Abstract
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and a major public health problem worldwide. Hepatocarcinogenesis is a complex multistep process at molecular, cellular, and histologic levels with key alterations that can be revealed by noninvasive imaging modalities. Therefore, imaging techniques play pivotal roles in the detection, characterization, staging, surveillance, and prognosis evaluation of HCC. Currently, ultrasound is the first-line imaging modality for screening and surveillance purposes. While based on conclusive enhancement patterns comprising arterial phase hyperenhancement and portal venous and/or delayed phase wash-out, contrast enhanced dynamic computed tomography and magnetic resonance imaging (MRI) are the diagnostic tools for HCC without requirements for histopathologic confirmation. Functional MRI techniques, including diffusion-weighted imaging, MRI with hepatobiliary contrast agents, perfusion imaging, and magnetic resonance elastography, show promise in providing further important information regarding tumor biological behaviors. In addition, evaluation of tumor imaging characteristics, including nodule size, margin, number, vascular invasion, and growth patterns, allows preoperative prediction of tumor microvascular invasion and patient prognosis. Therefore, the aim of this article is to review the current state-of-the-art and recent advances in the comprehensive noninvasive imaging evaluation of HCC. We also provide the basic key concepts of HCC development and an overview of the current practice guidelines.
Keywords: Magnetic resonance imaging, Surveillance, Computed tomography, Staging, Ultrasound, Guidelines, Diagnosis, Hepatocellular carcinoma, Prognosis
Core tip: Noninvasive imaging modalities allow diagnosis, characterization, staging, surveillance, and prognosis evaluation of hepatocellular carcinoma (HCC). Functional magnetic resonance imaging techniques show promise in providing further important information of tumor biological behaviors, and, thus, improve the early detection ability and characterization accuracies for HCC. Development of prediction model comprising serological, imaging, texture, and radiogenomic parameters may facilitate preoperative evaluation of tumor recurrence and patient survival. Here, we reviewed recent advances in imaging techniques for noninvasive HCC assessment, basic key concepts of HCC development, and current practice guidelines for HCC management.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a malignant epithelial tumor derived from hepatocytes. HCC is the fifth most common type of cancer and the second leading cause of cancer-related death worldwide[1]. Approximately 70%-90% of HCCs are developed on the background of established liver cirrhosis or advanced fibrosis[2]. Hepatitis B virus (HBV) and/or hepatitis C virus (HCV) infection, alcohol, and nonalcoholic fatty liver disease (NAFLD) are the most predominant risk factors for HCC worldwide[3-6].
Currently, the most effective curative treatment for HCC is liver transplantation[7-16]. However, its wide application is limited by the shortage of liver grafts and the possibility of tumor recurrence. Other major treatment options include hepatic resection, local ablation, transarterial chemoembolization, systematic therapy, and best supportive care[7-16]. However, the prognosis of HCC is largely dependent on the stage at which the tumor is detected, with complete curative treatment of the early-stage HCCs being the most effective way to improve long-term patient survival.
Noninvasive imaging modalities, including ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI), have played pivotal roles in assessing HCC in recent decades. Several clinical practice guidelines[7-16] have recommended noninvasive imaging approaches as the first-line tools for the screening, diagnosis, staging, and surveillance of HCC. Unlike many other solid tumors, the diagnosis of HCC can be established according to characteristic enhancement patterns on dynamic multiphasic CT or MRI, without further requirements for histopathologic confirmation. In addition, microbubble enhanced US[17], perfusion and dual-energy CT[18], and other evolving imaging techniques have led to improved diagnostic accuracies and better characterization of HCC[19]. Moreover, functional MR imaging techniques, including diffusion weighted MR imaging (DWI)[20], MR hepatobiliary contrast agents[21] etc., show promise in providing further biological information of HCC, enabling personalized treatment decisions in the era of precision medicine.
In this review, we discuss the current state-of-the-art and recent advances in the noninvasive imaging assessment of HCC. The basic key concepts of HCC development and an overview of current practice guidelines are illustrated as well. Treatment assessment of HCC, however, involves another complex system of different imaging techniques and features and, thus, is not within the scope of this review.
HEPATOCARCINOGENESIS
Hepatocarcinogenesis is a complex multistep process at molecular, cellular, and histologic levels. Chronic liver inflammation can result in repeated cell injury, death, and regeneration cycles, leading to subsequent epigenetic and genetic alterations of hepatocytes. At the molecular level, important oncogenes (e.g. MYC) and tumor suppressor genes (e.g. TP53, E-cadherin, RASSF1, and PTEN) are either mutated or aberrantly regulated due to structural genetic alterations[22-24], and several abnormally functioned signaling pathways (e.g., Ras, epithelial growth factor receptor, and insulin-like growth factor receptor 1 signaling)[2]. At the cellular level, HCC can develop after malignant transformation of mature hepatocytes or, as suggested by emerging data, intrahepatic stem cells[25,26]. At the histologic level, phenotypically abnormal precursor hepatic lesions, including cirrhotic nodules, low-grade dysplastic nodules (LGDN), and high-grade dysplastic nodules (HGDN), dedifferentiate and gradually evolve to form early and eventually progressed HCCs[27]. This process is a biologic continuum and may occur simultaneously at various rates in different parts of the liver.
Several key alterations happen during hepatocarcinogenesis. First, distinct hemodynamic changes take place during the multistep process. The blood supply of the nodules changes along with malignant transformation: the number of intranodular portal tracts decreases gradually, while the number of unpaired arteries increases. Eventually, HCC is supplied mostly by the hepatic artery system via abnormal unpaired arteries[27]. This results in the characteristic enhancing pattern of hepatic arterial phase hyperenhancement and portal venous and/or delayed phases wash-out relative to the background liver on contrast enhanced multiphasic CT and MRI. In addition, venous drainage of nodules evolves from hepatic veins to sinusoids and portal veins, which may explain why HCC mostly spreads through the portal venous system rather than hepatic veins.
Second, the most morphologically prominent features during hepatocarcinogenesis are the formation of a fibrous tumor capsule (a smooth, uniform and enhancing rim surrounding most or all of a nodule) and the “nodule-in-nodule” (smaller nodules with different imaging features within a larger outer nodule) architecture. Notably, since cirrhotic nodules, dysplastic nodules, and early HCC rarely present with a fibrous capsule, the capsule appearance is more suggestive of progressive HCC. The “nodule-in-nodule” architecture can be the result of successively dedifferentiated clonal populations or nodules with more fat development within a larger outer nodule. Fat can accumulate in dysplastic nodules and early HCC; however, the fat content usually regresses as the nodule grows or in progressed HCC[28,29].
Third, the expression of several important protein transporters is altered significantly during hepatocarcinogenesis. The organic anionic transporting polypeptides (OATP) family includes proteins that are expressed on the sinusoidal membranes of hepatocytes and involved in the transportation of bile salts[30]. MRI hepatobiliary contrast agents are predominantly taken up by human hepatocytes via OATP8 (also known as OATP1B1/3)[30]. During hepatocarcinogenesis, the expression of OATP8 decreases progressively[31]. Most HCCs, many HGDNs, and some LGDNs present with OATP8 underexpression relative to the background liver, resulting in hypointense nodules observed in the hepatobiliary phase (HBP) on MRI[32]. Moreover, emerging data suggest that OATP8 expression decreases before the reduction in portal tracts and increases in unpaired arteries[31,33]. Therefore, hepatobiliary agents can help improve the sensitivity in detecting early small HCCs[34]. In addition, OATP8 expression has been shown to be inversely correlated with HCC tumor grade and aggressiveness[33,35].
NONINVASIVE DETECTION, CHARACTERIZATION, AND DIAGNOSIS OF HCC
The prognosis of HCC is highly dependent on the stage at which the tumor is detected[36]. Therefore, early detection and accurate characterization are pivotal in the management of HCC. Imaging modalities, including US, CT, and MRI, play crucial roles in noninvasive HCC diagnosis.
Imaging modalities
US: US has been widely used as the screening test for HCC, with a sensitivity ranging from 51%-87% and a specificity from 80%-100%[37-39]. HCC is usually characterized as a hypoechoic nodule with hyperenhancement in the arterial phase and washout in the late phase on US[40]. Contrast-enhanced US (CEUS) with microbubble contrast agents is useful for characterizing focal liver lesions and has added diagnostic value to conventional US[40-42]. More recently, the introduction of Sonazoid (gaseous perflubutane, GE Healthcare, Oslo, Norway) microbubbles has led to improved diagnostic capability of CEUS, enabling the imaging of Kupffer cells[43]. CEUS has similar performance to CT and MRI for characterizing focal liver lesions[44-46].
Relative to dynamic CT and MRI, US can be performed real-time and is less expensive with no associated nephrotoxicity[47] or ionizing radiation. In addition, CEUS allows a continuous imaging and characterization of the dynamic wash-in and wash-out of contrast agents and can be useful to resolve indeterminate vascular shunts detected by CT or MRI. However, US is more prone to inter-and intraobserver variabilities and requires recognized expertise to perform good examinations. Besides, the application of US is limited in obese patients and patients with very cirrhotic heterogeneous livers. In addition, the performance of US is usually deteriorated for deep, subdiaphragmatic, multiple, and treated lesions. In general, US is less accurate for diagnosing HCC than CT or MRI[37,48]. Therefore, US is not yet recommended as the first-line diagnostic tool for HCC, according to current guidelines[7-16].
