Abstract
Objective(s):
Iron is an essential element for living organisms. Iron overload can have detrimental effects on health. This study pertains to the protective role of berberine against ferrous sulfate-induced hepatic and renal functional disorders and histological damages in rats.
Materials and Methods:
The rats were divided into four groups (n=7): Sham, Ber (10 mg/kg/day for 14 days, by gavage), FS (ferrous sulfate, 30 mg/kg/day for 14 days, intraperitoneally), FS + Ber (ferrous sulfate, 30 mg/kg/day for 14 days; berberine, 10 mg/kg/day for 11 days from fourth day of ferrous sulfate injection). After 24 hr, blood, urine, and tissue samples were collected.
Results:
Compared with sham and Ber groups, administration of ferrous sulfate resulted in liver and kidney dysfunction as evidenced by significantly higher levels of serum hepatic markers and bilirubin, and lower levels of serum albumin, total protein, triglyceride, cholesterol, and glucose, as well as lower creatinine clearance and higher fractional excretion of sodium. This was accompanied by increased malondialdehyde levels and histological damages. Berberine treatment significantly reversed the levels of serum hepatic markers, renal functional markers and lipid peroxidation marker in the FS + Ber group. Furthermore, it restored the levels of serum total protein, albumin, glucose, triglycerides, and cholesterol with a decrease in bilirubin concentration in the blood. All these changes were corroborated by histological observations of the liver and kidney.
Conclusion:
Berberine protects the liver and kidneys against ferrous sulfate-induced toxicity by reduction in lipid peroxidation and ability to chelate iron.
Keywords: Berberine, Creatinine, Ferrous sulfate, Glucose, Kidney, Liver
Introduction
Iron is an essential micronutrient (1) that can cycle reversibly between its ferrous (Fe2+) and ferric (Fe3+) oxidation states (2). This property is essential for iron functions, but makes it very dangerous, since free iron is a powerful catalyst for hydroxyl radical formation and lipid peroxidation (3). Iron-overload conditions cause iron deposition in different body organs including liver, which is the major site of iron storage (4). Iron is stored in the liver as ferritin, in which iron is not available to participate in Fenton reaction and, with heavy iron loading, as hemosiderin (5).
Evidence has shown that iron has an important role in various tissue injuries of the body involving reactive oxygen species (6). It has been suggested that iron can exert renal toxicity (7) and it has been implicated in renal tubular injury due to the formation of hydroxyl (•OH) radical (8). Also, the critical role of iron in mediating tissue injury in several models of acute kidney injury induced by rhabdomyolysis (9), cisplatin (10), and ischemia/reperfusion (11) is well established.
Extracts obtained from the roots and barks of various Berberis species are used as folk remedies worldwide for the treatment of various inflammatory ailments (12). Berberine is an isoquinoline alkaloid, which could be found in the root, rhizome, and stem bark of several plant species, such as Berberis vulgaris. Berberine exerts a wide range of pharmacological properties, including anti-inflammatory (12), antihypercholesterolemic (13), antihyperlipidaemic (14), antiemetic (15), and antitoxic (1). Nevertheless, the protective role of berberine against ferrous sulfate (FS)-induced hepato- and nephrotoxicity has not been investigated. Thus, the goal of the current report is to evaluate the ability of berberine in protection against FS-induced functional disorders and liver and kidney tissue damage in rats.
Materials and Methods
Experimental procedure
Male Wistar rats (250–300 g) were purchased from Razi Institute, Shiraz, Iran. They were maintained at 25 ± 2 °C with a 12:12 hr light/dark cycle. The animals had free access to a standard pellet diet and water ad libitum. The Ethics committee of Shiraz University approved the study. The rats were divided into four groups (7 each): Sham (1 ml distilled water was given intraperitoneally), Ber (berberine, 10 mg/kg/day dissolved in 1 ml distilled water and given by gavage for 14 days), FS (ferrous sulfate, 30 mg/kg/day dissolved in 1 ml distilled water and given intraperitoneally (IP) for14 days), FS + Ber (ferrous sulfate, 30 mg/kg/day for 14 days; berberine, 10 mg/kg/day for 11 days from fourth day of ferrous sulfate injection). After14 days of ferrous sulfate/berberine treatment, rats were placed in metabolic cages and 24-hr urine was collected. Thereafter, rats were anesthetized with ketamine (60 mg/kg, IP) and xylazine (5 mg/kg, IP) and blood samples were obtained from heart ventricles. Then the liver and the left kidney were quickly isolated. Parts of liver and kidney were preserved for future histological examination and the rest was immediately snap-frozen in liquid nitrogen and stored at -70 °C until further use. Rats were killed by injecting an overdose of anesthetics.
