Abstract
Background
Henoch-Schӧnlein purpura (HSP) is a common vasculitis of childhood. Though HSP is usually self-limiting, severe complications can occur. The management for this condition has not been established yet. Thus, this nationwide study aimed at investigating epidemiological characteristics of children with HSP in Korea. The patterns of clinical practice with regard to the complications of the condition were also investigated.
Methods
This is a national population-based study that used the National Health Insurance Database. Children below 18 years who were diagnosed with HSP in Korea between 2006 and 2015 were enrolled. Data, such as age, sex, yearly and monthly distribution of HSP, hospitalization, re-hospitalization, comorbidities, and interventions were obtained. The use of steroids was also analyzed.
Results
A total of 56,841 children were enrolled. The annual incidence of HSP was 55.9 per 100,000 children. The peak age was 5 years. Spring was the most prevalent season. Sex (male) and young age (< 9 years) were risk factors of hospitalization. Younger children were more likely to be re-hospitalized and suspected with intussusception, arthritis, and nephritis. Only 4 children received laparotomy. In total, 57% were managed with steroids, and mean durations of medication were 4–5 days. Children who were hospitalized and those with comorbidities used steroids more frequently (P < 0.001).
Conclusion
The annual incidence of HSP is 55.9 per 100,000 children which is higher in Korea than that in other countries. Younger children can have a more severe clinical course. This nationwide survey provides valuable information to understand HSP in children and to inspire further research on HSP.
Keywords: Henoch-Schӧnlein Purpura, Epidemiology, Child, Steroids, Korea
Graphical Abstract
INTRODUCTION
Henoch-Schӧnlein purpura (HSP), or recently called immunoglobulin A vasculitis,1 is the most common vasculitis of childhood, and it involves the small vessels in the skin, joints, gastrointestinal tract, and kidneys. Moreover, it is characterized by leukocytoclastic vasculitis and immunoglobulin A deposition. The diagnosis is based on the criteria by the American College of Rheumatology (ACR), and at least two of the following must be observed: palpable purpura without thrombocytopenia, age < 20 years at the onset of symptoms, biopsy showing granulocytes around arterioles or venules, or gastrointestinal bleeding.2 Most Korean pediatricians have been using the ACR diagnostic criteria, rather than the EULAR/PRINTO/PRES Ankara 2008 classification.3
HSP is usually self-limiting. Abdominal pain is the most common gastrointestinal symptom. However, intestinal bleeding and intussusception that require interventions or repeated hospitalization may occur.4 HSP nephritis is a major long-term complication. In relation to complications and prognosis, the management for this condition, which includes steroid use, has not been established yet.
Nationwide epidemiology data on HPS are limited worldwide. In cases of reported data, most were obtained from hospital-based studies or survey questionnaires.5,6,7,8,9 No nationwide surveys are available in Korea. As the Korean government operates a mandatory nationwide insurance system (National Health Insurance [NHI]), all health care utilization information is registered in a comprehensive database. The database includes all medical claims from approximately 50 million Korean residents.
This nationwide study assessed the epidemiological characteristics of children with HSP in Korea, which include annual incidence by age, sex, region, income level, and seasonal distribution. In addition, the patterns of clinical practice with regard to complications were investigated, which include hospitalization and steroid use.
METHODS
This is a national population-based study that utilized information from the database of the Korean NHI Sharing Service from 2006 to 2015. This database used the International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes. The medical claim records include a patient's identification number that can be used to identify multiple medical visits, date of birth, sex, socioeconomic status (income and region), primary diagnosis and comorbidity codes of the ICD-10, date of visit, hospitalization or ambulatory care, length of hospitalization, prescriptions, and interventions. Data on the signs and symptoms, and medical test results were not available in this database.