CT and MRI: Dynamic multiphasic CT and MRI are considered the first-line diagnostic modalities for HCC[7-16]. Characteristic imaging features of HCC include hyperenhancement in the hepatic arterial phase and wash-out appearance in the portal venous and/or delayed phases relative to the background liver[7-16] (Figures 1 and 2). Upon visualization of the above-mentioned imaging features, diagnosis of HCC can be established without further histopathologic confirmation. According to recent meta-analyses[34,37,49-51], the sensitivities of dynamic CT and of MRI were 63%-76% and 77%-90%, respectively, and the specificities were 87%-98% and 84%-97%, respectively. Recently, several functional MR imaging techniques have been developed to improve the noninvasive evaluation of HCC. Among them, the most significant techniques are DWI and hepatobiliary contrast agents.
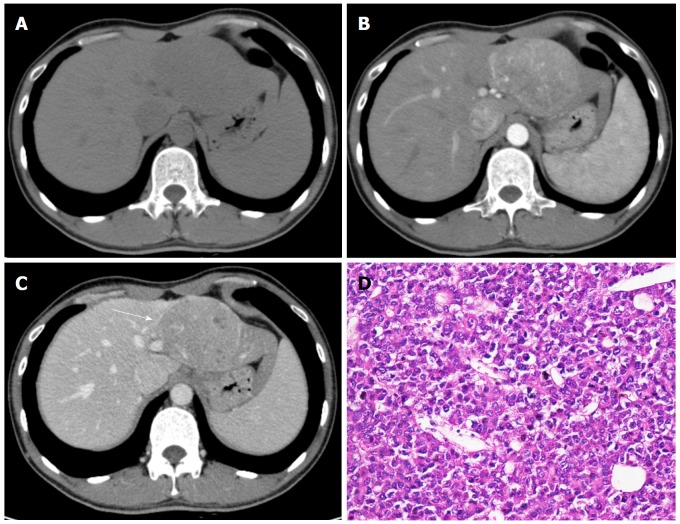
Figure 1.
Hepatocellular carcinoma in a 32-year-old male with chronic hepatitis B. Axial dynamic non-enhanced (A), late arterial phase (B), and portal venous phase (C) CT images show the 8.5 cm mass with arterial phase hyperenhancement and portal venous phase wash-out appearance. The capsule is seen as a hyperattenuating ring on portal venous phase (C, white arrow). The hematoxylin-eosin (HE) staining of the mass at 200 × magnification proved it to be Edmonson-Steiner grade II (D).
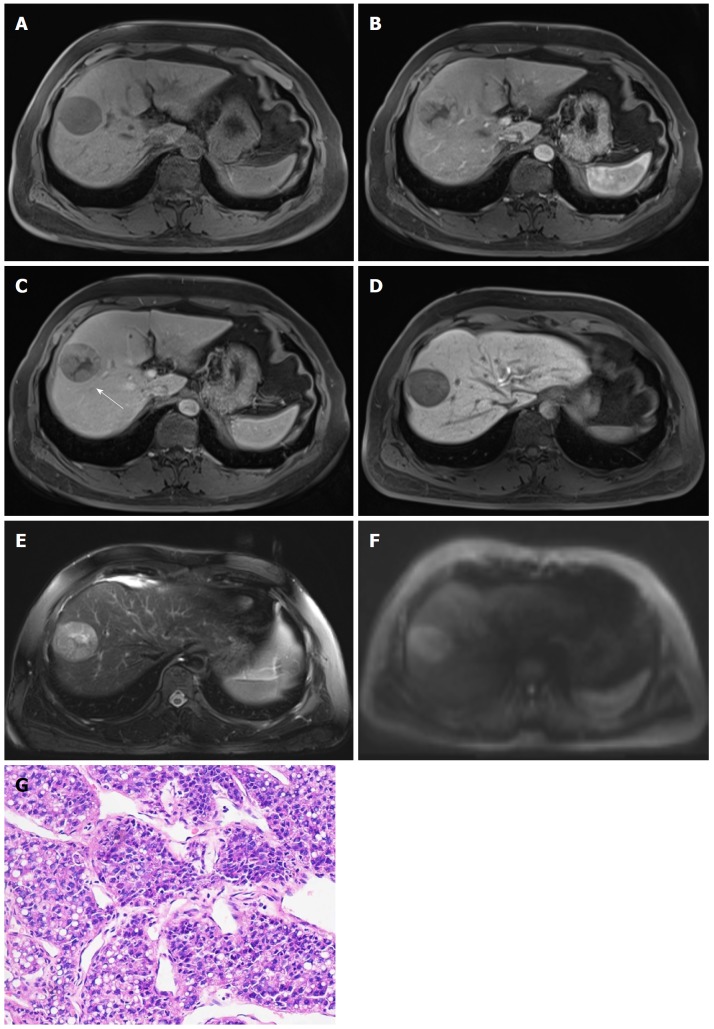
Figure 2.
Hepatocellular carcinoma in 47-year-old male with chronic hepatitis B. 4.7-cm-sized mass in right anterior hepatic section shows hypointensity on unenhanced T1-weighted image (A), hyperenhancement in arterial phase (B), hypointensity relative to the surrounding liver parenchyma in portal venous phase (C), and 20 min hepatobiliary phase (D). An enhancing capsule (white arrow, the peripheral rim of smooth enhancement) in portal venous phase, mosaic architecture, intermediate hyperintensity on T2-weighted images (E), and restricted diffusion (F) are also visible. The mass was confirmed as Edmonson-Steiner grade II at 200 × magnification with hematoxylin-eosin (HE) staining (D).
DWI is a functional MRI technique that allows quantitative measurements of proton diffusion in tissues[52]. HCC and other malignancies are usually characterized by increased cellularity and, thus, have restricted water proton diffusion[52,53]. Therefore, most HCCs are observed as a hyperintense lesion on high b value DWI with low apparent diffusion coefficient (ADC) value on quantitative maps compared with background liver. Intravoxel incoherent motion (IVIM) is a recently developed DWI-derived approach. IVIM can separate the effects of perfusion-related diffusion from pure molecular diffusion[53]. DWI and IVIM enable improved detection of HCC and better characterization of small lesions[51,54,55]. Therefore, “restricted diffusion” on DWI has been incorporated in the Liver Imaging Reporting and Data System (LI-RADS) endorsed by the American College of Radiology (ACR) as an ancillary imaging feature that favors malignancies[9]. Moreover, both ADC and IVIM-derived parameters are significantly correlated with HCC histologic grade[56-59].
However, DWI and DWI-based techniques have several limitations. First, the performance of DWI for diagnosing HCC may be degraded due to unstandardized DWI techniques and imaging protocols, including the determination of optimal b values and breathing techniques (respiratory-triggered, free-breathing, breath-hold, respiratory-cardiac triggering, etc.) across different modalities and medical centers. Therefore, universal thresholds for ADC and other quantitative parameters may not be acquirable. Second, DWI is sensitive to motion artifact; thus, detection and characterization of HCC can be greatly affected in the presence of motion artifacts[60]. Most importantly, DWI demonstrates restricted specificity for HCC, because many lesions, including hemangioma and other non-HCC malignancies, can show restricted diffusion on DWI[61,62]. Therefore, diffusion-weighted images should be interpreted with images from other sequences with caution.
Hepatobiliary contrast agents, including gadobenate dimeglumine (Gd-BOPTA) and gadoxetate disodium (Gd-EOB-DTPA), can provide information regarding tumor vasculature and hepatocyte function in a single examination[63]. As discussed above, hepatobiliary agents are taken up by hepatocytes via OATP8, and its expression decreases progressively during hepatocarcinogenesis. Therefore, most HCCs are hypointense relative to the surrounding liver parenchyma in the HBP.
As the expression of OATP8 decreases prior to neoangiogenesis, MRI with hepatobiliary contrast agents demonstrated superior capacity for detecting early HCC lesions compared with dynamic multiphasic CT and MRI with extracellular contrast agents[34,51]. Recently, Gd-EOB-DTPA-enhanced MRI has been recommended by several Asian guidelines as the first-line diagnostic modality for HCC[7,11,12]. Besides, hypointensity in the HBP has been endorsed as an important ancillary imaging feature that favors malignancies, although not exclusively HCC, by LI-RADS[9]. Moreover, Gd-EOB-DTPA can help differentiate early HCCs from several types of benign or borderline nodules, including cirrhosis-associated regenerative or dysplastic nodules, focal nodular hyperplasia, and hypervascular pseudolesions[64-67]. In addition, it has also been shown that the lower relative enhancement ratio on Gd-EOB-DTPA-enhanced MRI, which is defined as the ratio of the relative signal intensity of HCC versus the surrounding liver parenchyma on the HBP images to that on unenhanced images, can be indicative of a higher histologic grade[68-70].
However, 5%-20% HCCs can be iso- or even hyperintense in the HBP probably due to overexpression of OATP8[7,31,35]. In addition, any lesion with abnormally functioned hepatocytes, including benign lesions such as hemangiomas and other non-HCC malignancies, can appear hypointense on the HBP images. This results in relatively low specificity of hepatobiliary agents for HCC diagnosis. The specificity can be improved, however, when incorporating images obtained from dynamic phases and other sequences. Another important limitation is that the delayed phase, which can better depict the wash-out appearance than the portal venous phase, is absent on Gd-EOB-DTPA-enhanced MRI. Instead, it provides a transitional phase revealing the transition from extracellular to intracellular-dominant enhancement[71]. Therefore, the wash-out appearance should only be recognized in the portal venous phase on Gd-EOB-DTPA-enhanced MRI, and caution is warranted when interpreting the HBP images, particularly when the enhancement patterns are atypical of HCCs in the dynamic phases.