Renal functional assessments
Urine samples were collected at the end of the 24-hr period and total volume was recorded. Urinary creatinine was measured by colorimetric methods (Prestige, Biolis24I, Japan) and used in conjunction with serum creatinine concentration and urine flow to calculate creatinine clearance (CCr) as an indicator of glomerular function. Urinary Na+ was measured at the end of the 24-hr period and used in conjunction with serum Na+ to estimate the fractional excretion of Na+ (FENa) as an indicator of tubular dysfunction.
Biochemical estimation
Alanine aminotransferase (ALT), aspartate amino-transferase (AST), lactate dehydrogenase (LDH), and alkaline phosphatase (ALP) activities in serum and liver tissue samples (homogenized in a citrate buffer, pH 4.8, centrifuged at 10000 × g for 15 min at 4 °C, the supernatant was removed and stored on ice) were measured by commercially available kits. Also, total protein, albumin, glucose, triglycerides, and cholesterol concentrations in serum were measured by commercially available kits.
Lipid peroxidation assay
Malondialdehyde (MDA) levels were determined spectrophotometrically in tissue samples (homogenized in 5 ml of trichloroacetic acid per 0.1 g of tissue) (16). Malondialdehyde reacts with thiobarbituric acid (TBA) to produce a pink color with an absorption maximum at 532 nm.
Histopathological examinations
Liver and kidney samples were fixed in the buffered 10 % formaldehyde. After dehydration through a graded alcohol series, the samples were cleared in xylol. Then, liver and kidney samples were embedded in paraffin and 5-μm sections were obtained using a microtome (Erma, Japan). Routine staining with Prussian blue as well as hematoxylin and eosin were done for each liver and kidney section. In a blinded fashion, each section was examined in at least 10 randomly selected non-overlapping fields under a light microscope. In each liver section, the degree of the presence of congestion and cellular degenerative changes were examined. The renal histopathology was quantified for tubular necrosis, loss of brush borders, and formation of casts and vascular congestion. The level of each pathological manifestation was graded according to the observed changes as follow: none with 0, less than 20 % with 1, 21–40 % with 2, 41–60% with 3, 61–80 % with 4, and greater than 80 % with 5. The sum of all numerical scores in each group was taken as the total histopathological score.
Statistical analysis
Data are presented as mean±SEM. They were assessed by one-way analysis of variance followed by Tukey’s post hoc for comparison between groups. All data analyses were performed using SPSS (ver. 22) software package (SPSS Software, Chicago, IL, USA) and significance was considered at P<0.05.
Results
Effect of berberine on FS-induced changes in body weight
Figure 1 shows that body weight values of the FS group had no significant change in comparison with sham and Ber groups (P<0.05). In the FS+Ber group body weight value was statistically lower in comparison with sham (P<0.001) and Ber (P<0.01) groups, but it showed no significant change in comparison with the FS group.
Figure 1.

Berberine and ferrous sulfate-induced changes in body weight. Data are the mean±SEM (n=7 in each group). ***P<0.001 compared with the sham group; ††P<0.01 compared with the Ber group
Effect of berberine on FS-induced changes in hepatic functional markers
Figure 2 depicts the levels of serum hepatic markers in sham and experimental rats. In Fe-treated rats, the activities of AST, ALT, ALP, and LDH were significantly increased (P<0.01, P<0.05, P<0.001, P<0.001, respectively). Administration of berberine significantly reversed these changes (P<0.01, P<0.05, P<0.001, and P<0.001 for AST, ALT, ALP, and LDH, respectively).
Figure 2.
