Abstract
Objective(s):
Sepsis can result in severe organ injury by provoking inflammatory cascades and oxidative stress. Several studies are currently underway to find a drug with anti-inflammatory effects to prevent mortality and morbidity during sepsis. The present study was undertaken to assess the effects of metformin on oxidative stress and antioxidant status in sepsis induced by the Cecal Ligation and Puncture (CLP) method.
Materials and Methods:
Male Wistar rats were divided into 4 groups (n=10): sham, CLP, and 50 and 100 mg/kg metformin-treated CLP groups. After 12 hr, blood samples were collected and lung tissue was removed for histopathological study to detect tissue damage and degree of inflammation based on neutrophil infiltration and assay of the oxidative stress biomarkers superoxide dismutase (SOD), total antioxidant capacity (TAC), malondialdehyde (MDA), myeloperoxidase (MPO), glutathione peroxidase (GPx), and plasminogen activator inhibitor-1 (PAI-1).
Results:
The MPO activity and MDA level were decreased in the metformin-treated groups (P<0.05). Moreover, the groups receiving metformin showed lower inflammation scores than the CLP group (P<0.05). No significant differences in SOD, GPx, or PAI in the different groups were observed. The TAC level was reduced in the CLP group compared to the sham group (P<0.05), and interestingly, this value was reduced even further in the metformin-treated groups (P<0.05 compared with the CLP group).
Conclusion:
It was concluded that metformin protects lung tissue against sepsis-induced oxidative damage, and this protective effect may be more related to its anti-inflammatory and reduced neutrophil accumulation and less to its anti-oxidative properties.
Keywords: CLP, Inflammation, Metformin, Oxidative damages, Sepsis
Introduction
Sepsis is a systemic inflammatory response to infection, which is a major reason for morbidity and mortality in a critical care setting. Despite improvements in critical care management, sepsis is a considerable health problem worldwide (1). In the United States, one epidemiological study revealed that during 1979–2000, the total number of sepsis-related mortalities rose from 22 to 44 per 100,000 population (2). Although the pathophysiology of sepsis is not well understood, monocytes orchestrate the innate immune system response to various noxious insults, especially bacterial infections, by expressing a wide range of pro- and anti-inflammatory cytokines (3) and producing free radicals such as reactive oxygen species (ROS) (4). Moreover, the endotoxin derived from g-negative bacteria is associated with an oxidant-antioxidant imbalance that appears as endothelial damage, neutrophil reinforcement, and mitochondrial injury (5). In addition to Gram-negative bacteria-evoked direct tissue damage, it has been suggested that the most important component of injury during sepsis is mediated by the host’s inflammatory and hemostatic responses to the sepsis-induced infection (6, 7).
Metformin is an oral anti-hyperglycemic drug that is regularly prescribed as a first-line drug therapy for type 2 diabetes mellitus. Metformin is an insulin sensitizer that reduces blood sugar through the induction of peripheral glucose uptake and decreasing hepatic gluconeogenesis (8, 9). The principal molecular target of metformin is the intracellular adenosine monophosphate-activated protein kinase (AMPK) enzyme (10). AMPK is a serine/threonine kinase that is activated by an increase in the AMP/ATP ratio and involved in the metabolism of carbohydrates and fat (11). It is activated by exercise, oxidative stress, hypoxia, and ischemia (12). In addition to its catabolic effects, AMPK has been described to play an important role in inflammation and the treatment of inflammation-related disorders (13-16). Different lines of evidence have indicated that through AMPK activation, metformin produces a potent anti-inflammatory response both in vivo and in vitro (16-19). It has also been reported that metformin attenuated hepatic inflammation in human and animal models of nonalcoholic steatohepatitis with attenuating tumor necrotic factor-alpha (TNF-α)-induced pro-inflammatory response in the liver (20-22). In addition, researchers reported that metformin protected the liver against early damage caused by post-surgical sepsis and blunted LPS-induced damage (16). Interestingly, Tsoyi et. al. showed that metformin decreased the expression of LPS-induced inflammatory genes and pro-inflammatory cytokines in vitro (18). Furthermore, the protective effects of metformin on LPS-induced innate immunity in rat hearts were recently demonstrated (23).
