of the mice treated with olanzapine was significantly higher than that of the control mice (olanzapine: 22.80±0.40 g versus control: 21.86±0.11 g, P<0.05) (Figure 1A), and so was the weekly body weight gain from the second to the seventh week (Figure 1B). As expected, mice treated with olanzapine exhibited significantly increased average food intake (measured per mouse per day; olanzapine: 5.56±0.02 g versus control 4.93±0.08 g, P<0.05) (Figure 1C). Food efficiency, measured as average change in weekly body weight (g) divided by food intake (g), mildly increased (nonsignificantly) in the olanzapine-treated mice (olanzapine: 0.054±0.007 g versus control 0.031± 0.006 g, P<0.05) (Figure 1D). The hyperphagia effects of olanzapine may partially explain its ability to induce weight gain.
Figure 1.
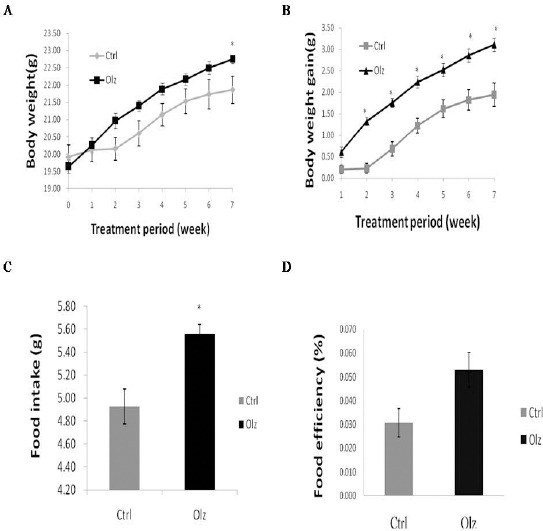
Effect of olanzapine in mice on (A) body weight and (B) body weight gain; effect on (C) average food intake per mouse per day; and effect on (D) food efficiency (average change in weekly body weight (g)/food intake (g)) after 7 weeks of treatment. All data are represented as the mean ± SEM (n=11 in each group). Statistical significance was set at *P<0.05
Blood glucose, triglyceride, and serum hormone analyses
The concentrations of plasma glucose, TG, serum insulin, and leptin are listed in Table 1. Blood glucose levels did not differ significantly between the two groups; however, the mice treated with olanzapine had significantly greater concentrations of blood TG (206.60 ±1.80 mg/dl in the olanzapine group versus 189.70 ±3.40 mg/dl in the control group, P<0.05), insulin (669.70±80.20 pg/ml in the olanzapine group versus 434.90±50.90 pg/ml in the control group, P<0.05), and leptin (3.69±1.76 ng/ml in the olanzapine group versus 1.69±0.66 ng/ml in the control group, P<0.05). The foregoing data for the control group are within or close to the normal ranges for each measured parameter (supplementary Table 1).
Table 1.
Effects of olanzapine on glucose, triglyceride, insulin, and
| Control (n = 11) | Olanzapine (n = 11) | |
|---|---|---|
| Glucose (mg/dL) | 81.50 ± 6.88 | 94.60 ± 3.45 |
| Triglyceride (mg/dL) | 189.70 ± 3.40 | 206.60 ± 1.80* |
| Insulin (pg/ml) | 434.90 ± 50.90 | 669.70 ± 80.20* |
| Leptin (ng/ml) | 1.69± 0.66 | 3.69 ± 1.76* |
All data are represented as the mean±SEM (n = 11 in each group). Statistical significance was set at
P<0.05
Effects of olanzapine on hepatic triglyceride
The measured TG concentration in the liver is shown in Figure 2. The concentration of hepatic TG wassignificantly elevated in the olanzapine group (14.38± 0.57 mmol/l in the olanzapine group versus 12.41± 0.99 mmol/l in the control group, P<0.05).
Figure 2.