Perfusion imaging permits quantitative evaluation of the microcirculation within tissues[19]. After administration of an iodinated tracer, serial images revealing the variations in tracer concentrations over time are rapidly acquired, and perfusion parameters are then extracted and adjusted. Perfusion imaging can be performed based on several modalities, including CEUS, CT (perfusion CT), and MRI (dynamic contrast-enhanced MRI). Perfusion imaging shows potential in the detection and characterization of HCC by providing important quantitative information regarding tumor vasculature and angiogenesis. Several studies have reported that the arterial hepatic blood flow and hepatic perfusion index of HCC are significantly higher than the surrounding liver parenchyma, while the portal venous hepatic blood flow is lower[72-75]. In addition, perfusion CT parameters are correlated with the tumor histologic grade, with higher perfusion values being indicative of well-differentiated HCC[76]. In addition to its role in HCC detection and characterization, perfusion imaging is most frequently used to assess the treatment response to several therapies, including locoregional treatment[77,78], transarterial chemoembolization[79,80], and anti-angiogenic therapies[81,82].
Major limitations of perfusion include the following. First, patients are exposed to a large amount of radiation with high-dose contrast agents when undergoing perfusion CT examinations. For dynamic contrast enhanced MRI, image quality can be degraded by motion artifacts and poor spatial resolution. In addition, imaging protocols and MR techniques lack standardization across centers and modalities. Therefore, low-dose perfusion imaging with better image quality and more standardized protocols should be explored in future studies.
Magnetic resonance elastography (MRE) allows the noninvasive measurement of tissue stiffness and has been widely used in the evaluation of liver fibrosis[83]. Recently, Motosugi reported that higher liver stiffness measured by MRE was a risk factor for HCC development[84]; however, this finding was not in line with Anaparthy’s study, which showed no significant correlation between liver stiffness and HCC development in patients with compensated cirrhosis[85]. In addition, it has been shown that MRE can help to differentiating malignant focal liver lesions from benign ones, with malignant liver lesions, including HCC, demonstrating significantly higher mean shear stiffness than benign lesions[86,87]. However, the evidence for MRE in HCC remains scarce; thus, more studies are needed to refine and validate the performance of MRE for diagnosing and evaluating HCC.
Tumor metabolism imaging: positron emission tomography
Positron emission tomography (PET) using 18F-fluorodeoxyglucose (18F-FDG) as a radiotracer can provide information regarding tissue gluconeogenesis, which is elevated in most malignancies, including HCC. 18F-FDG PET is not used routinely in HCC imaging because of its limited sensitivity (about 50%-70%)[88,89]. However, 18F-FDG PET was reported to be useful in patient selection before liver transplantation[90], detection of extrahepatic metastases[91], and evaluation of tumor recurrence[92]. The introduction of novel radiotracers shows promise in optimizing the sensitivity of PET for HCC, among which choline has been studied the most. It has been reported that the detection rate of 18F/11C-choline for HCC was 84%[93], and 18F-fluorocholine demonstrated significantly higher sensitivity for HCC detection than 18F-FDG[94]. Major imaging modalities and their reported diagnostic performances, advantages, and limitations are illustrated in Table 1.
Table 1.
Major Imaging modalities for the diagnosis of hepatocellular carcinoma
| Modality | Role | Imaging features of HCC |
Sensitivity |
Specificity |
Advantages | Disadvantages | |||
| All size | ≤ 20 mm | All size | ≤ 20 mm | ||||||
| US | B-mode US | Screening and surveillance | Nodules with altered echogenicity (hypo- or hyperechoic) and abnormal portal venous and/or arterial blood flow compared with background liver. | 51%-67% | 26%-49% | 80%-100% | 67%-80% | 1. Real-time, less expensive, no ionizing radiation. 2. CEUS Allows real-time continuous imaging and characterization of the dynamic washin of contrast agents. 3. CEUS Can help resolve indeterminate vascular shunts detected by CT or MRI. | 1. Requires recognized expertise to perform good examinations. 2. Sensitive to inter- and intraobserver variabilities. 3. Limited application in obese patients and patients with very cirrhotic heterogeneous livers. 4. US is less accurate compared with CT and MRI for HCC diagnosis. 5. CEUS may demonstrate deteriorated performance for deep, subdiaphragmatic, multiple and treated lesions. |
| CEUS | Focal liver lesion characterization, rapid diagnosis | Hyperenhancement in the hepatic arterial phase and wash-out appearance in the portal venous and/or delayed phases. | 80%-94% | 55%-76% | 82%-98% | 80%-98% | |||
| CT | Diagnostic | Hyperenhancement in the hepatic arterial phase and wash-out appearance in the portal venous and/or delayed phases. | 63%-76% | 63%-70% | 87%-98% | 89%-93% | 1. Widely available and well validated worldwide. 2. Enables full cross-sectional evaluation of the liver and can provide important staging information. 3. Demonstrates high specificity for HCC diagnosis. | 1. Ionizing radiation exposure. 2. Requires application intravenous contrast agents. 3. Less sensitive for early and small lesions. | |
| MRI | All | Diagnostic | Hyperenhancement in the hepatic arterial phase and wash-out appearance in the portal venous and/or delayed phases. | 77%-90% | 68%-85% | 84%-97% | 88%-95% | 1. No ionizing radiation exposure; 2. Widely available and well validated worldwide; 3. Enables full cross-sectional evaluation of the liver and can provide important staging information; 4. Demonstrate high specificity for HCC diagnosis; 5. Better depiction of tumor intrinsic characteristics than CT. | 1. More Sensitive to motion and susceptibility artifact. 2. Requires injection of potentially nephrotoxic contrast agents. 3. More time-consuming than CT or US. 4. Limited sensitivity for early and small lesions. |
| Gadolinium-enhanced MRI | 67%-82% | 57%-75% | 68%-95% | 86%-94% | |||||
| Gadoxetate-enhanced MRI | Arterial phase hyperenhancement, portal venous phase wash-out appearance and hepatobiliary phase hypointensity. | 79%-93% | 90%-93% | 90%-97% | 87%-91% | 1. Permits evaluation of hepatocyte functions. 2. Very sensitive for early and small lesions. 3. Hepatobiliary phase signal intensity is well correlated with HCC histologic grade. 4. Can help differentiate early HCCs from cirrhosis-associated benign nodules. | 1. Prolonged examination time and increased cost. 2. Less available and validated than CT or conventional MR. 3. Some HCCs can appear iso- or even hyperintense on hepatobiliary phase images. 4. Hepatobiliary phase hypointensity may be appreciated in a wide spectrum of diseases with both benign and malignant entities. 5. Delayed phase, which can better depict washout appearance, is absent. | ||
US: Ultrasound; CEUS: Contrast-enhanced ultrasound; CT: Computed tomography; MRI: Magnetic res-onance imaging; HCC: Hepatocellular carcinoma.
Diagnostic algorithms and comparison of guidelines
According to the advantages and limitations of different imaging techniques for HCC, various diagnostic algorithms have been recommended by guidelines from different regions and countries. Among them, guidelines proposed by the American Association for the Study of Liver Diseases (AASLD)[14], the ACR[9], the European Association for the Study of the Liver and the European Organization for Research and Treatment of Cancer (EASL-EORTC)[15], the Asian Pacific Association for the Study of the Liver (APASL)[7], the Japan Society of Hepatology and the Liver Cancer Study Group of Japan (JSH-LCSG)[11], the Korean Liver Cancer Study Group and the National Cancer Center (KLCSG-NCC)[12], China[95], and the Indian National Association for study of the Liver (INASL)[16] are the most influential ones. Notably, these diagnostic algorithms should only be applied to patients with increased risks of developing HCC, including adult patients with cirrhosis, chronic HBV and/or HCV infections, or current or prior HCC. The size-based diagnostic algorithms were endorsed by five guidelines (KLCSG-NCC, INASL, China, EASL-EORTC, and AASLD). When a nodule is detected in a high-risk patient, further evaluation and diagnosis should be made according to the nodule diameter. The diagnostic cut-off size of 1 cm is recommended by most guidelines, except for the Chinese guideline, in which a cut-off size of 2 cm is adopted. The non-size-based diagnostic algorithms stratify nodules by their typical enhancement patterns on dynamic CT or MRI regardless of the nodule sizes, as recommended by the remaining two guidelines (JSH-LCSG and APASL). Generally, contrast-enhanced dynamic CT and/or MR are the recommended noninvasive diagnostic modalities, and the prime diagnostic criteria endorsed by most guidelines with typical patterns comprising arterial phase hyperenhancement and portal venous and/or delayed phase wash-out. Biopsy is considered only for suspicious nodules without typical dynamic enhancement pattern, or nodules still inconclusive after a second alternative imaging technique.
Despite similarities in the diagnostic criteria for HCC across different guidelines, discrepancies also exist in the use of tumor markers such as alpha-fetoprotein (AFP), the number of required imaging modalities, and the use of CEUS and/or the hepatobiliary contrast agent Gd-EOB-DTPA. Diagnostic systems advocated by Western guidelines, including the EASL-EORTC and AASLD, are more intended to improve the specificity of HCC diagnosis, while algorithms proposed by Asian guidelines tend to attain maximum sensitivity for HCC detection. For example, most Asian guidelines included the use of CEUS in their diagnostic algorithms, and two of them (KLCSG-NCC and China) included serum AFP levels. In contrast, AFP levels and CEUS were considered less valuable or even removed in guidelines from Europe and the United States. The use of Gd-EOB-DTPA-enhanced MRI is also controversial. All the Asian algorithms recommended performing Gd-EOB-DTPA-enhanced MRI, and it was even endorsed as the first-line diagnostic modality for HCC in the JSH-LCSG and APASL guidelines. However, Gd-EOB-DTPA-enhanced MRI is not yet used as a primary diagnostic test in guidelines from Europe or America but rather as a complementary technique to dynamic images. Similarities and differences of different guidelines are illustrated in Table 2.
Table 2.