The experimental model of sepsis is a useful tool for investigating the beneficial effects of drugs during sepsis and for identifying potential new therapies to reduce sepsis-associated morbidity and mortality. Cecal Ligation and Puncture (CLP) is a model of polymicrobial sepsis, characterized by the presence of multiple enteric species within 18–20 hr, which closely emulates human polymicrobial sepsis (24). The present study investigated the influence of metformin on oxidative-stress biomarkers and histopathological changes of lung tissue in the rat model of CLP.
Materials and Methods
Animals
Male Wistar rats (weight range: 270–300 g) were bought from Razi Institute, Tehran, Iran. The animals were kept at controlled ambient temperature (23±2 °C) in a standard polypropylene cage (four rats per cage), and housed under a 12 hr light/dark cycle with access to food and water ad libitum. Afterward, the rats were randomly allocated into four experimental groups (n=10). All experiments were carried out according to guidelines for the care of laboratory animals by the research affairs of Tabriz Medical Sciences University.
Grouping
Forty male Wistar rats were randomly allocated into four groups: sham-operated group, CLP group, and CLP operated group receiving metformin (50 and 100 mg/kg). Needless to say that the chosen dose of metformin was selected based on the previous studies (17, 25).
Drug administration
Metformin (Osveh Pharmaceutical Inc., Tehran, Iran) was dissolved in normal saline and injected intraperitoneally (IP) at a volume of 0.5 ml 2 hr after completion of the surgery. Under the same conditions, the sham-operated and the CLP groups received 0.5 ml normal saline.
Cecal ligation and puncture
For the CLP operation, animals were anesthetized with IP administration of ketamine and xylazine (90 and 10 mg/kg body weight, respectively). A 2-cm incision was made in the lower abdomen to expose the cecum. The cecum was externalized and ligated with 4-0 silk just below the ileocecal valve. The cecum was punctured once in the middle with a sterile 18-gauge needle. In order to ensure that the wound was patented, the cecum was squeezed to extrude a small amount of stool into the peritoneal cavity. The cecum was placed back and the abdominal cavity was then closed with sutures. Rats were resuscitated with subcutaneous injection of normal saline (3 ml/100 g body weight, 37 °C) follow by 15 min incubation at 37 °C. The sham-operated control animals were treated in an identical manner, but no surgical manipulation of the cecum was performed (24). 12 hr after CLP, rats were euthanized by an overdose of pentobarbital and after blood sampling through cardiac puncture, the lungs were harvested and rinsed in cool normal saline. One part of the harvested lungs was used for histological examination and the other parts were kept at -70 °C for biochemical analysis.
Histopathological study
For the histopathological study, immediately after harvesting the pulmonary tissue, it was fixed in a 10% formalin solution for 24 hr, dehydrated by an increasing concentration of alcohol series, and cleared by xylene series. After histological processing, the tissue was fixed in paraffin and split to a thickness of 4 μm using a microtome. The lung sections were stained with hematoxylin-eosin (H&E). After staining, an experienced pathologist blinded to treatment evaluated the lung processed samples under light microscopy and scored them based on the degree of inflammation and neutrophil infiltration. The severity of tissue injury was scored as follows: 1: normal; 2: mild; 3: moderate; and 4: severe.
Determination of plasma plasminogen activator inhibitor (PAI) level
Plasma plasminogen activator inhibitor (PAI) level sustainably rises following sepsis and confers a worse prognosis. In order to evaluate the PAI plasma level, blood samples were collected in heparinized tubes 12 hr after the CLP via cardiac puncture. The samples were centrifuged at 2000 rpm for 10 min and the plasma separated and stored at –70 °C. PAI-1 levels were quantified by a highly sensitive rat ELISA kit (Glory Sciences, USA), according to the manufacturer’s instructions and the results were presented as ng/ml.
Determination of malondialdehyde (MDA) level of plasma and lung tissue
MDA, which is the main by-product of polyunsaturated fatty acid oxidation, is a known biomarker of lipid peroxidation principally induced by ROS. The MDA concentration in plasma and lung tissue was determined using the Thiobarbituric Acid-Reactive Substance (TBARS) assay. In this method, MDA reacts with thiobarbituric acid to produce a pink color and its absorbance is measured spectrophotometrically at 532 nm. The MDA level was expressed as nmol/ml tissue extract normalized to protein content of the sample (nmol/ml tissue extract/mg protein) for tissue MDA and nmol/ml of plasma for plasma MDA (26).