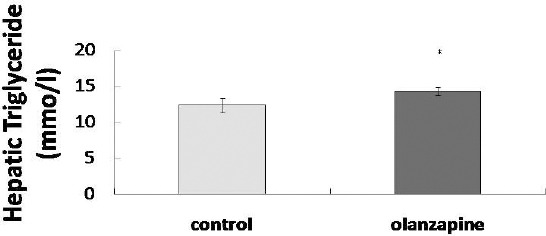
Effects of olanzapine on hepatic triglycerides in mice. All data are represented as the mean±SEM (n=11 in each group). Statistical significance was set at *P<0.05
Effects of olanzapine on fat mass and histological change in abdominal fat
At the end of the 7-week study period, the abdominal fat mass of mice was assessed (Figure 3). In the olanzapine group, the abdominal fat mass was significantly increased (0.20±0.06 grams in the olanzapine group versus 0.13±0.03 grams in the control group, P<0.05). In the olanzapine group, H&E staining revealed considerable fat cell enlargement in the abdominal fat tissues. These results suggest that olanzapine increased both abdominal fat mass and the size of fat cells.
Figure 3.

(A) effects of olanzapine on abdominal fat in mice. All data are represented as the mean ± SEM (n = 11 in each group). Statistical significance was set at *P<0.05. (B) photomicrographs (×400) of hematoxylin and eosin (H&E)-stained abdominal white adipose tissues harvested from mice after 7 weeks of treatment with olanzapine
Effects of olanzapine on liver stearoyl-CoA desaturase-1 and fatty acid synthetase expression
SCD-1 and FAS are key enzymes in TG synthesis and lipid homeostasis. Western blotting of the liver tissues revealed significantly increased SCD-1 expression (1.6-fold) in the olanzapine group relative to the control group (Figure 4A). By contrast, the FAS levels of the two groups did not differ significantly (Figure 4B).
Figure 4.
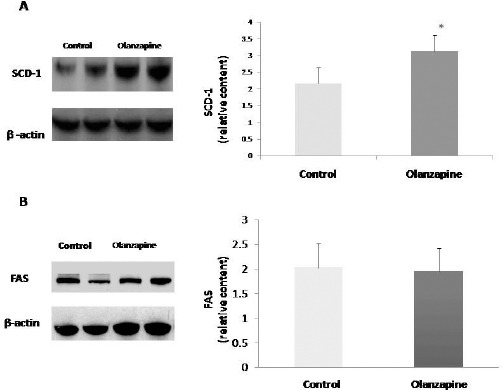
Effects of olanzapine on the expression of (A) stearoyl-CoA desaturase-1 (SCD-1) and (B) fatty acid synthase (FAS) in the liver. All data are represented as the mean±SEM (n=11 in each group). Statistical significance was set at *P<0.05
Discussion
The results of the present study indicate that olanzapine treatment may induce weight gain and metabolic changes in female C57BL/6J mice. Chronic exposure to olanzapine significantly increased abdominal fat, leptin, TG levels, and hepatic SCD-1 protein levels.
In this study, chronic administration of olanzapine significantly increased weight gain, a result consistent with related animal studies (31, 32). SGAs possess binding affinities for numerous neurotransmitter receptors (e.g., dopamine D2, serotonin 5HT2A and 5HT2C, muscarinic M1 and M3, and histamine H1 receptors) (33). Previous studies have revealed that the effects of drugs on histamine H1 receptors, serotonin 5-HT2C receptors, muscarinic receptors (34, 35), and hypothalamic AMP-activated protein kinase (36) may be attributable to the adverse metabolic effects of the drugs. Furthermore, H1 receptor antagonism was reported to provide the primary contribution to olanzapine-induced hyperphagia and weight gain (37). Similarly, we found increased food intake in the olanzapine-treated group, suggesting that hyperphagia is one cause for body weight gain.
Data on the association of leptin levels with atypical antipsychotic use are controversial: Some studies have reported weight gain with low leptin levels after olanzapine treatment (38, 39), suggesting that low levels of leptin decrease satiety signaling to the hypothalamus, which in turn increases appetite and leads to weight gain. Other studies have demonstrated increased leptin levels during atypical antipsychotic treatment, suggesting that increased leptin levels are due to weight gain and not a direct, physiological effect of atypical antipsychotics on leptin (40, 41). Our findings corroborate those of the latter studies, indicating that increased leptin levels result from weight gain.
The change in body composition attributable to olanzapine-induced weight gain is not yet fully understood. A study found that most of the olanzapine-induced gain is in the form of body fat (42). Other studies have argued that olanzapine increases the circumference of the waist and the deposition patterns of truncal fat (43, 44). Researchers (45) reported that the subcutaneous and intra-abdominal fat of schizophrenic patients increased significantly after treatment with olanzapine. Overall, these reports indicate that the increase in body weight induced by SGA is—at least partially—related to increased fat mass and that olanzapine appears to affect the abdominal fat.