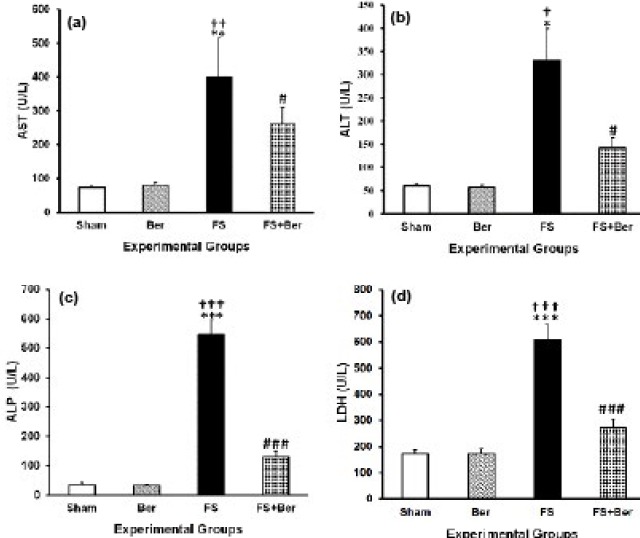
Berberine and ferrous sulfate-induced changes in hepatic enzyme activities. Data are the mean±SEM (n=7 in each group). *P<0.05, **P<0.01, and *** P<0.001 compared with the sham group; † P<0.05, †† P<0.01, and ††† P<0.001 compared with the Ber group; #P<0.05 and ### P<0.001 compared with the ferrous sulfate (FS) group
Table 1 shows that serum levels of total protein (P<0.05), albumin (P<0.01), triglycerides (P<0.001, P<0.01 compared with sham and Ber groups, respectively), cholesterol (P<0.01), and glucose (P<0.001, P<0.01 compared with sham and Ber groups, respectively) in the FS group were statistically lower than those of sham and Ber groups. It also shows that serum level of total bilirubin in the FS group was statistically higher than those of sham and Ber groups (P<0.001). Berberine treatment significantly reversed these changes (P<0.05 for all except P<0.001 for total bilirubin, P <0.01 for triglyceride and cholesterol).
Table 1.
Effect of berberine on FS-induced changes in the levels of rat serum biochemical parameters
| Groups | Sham | Ber | FS | FS + Ber |
|---|---|---|---|---|
| Total bilirubin (U/l) | 0.62±0.11 | 0.60±0.11 | 3.28±0.38***††† | 2.25±0.30***†††♯♯♯ |
| Total protein (mg/dl) | 6.58±0.44 | 6.72±0.29 | 5.24±0.29*† | 6.40±0.14♯ |
| Albumin (mg/dl) | 4.34±0.22 | 4.11±0.30 | 2.74±0.18**†† | 3.44±0.23♯ |
| Triglyceride (mg/dl) | 75.28 ± 6.09 | 61.85 ± 7.10 | 31.42 ± 4.94***†† | 67.71 ± 4.67♯♯ |
| Cholestrol (mg/dl) | 47.42 ± 2.99 | 47.00 ± 2.74 | 31.28 ± 1.74**†† | 46.14 ± 4.63♯ |
| Glucose (mg/dl) | 127.71 ± 9.30 | 132.42 ± 8.93 | 68.14 ± 3.63**††† | 126.28 ± 13.56♯♯ |
P<0.05,
P<0.01, and
P<0.001 as compared to the sham group;
P<0.05,
P<0.01, and
P<0.001 as compared to the Ber group;
P<0.05,
P<0.01, and
P<0.00, as compared to the FS group
Ber: berberine; FS: ferrous sulfate
Table 2 shows that AST, ALT, ALP, and LDH levels of liver tissue in the FS group were statistically higher than those of sham and Ber groups (P<0.01, P<0.05, P<0.001, and P<0.001, respectively). Berberine treatment reduced the levels of AST, ALT, ALP, and LDH in FS + Ber in comparison to the FS group (P<0.05, P<0.05, P<0.001, and P<0.001, respectively).
Table 2.