Comparison of guidelines of different countries and regions
| Region | Year | Country/society | Stratification | Cut-off size | AFP | CEUS | EOB-MRI |
| Asia | 2014 | Japan/JSH-LCSG | Dynamic pattern | / | Not included | Included | First-line |
| 2014 | Korea/KLCSG-NCC | Size-based | 1 cm | Included | Not included | Preferentially recommended | |
| 2014 | India/INASL | Size-based | 1 cm | Not included | Included | According to availability | |
| 2017 | China/NHFPCPRC | Size-based | 2 cm | Included | Included | Optional | |
| 2017 | APASL | Dynamic pattern | / | Not included | Included | First-line | |
| Europe | 2012 | EASL-EORTC | Size-based | 1 cm/ 2 cm | Not included | ||
| America | 2011 | USA/AASLD | Size-based | 1 cm | Not included |
AFP: Alpha fetoprotein; CEUS: Contrast-enhanced ultrasonography; EOB-MRI: Gadoxetic acid-enhanced MRI; JSH-LCSG: Japan Society of Hepatology - Liver Cancer Study Group of Japan; KLCSG-NCC: Korean Liver Cancer Study Group - National Cancer Center; INASL: The Indian National Associa-tion for Study of the Liver; NHFPCPRC: National Health and Family Planning Commission of the Peo-ple’s Republic of China; APASL: Asia-Pacific Association for the Study of the Liver; EASL-EORTC: Eu-ropean Association for the Study of the Liver - European Organization for Research & Treatment of Can-cer; AASLD: The American Association for the Study of Liver Diseases.
Notably, the LI-RADS system proposed by the ACR provided a separate algorithm for US screening and surveillance of HCC and guidance on the diagnosis of non-HCC malignancies and macrovascular invasion, which have been beyond the scope of other diagnostic systems thus far. Moreover, several ancillary imaging features were incorporated into the LI-RADS algorithm. Although the levels of evidence supporting these ancillary features remain relatively low, these features demonstrate great potential for improving detection and characterization of liver nodules. In addition, a treatment response assessment algorithm for malignant hepatic lesions treated with locoregional therapies has been advocated in the most recent LI-RADS system, allowing a comprehensive assessment of treatment response to different locoregional therapies. Therefore, the LI-RADS system shows promise in optimizing the diagnostic accuracies of current noninvasive modalities for liver nodules, but more studies are needed to validate further the performance of this algorithm in prospective cohorts.
STAGING OF HCC
Several staging systems based on dynamic contrast-enhanced multiphasic CT and MRI have been developed for HCC. The Barcelona Clinic Liver Cancer (BCLC) classification is currently the most widely used staging system and has been endorsed by the EASL-EORTC[15] and the AASLD[14] as the standard staging system for HCC. The BCLC classification incorporates radiologic staging of HCC (size and number of HCC nodules, presence of nodal and macrovascular invasion and extrahepatic metastases), with liver function and physiologic status[96]. A corresponding treatment schedule, from liver resection and transplantation to best supportive care, is recommended for each stage. The BCLC system demonstrated superior capacity in treatment guidance, particularly for patients with early stage HCC[14], and prognosis prediction[97-99]. However, the performance of this system may be affected due to considerable heterogeneity of disease severity within the intermediate stage (BCLC-B)[71]. In addition, a worldwide consensus on the optimal staging system for HCC has not been reached yet.
SURVEILLANCE OF HCC
Several guidelines have recommended US as the first-line surveillance modality for HCC in high-risk patients[7-16], including cirrhotic patients and noncirrhotic patients with chronic HBV and/or HCV infection or high HBV-DNA levels. Noncirrhotic patients with family history of HCC and NAFLD should also undergo routine surveillance[7-16]. US demonstrated a sensitivity of 40%-81% and specificity of 80%-100% for surveillance purposes[38,100-102]. The recommended screening interval for HCC is 6 months. In cirrhotic patients, subcentimeter nodules (< 1 cm) detected by US should be followed every 3[14]-4[15] months during the first year and every 6 months thereafter, while nodules over 1 cm should undergo further imaging work-ups and/or biopsy for characterization.
US is a noninvasive, real-time imaging technique with good patient acceptance, relatively low cost, and no radiation exposure. However, the performance of US is highly dependent on the equipment quality and operator expertise. In addition, US may not be sensitive enough to detect early-stage HCCs, with a pooled sensitivity of 63%[101]. To improve the sensitivity of US in HCC surveillance, the 2017 APASL guideline endorsed the combination of AFP levels and US as the standard surveillance strategy for HCC[7]. However, emerging data have shown that AFP provided limited diagnostic benefits of a low (6%-8%) additional detection rate with increased false positive results and surveillance cost when combined with US[15,101]. In addition, AFP levels can be within the normal range in up to 35% of small HCCs and elevated in patients with active hepatocyte regeneration due to various etiologies[12]. Thus, the measurement of AFP levels is not within the recommended surveillance or diagnostic scheme in the most recent AASLD[14] or EASL-EORTC[15] guidelines.
NONINVASIVE PROGNOSTIC EVALUATION
Tumor characteristics evaluated by noninvasive imaging techniques can be indicative of HCC prognosis after treatment. It has been shown that presence of cirrhosis, large tumor size (> 3 cm), multifocality, vascular invasion, and Eggel’s growth classification (type 2 and 3) were associated with higher recurrence rates and worse prognosis after liver resection[103-107] or liver transplantation[108-110].
Microvascular invasion (MVI), defined as microscopically detected tumor thrombi within small tumor or peritumoral vessels (central hepatic vein, portal vein branches, and venous vessels in tumor capsule and/or fibrous septa)[111], has drawn worldwide attention in recent years. MVI is indicative of early infiltration of tumor cells into the tumor vessels. The presence of MVI should be recognized based on microscopy, but several imaging features may be predictive of MVI. The presence of non-smooth tumor margins, no or incomplete capsule, multifocality, intratumoral arteries, and large tumor size detected on contrast-enhanced dynamic CT and MRI were reported to be associated with an increased risk for MVI[112-118]. In addition, Eggel’s growth classification type 2 (single nodule with extranodular growth) and type 3 (multiple confluent nodules) were also correlated with higher MVI risk[119-121]. Moreover, it has been shown that arterial phase peritumoral enhancement, possibly due to compensatory arterial hyperperfusion after decreased portal venous flow caused by tumor thrombi occlusion of the minute portal venules around the tumor, was also indicative of a higher risk of MVI[122,123].
Apart from conventional dynamic CT and MRI, functional MR techniques play a pivotal role in evaluating MVI. Studies have shown that higher tumor-to-liver signal intensity ratio and lower ADCs value measured on DWI can predict MVI in HCC[124-126]. This may be due to higher cellularity with restricted diffusion and decreased perfusion in MVI-positive HCCs compared with MVI-negative ones. In addition, it has also been shown that increased mean kurtosis values measured by diffusion kurtosis imaging, which is a DWI-based MR technique, were independent risk factors for MVI[127].
Gd-EOB-DTPA-enhanced MRI is another useful tool for assessing MVI in HCC patients. The presence of a faint hypointense halo around the tumor in the HBP can predict the presence of MVI[128-131] (Figure 3). One possible explanation for this specific imaging feature is that MVI can cause hemodynamic changes surrounding the tumor due to obstruction of minute vessels, resulting in impaired hepatocytic function and decreased uptake of Gd-EOB-DTPA in these areas.
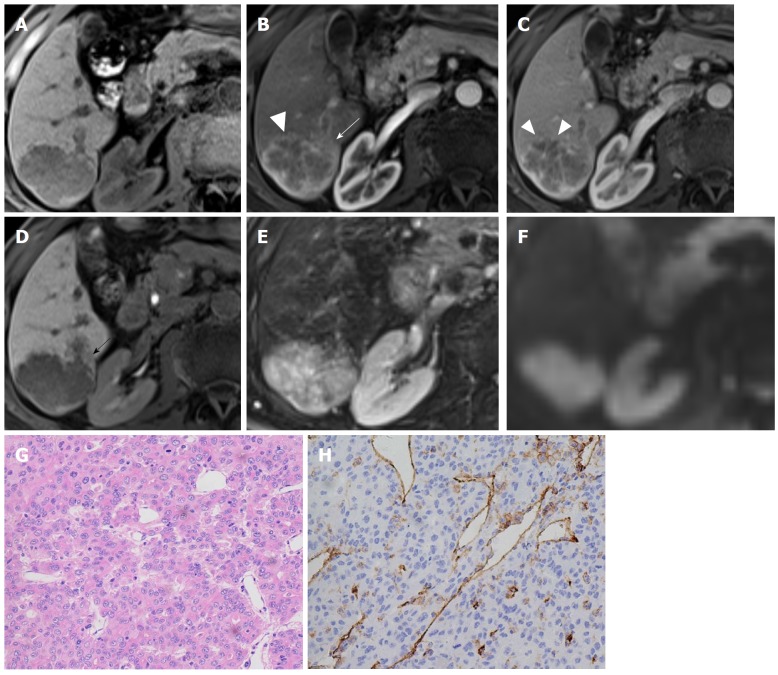
Figure 3.
Hepatocellular carcinoma in a 71-year-old male with recognized cirrhosis. Gd-EOB-DTPA-enhanced MR image demonstrates a 5.3 cm lobulated HCC in right posterior section of liver. The lesion shows peritumor enhancement in arterial phase (B, white arrow) and peritumor hypointense (D, black arrow) in hepatobiliary phase. Capsular disruption and non-smooth tumor margin are present (white triangles) in arterial phase (B) and portal venous phase (C). The lesion was histopathologically proven to be Edmonson-Steiner III grade with hematoxylin-eosin (HE) staining at 200 × magnification (G). Prominent microvascular invasion was detected at 200 × magnification with CD31 immunohistochemical staining (H).