Determination of myeloperoxidase (MPO) activity of lung tissue
MPO activity, a unique marker of neutrophil inflammation, was measured according to the method of Bradley et al. (27). In brief, the lung tissue was homogenized in a solution containing 0.5% (w/v) hexadecyl-trimethyl-ammonium-bromide (HTAB) dissolved in 50 mM potassium phosphate buffer (pH=6) in an ice bath using a Polytron homogenizer (50 mg tissue/ml; 3 min at 8500 rpm). The homogenate was freeze-thawed three times and sonicated for 10 sec, then centrifuged at 4500 rpm at 4 °C for 45 min. The supernatant (100 μl) or standard (Sigma, Germany) was added to 2.9 ml of phosphate buffer (50 mM; pH=6) including 0.167 mg/ml of O-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. Five mins later, the reaction was stopped after addition of 0.1 ml of 1.2 M hydrochloric acid and absorbance was measured spectrophotometrically (Cecil 9000, Cambridge, UK) at 400 nm. MPO activity was defined as the quantity of enzyme degrading 1 μmol peroxide/min at 37 °C. Values are expressed as units of MPO activity per mg weight of wet tissue (U/mg).
Determination of superoxide dismutase (SOD) level of lung tissue
Superoxide is a component of antioxidant response and catalyzes the conversion of superoxide anions to hydrogen peroxide. To determine the SOD level, the rat lung tissues were homogenized in a KCl solution and then centrifuged and the supernatants were used for SOD assay according to the method of Paoletti et al. (28). In this method, superoxide anion is generated from molecular oxygen in the presence of Ethylenediaminetetraacetic acid (EDTA), manganese (II) chloride, and mercaptoethanol. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidation is linked to the availability of superoxide anions in the medium. The change in absorbance at 340 nm was monitored spectrophotometrically. The enzyme activity was reported as units per ml of extract normalized to protein content of the sample (unit/ml tissue extract/mg protein).
Determination of glutathione peroxidase (GPx) level of lung tissue
GPx plays a major role in the regulation of inflammatory processes, including protection against ROS. GPx activity was measured according to the method of Paglia and Valentine (29). The lung tissues were homogenized in a KCl solution and were then centrifuged and the supernatants were used for GPx assay. GPx catalyzes the oxidation of glutathione by cumene hydroperoxide. In the presence of glutathione reductase and NADPH, the oxidized glutathione is reduced with a concomitant oxidation of NADPH to NADP+. Tissue GPx was measured by the Randox commercial kit and the change in the absorbance at 340 nm was monitored spectrophotometrically. Activity was given in units per ml of extract normalized to protein content of the sample (unit/ml tissue extract/mg protein).
Determination of total antioxidant capacity (TAC) of whole blood
In this assay, 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonate) (ABTS) is incubated with a peroxidase (metmyoglobin) and H2O2 to produce the radical cation of ABTS. This species has a relatively stable blue-green color, which is measured at 660 nm. Sample antioxidants cause suppression of this color production to a degree that is proportional to antioxidant concentration (30).
Statistical evaluation
Data are expressed as mean±SD. The normal distribution of data was evaluated by Kolmogorov–Smirnov test. One-way ANOVA was used to find out differences between the groups. In the case of significant difference in ANOVA analysis, Tukey post hoc test was performed. Differences were considered significant at P<0.05.
Results
Histopathological study
Semi-quantitative data analysis of histopathological examinations of sections taken from lung tissue is demonstrated in Figure 1. Significant differences were found in the control group in comparison to the sham group, which represent the CLP procedure-induced tissue damage and inflammation (P<0.05). Metformin treatments (50 and 100 mg/kg) reduced the inflammation score compared to the control group (P<0.05).
Figure 1.