Children younger than 18 years who were diagnosed with HSP in Korea between 2006 and 2015 were enrolled in this study. Incidence case was defined as the first visit with a primary code of HSP in a patient. The wash-out period was 4 years. Patient characteristics, such as age, sex, the year of diagnosis, monthly distribution of HSP were analyzed. The age group was classified into two (< 9 years of age vs. 9–17 years). Data on initial diagnosis upon hospitalization and length of hospital stay were obtained. Moreover, re-hospitalization was obtained to predict recurrence. Re-hospitalization was defined as hospitalization with the primary code of HSP after 3, 6, and 12 months after the first diagnosis. Among incidence cases from 2006 to 2013, comorbidities according to the age and sex were analyzed, which include intussusception, abdominal interventions (barium reduction or abdominal surgery), HSP nephritis, and HSP arthritis. Children with HSP nephritis were followed-up for at least 2 years until 2015. The use of steroids and mean durations with regard to age, sex, length of hospitalization and comorbidities were analyzed.
Statistical analysis
Data are expressed as the number of participants. Demographic differences between the participant were examined using χ2 tests, and P < 0.05 was considered statistically significant. To estimate the differences in hospitalization, re-hospitalization, steroid use, and comorbidities in terms of age and sex, the hazard ratios (HRs) and 95% confidence intervals (CIs) were analyzed. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for statistical analysis.
Ethics statement
Informed consent was not required because the data were obtained from NHI Sharing Service after deleting personally identifiable information. The Institutional Review Board of Korea University Guro Hospital approved this study (approval No. 2016GR0356).
RESULTS
Demographic findings
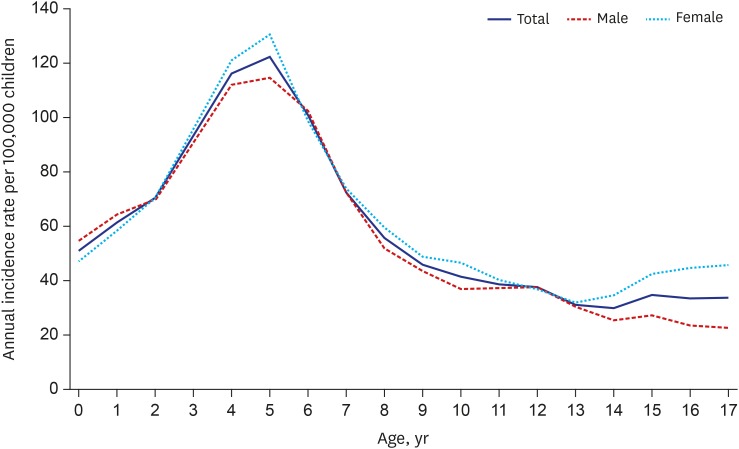
The eligible population included 9,138,868–10,925,900 people under 18 years in Korea each year (Supplementary Table 1). A total of 56,841 children who were diagnosed with HSP during the 10-year study period were enrolled. The overall annual incidence was 55.9 per 100,000 children under 18 years and it ranged from 52.6 to 60.5 without significant changes during the study period (2006–2015). The peak age was 5 years, and the annual incidence was 121.6 per 100,000 children. The annual incidence has increased from 0 to 5 years and decreased thereafter. Most incidence cases occurred in children under the age of 9. The male-to-female ratio was 1:1.1. The annual incidences by age and sex are described in Fig. 1 and Supplementary Fig. 1.
Fig. 1. Annual incidence by age and sex in children with HSP in Korea from 2006 to 2015.
HSP = Henoch-Schӧnlein purpura.
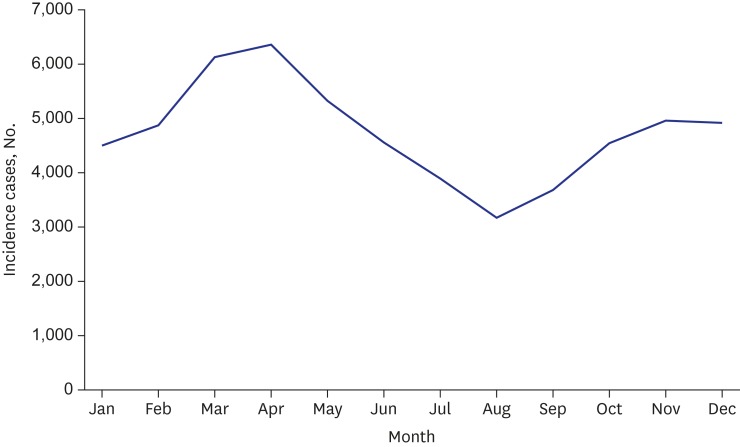
HSP occurred throughout the year, and it was most prevalent during the spring season, that is, between March and May (Fig. 2 and Supplementary Fig. 2). Socioeconomic status was not associated with HSP. No significant differences were observed between the four levels of family income and the regions of the 17 provinces and metropolitan areas in Korea (P > 0.05, Supplementary Fig. 3).