Comprehensive predictive models incorporating several imaging and serological parameters have been developed recently to predict the presence of MVI. Lei et al[132] conducted a retrospective study including 1004 consecutive HBV-related HCC patients and developed a nomogram for preoperative estimation of MVI. A large tumor diameter, multiple nodules, incomplete capsule, typical dynamic pattern of HCC on contrast-enhanced MRI, elevated AFP levels, elevated HBV DNA load, and decreased platelet count were incorporated into the predictive model. The sensitivities of the training and validation cohorts were 74% and 62%, respectively, while the specificities were 77% and 81%, respectively. Another study demonstrated that radiogenomic venous invasion, which was derived from a 91-gene HCC “venous invasion” gene expression signature consisting of three major imaging features (“internal arteries”, “hypodense halo” and “tumor-liver difference”) on contrast-enhanced CT, was able to predict MVI with a sensitivity and specificity of 76% and 94%, respectively[114].
Although substantial progress has been made in the noninvasive evaluation of HCC prognosis with combinations of various imaging features and modalities, there is still debate over the optimal predictive model for tumor recurrence and patient survival. In addition, the current data are mostly limited to single-center experiences with relatively small numbers of HCC cases. Therefore, further large-scale multicentered studies are necessary to develop the comprehensive predictive models to assess HCC prognosis, particularly those that use artificial intelligence techniques in the era of radiomics and radiogenomics.
CONCLUSION
HCC is a major public health problem worldwide. Noninvasive imaging techniques play significant roles in the surveillance, diagnosis, characterization, staging, and prognosis evaluation of HCC. Currently, US is the first-line imaging modality for screening and surveillance purposes. Contrast enhanced dynamic multiphasic CT and MRI are diagnostic tools for HCC based on characteristic enhancement patterns that do not require histopathologic confirmation. Functional MRI techniques, including DWI, MRI with hepatobiliary contrast agents, perfusion imaging, and MRE, show promise in providing further important information regarding tumor biological behavior. In addition, several imaging features based on different imaging modalities enable the prediction of MVI, patient recurrence, and survival.
However, as the prognosis of HCC is largely dependent on the stage at which the tumor is detected; early detection and accurate assessment are critical for patient management and survival. Nevertheless, early diagnosis of HCC is still one of the most challenging areas in liver imaging. Moreover, comprehensive prediction models for preoperative evaluation of HCC prognosis are still urgently needed. Therefore, more multicentered, large-scale, prospective studies are encouraged to explore the biological behaviors of HCC and to develop comprehensive diagnostic and predictive models based on serological, imaging, texture, and radiogenomic parameters.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: All authors declared no potential conflict of interests related to this publication.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 27, 2018
First decision: April 11, 2018
Article in press: May 5, 2018
P- Reviewer: Berkane S, Cerwenka H, Facciorusso A, Tsoulfas G S- Editor: Gong ZM L- Editor: A E- Editor: Yin SY
Contributor Information
Han-Yu Jiang, Department of Radiology, Sichuan University West China Hospital, Chengdu 610041, Sichuan Province, China.
Jie Chen, Department of Radiology, Sichuan University West China Hospital, Chengdu 610041, Sichuan Province, China.
Chun-Chao Xia, Department of Radiology, Sichuan University West China Hospital, Chengdu 610041, Sichuan Province, China.
Li-Kun Cao, Department of Radiology, Sichuan University West China Hospital, Chengdu 610041, Sichuan Province, China.
Ting Duan, Department of Radiology, Sichuan University West China Hospital, Chengdu 610041, Sichuan Province, China.
Bin Song, Department of Radiology, Sichuan University West China Hospital, Chengdu 610041, Sichuan Province, China. anicesong@vip.sina.com..
References
- 1.International Agency for Research on Cancer. GLOBOCAN 2012: Esti-mated cancer incidence, mortaloty and prevalence worldwide in 2012. Accessed to 2018-02-28. Available from: http://globocan.iarc.fr/old/FactSheets/cancers/liver-new.asp.
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Dimitroulis D, Damaskos C, Valsami S, Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D, Sakellariou S, Kykalos S, et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol. 2017;23:5282–5294. doi: 10.3748/wjg.v23.i29.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 6.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 9.Elsayes KM, Hooker JC, Agrons MM, Kielar AZ, Tang A, Fowler KJ, Chernyak V, Bashir MR, Kono Y, Do RK, et al. 2017 Version of LI-RADS for CT and MR Imaging: An Update. Radiographics. 2017;37:1994–2017. doi: 10.1148/rg.2017170098. [DOI] [PubMed] [Google Scholar]
- 10.Marrero JA, Ahn J, Rajender Reddy K; Americal College of Gastroenterology. ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol. 2014;109:1328–1347; quiz 1348. doi: 10.1038/ajg.2014.213. [DOI] [PubMed] [Google Scholar]
- 11.Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458–468. doi: 10.1159/000343875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korean Liver Cancer Study Group (KLCSG) National Cancer Center, Korea (NCC). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015;16:465–522. doi: 10.3348/kjr.2015.16.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korean Society of Abdominal Radiology. Diagnosis of Hepatocellular Carcinoma with Gadoxetic Acid-Enhanced MRI: 2016 Consensus Recommendations of the Korean Society of Abdominal Radiology. Korean J Radiol. 2017;18:427–443. doi: 10.3348/kjr.2017.18.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Association for Study of Liver. European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48:599–641. doi: 10.1016/j.ejca.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Acharya SK, Singh SP, Saraswat VA, Arora A, Duseja A, Goenka MK, Jain D, Kar P, Kumar M, Kumaran V, Mohandas KM, Panda D, Paul SB, Ramachandran J, Ramesh H, Rao PN, Shah SR, Sharma H, Thandassery RB; (The INASL Task-Force on Hepatocellular Carcinoma) The Indian National Association for Study of the Liver (INASL) Consensus on Prevention, Diagnosis and Management of Hepatocellular Carcinoma in India: The Puri Recommendations. J Clin Exp Hepatol. 2014;4:S3–S26. doi: 10.1016/j.jceh.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jo PC, Jang HJ, Burns PN, Burak KW, Kim TK, Wilson SR. Integration of Contrast-enhanced US into a Multimodality Approach to Imaging of Nodules in a Cirrhotic Liver: How I Do It. Radiology. 2017;282:317–331. doi: 10.1148/radiol.2016151732. [DOI] [PubMed] [Google Scholar]
- 18.Gordic S, Puippe GD, Krauss B, Klotz E, Desbiolles L, Lesurtel M, Müllhaupt B, Pfammatter T, Alkadhi H. Correlation between Dual-Energy and Perfusion CT in Patients with Hepatocellular Carcinoma. Radiology. 2016;280:78–87. doi: 10.1148/radiol.2015151560. [DOI] [PubMed] [Google Scholar]
- 19.Ronot M, Clift AK, Vilgrain V, Frilling A. Functional imaging in liver tumours. J Hepatol. 2016;65:1017–1030. doi: 10.1016/j.jhep.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 20.Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254:47–66. doi: 10.1148/radiol.09090021. [DOI] [PubMed] [Google Scholar]
- 21.Motosugi U, Bannas P, Sano K, Reeder SB. Hepatobiliary MR contrast agents in hypovascular hepatocellular carcinoma. J Magn Reson Imaging. 2015;41:251–265. doi: 10.1002/jmri.24712. [DOI] [PubMed] [Google Scholar]
- 22.Calderaro J, Couchy G, Imbeaud S, Amaddeo G, Letouzé E, Blanc JF, Laurent C, Hajji Y, Azoulay D, Bioulac-Sage P, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol. 2017;67:727–738. doi: 10.1016/j.jhep.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Niu ZS, Niu XJ, Wang WH. Genetic alterations in hepatocellular carcinoma: An update. World J Gastroenterol. 2016;22:9069–9095. doi: 10.3748/wjg.v22.i41.9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M, Degos F, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–698. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sia D, Villanueva A, Friedman SL, Llovet JM. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology. 2017;152:745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moeini A, Sia D, Zhang Z, Camprecios G, Stueck A, Dong H, Montal R, Torrens L, Martinez-Quetglas I, Fiel MI, et al. Mixed hepatocellular cholangiocarcinoma tumors: Cholangiolocellular carcinoma is a distinct molecular entity. J Hepatol. 2017;66:952–961. doi: 10.1016/j.jhep.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272:635–654. doi: 10.1148/radiol.14132361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International Consensus Group for Hepatocellular NeoplasiaThe International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 29.Kutami R, Nakashima Y, Nakashima O, Shiota K, Kojiro M. Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J Hepatol. 2000;33:282–289. doi: 10.1016/s0168-8278(00)80369-4. [DOI] [PubMed] [Google Scholar]