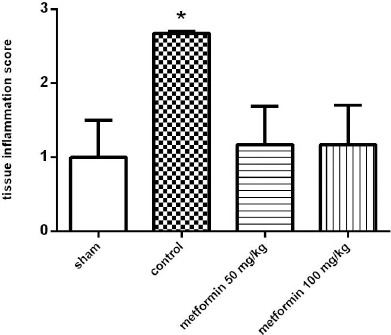
The histopathological examination of the lung tissue based on inflammation scores 12 hr after CLP. Metformin was administrated 2 hr after completion of the CLP surgery (50 and 100 mg/kg, IP). Data are expressed as mean±SD (n=10). *P<0.01 from the sham group; #P<0.05 from control group using one way ANOVA with Tukey’s post-hoc test
Plasma concentration of PAI-1
The plasma concentrations of PAI-1 in the different groups were determined using the ELISA method 12 hr following the CLP. As depicted in Figure 2, there were no significant differences between the different groups in PAI-1 plasma levels.
Figure 2.
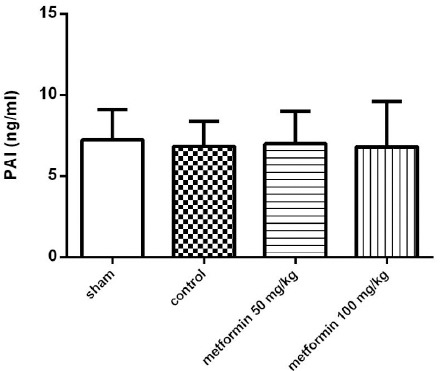
The plasma concentration of PAI-1 12 hr after CLP. Metformin was administrated 2 hr after completion of the CLP surgery (50 and 100 mg/kg, IP). Data are expressed as mean±SD (n=10) and analyzed by one way ANOVA
Plasma and pulmonary MDA concentrations
MDA concentrations in the lung tissues of different groups and in plasma are presented in Figure 3. CLP in the control group significantly increased the MDA content in tissues as compared with the sham group (P<0.05, Figure 3a). Metformin (in both doses) attenuated the CLP-induced increase in MDA levels compared to the control group; however, the reduction did not reach a significant level (P>0.05, Figure 3a). There was no difference in MDA plasma concentration between the groups (Figure 3b).
Figure 3.
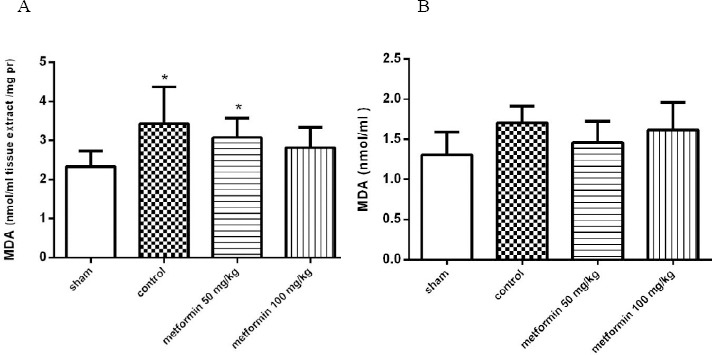
A: The MDA concentration in lung tissues 12 hr after CLP. Metformin was administrated 2 hr after completion of the CLP surgery (50 and 100 mg/kg, IP). B: The plasma level of MDA 12 hr after CLP. There were not any differences in MDA plasma concentration among groups. Data are expressed as mean±SD (n=10). *P<0.01 from the sham group using one way ANOVA with Tukey’s post-hoc test
Pulmonary MPO activity levels
The CLP-induced pulmonary injury was accompanied by increased MPO activity, which is a sign of neutrophil infiltration. 12 hr after the operation, MPO levels were significantly higher in the control group than in the sham group (P<0.05, Figure 4). The effect of metformin on MPO activity was also examined at this time point. Metformin at the doses of 50 and 100 mg/kg significantly suppressed the elevation of MPO activity (P<0.05).
Figure 4.