Effect of berberine on FS-induced changes in the activities of hepatic and lipid peroxidation markers in liver tissue homogenates in experimental groups (each n=7)
| Groups | Sham | Ber | FS | FS + Ber |
|---|---|---|---|---|
| AST (U/l) | 761.14 ± 31.67 | 715.00± 67.00 | 1818.28 ± 41.94**†† | 1584.57± 97.31♯ |
| ALT (U/l) | 665.71± 35.96 | 687.00 ± 16.93 | 1797.00 ± 118.99*† | 1483.42±39.39♯ |
| ALP (U/l) | 685.85 ± 40.71 | 678.28 ± 51.15 | 1532.00 ± 36.62***††† | 1387.00±59.20*†♯♯♯ |
| LDH (U/l) | 692.42 ± 48.81 | 693.001 ± 43.08 | 3243.71 ± 270.07***††† | 2363 ± 195.65♯♯♯ |
| MDA (mol/g tissue) | 0.42 ± 0.04 | 0.55 ± 0.09 | 1.40 ± 0.20***††† | 0.84 ± 0.10♯♯♯ |
P<0.05,
P<0.01, and
P<0.001 as compared to the sham group;
P<0.05,
P<0.01, and
P<0.001 as compared to the Ber group;
P<0.05,
##P<0.01, and
P<0.001, as compared to the ferrous sulfate (FS) group
Effect of berberine on FS-induced changes in renal function parameters
In Fe-treated rats, renal functional markers such as creatinine, urea nitrogen (Table 3), and fractional Na+-excretion (FENa) (Figure 3) were significantly increased (P<0.01, P<0.001, and P<0.01, respectively), while creatinine clearance was significantly (P<0.05) decreased (Figure 3). Administration of berberine significantly reversed these changes (P<0.001, P<0.01, P<0.001, and P<0.01 for creatinine, urea nitrogen, fractional Na+-excretion, and creatinine clearance, respectively).
Table 3.
Effect of berberine (Ber) on the levels of renal function parameters and lipid peroxidation marker in experimental groups (n=7)
| Groups | Sham | Ber | FS | FS + Ber |
|---|---|---|---|---|
| Creatinine (mg/dl) | 0.45± 0.02 | 0.47± 0.01 | 0.78 ± 0.05***††† | 0.52 ± 0.04♯♯♯ |
| Urea nitrogen (mg/dl) | 25.42 ± 2.23 | 26.28 ± 2.69 | 36.42 ± 2.05**†† | 26.42 ± 1.25♯♯ |
| MDA (mol/g tissue) | 0.42 ± 0.04 | 0.55 ± 0.10 | 1.25 ± 0.21***†† | 0.75 ± 0.07♯ |
*P<0.05,
P<0.01,
P<0.001 vs sham group;
†P<0.05,
P<0.01,
P<0.001 vs Ber group;
P<0.05,
P<0.01,
P<0.00, vs FS group
Figure 3.

Berberine and ferrous sulfate-induced changes in renal function parameters (CCr and FENa). Data are the mean±SEM (n=7 in each group). *** P<0.001 compared with the sham group; † P<0.05 and ††† P<0.001 compared with the Ber group; ## P<0.01 and ### P<0.001 compared with the FS group
Effect of berberine on FS-induced oxidative stress in the liver and kidney
Tables 2 and 3 show that ferrous sulfate treatment resulted in significant increases in MDA contents of the liver (P<0.001) and kidney (P<0.001) compared with the sham and Ber groups. Berberine treatment reduced the levels of MDA contents of the liver (P<0.001) and kidney (P<0.05) in FS + Ber in comparison to the FS group.
Effect of berberine on FS-induced histological damages in the liver and kidney
Results of the histological studies of the liver were in agreement with the measured activities of hepatic enzymes. There were no abnormalities or histological changes in the livers of sham and Ber groups (Figures 4 and 5). In the FS group (Figure 3), iron deposition which indicates accumulation of iron (grade 2) was observed. In the FS + Ber group (Figure 3) less iron deposition (grade 1) was noticed in comparison with the FS group. In the FS group the most prominent lesions were hepatocellular ballooning (grade 2, Figure 5a), apoptosis of the hepatic cells (grade 2, Figure 5b), infiltration of inflammatory cells in the portal space (grade 3, Figure 5c), and vacuolization of the hepatocyte cytoplasm (grade 2, Figure 5d). In the FS + Ber group, less intense lesions were noticed in comparison with the FS group (Figures 5e and 5f).
Figure 4.

Representative light microphotographs of the livers. Prussian blue-stained sections were evaluated by light microscopy. The histological changes observed included iron deposition
Figure 5.