Fig. 2. Monthly distribution of children with HSP in Korea from 2006 to 2015.
HSP = Henoch-Schӧnlein purpura.
Hospitalization
Not all children who were diagnosed with HSP needed to be hospitalized. Of them, 11.3% children were hospitalized upon initial diagnosis, that is, 20.6 per 1,000 children with HSP. Boys are more at risk for hospitalization than girls. The HR for girls was 0.856 (95% CI, 0.811–0.904). Young age (< 9 years) was the risk factor for hospitalization. The HR of older children (9–17 years) was 0.455 (95% CI, 0.426–0.486).
We could not distinguish the recurrence of HSP using this database during routine follow-up in out-patient clinic. Thus the re-hospitalization rate was evaluated to predict severely recurring cases, that is, 2.0%, 1.61%, and 1.14% after 3, 6, and 12 months, respectively, from the initial diagnosis. The younger age group (< 9 years) was more likely to be re-hospitalized; the HR of children aged 9–17 years after 3, 6, and 12 months from the initial diagnosis were 0.868 (95% CI, 0.758–0.993), 0.899 (0.773–1.045), and 0.822 (0.686–0.985), respectively (Table 1).
Table 1. Clinical severity according to age and sex in children with HSP.
| Incidence (per 1,000 children with HSP) | HR | 95% CIs | |||
|---|---|---|---|---|---|
| Hospitalization | |||||
| Total | 20.556 | ||||
| Male | 22.511 | ||||
| Female | 18.664 | 0.856 | 0.811–0.904 | ||
| 0–8 yr | 26.484 | ||||
| 9–17 yr | 11.408 | 0.455 | 0.426–0.486 | ||
| Re-hospitalization | |||||
| After 3 months | |||||
| Total | 3.449 | ||||
| Male | 3.448 | ||||
| Female | 3.450 | 0.999 | 0.879–1.135 | ||
| 0–8 yr | 3.644 | ||||
| 9–17 yr | 3.124 | 0.868 | 0.758–0.993 | ||
| After 6 months | |||||
| Total | 2.867 | ||||
| Male | 2.857 | ||||
| Female | 2.876 | 1.003 | 0.869–1.158 | ||
| 0–8 yr | 2.991 | ||||
| 9–17 yr | 2.660 | 0.899 | 0.773–1.045 | ||
| After 12 months | |||||
| Total | 2.222 | ||||
| Male | 2.197 | ||||
| Female | 2.246 | 1.022 | 0.862–1.212 | ||
| 0–8 yr | 2.387 | ||||
| 9–17 yr | 1.946 | 0.822 | 0.686–0.985 | ||
| Comorbidity | |||||
| Intussusception | |||||
| Male | 0.643 | ||||
| Female | 0.450 | 0.733 | 0.534–1.007 | ||
| 0–8 yr | 0.813 | ||||
| 9–17 yr | 0.102 | 0.129 | 0.070–0.238 | ||
| Abdominal intervention | |||||
| Male | 0.153 | ||||
| Female | 0.110 | 0.750 | 0.394–1.428 | ||
| 0–8 yr | 0.189 | ||||
| 9–17 yr | 0.037 | 0.202 | 0.072–0.569 | ||
| HSP arthritis | |||||
| Male | 0.433 | ||||
| Female | 0.463 | 1.103 | 0.781–1.558 | ||
| 0–8 yr | 0.600 | ||||
| 9–17 yr | 0.195 | 0.328 | 0.205–0.523 | ||
| HSP nephritis | |||||
| Male | 2.413 | ||||
| Female | 2.043 | 0.849 | 0.726–0.993 | ||
| 0–8 yr | 2.413 | ||||
| 9–17 yr | 1.916 | 0.809 | 0.685–0.955 | ||
HSP = Henoch-Schӧnlein purpura, HR = hazard ratio, CI = confidence interval.