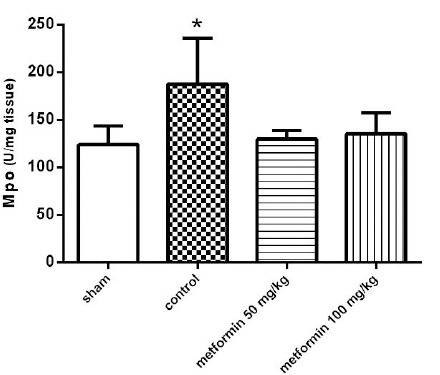
The myeloperoxidase activity (MPO) of lung tissues 12 hr after CLP. Metformin was administrated 2 hr after completion of the CLP surgery (50 and 100 mg/kg, IP). Data are expressed as mean± SD (n=10). *P<0.05 from the sham group; #P<0.05 from control group using one way ANOVA with Tukey’s post-hoc test
Pulmonary GPx and SOD Levels
The effect of the CLP procedure and metformin treatments on the tissue levels of GPX and SOD activity were evaluated. As is summarized in Figures 5 and 6, respectively, there were no significant differences between groups in tissue SOD and GPx enzyme activity (P>0.05).
Figure 5.
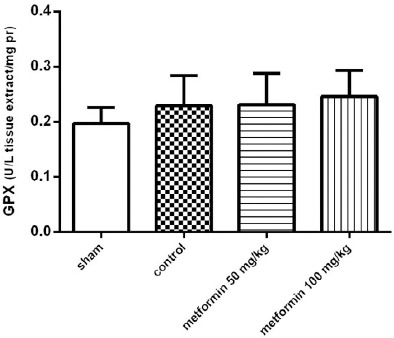
The Glutathione Peroxidase (GPx) activity of lung tissues 12 hr after CLP. Metformin was administrated 2 hr after completion of the CLP surgery (50 and 100 mg/kg, IP). Data are expressed as mean±SD (n=10) and analyzed using one way ANOVA
Figure 6.
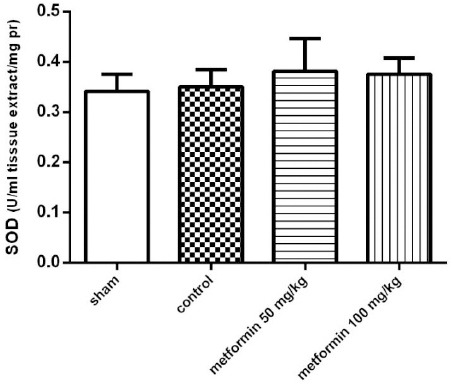
The Superoxide Dismutase (SOD) activity of lung tissues 12 hr after CLP. Metformin was administrated 2 hr after completion of the CLP surgery (50 and 100 mg/kg, IP). Data are expressed as mean ± SD (n=10) and analyzed using one way ANOVA
TAC of blood
The results revealed a significant decrease in the blood TAC levels of the control group compared to the sham group (P<0.05, Figure 6). Interestingly, TAC levels were significantly reduced in the blood of rats treated with metformin compared to the control group (P<0.05, Figure 7).
Figure 7.
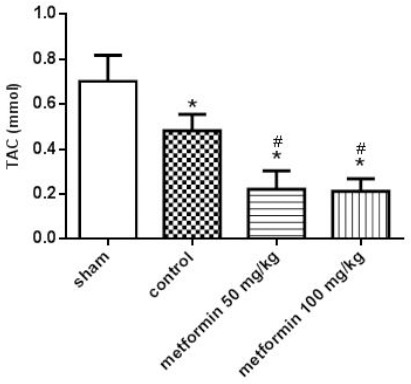
Total antioxidant capacity (TAC) of blood 12 hr after CLP. Metformin was administrated 2 hr after completion of the CLP surgery (50 and 100 mg/kg, IP). Data are expressed as mean±SD (n=10). *P<0.05 from the sham group; #P<0.05 from control group using one way ANOVA with Tukey’s post-hoc test
Discussion
In line with our efforts to find new indications for old drugs (31, 32) in sepsis management, the effects of two different doses of metformin (50 and 100 mg/kg) on a rat model of polymicrobial sepsis were investigated. The results demonstrated the salutary properties of metformin in an animal model of sepsis. Metformin significantly improved CLP-induced pulmonary histological damage, hampered lipid peroxidation, and diminished neutrophil infiltration, which is indexed as decreased MPO activity. Moreover, the reduction of CLP-induced pulmonary injury by metformin was associated with the decreased leucocyte infiltration observed in the histological inspection. The most suitable animal model of sepsis, the CLP, was used in this study, as it appears to be the most representative of the clinical situation of human sepsis (24).