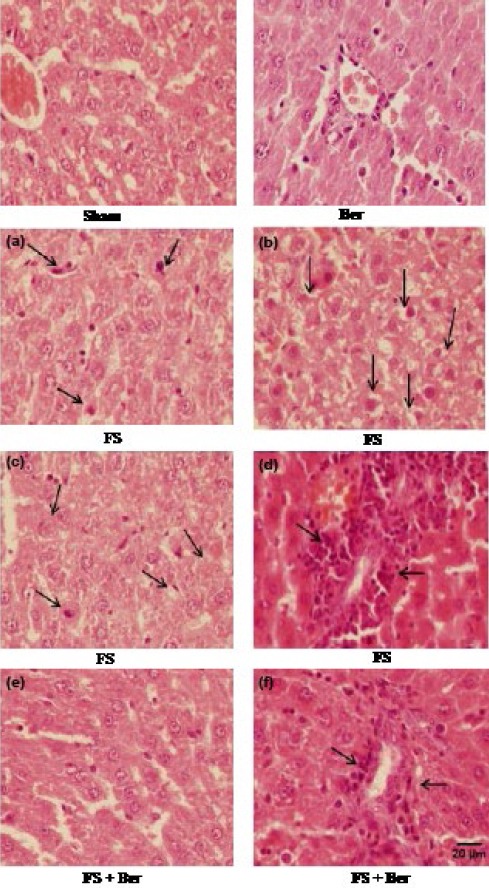
Representative light microphotographs of the livers. Haematoxylin and eosin-stained sections were evaluated by light microscopy. The histological changes observed included apoptosis (a), swelling (b), vacuolization (c), and inflammatory cell infiltration (d)
Renal sections from the sham (Figures 6a1, 6a2, 6a3, and 6a4) and Ber groups (Figures 6b1, 6b2, 6b3, and 6b4) exhibited only minimal or no changes. In the FS group, iron deposition (grade 2) was observed in the cortex (Figure 6c1) and medulla (Figure 6c2). However, in the FS + Ber group (Figures 6d1 and 6d2) less iron deposition (grade 1) was noticed in comparison with the FS group. Furthermore, in the FS group, there was congestion (grade 2) in glomerular capillaries (Figure 6c3) and medullary vessels (Figure 6c4). However, in the FS + Ber group (Figures 6d3 and 6d4) less congestion (grade 1) was observed in comparison with the FS group. The sum of histopathological grades, marking the changes described above, is mentioned in Table 4.
Figure 6.
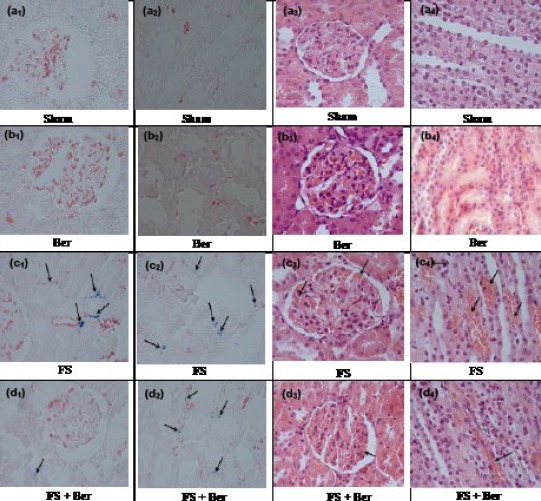
Representative light microphotographs of the kidneys. Prussian blue- and hematoxylin & eosin-stained sections were evaluated by light microscopy. The histological changes observed included iron deposition in the cytoplasm of: cortical (c1, d1) and medullary (c2, d2) tubular cells, Prussian blue staining; glomerular capillary congestion (c3, d3) and medullary vascular congestion (c4, d4), haematoxylin-eosin staining
Table 4.
Total histopathological score in Sham, berberine (Ber), ferrous sulfate FS, and FS + Ber groups (n=7) at the end of the experiment
| Experimental groups | Liver | Kidney |
|---|---|---|
| Sham | 0.0 ± 0.00 | 0.0 ± 0.00 |
| Ber | 0.0 ± 0.00 | 0.0 ± 0.00 |
| FS | 11.25 ± 0.55*** | 7.62 ± 0.37*** |
| FS + Ber | 4.25 ± 0.36††† | 3.75 ± 0.25††† |
Values (sum of histopathological scores in each group) are expressed as mean±xsSEM
P<0.001 as compared to the Sham and Ber groups
P<0.001 as compared to the FS groups
Discussion
The present study demonstrated that ferrous sulfate (FS) leads to functional disorders and histological damages to the liver and kidney. Our findings suggest that berberine treatment attenuates FS-induced liver and kidney functional injury and MDA levels in hepatic and renal tissues compared to the FS group.