Comorbidity
The intussusception code was used in 0.34% of children with HSP upon initial diagnosis, with an incidence rate of 0.54 per 1,000 children. Younger children (< 9 years) were more likely to be suspected with intussusception. The HR of the older children group was 0.129 (95% CI, 0.07–0.238). Sex was not associated with intussusception. Approximately 0.08% of children with HSP underwent abdominal interventions, such as barium reduction or surgical laparotomy, with an incidence of 0.132 per 1,000 children with HSP. Younger children are more at risk of abdominal interventions. The HR of older children was 0.202 (95% CI, 0.072–0.569). Only 4 children with HSP underwent surgical laparotomy, and 3 out of 4 were older than 9 years.
The HSP arthritis code was used in 0.28% of children with HSP upon initial diagnosis, with an incidence of 0.448 per 1,000 children. Young children are more at risk of developing arthritis: the HR of older children was 0.328 (95% CI, 0.205–0.523).
The HSP nephritis code was utilized in 1.36% of children with HSP during the follow-up period of at least 2 years (2–9 years), with an incidence of 2.227 per 1,000 children. Young children are more at risk of developing HSP nephritis. The HR of older children was 0.809 (95% CI, 0.685–0.955; Table 1).
Steroid use
Physicians in Korea prescribed steroids in 56.6% of children with HSP upon initial diagnosis. Boys were more often treated with steroids than girls (57.1% vs. 56.1%, P = 0.024). Older children are more likely to receive steroid therapy than younger children (60.1% vs. 54.5%, P < 0.001). The mean durations of steroid use were 5 days in boys, 4 days in girls, 5 days in young children, and 4 days in older children.
Approximately 77.4% of hospitalized children and 51.0% of children in out-patient clinic received steroid therapy (P < 0.001). Children with suspected intussusception received steroid therapy more frequently than those who were not suspected with intussusception (88.9% vs. 56.5%, P < 0.001). Children with HSP arthritis received steroid therapy more frequently than those without HSP arthritis (77.4% vs. 56.5%, P < 0.001) (Table 2). Steroid use upon initial diagnosis was associated with HSP nephritis during the follow-up periods (1 month after the diagnosis and until maximum of 9 years thereafter) with an incidence of 2.101 per 1,000 children with HSP in the steroid group and 0.776 per 1,000 children with HSP without steroid group. The HR was 2.714 (95% CI, 2.159–3.412).
Table 2. Steroid use and characteristics of children with HSP.
| Characteristics | Steroid use, % | P value | ||
|---|---|---|---|---|
| Sex | 0.024 | |||
| Total | 56.6 | |||
| Male | 57.1 | |||
| Female | 56.1 | |||
| Age, yr | < 0.001 | |||
| 0–8 | 54.5 | |||
| 9–17 | 60.1 | |||
| Hospitalization | < 0.001 | |||
| Admitted | 77.4 | |||
| Out-patient | 51.0 | |||
| Comorbidity | ||||
| Intussusception | < 0.001 | |||
| Positive | 88.9 | |||
| Negative | 56.5 | |||
| Abdominal interventions | 0.098 | |||
| Positive | 76.47 | |||
| Negative | 56.55 | |||
| HSP arthritis | < 0.001 | |||
| Positive | 77.4 | |||
| Negative | 56.5 | |||
HSP = Henoch-Schӧnlein purpura.
DISCUSSION
This 10-year survey is the first nationwide epidemiologic study on children with HSP in Korea, and it is also the largest in the world, which included 56,841 cases. Hospital-based surveys for annual incidences in children varied between countries: 6.1 per 100,000 children in the Netherlands,5 6.7 in Saudi Arabia,6 17.6 in Sweden,7 18.6 in France,10 20.3–26.7 in Scotland,8 and 20.4 in the UK.9 Recently, a nationwide survey in Taiwan reported annual incidence of 12.9 per 100,000 children.11 In a study conducted in France, the undercount-corrected annual incidence was 30 per 100,000 children.10 In a study conducted in the UK, Asians had a higher incidence than Black. Only 10% of the study populations in the UK involved Asians, and most of them were from India, Pakistan, or Bangladesh. The annual incidence in our study was one of the highest compared to that reported in other countries. This result might be attributed to the fact that this study covered all children in Korea using nationwide claims data. Thus the number of patients was not undercounted, unlike when data are obtained from questionnaires or hospital-based studies. Korean ethnicity might be a risk factor for HSP. HSP was not uncommon in Korea. Ethnic differences might provide important information for the assessment of genetic causes.