Sepsis results from the immense triggering of the body’s immunity mechanisms by invading micro-organisms which is characterized by an extensive inflammatory response (33), multiorgan dysfunction, and tissue ischemia/reperfusion injuries (34). Previously, it has been reported that metformin has beneficial effects on endotoxin-induced liver and heart injuries (16, 23) and improves survival in a mouse model of lethal endotoxemia (18). The current findings were in accordance with the results of studies on the anti-inflammatory effects of metformin. Despite accumulating data concerning the anti-inflammatory role of metformin, its exact mechanism(s) at the cellular level is not clearly known.
Increased rates of lipid peroxidation and MPO are found in rats with sepsis. MPO is stored in some immune cells, such as neutrophils. The infiltration and activation of neutrophils produce a large number of detrimental components (e.g., ROS), leading to the exacerbation of inflammation. With respect to the current findings, metformin is able to significantly reduce MPO activity in pulmonary tissue. This result is inconsistent with those of another study, which indicated that metformin reduced MPO activity in the left ventricle of rats following an isoproterenol-induced myocardial infarction (17). Another role of ROS in the progression of inflammation and detrimental tissue injury was expressed on the raised tissue MDA level, which was significantly reduced by metformin. This inhibition of MDA generation and lipid peroxidation may help to ameliorate pulmonary damage induced by sepsis.
SOD over-activation in conditions like inflammation and sepsis causes the accumulation of H2O2, which can generate hydroxyl radicals (OH) (35). However, in the current study, SOD was not increased following 12 hr after CLP in the septic group compared to the sham group. Moreover, GPx protects cytosolic organelles from oxidative damage by converting H2O2 to water and preventing lipid peroxidation (36). The absence of any change in GPx levels found in this study may correlate with the SOD results. It is supposed that, since the amount of H2O2 was not increased because of any changes in SOD levels, the GPx values remained unchanged. Considering these results, it seems that the mechanism of metformin in reducing sepsis-induced lung injuries is based more on reducing the accumulation of neutrophils and MPO activity and less on antioxidative and radical scavenging effects.
In the present study, TAC, an index of antioxidant defense, was used for the widest assessment of oxidative status. Changes in TAC levels in patients with severe sepsis remain controversial. Some studies have suggested that the TAC of serum reflects the severity of illness in patients with severe sepsis (37-39). According to the current results, serum TAC levels declined in both the control and the metformin-treated groups. In sepsis, the major pathophysiological challenge, rather than the pathogen itself, is the host’s inflammatory response to the pathogen (37, 39, 40). The reason for lower TAC is not clear but it seems that as inflammatory and oxidative factors increase, TAC also increases in parallel in order to compensate (39). The decrease in TAC amount in metformin-treated groups may be justified because of lower oxidative and inflammatory stress.
PAI levels were significantly higher in septic shock and severe sepsis patients (41). Metformin was demonstrated to be a potent inhibitor of the expression of PAI (42); however, this result is not supported by the current study. This difference in results can be attributed to differences in metformin dose, time, and route of administration or differences in the sepsis induction method.
Based on the results obtained in different studies, metformin has been proven to be beneficial against inflammatory conditions (19, 43). AMPK dependent/independent pathways are involved in the beneficial effect of metformin. A study demonstrated that in macrophages, the activation of AMPK by metformin resulted in the attenuation of the expression of LPS-induced pro-inflammatory cytokines and mediators (14).
It is known that sepsis interrupts mitochondrial ATP production in response to mitochondrial oxidative damage (44), and metformin can modulate this process by activating AMPK. Hence, AMPK is a potential target that protects cells from mitochondrial injury and attenuates inflammation. Further studies are necessary to determine whether these early promising results utilizing metformin and other AMPK activators are beneficial in controlling sepsis in clinical application.
The anti-inflammatory effects of metformin were presented by this study and other research however, because of the risk of metabolic acidosis induced by metformin especially in sepsis status, we will need more evidence about the safety issue of metformin in sepsis to use this drug clinically.
Conclusion
It is concluded that metformin protects lung tissue against sepsis-induced oxidative damage, and this protective effect may be related more to its anti-inflammatory and decreasing neutrophil accumulation and less to its anti-oxidative properties.