It has been suggested that chronic deposition of excess iron in hepatic parenchymal cells can lead to hepatic injury (5). In the present study, changes in AST, ALT, ALP, and LDH in serum and liver tissue following FS administration are indicators of liver damage. Induction of oxidative stress and the increased production of reactive oxygen species, as a result of excessive iron deposition in the liver, are thought to play key roles in triggering the damage (17). In the FS group, administration of berberine (10 mg/kg/day for 14 days) significantly protected hepatocytes against toxic effects of iron, as shown by improvement in histology and prevention of increase in plasma AST, ALT, ALP, and LDH levels and liver lipid peroxidation. In this regard, the membrane protective effect of berberine has already been reported (18).
One of the most important functions of the liver is protein synthesis. From the results obtained, there was a significant decrease in total protein and albumin levels in the FS group compared to that of the sham group. In agreement with our results, it has been shown that total protein level is decreased in FS-induced hepatotoxicity (17, 19). The berberine-induced rise in the levels of total protein and albumin demonstrated that berberine could improve liver function.
In this work, ferrous sulfate treatment resulted in decreased serum glucose concentration. The liver releases glucose both by glycogenolysis and gluconeogenesis (20). It has been suggested that imperfect energy-linked mitochondrial function impairs gluconeogenesis, and depletion of hepatic glycogen stores following uncoupling of oxidative phosphorylation leads to hypoglycemia (21). Berberine treatment significantly inversed the ferrous sulfate induced peroxidative damage in the liver as evidenced by the lower levels of MDA, probably due to the antioxidative effect of berberine. Besides, berberine raised glucose concentration to the normal level due to its ability to reduce lipid peroxidation. In this regard, earlier experimental findings have suggested that berberine induces glycolysis as a consequence of inhibition of glucose oxidation in mitochondria (22). Protecting the ability of berberine against Fe2+-induced lipid peroxidation has been already shown (23). Light microscopy for experimental groups showed that accumulation of iron in the liver was effectively reduced by berberine, which reveals that berberine chelates the iron (18). It is well noticed that berberine possesses antioxidant action (18), which can scavenge the excess iron in biological systems.
Oxidative stress is frequently stated to be a central mechanism of hepatocellular injury. In the present study, other considerable pathological features of FS-induced hepatotoxicity were leukocyte infiltration and apoptosis. It might be due to the formation of ROS due to iron-induced oxidative stress (24). Administration of berberine reduced the histological alterations induced by ferrous sulfate and restored normal physiological functions. This is in accordance with the result of Ghareeb et al. (2015) who found that berberine attenuated inflammatory cell infiltrations induced by CCL4 in the liver (25).
Light microscopy for the FS group showed hepatocyte ballooning and cytoplasmic vacuolization. High dose of ferrous sulfate led to increased levels of serum concentrations of cholesterol and TG in the FS group. The Fe-induced rise of cholesterol in serum may be due to changes in the gene expression of hepatic enzymes mainly HMG-COA reductase as was suggested by others (26). Berberine attenuated the increased blood levels of these lipids in the FS+Ber group. Taken together, the therapeutic effect of berberine against hyperlipidemia, observed in this study, is probably due to the combined effect of its inhibitory activity of HMG-CoA reductase (25) and its antioxidant capacity (18). Besides, Brusq et al. have demonstrated that berberine inhibits cholesterol (27, 28) and TG (28) synthesis in hepatic cells.
Histopathological evaluations of the FS group kidneys showed mild congestion of glomerular capillaries and medullary vessels. Besides, moderate iron deposition in the cytoplasm of tubular cells in the FS group was the basis for cellular damage. The prolonged keeping of Fe in the tissues elevates oxidative stress that ends in pathological changes in the kidney (1). Administration of berberine reduced the renal histological alterations induced by ferrous sulfate.
It is well known that oxidative stress plays a major role in the renal structural and functional deterioration by altering biochemical indicators. In this study, FS-induced nephrotoxicity was manifested by elevated serum creatinine and BUN levels (Table 3). These results are in accordance with the findings of Pari et al. (2015) in male rats (29). It is recognized that elevated blood urea is correlated with increased protein catabolism in mammals (30). Our results show that serum concentrations of total protein were decreased in the FS group (Table 1). Also, the increase in serum urea and creatinine concentrations as well as FENa in this work may be the result of kidney dysfunction. In our model of FS-induced nephrotoxicity, berberine showed remarkable useful effects, with the almost complete restoration of CCr and FENa to normal levels. This prevention of the CCr decrease and FENa increase was along with fewer histological damages. Berberine administration significantly inversed the ferrous sulfate induced peroxidative damage in the kidney which is evident from the lowered levels of MDA, probably due to its antioxidant feature (1, 31). In this regard, the nephroprotective effect of berberine against renal damage induced by cisplatin (32) and gentamicin (33) through its antioxidant, anti-inflammatory, and anti-apoptotic properties is well documented.