Although total body composition was not evaluated in our study, we found that abdominal fat mass increased significantly and that it may contribute to the final body weight gain in olanzapine-treated animals. This result is consistent with prior studies that have demonstrated increased visceral fat or intra-abdominal fat mass after olanzapine administration in animals (46, 47), which suggests that adipose tissue may be a peripheral target of olanzapine-induced metabolic deregulation. Furthermore, in our study, olanzapine-induced fat deposition occurred in conjunction with adipocyte hypertrophy. This result is consistent with a previous report of adipocyte hypertrophy in response to olanzapine treatment (48); however, our result is inconsistent with those of earlier studies that have found adipocyte hyperplasia as an effect of SGAs (49, 50). This inconsistency is potentially attributable to variation in the metabolic response of rodents to SGAs.
In the current study, the levels of serum TG and hepatic TG increased significantly after 7 weeks of olanzapine treatment. Several studies on SGAs have found that increases in serum TG and weight gain occur independently of each other (51-53). SGAs have been hypothesized to directly affect lipid levels through a yet unknown mechanism (54). An in vitro study (55) demonstrated that olanzapine influences lipolysis and lipogenesis directly, which in turn results in the accumulation of lipids and the enlargement of adipocytes.
To further understand the effects of olanzapine on lipid metabolism, we evaluated liver SCD-1 and FAS, which are enzymes related to lipid metabolism. Regulating the oxidation, synthesis, and storage of fatty acids is crucial for maintaining normal lipid concentrations. SCD-1—an endoplasmic-reticulum-bound enzyme that converts various saturated fatty acids into monounsaturated fatty acid—is likely involved in regulating lipid and metabolic homeostasis, as evidenced in the literature (56). Animal studies have yielded evidence for an association between SCD-1 and obesity (15). Furthermore, some in vitro studies have revealed that at the transcriptional level, antipsychotic medications upregulate lipogenesis through the effect of SREBPs, including SCD-1 (17-19). In the present study, the olanzapine group exhibited significantly elevated SCD-1 protein expression. These preclinical observations suggest that the elevation in TG and increased fat mass following chronic olanzapine treatment are associated with SCD-1 protein levels.
Our results show that insulin levels significantly increased after 7 weeks of olanzapine treatment, which is consistent with several reports of chronic treatment with olanzapine both increasing insulin levels and inducing insulin resistance (57, 58). However, according to one study (59), a single olanzapine dose can decrease insulin levels significantly and induce hyperglycemia. Other studies have shown that insulin levels decrease after 14 days of olanzapine treatment (60, 61). These results indicate the time-dependent nature of insulin response to olanzapine. In addition, numerous studies have demonstrated that short-term olanzapine intake might hinder beta cell function in the pancreas and that this effect dissipates over time (59, 62, 63). Moreover, olanzapine-induced insulin resistance might trigger compensatory hyperinsulinemia. For example, temporal variation in insulin secretion has been reported among patients with schizophrenia prescribed olanzapine (64).
Consistent with some earlier studies (65, 66), the blood glucose levels in the two groups in this study did not differ significantly, evidencing that insulin resistance was not accompanied by hyperglycemia during olanzapine treatment. Furthermore, patients treated with olanzapine have been reported to develop diabetes (67, 68). Hyperinsulinemia likely maintains normal plasma glucose levels in the early phase of treatment; consequently, any ineffectiveness in maintaining compensatory hyperinsulinemia would result in the development of diabetes. The exact mechanisms underlying the association between antipsychotics and diabetes are unclear; hypothesized mechanisms include stronger insulin resistance secondary to weight gain and direct damage to pancreatic islet cells (69, 70). Given that metabolic dysregulation follows within a few weeks of olanzapine administration, we recommend that clinicians closely monitor glucose homeostasis, especially during early-stage treatment.
As listed in supplementary Table 1, in the control group, the value of each investigated parameter is within (or close to) the normal range, whereas the olanzapine group exhibited significantly higher blood concentrations of TG, insulin, and leptin.
This study had a few limitations. First, only one type of antipsychotic, olanzapine, was investigated. Olanzapine is one of the most potent SGAs given its ability to induce weight gain and dyslipidemia. Moreover, in clinical practice, olanzapine is quite effective and is widely used to treat psychiatric disorders. Hence, olanzapine was chosen as the drug of treatment in our study. However, outcomes may differ for other antipsychotic medications. Second, the effects of olanzapine were examined over a rather short time period. Olanzapine is mostly prescribed chronically; therefore, its metabolic side effects may occur only after several weeks of use. Studies with longer and varying durations will help further explore the metabolic side effects of antipsychotics.