Acknowledgment
The results presented in this paper were part of a student thesis. The authors would like to thank Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran, for financial support.
References
- 1.Kissoon N, Carcillo JA, Espinosa V, Argent A, Devictor D, Madden M, et al. World Federation of Pediatric Intensive Care and Critical Care Societies:Global Sepsis Initiative. Pediatric critical care medicine: Pediatr Crit Care Med. 2011;12:494–503. doi: 10.1097/PCC.0b013e318207096c. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170:1435–1444. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chabot F, Mitchell JA, Gutteridge JM, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J. 1998;11:745–757. [PubMed] [Google Scholar]
- 5.Andrades ME, Ritter C, Dal-Pizzol F. The role of free radicals in sepsis development. Front Biosci. 2009;1:277–287. doi: 10.2741/E27. [DOI] [PubMed] [Google Scholar]
- 6.Strassheim D, Park JS, Abraham E. Sepsis:current concepts in intracellular signaling. Int J Biochem Cell Biol. 2002;34:1527–1533. doi: 10.1016/s1357-2725(02)00049-3. [DOI] [PubMed] [Google Scholar]
- 7.Jagneaux T, Taylor DE, Kantrow SP. Coagulation in sepsis. Am J Med Sci. 2004;328:196–204. doi: 10.1097/00000441-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Galuska D, Nolte LA, Zierath JR, Wallberg-Henriksson H. Effect of metformin on insulin-stimulated glucose transport in isolated skeletal muscle obtained from patients with NIDDM. Diabetologia. 1994;37:826–832. doi: 10.1007/BF00404340. [DOI] [PubMed] [Google Scholar]
- 9.Hundal HS, Ramlal T, Reyes R, Leiter LA, Klip A. Cellular mechanism of metformin action involves glucose transporter translocation from an intracellular pool to the plasma membrane in L6 muscle cells. Endocrinology. 1992;131:1165–1173. doi: 10.1210/endo.131.3.1505458. [DOI] [PubMed] [Google Scholar]
- 10.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardie DG. AMP-activated protein kinase:an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, McCullough LD. Effects of AMP-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:480–492. doi: 10.1038/jcbfm.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5'-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol. 2009;182:8005–8014. doi: 10.4049/jimmunol.0803563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 16.Bergheim I, Luyendyk JP, Steele C, Russell GK, Guo L, Roth RA, et al. Metformin prevents endotoxin-induced liver injury after partial hepatectomy. J Pharmacol Exp Ther. 2006;316:1053–61. doi: 10.1124/jpet.105.092122. [DOI] [PubMed] [Google Scholar]
- 17.Soraya H, Farajnia S, Khani S, Rameshrad M, Khorrami A, Banani A, et al. Short-term treatment with metformin suppresses toll like receptors (TLRs) activity in isoproterenol-induced myocardial infarction in rat:are AMPK and TLRs connected? Int Immunopharmacol. 2012;14:785–791. doi: 10.1016/j.intimp.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Tsoyi K, Jang HJ, Nizamutdinova IT, Kim YM, Lee YS, Kim HJ, et al. Metformin inhibits HMGB1 release in LPS-treated RAW 264.7 cells and increases survival rate of endotoxaemic mice. Br J Pharmacol. 2011;162:1498–1508. doi: 10.1111/j.1476-5381.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, et al. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26:611–617. doi: 10.1161/01.ATV.0000201938.78044.75. [DOI] [PubMed] [Google Scholar]
- 20.Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 21.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 22.Nair S, Diehl AM, Wiseman M, Farr GH, Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis:a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 23.Vaez H, Rameshrad M, Najafi M, Barar J, Barzegari A, Garjani A. Cardioprotective effect of metformin in lipopolysaccharide-induced sepsis via suppression of toll-like receptor 4 (TLR4) in heart. Europ jof pharmacol. 2015;772:115–123. doi: 10.1016/j.ejphar.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 24.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nature protocols. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soraya H, Khorrami A, Garjani A, Maleki-Dizaji N, Garjani A. Acute treatment with metformin improves cardiac function following isoproterenol induced myocardial infarction in rats. Pharmacol Rep. 2012;64:1476–1484. doi: 10.1016/s1734-1140(12)70945-3. [DOI] [PubMed] [Google Scholar]