Conclussion
In summary, this study demonstrates for the first time that berberine has potent protective effects against iron-induced hepato- and nephrotoxicity. Here we demonstrate that berberine administration attenuated serum and tissue hepatic markers, bilirubin, creatinine, BUN, lipid peroxidation marker, iron deposition, renal functional disorders, and renal histological damages, and normalized the hepatic cells’ architecture. Berberine exerts its hepato- and nephroprotective effects via its antioxidant potential by scavenging the free radicals and chelating iron through binding to iron and reducing the concentration of the catalyzing iron in lipid peroxidation. Since berberine itself did not exert harmful effects in normal rats, it should be considered as a hepato- and nephroprotective agent in iron-induced hepato- and nephrotoxicity. However, clinical experiments to acknowledge the efficacy of berberine treatment in patients are necessary.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Li Z, Geng YN, Jiang JD, Kong WJ. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid Based Complement Alternat Med. 2014;2014:289264. doi: 10.1155/2014/289264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bresgen N, Eckl PM. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules. 2015;5:808–847. doi: 10.3390/biom5020808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci. 2017;38:592–607. doi: 10.1016/j.tips.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Papanastasiou DA, Vayenas DV, Vassilopoulos A, Repanti M. Concentration of iron and distribution of iron and transferrin afterexperimental iron overload in rat tissues in vivo:study of the liver,the spleen, the central nervous system and other organs. Pathol ResPract. 2000;1:47–54. doi: 10.1016/S0344-0338(00)80021-7. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GJ, Frazer DM. Hepatic iron metabolism. Semin Liver Dis. 2005;25:420–432. doi: 10.1055/s-2005-923314. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease:an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 7.Zager RA, Johnson ACM, Hanson SY. Parenteral iron nephrotoxicity:potential mechanisms and consequences. Kidney Int. 2004;66:144–156. doi: 10.1111/j.1523-1755.2004.00716.x. [DOI] [PubMed] [Google Scholar]
- 8.Madhusudhan KS, Oberoi R. Renal iron deposition in aplastic ane-mia:magnetic resonance imaging appearance. Indian J Nephrol. 2011;21:134–135. doi: 10.4103/0971-4065.82145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO, Arosio P, et al. Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest. 2013;123:4423–4434. doi: 10.1172/JCI67867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998;53:394–401. doi: 10.1046/j.1523-1755.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- 11.de Vries B, Walter SJ, von Bonsdorff L, Wolfs TG, van Heurn LW, Parkkinen J, et al. Reduction of circulating redox-active iron by apotransferrin protects against renal ischemia-reperfusion injury. Transplantation. 2004;77:669–675. doi: 10.1097/01.tp.0000115002.28575.e7. [DOI] [PubMed] [Google Scholar]
- 12.Yeşilada E, Küpeli E. Berberis crataegina DC. root exhibits potent anti-inflammatory, analgesic and febrifuge effects in mice and rats. J Ethnopharmacol. 2002;79:237–248. doi: 10.1016/s0378-8741(01)00387-7. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Dong B, Park SW, Lee HS, Chen W, Liu J. Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J Biol Chem. 2009;284:28885–28895. doi: 10.1074/jbc.M109.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel-Rahman M, Moawad Mahmoud A, Bastawy N, Eissa H. Anti-hyperlipidemic and myocardial enhancing effects of berberine in high fat diet/streptozotocin-induced diabetic rats;Possible role of adiponectin. Nutri Food Sci Int J. 2016;2:1–7. [Google Scholar]