Conclusion
This in vivo study suggests that olanzapine treatment may increase food intake and body weight gain and trigger the accumulation of abdominal fat. In addition, olanzapine increased plasma TG, hepatic TG levels, plasma insulin, and hepatic SCD-1 protein levels, but it did not influence plasma glucose levels. These results suggest a peripheral mechanism of olanzapine-induced dyslipidemia, involving enhanced lipogenesis through increased hepatic SCD-1 activity. Furthermore, our results evidenced that olanzapine may induce adverse metabolic effects during treatment, some of which may be related to the disturbance of lipid homeostasis in the liver. Owing to the association of these side effects with cardiovascular disease, clinicians should regularly monitor metabolic parameters in patients being treated with olanzapine. Additional study is required to understand and ameliorate the mechanisms underlying olanzapine-induced adverse metabolic effects.
Acknowledgment
The results reported and discussed in this paper are part of a student thesis. This work was supported in part by the Ministry of Education, Taiwan, under the Aim for the Top University plan.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.de Leon J, Susce MT, Johnson M, Hardin M, Pointer L, Ruano G, et al. A clinical study of the association of antipsychotics with hyperlipidemia. Schizophr Res. 2007;92:95–102. doi: 10.1016/j.schres.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Gebhardt S, Haberhausen M, Heinzel-Gutenbrunner M, Gebhardt N, Remschmidt H, Krieg JC, et al. Antipsychotic-induced body weight gain:predictors and a systematic categorization of the long-term weight course. J Psychiatr Res. 2009;43:620–626. doi: 10.1016/j.jpsychires.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Rzewuska M. Metabolic risk during antipsychotic treatment in patients with schizophrenia. Psychiatr Pol. 2007;41:457–472. [PubMed] [Google Scholar]
- 4.Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, et al. Antipsychotic-induced weight gain:a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 5.Kluge M, Schuld A, Himmerich H, Dalal M, Schacht A, Wehmeier PM, et al. Clozapine and olanzapine are associated with food craving and binge eating:results from a randomized double-blind study. J Clin Psychopharmacol. 2007;27:662–626. doi: 10.1097/jcp.0b013e31815a8872. [DOI] [PubMed] [Google Scholar]
- 6.McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, et al. Prevalence of the metabolic syndrome in patients with schizophrenia:baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80:19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Meyer JM, Nasrallah HA, McEvoy JP, Goff DC, Davis SM, Chakos M, et al. The Clinical Antipsychotic Trials Of Intervention Effectiveness (CATIE) Schizophrenia Trial:clinical comparison of subgroups with and without the metabolic syndrome. Schizophr Res. 2005;80:9–18. doi: 10.1016/j.schres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia:a systematic review and meta-analysis. Schizophr Res. 2010;123:225–233. doi: 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haro JM, Novick D, Suarez D, Roca M. Antipsychotic treatment discontinuation in previously untreated patients with schizophrenia:36-month results from the SOHO study. J Psychiatr Res. 2009;43:265–273. doi: 10.1016/j.jpsychires.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Kurzthaler I, Fleischhacker WW. The clinical implications of weight gain in schizophrenia. J Clin Psychiatry. 2001;62(Suppl7):32–37. [PubMed] [Google Scholar]
- 11.Tighe S, Dinan T. An overview of the central control of weight regulation and the effect of antipsychotic medication. J Clin Psychopharmacol. 2005;19:36–46. doi: 10.1177/0269881105058679. [DOI] [PubMed] [Google Scholar]
- 12.Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, et al. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res. 2002;43:1899–1907. doi: 10.1194/jlr.m200189-jlr200. [DOI] [PubMed] [Google Scholar]