- 30.Nassif A, Jia J, Keiser M, Oswald S, Modess C, Nagel S, Weitschies W, Hosten N, Siegmund W, Kühn JP. Visualization of hepatic uptake transporter function in healthy subjects by using gadoxetic acid-enhanced MR imaging. Radiology. 2012;264:741–750. doi: 10.1148/radiol.12112061. [DOI] [PubMed] [Google Scholar]
- 31.Kitao A, Matsui O, Yoneda N, Kozaka K, Shinmura R, Koda W, Kobayashi S, Gabata T, Zen Y, Yamashita T, et al. The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging. Eur Radiol. 2011;21:2056–2066. doi: 10.1007/s00330-011-2165-8. [DOI] [PubMed] [Google Scholar]
- 32.Vilgrain V, Van Beers BE, Pastor CM. Insights into the diagnosis of hepatocellular carcinomas with hepatobiliary MRI. J Hepatol. 2016;64:708–716. doi: 10.1016/j.jhep.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Kogita S, Imai Y, Okada M, Kim T, Onishi H, Takamura M, Fukuda K, Igura T, Sawai Y, Morimoto O, et al. Gd-EOB-DTPA-enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading and portal blood flow. Eur Radiol. 2010;20:2405–2413. doi: 10.1007/s00330-010-1812-9. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Jiang H, Chen J, Zhou Y, Huang Z, Song B. Gadoxetic acid disodium-enhanced magnetic resonance imaging outperformed multidetector computed tomography in diagnosing small hepatocellular carcinoma: A meta-analysis. Liver Transpl. 2017;23:1505–1518. doi: 10.1002/lt.24867. [DOI] [PubMed] [Google Scholar]
- 35.Tsuboyama T, Onishi H, Kim T, Akita H, Hori M, Tatsumi M, Nakamoto A, Nagano H, Matsuura N, Wakasa K, et al. Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid-enhanced MR imaging--correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology. 2010;255:824–833. doi: 10.1148/radiol.10091557. [DOI] [PubMed] [Google Scholar]
- 36.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11:e1001624. doi: 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanna RF, Miloushev VZ, Tang A, Finklestone LA, Brejt SZ, Sandhu RS, Santillan CS, Wolfson T, Gamst A, Sirlin CB. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol (NY) 2016;41:71–90. doi: 10.1007/s00261-015-0592-8. [DOI] [PubMed] [Google Scholar]
- 38.Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography -- a randomised study. Aliment Pharmacol Ther. 2013;38:303–312. doi: 10.1111/apt.12370. [DOI] [PubMed] [Google Scholar]
- 39.Yoon JH, Park JW, Lee JM. Noninvasive Diagnosis of Hepatocellular Carcinoma: Elaboration on Korean Liver Cancer Study Group-National Cancer Center Korea Practice Guidelines Compared with Other Guidelines and Remaining Issues. Korean J Radiol. 2016;17:7–24. doi: 10.3348/kjr.2016.17.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187–210. doi: 10.1016/j.ultrasmedbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 41.D’Onofrio M, Crosara S, De Robertis R, Canestrini S, Mucelli RP. Contrast-Enhanced Ultrasound of Focal Liver Lesions. AJR Am J Roentgenol. 2015;205:W56–W66. doi: 10.2214/AJR.14.14203. [DOI] [PubMed] [Google Scholar]
- 42.Kim TK, Jang HJ. Contrast-enhanced ultrasound in the diagnosis of nodules in liver cirrhosis. World J Gastroenterol. 2014;20:3590–3596. doi: 10.3748/wjg.v20.i13.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kudo M. Defect Reperfusion Imaging with Sonazoid®: A Breakthrough in Hepatocellular Carcinoma. Liver Cancer. 2016;5:1–7. doi: 10.1159/000367760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trillaud H, Bruel JM, Valette PJ, Vilgrain V, Schmutz G, Oyen R, Jakubowski W, Danes J, Valek V, Greis C. Characterization of focal liver lesions with SonoVue-enhanced sonography: international multicenter-study in comparison to CT and MRI. World J Gastroenterol. 2009;15:3748–3756. doi: 10.3748/wjg.15.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strobel D, Bernatik T, Blank W, Schuler A, Greis C, Dietrich CF, Seitz K. Diagnostic accuracy of CEUS in the differential diagnosis of small (≤ 20 mm) and subcentimetric (≤ 10 mm) focal liver lesions in comparison with histology. Results of the DEGUM multicenter trial. Ultraschall Med. 2011;32:593–597. doi: 10.1055/s-0031-1271114. [DOI] [PubMed] [Google Scholar]
- 46.Sporea I, Badea R, Popescu A, Spârchez Z, Sirli RL, Dănilă M, Săndulescu L, Bota S, Calescu DP, Nedelcu D, et al. Contrast-enhanced ultrasound (CEUS) for the evaluation of focal liver lesions - a prospective multicenter study of its usefulness in clinical practice. Ultraschall Med. 2014;35:259–266. doi: 10.1055/s-0033-1355728. [DOI] [PubMed] [Google Scholar]
- 47.Ferraioli G, Meloni MF. Contrast-enhanced ultrasonography of the liver using SonoVue. Ultrasonography. 2018;37:25–35. doi: 10.14366/usg.17037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Martino M, De Filippis G, De Santis A, Geiger D, Del Monte M, Lombardo CV, Rossi M, Corradini SG, Mennini G, Catalano C. Hepatocellular carcinoma in cirrhotic patients: prospective comparison of US, CT and MR imaging. Eur Radiol. 2013;23:887–896. doi: 10.1007/s00330-012-2691-z. [DOI] [PubMed] [Google Scholar]
- 49.Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, Han JK, Choi BI. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015;275:97–109. doi: 10.1148/radiol.14140690. [DOI] [PubMed] [Google Scholar]
- 50.Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, Murad MH, Mohammed K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology. 2018;67:401–421. doi: 10.1002/hep.29487. [DOI] [PubMed] [Google Scholar]
- 51.Kierans AS, Kang SK, Rosenkrantz AB. The Diagnostic Performance of Dynamic Contrast-enhanced MR Imaging for Detection of Small Hepatocellular Carcinoma Measuring Up to 2 cm: A Meta-Analysis. Radiology. 2016;278:82–94. doi: 10.1148/radiol.2015150177. [DOI] [PubMed] [Google Scholar]
- 52.Kele PG, van der Jagt EJ. Diffusion weighted imaging in the liver. World J Gastroenterol. 2010;16:1567–1576. doi: 10.3748/wjg.v16.i13.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iima M, Le Bihan D. Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology. 2016;278:13–32. doi: 10.1148/radiol.2015150244. [DOI] [PubMed] [Google Scholar]
- 54.Park MS, Kim S, Patel J, Hajdu CH, Do RK, Mannelli L, Babb JS, Taouli B. Hepatocellular carcinoma: detection with diffusion-weighted versus contrast-enhanced magnetic resonance imaging in pretransplant patients. Hepatology. 2012;56:140–148. doi: 10.1002/hep.25681. [DOI] [PubMed] [Google Scholar]
- 55.Park MJ, Kim YK, Lee MW, Lee WJ, Kim YS, Kim SH, Choi D, Rhim H. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012;264:761–770. doi: 10.1148/radiol.12112517. [DOI] [PubMed] [Google Scholar]
- 56.Woo S, Lee JM, Yoon JH, Joo I, Han JK, Choi BI. Intravoxel incoherent motion diffusion-weighted MR imaging of hepatocellular carcinoma: correlation with enhancement degree and histologic grade. Radiology. 2014;270:758–767. doi: 10.1148/radiol.13130444. [DOI] [PubMed] [Google Scholar]
- 57.Chang WC, Chen RC, Chou CT, Lin CY, Yu CY, Liu CH, Chou JM, Hsu HH, Huang GS. Histological grade of hepatocellular carcinoma correlates with arterial enhancement on gadoxetic acid-enhanced and diffusion-weighted MR images. Abdom Imaging. 2014;39:1202–1212. doi: 10.1007/s00261-014-0168-z. [DOI] [PubMed] [Google Scholar]
- 58.Granata V, Fusco R, Catalano O, Guarino B, Granata F, Tatangelo F, Avallone A, Piccirillo M, Palaia R, Izzo F, et al. Intravoxel incoherent motion (IVIM) in diffusion-weighted imaging (DWI) for Hepatocellular carcinoma: correlation with histologic grade. Oncotarget. 2016;7:79357–79364. doi: 10.18632/oncotarget.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le Moigne F, Boussel L, Haquin A, Bancel B, Ducerf C, Berthezène Y, Rode A. Grading of small hepatocellular carcinomas (≤2 cm): correlation between histology, T2 and diffusion-weighted imaging. Br J Radiol. 2014;87:20130763. doi: 10.1259/bjr.20130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McNamara MM, Thomas JV, Alexander LF, Little MD, Bolus DN, Li YE, Morgan DE. Diffusion-weighted MRI as a screening tool for hepatocellular carcinoma in cirrhotic livers: correlation with explant data-a pilot study. Abdom Radiol (NY) 2018 doi: 10.1007/s00261-018-1535-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 61.Miller FH, Hammond N, Siddiqi AJ, Shroff S, Khatri G, Wang Y, Merrick LB, Nikolaidis P. Utility of diffusion-weighted MRI in distinguishing benign and malignant hepatic lesions. J Magn Reson Imaging. 2010;32:138–147. doi: 10.1002/jmri.22235. [DOI] [PubMed] [Google Scholar]
- 62.Galea N, Cantisani V, Taouli B. Liver lesion detection and characterization: role of diffusion-weighted imaging. J Magn Reson Imaging. 2013;37:1260–1276. doi: 10.1002/jmri.23947. [DOI] [PubMed] [Google Scholar]
- 63.Van Beers BE, Pastor CM, Hussain HK. Primovist, Eovist: what to expect? J Hepatol. 2012;57:421–429. doi: 10.1016/j.jhep.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 64.Ichikawa T, Saito K, Yoshioka N, Tanimoto A, Gokan T, Takehara Y, Kamura T, Gabata T, Murakami T, Ito K, et al. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Invest Radiol. 2010;45:133–141. doi: 10.1097/RLI.0b013e3181caea5b. [DOI] [PubMed] [Google Scholar]