- 26.Olgen S, Coban T. Antioxidant evaluations of novel N-H and N-substituted indole esters. Biol Pharm Bull. 2003;26:736–738. doi: 10.1248/bpb.26.736. [DOI] [PubMed] [Google Scholar]
- 27.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation:estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 28.Paoletti F, Aldinucci D, Mocali A, Caparrini A. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal biochem. 1986;154:536–541. doi: 10.1016/0003-2697(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 30.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Zolali E, Asgharian P, Hamishehkar H, Kouhsoltani M, Khodaii H, Hamishehkar H. Effects of gamma oryzanol on factors of oxidative stress and sepsis-induced lung injury in experimental animal model. Iran J Basic Med Sci. 2015;18:1257–1263. [PMC free article] [PubMed] [Google Scholar]
- 32.Zolali E, Hamishehkar H, Maleki-Dizaji N, Majidi Zolbanin N, Ghavimi H, Kouhsoltani M, et al. Selenium effect on oxidative stress factors in septic rats. Adv Pharm Bull. 2014;4:289–293. doi: 10.5681/apb.2014.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall JC. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 2001;29:99–106. doi: 10.1097/00003246-200107001-00032. [DOI] [PubMed] [Google Scholar]
- 35.Andrades M, Ritter C, Moreira JC, Dal-Pizzol F. Oxidative parameters differences during non-lethal and lethal sepsis development. J Surg Res. 2005;125:68–72. doi: 10.1016/j.jss.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Ozturk E, Demirbilek S, Begec Z, Surucu M, Fadillioglu E, Kirimlioglu H, et al. Does leflunomide attenuate the sepsis-induced acute lung injury? Pediatr Surg Int. 2008;24:899–905. doi: 10.1007/s00383-008-2184-y. [DOI] [PubMed] [Google Scholar]
- 37.Tsai K, Hsu T, Kong C, Lin K, Lu F. Is the endogenous peroxyl-radical scavenging capacity of plasma protective in systemic inflammatory disorders in humans? Free Radic Biol Med. 2000;28:926–933. doi: 10.1016/s0891-5849(00)00180-5. [DOI] [PubMed] [Google Scholar]
- 38.MacKinnon KL, Molnar Z, Lowe D, Watson ID, Shearer E. Measures of total free radical activity in critically ill patients. Clin Biochem. 1999;32:263–268. doi: 10.1016/s0009-9120(98)00109-x. [DOI] [PubMed] [Google Scholar]
- 39.Pascual C, Karzai W, Meier-Hellmann A, Oberhoffer M, Horn A, Bredle D, et al. Total plasma antioxidant capacity is not always decreased in sepsis. Crit Care Med. 1998;26:705–709. doi: 10.1097/00003246-199804000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Opal SM. Clinical trial design and outcomes in patients with severe sepsis. Shock. 2003;20:295–302. doi: 10.1097/01.shk.0000084343.58020.57. [DOI] [PubMed] [Google Scholar]
- 41.Zeerleder S, Schroeder V, Hack CE, Kohler HP, Wuillemin WA. TAFI and PAI-1 levels in human sepsis. Thromb Res. 2006;118:205–212. doi: 10.1016/j.thromres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 42.He G, Pedersen SB, Bruun JM, Lihn AS, Richelsen B. Metformin, but not thiazolidinediones, inhibits plasminogen activator inhibitor-1 production in human adipose tissue in vitro. Horm Metab Res. 2003;35:18–23. doi: 10.1055/s-2003-38386. [DOI] [PubMed] [Google Scholar]
- 43.Caballero AE, Delgado A, Aguilar-Salinas CA, Herrera AN, Castillo JL, Cabrera T, et al. The differential effects of metformin on markers of endothelial activation and inflammation in subjects with impaired glucose tolerance:a placebo-controlled, randomized clinical trial. J Clin Endocrinol Metab. 2004;89:3943–3948. doi: 10.1210/jc.2004-0019. [DOI] [PubMed] [Google Scholar]
- 44.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. 2007;35:2408–2416. doi: 10.1097/01.ccm.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]