- 13.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;275:30132–30138. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 14.Issandou M, Bouillot A, Brusq JM, Forest MC, Grillot D, Guillard R, et al. Pharmacological inhibition of stearoyl-CoA desaturase 1 improves insulin sensitivity in insulin-resistant rat models. Eur J Pharmacol. 2009;618:28–36. doi: 10.1016/j.ejphar.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Flowers MT, Ntambi JM. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochim Biophys Acta. 2009;1791:85–91. doi: 10.1016/j.bbalip.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paillard F, Catheline D, Duff FL, Bouriel M, Deugnier Y, Pouchard M, et al. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr Metab Cardiovasc Dis. 2008;18:436–440. doi: 10.1016/j.numecd.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Polymeropoulos MH, Licamele L, Volpi S, Mack K, Mitkus SN, Carstea ED, et al. Common effect of antipsychotics on the biosynthesis and regulation of fatty acids and cholesterol supports a key role of lipid homeostasis in schizophrenia. Schizophr Res. 2009;108:134–142. doi: 10.1016/j.schres.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Lauressergues E, Staels B, Valeille K, Majd Z, Hum DW, Duriez P, et al. Antipsychotic drug action on SREBPs-related lipogenesis and cholesterogenesis in primary rat hepatocytes. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:427–439. doi: 10.1007/s00210-010-0499-4. [DOI] [PubMed] [Google Scholar]
- 19.Ferno J, Raeder MB, Vik-Mo AO, Skrede S, Glambek M, Tronstad KJ, et al. Antipsychotic drugs activate SREBP-regulated expression of lipid biosynthetic genes in cultured human glioma cells:a novel mechanism of action? Pharmacogenomics J. 2005;5:298–304. doi: 10.1038/sj.tpj.6500323. [DOI] [PubMed] [Google Scholar]
- 20.Vik-Mo AO, Birkenaes AB, Ferno J, Jonsdottir H, Andreassen OA, Steen VM. Increased expression of lipid biosynthesis genes in peripheral blood cells of olanzapine-treated patients. Int J Neuropsychopharmacol. 2008;11:679–684. doi: 10.1017/S1461145708008468. [DOI] [PubMed] [Google Scholar]
- 21.Ferno J, Vik-Mo AO, Jassim G, Havik B, Berge K, Skrede S, et al. Acute clozapine exposure in vivo induces lipid accumulation and marked sequential changes in the expression of SREBP, PPAR, and LXR target genes in rat liver. Psychopharmacology. 2009;203:73–84. doi: 10.1007/s00213-008-1370-x. [DOI] [PubMed] [Google Scholar]
- 22.Coccurello R, Brina D, Caprioli A, Conti R, Ghirardi O, Schepis F, et al. 30 days of continuous olanzapine infusion determines energy imbalance, glucose intolerance, insulin resistance, and dyslipidemia in mice. J Clin Psychopharmacol. 2009;29:576–583. doi: 10.1097/JCP.0b013e3181bfe13e. [DOI] [PubMed] [Google Scholar]
- 23.Shertzer HG, Kendig EL, Nasrallah HA, Johansson E, Genter MB. Protection from olanzapine-induced metabolic toxicity in mice by acetaminophen and tetrahydroindenoindole. Int J Obes. 2010;34:970–979. doi: 10.1038/ijo.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon S, Noh JS, Choi SY, Baik JH. Effects of atypical antipsychotic drugs on body weight and food intake in dopamine D2 receptor knockout mice. Biochem Biophys Res Commun. 2010;393:235–241. doi: 10.1016/j.bbrc.2010.01.108. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt RH, Jokinen JD, Massey VL, Falkner KC, Shi X, Yin X, et al. Olanzapine activates hepatic mammalian target of rapamycin:new mechanistic insight into metabolic dysregulation with atypical antipsychotic drugs. J Pharmacol Exp Ther. 2013;347:126–135. doi: 10.1124/jpet.113.207621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spisni E, Tugnoli M, Ponticelli A, Mordenti T, Tomasi V. Hepatic steatosis in artificially fed marine teleosts. J Fish Dis. 1998;21:177–184. doi: 10.1046/j.1365-2761.1998.00089.x. [DOI] [PubMed] [Google Scholar]