- 65.Motosugi U, Ichikawa T, Sou H, Sano K, Tominaga L, Muhi A, Araki T. Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging. Radiology. 2010;256:151–158. doi: 10.1148/radiol.10091885. [DOI] [PubMed] [Google Scholar]
- 66.Sano K, Ichikawa T, Motosugi U, Sou H, Muhi AM, Matsuda M, Nakano M, Sakamoto M, Nakazawa T, Asakawa M, et al. Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging. Radiology. 2011;261:834–844. doi: 10.1148/radiol.11101840. [DOI] [PubMed] [Google Scholar]
- 67.Wang YC, Chou CT, Lin CP, Chen YL, Chen YF, Chen RC. The value of Gd-EOB-DTPA-enhanced MR imaging in characterizing cirrhotic nodules with atypical enhancement on Gd-DTPA-enhanced MR images. PLoS One. 2017;12:e0174594. doi: 10.1371/journal.pone.0174594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim HY, Choi JY, Kim CW, Bae SH, Yoon SK, Lee YJ, Rha SE, You YK, Kim DG, Jung ES. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging predicts the histological grade of hepatocellular carcinoma only in patients with Child-Pugh class A cirrhosis. Liver Transpl. 2012;18:850–857. doi: 10.1002/lt.23426. [DOI] [PubMed] [Google Scholar]
- 69.Jin YJ, Cho SG, Lee KY, Kim JM, Lee JW. Association between relative liver enhancement on gadoxetic acid enhanced magnetic resonance images and histologic grade of hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e7580. doi: 10.1097/MD.0000000000007580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tong HF, Liang HB, Mo ZK, Guan TP, Yang J, Fang CH. Quantitative analysis of gadoxetic acid-enhanced magnetic resonance imaging predicts histological grade of hepatocellular carcinoma. Clin Imaging. 2017;43:9–14. doi: 10.1016/j.clinimag.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273:30–50. doi: 10.1148/radiol.14132362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fischer MA, Kartalis N, Grigoriadis A, Loizou L, Stål P, Leidner B, Aspelin P, Brismar TB. Perfusion computed tomography for detection of hepatocellular carcinoma in patients with liver cirrhosis. Eur Radiol. 2015;25:3123–3132. doi: 10.1007/s00330-015-3732-1. [DOI] [PubMed] [Google Scholar]
- 73.Taouli B, Johnson RS, Hajdu CH, Oei MT, Merad M, Yee H, Rusinek H. Hepatocellular carcinoma: perfusion quantification with dynamic contrast-enhanced MRI. AJR Am J Roentgenol. 2013;201:795–800. doi: 10.2214/AJR.12.9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ippolito D, Sironi S, Pozzi M, Antolini L, Ratti L, Meloni F, Invernizzi F, Valsecchi MG, Fazio F. Perfusion computed tomographic assessment of early hepatocellular carcinoma in cirrhotic liver disease: initial observations. J Comput Assist Tomogr. 2008;32:855–858. doi: 10.1097/RCT.0b013e318161dc58. [DOI] [PubMed] [Google Scholar]
- 75.Ippolito D, Sironi S, Pozzi M, Antolini L, Ratti L, Alberzoni C, Leone EB, Meloni F, Valsecchi MG, Fazio F. Hepatocellular carcinoma in cirrhotic liver disease: functional computed tomography with perfusion imaging in the assessment of tumor vascularization. Acad Radiol. 2008;15:919–927. doi: 10.1016/j.acra.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 76.Sahani DV, Holalkere NS, Mueller PR, Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue--initial experience. Radiology. 2007;243:736–743. doi: 10.1148/radiol.2433052020. [DOI] [PubMed] [Google Scholar]
- 77.Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, Reig M, Bianchi L, Llovet JM, Bruix J. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616–623. doi: 10.1002/cncr.24050. [DOI] [PubMed] [Google Scholar]
- 78.Meijerink MR, van Waesberghe JH, van der Weide L, van den Tol P, Meijer S, Comans EF, Golding RP, van Kuijk C. Early detection of local RFA site recurrence using total liver volume perfusion CT initial experience. Acad Radiol. 2009;16:1215–1222. doi: 10.1016/j.acra.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 79.Marquez HP, Karalli A, Haubenreisser H, Mathew RP, Alkadhi H, Brismar TB, Henzler T, Fischer MA. Computed tomography perfusion imaging for monitoring transarterial chemoembolization of hepatocellular carcinoma. Eur J Radiol. 2017;91:160–167. doi: 10.1016/j.ejrad.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 80.Tamandl D, Waneck F, Sieghart W, Unterhumer S, Kölblinger C, Baltzer P, Ba-Ssalamah A, Loewe C. Early response evaluation using CT-perfusion one day after transarterial chemoembolization for HCC predicts treatment response and long-term disease control. Eur J Radiol. 2017;90:73–80. doi: 10.1016/j.ejrad.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 81.Chen BB, Hsu CY, Yu CW, Liang PC, Hsu C, Hsu CH, Cheng AL, Shih TT. Early perfusion changes within 1 week of systemic treatment measured by dynamic contrast-enhanced MRI may predict survival in patients with advanced hepatocellular carcinoma. Eur Radiol. 2017;27:3069–3079. doi: 10.1007/s00330-016-4670-2. [DOI] [PubMed] [Google Scholar]
- 82.Ippolito D, Querques G, Okolicsanyi S, Franzesi CT, Strazzabosco M, Sironi S. Diagnostic value of dynamic contrast-enhanced CT with perfusion imaging in the quantitative assessment of tumor response to sorafenib in patients with advanced hepatocellular carcinoma: A feasibility study. Eur J Radiol. 2017;90:34–41. doi: 10.1016/j.ejrad.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 83.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37:544–555. doi: 10.1002/jmri.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Motosugi U, Ichikawa T, Koshiishi T, Sano K, Morisaka H, Ichikawa S, Enomoto N, Matsuda M, Fujii H, Araki T. Liver stiffness measured by magnetic resonance elastography as a risk factor for hepatocellular carcinoma: a preliminary case-control study. Eur Radiol. 2013;23:156–162. doi: 10.1007/s00330-012-2571-6. [DOI] [PubMed] [Google Scholar]
- 85.Anaparthy R, Talwalkar JA, Yin M, Roberts LR, Fidler JL, Ehman RL. Liver stiffness measurement by magnetic resonance elastography is not associated with developing hepatocellular carcinoma in subjects with compensated cirrhosis. Aliment Pharmacol Ther. 2011;34:83–91. doi: 10.1111/j.1365-2036.2011.04673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Venkatesh SK, Yin M, Glockner JF, Takahashi N, Araoz PA, Talwalkar JA, Ehman RL. MR elastography of liver tumors: preliminary results. AJR Am J Roentgenol. 2008;190:1534–1540. doi: 10.2214/AJR.07.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hennedige TP, Hallinan JT, Leung FP, Teo LL, Iyer S, Wang G, Chang S, Madhavan KK, Wee A, Venkatesh SK. Comparison of magnetic resonance elastography and diffusion-weighted imaging for differentiating benign and malignant liver lesions. Eur Radiol. 2016;26:398–406. doi: 10.1007/s00330-015-3835-8. [DOI] [PubMed] [Google Scholar]
- 88.Hennedige T, Venkatesh SK. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer Imaging. 2013;12:530–547. doi: 10.1102/1470-7330.2012.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paudyal B, Paudyal P, Oriuchi N, Tsushima Y, Nakajima T, Endo K. Clinical implication of glucose transport and metabolism evaluated by 18F-FDG PET in hepatocellular carcinoma. Int J Oncol. 2008;33:1047–1054. [PubMed] [Google Scholar]
- 90.Hong G, Suh KS, Suh SW, Yoo T, Kim H, Park MS, Choi Y, Paeng JC, Yi NJ, Lee KW. Alpha-fetoprotein and (18)F-FDG positron emission tomography predict tumor recurrence better than Milan criteria in living donor liver transplantation. J Hepatol. 2016;64:852–859. doi: 10.1016/j.jhep.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 91.Sugiyama M, Sakahara H, Torizuka T, Kanno T, Nakamura F, Futatsubashi M, Nakamura S. 18F-FDG PET in the detection of extrahepatic metastases from hepatocellular carcinoma. J Gastroenterol. 2004;39:961–968. doi: 10.1007/s00535-004-1427-5. [DOI] [PubMed] [Google Scholar]
- 92.Lin CY, Chen JH, Liang JA, Lin CC, Jeng LB, Kao CH. 18F-FDG PET or PET/CT for detecting extrahepatic metastases or recurrent hepatocellular carcinoma: a systematic review and meta-analysis. Eur J Radiol. 2012;81:2417–2422. doi: 10.1016/j.ejrad.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 93.Bertagna F, Bertoli M, Bosio G, Biasiotto G, Sadeghi R, Giubbini R, Treglia G. Diagnostic role of radiolabelled choline PET or PET/CT in hepatocellular carcinoma: a systematic review and meta-analysis. Hepatol Int. 2014;8:493–500. doi: 10.1007/s12072-014-9566-0. [DOI] [PubMed] [Google Scholar]
- 94.Talbot JN, Fartoux L, Balogova S, Nataf V, Kerrou K, Gutman F, Huchet V, Ancel D, Grange JD, Rosmorduc O. Detection of hepatocellular carcinoma with PET/CT: a prospective comparison of 18F-fluorocholine and 18F-FDG in patients with cirrhosis or chronic liver disease. J Nucl Med. 2010;51:1699–1706. doi: 10.2967/jnumed.110.075507. [DOI] [PubMed] [Google Scholar]
- 95.Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. Critical appraisal of Chinese 2017 guideline on the management of hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2017;6:387–396. doi: 10.21037/hbsn.2017.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 97.Kim BK, Kim SU, Park JY, Kim DY, Ahn SH, Park MS, Kim EH, Seong J, Lee DY, Han KH. Applicability of BCLC stage for prognostic stratification in comparison with other staging systems: single centre experience from long-term clinical outcomes of 1717 treatment-naïve patients with hepatocellular carcinoma. Liver Int. 2012;32:1120–1127. doi: 10.1111/j.1478-3231.2012.02811.x. [DOI] [PubMed] [Google Scholar]