- 15.Abd El-Wahab AE, Ghareeb DA, Sarhan EE, Abu-Serie MM, El Demellawy MA. In vitro biological assessment of Berberis vulgaris and its active constituent, berberine:antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement Altern Med. 2013;13:218. doi: 10.1186/1472-6882-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heath R, Packer L. Photoperoxidation in isolated chloroplast.I. Kinetics and stoichiometry of fatty acid peroxidation. J Biochem Biophs. 1986;125:189–190. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 17.Pawar N, Nimbalkar V, Gaikvad P. Hepatoprotective activity of a polyherbal mixture in ferrous sulphate and ethanol induced hepatotoxicity experimental animals. Der Pharmacia Sinica. 2012;3:594–597. [Google Scholar]
- 18.Shirwaikar A, Shirwaikar A, Rajendran K, Punitha IS. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol Pharm Bull. 2006;29:1906–1910. doi: 10.1248/bpb.29.1906. [DOI] [PubMed] [Google Scholar]
- 19.Sowmya A, Nagarajan V. Hepatoprotective potential of Azima tetracantha and Tribulus terrestris on ferrous sulfate-induced toxicity in rat. RJPBCS. 2014;5:380–387. [Google Scholar]
- 20.Shrayyef MZ, Gerich JE. Normal glucose homeostasis. In: Poretsky L, editor. Principles of Diabetes mellitus. Springer; 2010. pp. 19–35. [Google Scholar]
- 21.Pingale S, Patil V, Patil S, Sangle D. Study of carbohydrate metabolism in severe acute malnutrition and correlations of weight and height with pp-sugar and BMI. IJTRA. 2015;3:334–342. [Google Scholar]
- 22.Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab. 2008;294:E148–E156. doi: 10.1152/ajpendo.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oboh G, Akinyemi AJ, Ademiluyi AO. Antioxidant and inhibitory effect of red ginger (Zingiber officinale var Rubra) and white ginger (Zingiber officinale Roscoe) on Fe(2+) induced lipid peroxidation in rat brain in vitro. Exp Toxicol Pathol. 2012;64:31–36. doi: 10.1016/j.etp.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Czaja MJ. Induction and regulation of hepatocyte apoptosis by oxidative stress. Antioxid Redox Signal. 2004;4:759–767. doi: 10.1089/152308602760598909. [DOI] [PubMed] [Google Scholar]
- 25.Ghareeb DA, Khalil S, Hafez HS, Bajorath J, Ahmed HE, Sarhan E, et al. Berberine reduces neurotoxicity related to nonalcoholic steatohepatitis in rats. Evid Based Complement Alternat Med. 2015;2015:361847. doi: 10.1155/2015/361847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima R, Randall JD, Ito E, Manshio H, Suzuki Y, Gullans SR. Regulation of expression of the stress response gene, Osp94 :identification of the tonicity response element and intracellular signalling pathways. Biochem J. 2004;380:783–794. doi: 10.1042/BJ20040313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, et al. Berberine is a novel cholesterol-lowering drug working through a uniqe mechanism distinc from statins. 2004;10:1344–1351. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 28.Brusq JM, Pascal Grondin NA, Guillard R, Martin S, Saintillan Y, Issandou M. Inhibition of lipid synthesis through activation of AMP kinase:an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res. 2006;47:1281–1288. doi: 10.1194/jlr.M600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Pari L, Asaithambi K, Paramasivam K, Ayyasamy R. Protective effects of hesperidin on oxidative stress,dyslipidaemia and histological changes in iron-inducedhepatic and renal toxicity in rats. Toxicol Rep. 2015;2:46–55. doi: 10.1016/j.toxrep.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harper HA, Rodwell VW, Mayes PA, Cochrum KC, Grodsky GM, Martin DWJ, et al. Review of physiological chemistry. 17th ed. Los Altos, California, USA: Lange Medical Publications; 1979. [Google Scholar]
- 31.Zhou JY, Zhou SW. Protective effect of berberine on antioxidant enzymes and positive transcription elongation factor b expression in diabetic rat liver. Fitoterapia. 2011;82:184–189. doi: 10.1016/j.fitote.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Domitrovic R, Cvijanovic O, Pernjak-Pugel E, Skoda M, Mikelic L, Crncevic-Orlic Z. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem Toxicol. 2013;62:397–406. doi: 10.1016/j.fct.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Adil M, Kandhare AD, Dalvi G, Ghosh P, Venkata S, Raygude KS, et al. Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Ren Fail. 2016;38:996–1006. doi: 10.3109/0886022X.2016.1165120. [DOI] [PubMed] [Google Scholar]