- 27.Chang GR, Wu YY, Chiu YS, Chen WY, Liao JW, Hsu HM, et al. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin Pharmacol Toxicol. 2009;105:188–198. doi: 10.1111/j.1742-7843.2009.00427.x. [DOI] [PubMed] [Google Scholar]
- 28.Chang GR, Chen PL, Hou PH, Mao FC. Resveratrol protects against diet-induced atherosclerosis by reducing low-density lipoprotein cholesterol and inhibiting inflammation in apolipoprotein E-deficient mice. Iran J Basic Med Sci. 2015;18:1063–1071. [PMC free article] [PubMed] [Google Scholar]
- 29.Chang GR, Chiu YS, Wu YY, Chen WY, Liao JW, Chao TH, et al. Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. J Pharmacol Sci. 2009;109:496–503. doi: 10.1254/jphs.08215fp. [DOI] [PubMed] [Google Scholar]
- 30.Chang GR, Chiu YS, Wu YY, Lin YC, Hou PH, Mao FC1. Rapamycin impairs HPD-induced beneficial effects on glucose homeostasis. Br J Pharmacol. 2015;172:3793–3804. doi: 10.1111/bph.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arjona AA, Zhang SX, Adamson B, Wurtman RJ. An animal model of antipsychotic-induced weight gain. Behav Brain Res. 2004;152:121–127. doi: 10.1016/j.bbr.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 32.Cope MB, Nagy TR, Fernandez JR, Geary N, Casey DE, Allison DB. Antipsychotic drug-induced weight gain:development of an animal model. Int J Obes. 2005;29:607–614. doi: 10.1038/sj.ijo.0802928. [DOI] [PubMed] [Google Scholar]
- 33.Nasrallah HA. Atypical antipsychotic-induced metabolic side effects:insights from receptor-binding profiles. Mol Psychiatry. 2008;13:27–35. doi: 10.1038/sj.mp.4002066. [DOI] [PubMed] [Google Scholar]
- 34.Kirk SL, Glazebrook J, Grayson B, Neill JC, Reynolds GP. Olanzapine-induced weight gain in the rat:role of 5-HT2C and histamine H1 receptors. Psychopharmacology. 2009;207:119–125. doi: 10.1007/s00213-009-1639-8. [DOI] [PubMed] [Google Scholar]
- 35.Weston-Green K, Huang XF, Lian J, Deng C. Effects of olanzapine on muscarinic M3 receptor binding density in the brain relates to weight gain, plasma insulin and metabolic hormone levels. Eur Neuropsychopharmacol. 2012;22:364–373. doi: 10.1016/j.euroneuro.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Kim SF, Huang AS, Snowman AM, Teuscher C, Snyder SH. From the Cover:Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci U S A. 2007;104:3456–3459. doi: 10.1073/pnas.0611417104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Correll CU. Monitoring and management of antipsychotic-related metabolic and endocrine adverse events in pediatric patients. Int Rev Psychiatry. 2008;20:195–201. doi: 10.1080/09540260801889179. [DOI] [PubMed] [Google Scholar]
- 38.Esen-Danaci A, Sarandol A, Taneli F, Yurtsever F, Ozlen N. Effects of second generation antipsychotics on leptin and ghrelin. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1434–1438. doi: 10.1016/j.pnpbp.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Zugno AI, Barcelos M, Oliveira L, Canever L, Luca RD, Fraga DB, et al. Energy metabolism, leptin, and biochemical parameters are altered in rats subjected to the chronic administration of olanzapine. Rev Bras Psiquiatr. 2012;34:168–175. doi: 10.1590/s1516-44462012000200009. [DOI] [PubMed] [Google Scholar]
- 40.Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Serum leptin and triglyceride levels in patients on treatment with atypical antipsychotics. J Clin Psychiatry. 2003;64:598–604. doi: 10.4088/jcp.v64n0516. [DOI] [PubMed] [Google Scholar]
- 41.Albaugh VL, Henry CR, Bello NT, Hajnal A, Lynch SL, Halle B, et al. Hormonal and metabolic effects of olanzapine and clozapine related to body weight in rodents. Obesity. 2006;14:36–51. doi: 10.1038/oby.2006.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eder U, Mangweth B, Ebenbichler C, Weiss E, Hofer A, Hummer M, et al. Association of olanzapine-induced weight gain with an increase in body fat. Am J Psychiatry. 2001;158:1719–1722. doi: 10.1176/appi.ajp.158.10.1719. [DOI] [PubMed] [Google Scholar]