- 98.Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, Lok AS. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–716. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 99.Cammà C, Di Marco V, Cabibbo G, Latteri F, Sandonato L, Parisi P, Enea M, Attanasio M, Galia M, Alessi N, et al. Survival of patients with hepatocellular carcinoma in cirrhosis: a comparison of BCLC, CLIP and GRETCH staging systems. Aliment Pharmacol Ther. 2008;28:62–75. doi: 10.1111/j.1365-2036.2008.03692.x. [DOI] [PubMed] [Google Scholar]
- 100.Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, Piscaglia F, Gramantieri L, Zanetti M, Sherman M. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48:251–259. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, Duca P. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513–523. doi: 10.1111/j.1572-0241.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 103.Cheng Z, Yang P, Lei Z, Zhang B, Si A, Yan Z, Xia Y, Li J, Wang K, Hartmann D, et al. Nomograms for prediction of long-term survival in elderly patients after partial hepatectomy for hepatocellular carcinoma. Surgery. 2017;162:1231–1240. doi: 10.1016/j.surg.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 104.Pais R, Fartoux L, Goumard C, Scatton O, Wendum D, Rosmorduc O, Ratziu V. Temporal trends, clinical patterns and outcomes of NAFLD-related HCC in patients undergoing liver resection over a 20-year period. Aliment Pharmacol Ther. 2017;46:856–863. doi: 10.1111/apt.14261. [DOI] [PubMed] [Google Scholar]
- 105.Sumie S, Nakashima O, Okuda K, Kuromatsu R, Kawaguchi A, Nakano M, Satani M, Yamada S, Okamura S, Hori M, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21:1002–1009. doi: 10.1245/s10434-013-3376-9. [DOI] [PubMed] [Google Scholar]
- 106.Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, Labow DM, Llovet JM, Schwartz ME. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 108.Parfitt JR, Marotta P, Alghamdi M, Wall W, Khakhar A, Suskin NG, Quan D, McAllister V, Ghent C, Levstik M, et al. Recurrent hepatocellular carcinoma after transplantation: use of a pathological score on explanted livers to predict recurrence. Liver Transpl. 2007;13:543–551. doi: 10.1002/lt.21078. [DOI] [PubMed] [Google Scholar]
- 109.Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 110.Dudek K, Kornasiewicz O, Remiszewski P, Kobryń K, Ziarkiewicz-Wróblewska B, Górnicka B, Zieniewicz K, Krawczyk M. Impact of tumor characteristic on the outcome of liver transplantation in patients with hepatocellular carcinoma. Transplant Proc. 2009;41:3135–3137. doi: 10.1016/j.transproceed.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 111.Gouw AS, Balabaud C, Kusano H, Todo S, Ichida T, Kojiro M. Markers for microvascular invasion in hepatocellular carcinoma: where do we stand? Liver Transpl. 2011;17 Suppl 2:S72–S80. doi: 10.1002/lt.22368. [DOI] [PubMed] [Google Scholar]
- 112.Reginelli A, Vanzulli A, Sgrazzutti C, Caschera L, Serra N, Raucci A, Urraro F, Cappabianca S. Vascular microinvasion from hepatocellular carcinoma: CT findings and pathologic correlation for the best therapeutic strategies. Med Oncol. 2017;34:93. doi: 10.1007/s12032-017-0949-7. [DOI] [PubMed] [Google Scholar]
- 113.Zhao H, Hua Y, Dai T, He J, Tang M, Fu X, Mao L, Jin H, Qiu Y. Development and validation of a novel predictive scoring model for microvascular invasion in patients with hepatocellular carcinoma. Eur J Radiol. 2017;88:32–40. doi: 10.1016/j.ejrad.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 114.Banerjee S, Wang DS, Kim HJ, Sirlin CB, Chan MG, Korn RL, Rutman AM, Siripongsakun S, Lu D, Imanbayev G, et al. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology. 2015;62:792–800. doi: 10.1002/hep.27877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chou CT, Chen RC, Lin WC, Ko CJ, Chen CB, Chen YL. Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR Am J Roentgenol. 2014;203:W253–W259. doi: 10.2214/AJR.13.10595. [DOI] [PubMed] [Google Scholar]
- 116.Chandarana H, Robinson E, Hajdu CH, Drozhinin L, Babb JS, Taouli B. Microvascular invasion in hepatocellular carcinoma: is it predictable with pretransplant MRI? AJR Am J Roentgenol. 2011;196:1083–1089. doi: 10.2214/AJR.10.4720. [DOI] [PubMed] [Google Scholar]
- 117.Kim H, Park MS, Choi JY, Park YN, Kim MJ, Kim KS, Choi JS, Han KH, Kim E, Kim KW. Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol. 2009;19:1744–1751. doi: 10.1007/s00330-009-1331-8. [DOI] [PubMed] [Google Scholar]
- 118.Lim JH, Choi D, Park CK, Lee WJ, Lim HK. Encapsulated hepatocellular carcinoma: CT-pathologic correlations. Eur Radiol. 2006;16:2326–2333. doi: 10.1007/s00330-006-0203-8. [DOI] [PubMed] [Google Scholar]
- 119.Eguchi S, Takatsuki M, Hidaka M, Soyama A, Tomonaga T, Muraoka I, Kanematsu T. Predictor for histological microvascular invasion of hepatocellular carcinoma: a lesson from 229 consecutive cases of curative liver resection. World J Surg. 2010;34:1034–1038. doi: 10.1007/s00268-010-0424-5. [DOI] [PubMed] [Google Scholar]
- 120.Nagano Y, Shimada H, Takeda K, Ueda M, Matsuo K, Tanaka K, Endo I, Kunisaki C, Togo S. Predictive factors of microvascular invasion in patients with hepatocellular carcinoma larger than 5 cm. World J Surg. 2008;32:2218–2222. doi: 10.1007/s00268-008-9585-x. [DOI] [PubMed] [Google Scholar]
- 121.Hui AM, Takayama T, Sano K, Kubota K, Akahane M, Ohtomo K, Makuuchi M. Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J Hepatol. 2000;33:975–979. doi: 10.1016/s0168-8278(00)80131-2. [DOI] [PubMed] [Google Scholar]
- 122.Nishie A, Yoshimitsu K, Asayama Y, Irie H, Tajima T, Hirakawa M, Ishigami K, Nakayama T, Kakihara D, Nishihara Y, et al. Radiologic detectability of minute portal venous invasion in hepatocellular carcinoma. AJR Am J Roentgenol. 2008;190:81–87. doi: 10.2214/AJR.07.2810. [DOI] [PubMed] [Google Scholar]
- 123.Miyata R, Tanimoto A, Wakabayashi G, Shimazu M, Nakatsuka S, Mukai M, Kitajima M. Accuracy of preoperative prediction of microinvasion of portal vein in hepatocellular carcinoma using superparamagnetic iron oxide-enhanced magnetic resonance imaging and computed tomography during hepatic angiography. J Gastroenterol. 2006;41:987–995. doi: 10.1007/s00535-006-1890-2. [DOI] [PubMed] [Google Scholar]
- 124.Suh YJ, Kim MJ, Choi JY, Park MS, Kim KW. Preoperative prediction of the microvascular invasion of hepatocellular carcinoma with diffusion-weighted imaging. Liver Transpl. 2012;18:1171–1178. doi: 10.1002/lt.23502. [DOI] [PubMed] [Google Scholar]
- 125.Okamura S, Sumie S, Tonan T, Nakano M, Satani M, Shimose S, Shirono T, Iwamoto H, Aino H, Niizeki T, et al. Diffusion-weighted magnetic resonance imaging predicts malignant potential in small hepatocellular carcinoma. Dig Liver Dis. 2016;48:945–952. doi: 10.1016/j.dld.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 126.Xu P, Zeng M, Liu K, Shan Y, Xu C, Lin J. Microvascular invasion in small hepatocellular carcinoma: is it predictable with preoperative diffusion-weighted imaging? J Gastroenterol Hepatol. 2014;29:330–336. doi: 10.1111/jgh.12358. [DOI] [PubMed] [Google Scholar]
- 127.Wang WT, Yang L, Yang ZX, Hu XX, Ding Y, Yan X, Fu CX, Grimm R, Zeng MS, Rao SX. Assessment of Microvascular Invasion of Hepatocellular Carcinoma with Diffusion Kurtosis Imaging. Radiology. 2018;286:571–580. doi: 10.1148/radiol.2017170515. [DOI] [PubMed] [Google Scholar]
- 128.Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol. 2017;67:526–534. doi: 10.1016/j.jhep.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 129.Shin SK, Kim YS, Shim YS, Choi SJ, Park SH, Jung DH, Kwon OS, Choi DJ, Kim JH. Peritumoral decreased uptake area of gadoxetic acid enhanced magnetic resonance imaging and tumor recurrence after surgical resection in hepatocellular carcinoma: A STROBE-compliant article. Medicine (Baltimore) 2017;96:e7761. doi: 10.1097/MD.0000000000007761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nishie A, Asayama Y, Ishigami K, Kakihara D, Nakayama T, Ushijima Y, Takayama Y, Shirabe K, Fujita N, Kubo Y, et al. Clinicopathological significance of the peritumoral decreased uptake area of gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid in hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:561–567. doi: 10.1111/jgh.12423. [DOI] [PubMed] [Google Scholar]
- 131.Kim KA, Kim MJ, Jeon HM, Kim KS, Choi JS, Ahn SH, Cha SJ, Chung YE. Prediction of microvascular invasion of hepatocellular carcinoma: usefulness of peritumoral hypointensity seen on gadoxetate disodium-enhanced hepatobiliary phase images. J Magn Reson Imaging. 2012;35:629–634. doi: 10.1002/jmri.22876. [DOI] [PubMed] [Google Scholar]
- 132.Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, Wang K, Wan X, Lau WY, Wu M, et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg. 2016;151:356–363. doi: 10.1001/jamasurg.2015.4257. [DOI] [PubMed] [Google Scholar]