- 43.Graham KA, Perkins DO, Edwards LJ, Barrier RC, Jr, Lieberman JA, Harp JB. Effect of olanzapine on body composition and energy expenditure in adults with first-episode psychosis. Am J Psychiatry. 2005;162:118–123. doi: 10.1176/appi.ajp.162.1.118. [DOI] [PubMed] [Google Scholar]
- 44.Oriot P, Feys JL, Mertens de Wilmars S, Misson A, Ayache L, Fagnart O, et al. Insulin sensitivity, adjusted beta-cell function and adiponectinaemia among lean drug-naive schizophrenic patients treated with atypical antipsychotic drugs:a nine-month prospective study. Diabetes Metab. 2008;34:490–496. doi: 10.1016/j.diabet.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Ryan MC, Flanagan S, Kinsella U, Keeling F, Thakore JH. The effects of atypical antipsychotics on visceral fat distribution in first episode, drug-naive patients with schizophrenia. Life Sci. 2004;74:1999–2008. doi: 10.1016/j.lfs.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 46.Fell MJ, Marshall KM, Williams J, Neill JC. Effects of the atypical antipsychotic olanzapine on reproductive function and weight gain in female rats. J Psychopharmacol. 2004;18:149–155. doi: 10.1177/0269881104042613. [DOI] [PubMed] [Google Scholar]
- 47.Chintoh AF, Mann SW, Lam TK, Giacca A, Remington G. Insulin resistance following continuous, chronic olanzapine treatment:an animal model. Schizophr Res. 2008;104:23–30. doi: 10.1016/j.schres.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Minet-Ringuet J, Even PC, Valet P, Carpene C, Visentin V, Prevot D, et al. Alterations of lipid metabolism and gene expression in rat adipocytes during chronic olanzapine treatment. Mol Psychiatry. 2007;12:562–571. doi: 10.1038/sj.mp.4001948. [DOI] [PubMed] [Google Scholar]
- 49.Victoriano M, de Beaurepaire R, Naour N, Guerre-Millo M, Quignard-Boulange A, Huneau JF, et al. Olanzapine-induced accumulation of adipose tissue is associated with an inflammatory state. Brain Res. 2010;1350:167–175. doi: 10.1016/j.brainres.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 50.Baptista T, Araujo de Baptista E, Ying Kin NM, Beaulieu S, Walker D, Joober R, et al. Comparative effects of the antipsychotics sulpiride or risperidone in rats. I:bodyweight, food intake, body composition, hormones and glucose tolerance. Brain Res. 2002;957:144–151. doi: 10.1016/s0006-8993(02)03616-8. [DOI] [PubMed] [Google Scholar]
- 51.Kang SH, Lee JI. Metabolic disturbances independent of body mass in patients with schizophrenia taking atypical antipsychotics. Psychiatry Investig. 2015;12:242–248. doi: 10.4306/pi.2015.12.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skrede S, Ferno J, Vazquez MJ, Fjaer S, Pavlin T, Lunder N, et al. Olanzapine, but not aripiprazole, weight-independently elevates serum triglycerides and activates lipogenic gene expression in female rats. Int J Neuropsychopharmacol. 2012;15:163–179. doi: 10.1017/S1461145711001271. [DOI] [PubMed] [Google Scholar]
- 53.Birkenaes AB, Birkeland KI, Engh JA, Faerden A, Jonsdottir H, Ringen PA, et al. Dyslipidemia independent of body mass in antipsychotic-treated patients under real-life conditions. J Clin Psychopharmacol. 2008;28:132–137. doi: 10.1097/JCP.0b013e318166c4f7. [DOI] [PubMed] [Google Scholar]
- 54.Almeras N, Despres JP, Villeneuve J, Demers MF, Roy MA, Cadrin C, et al. Development of an atherogenic metabolic risk factor profile associated with the use of atypical antipsychotics. J Clin Psychiatry. 2004;65:557–564. doi: 10.4088/jcp.v65n0417. [DOI] [PubMed] [Google Scholar]
- 55.Vestri HS, Maianu L, Moellering DR, Garvey WT. Atypical antipsychotic drugs directly impair insulin action in adipocytes:effects on glucose transport, lipogenesis, and antilipolysis. Neuropsychopharmacology. 2007;32:765–72. doi: 10.1038/sj.npp.1301142. [DOI] [PubMed] [Google Scholar]
- 56.Cohen P, Ntambi JM, Friedman JM. Stearoyl-CoA desaturase-1 and the metabolic syndrome. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:271–280. doi: 10.2174/1568008033340117. [DOI] [PubMed] [Google Scholar]
- 57.Perez-Iglesias R, Vazquez-Barquero JL, Amado JA, Berja A, Garcia-Unzueta MT, Pelayo-Teran JM, et al. Effect of antipsychotics on peptides involved in energy balance in drug-naive psychotic patients after 1 year of treatment. J Clin Psychopharmacol. 2008;28:289–295. doi: 10.1097/JCP.0b013e318172b8e6. [DOI] [PubMed] [Google Scholar]
- 58.Wu RR, Zhao JP, Guo XF, He YQ, Fang MS, Guo WB, et al. Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients:a double-blind, placebo-controlled study. Am J Psychiatry. 2008;165:352–358. doi: 10.1176/appi.ajp.2007.07010079. [DOI] [PubMed] [Google Scholar]
- 59.Chintoh AF, Mann SW, Lam L, Lam C, Cohn TA, Fletcher PJ, et al. Insulin resistance and decreased glucose-stimulated insulin secretion after acute olanzapine administration. J Clin Psychopharmacol. 2008;28:494–499. doi: 10.1097/JCP.0b013e318184b4c5. [DOI] [PubMed] [Google Scholar]
- 60.Chiu CC, Chen KP, Liu HC, Lu ML. The early effect of olanzapine and risperidone on insulin secretion in atypical-naive schizophrenic patients. J Clin Psychopharmacol. 2006;26:504–7. doi: 10.1097/01.jcp.0000237947.80764.d9. [DOI] [PubMed] [Google Scholar]
- 61.Weston-Green K, Huang XF, Deng C. Olanzapine treatment and metabolic dysfunction:a dose response study in female Sprague Dawley rats. Behav Brain Res. 2011;217:337–346. doi: 10.1016/j.bbr.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 62.Spoelstra JA, Stolk RP, Cohen D, Klungel OH, Erkens JA, Leufkens HG, et al. Antipsychotic drugs may worsen metabolic control in type 2 diabetes mellitus. J Clin Psychiatry. 2004;65:674–678. doi: 10.4088/jcp.v65n0512. [DOI] [PubMed] [Google Scholar]
- 63.van Winkel R, De Hert M, Wampers M, Van Eyck D, Hanssens L, Scheen A, et al. Major changes in glucose metabolism, including new-onset diabetes, within 3 months after initiation of or switch to atypical antipsychotic medication in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2008;69:472–429. doi: 10.4088/jcp.v69n0320. [DOI] [PubMed] [Google Scholar]
- 64.Chiu CC, Chen CH, Chen BY, Yu SH, Lu ML. The time-dependent change of insulin secretion in schizophrenic patients treated with olanzapine. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:866–870. doi: 10.1016/j.pnpbp.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 65.Smith RC, Lindenmayer JP, Davis JM, Kelly E, Viviano TF, Cornwell J, et al. Effects of olanzapine and risperidone on glucose metabolism and insulin sensitivity in chronic schizophrenic patients with long-term antipsychotic treatment:a randomized 5-month study. J Clin Psychiatry. 2009;70:1501–1513. doi: 10.4088/JCP.08m04446yel. [DOI] [PubMed] [Google Scholar]
- 66.Newcomer JW, Ratner RE, Eriksson JW, Emsley R, Meulien D, Miller F, et al. A 24-week, multicenter, open-label, randomized study to compare changes in glucose metabolism in patients with schizophrenia receiving treatment with olanzapine, quetiapine, or risperidone. J Clin Psychiatry. 2009;70:487–499. doi: 10.4088/jcp.08m04132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goff DC, Cather C, Evins AE, Henderson DC, Freudenreich O, Copeland PM, et al. Medical morbidity and mortality in schizophrenia:guidelines for psychiatrists. J Clin Psychiatry. 2005;66:183–94. doi: 10.4088/jcp.v66n0205. quiz 47, 273-274. [DOI] [PubMed] [Google Scholar]
- 68.Liao CH, Chang CS, Wei WC, Chang SN, Liao CC, Lane HY, et al. Schizophrenia patients at higher risk of diabetes, hypertension and hyperlipidemia:a population-based study. Schizophr Res. 2011;126:110–116. doi: 10.1016/j.schres.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Baptista T, Kin NM, Beaulieu S, de Baptista EA. Obesity and related metabolic abnormalities during antipsychotic drug administration:mechanisms, management and research perspectives. Pharmacopsychiatry. 2002;35:205–219. doi: 10.1055/s-2002-36391. [DOI] [PubMed] [Google Scholar]
- 70.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects:a comprehensive literature review. CNS drugs. 2005;19(Suppl1):1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]