The peak age was 5 (4–6) years (annual incidence of 121.6 per 100,000 children), which is similar to previous studies.9,10,11,12 In a study conducted in the UK, the incidence has increased to 70.3 in children aged 6 years.9 Lee et al.13 reported at the peak age of 6.93 years in Korean children with HSP. The peak season was spring, followed by winter, which is similar in France,10 and different in Taiwan (autumn and winter).11 Summer is a trough season in these studies, which is similar to our study. Previous upper respiratory infections were reported in patients with HSP.14,15 Exposure to viral infections among clustered children in daycare centers, or preschools might contribute to the fact that HSP is common in young children and rare during summer.
Boys were more frequently hospitalized than girls. Moreover, hospitalization and longer length of hospital stay were more frequently observed in the younger age group than in the older children group. Re-hospitalization was more common in the younger age group than in the older children group.
The primary diagnosis is mandatory. However, physicians are not obliged to make a secondary diagnosis to claim the NHI. Thus, HSP nephritis could be less estimated. Lee et al.13 reported that 26.9% of Korean children had renal involvement. Gastrointestinal involvement was reported in approximately 80% of children with HSP.13,16 The signs and symptoms, such as abdominal pain, gastrointestinal bleeding, hematuria, and proteinuria, and medical test results were not available in the NHI database. Nevertheless, the comorbidities and risk factors could be analyzed using the codes for secondary diagnosis and interventions or surgery. Younger children with HSP were more likely to be suspected with intussusception and undergo abdominal interventions. Surgical laparotomy is rarely performed. Young children are more at risk of developing arthritis and nephritis. Younger children might have a more severe clinical course; higher rate and longer duration of hospitalization; and higher rate of recurrences and comorbidities.
There is no consensus with regard the use of steroids for the management of HSP. No large-scale surveys are available. Some physicians use steroids in 89% of hospitalized patients in a single center study.13 Some reports have suggested that abdominal pain in children with HSP may respond to steroid use.4,16 A systematic review that included 3 prospective studies has suggested that early treatment with corticosteroids is associated with the increased odds of abdominal pain resolution within 24 hours.17 Niaudet and Habib18 in 1988 have reported that methylprednisolone pulse therapy was effective in patients who are at risk of nephropathy. Another study reported that the early administration of prednisone is useful in preventing HSP nephritis.19 A systematic review in 2009 reported heterogeneity among studies about the effect of early treatment with corticosteroids on cumulative (transient or persistent) renal disease, but has suggested an association with reduced odds of persistent renal disease.17 However, in a recent Cochrane database systematic review from RCTs, results showed no evidence on the benefit of using steroids to prevent persistent nephropathy in children with HSP.20
Our survey showed that steroids have been used in 57% of children with HSP in Korea. The mean duration of steroid therapy was 4–5 days. Physicians are more likely to use steroids in complex cases. Hospitalized children and those with suspected intussusception, arthritis, or nephritis have received steroids more often. Steroid use upon initial diagnosis of children with HSP was associated with HSP nephritis 1 month after diagnosis and thereafter. Steroid use upon initial diagnosis of children with HSP seems not to prevent HSP nephritis, which may support the results of the recent Cochrane review.20 However, this may be partly because physicians use steroids more frequently in children with HSP nephritis, then the secondary comorbidity code may have been entered late (after being transferred to a pediatric nephrologist) in the database, that is, after 1 month from diagnosis. Our data could not distinguish persistent renal disease from transient renal disease, and mean duration of steroid use was 4–5 days, that is, a relatively short period. Most studies have used steroids for about 2 weeks to evaluate the effect of steroids on HSP nephritis.17,19 It is hard to conclude that early use of steroids is not effective on HSP nephritis through this study. For the treatment of HSP, we showed the clinical practice patterns of physicians in Korea, and did not conclude causal relationship. A large-scale prospective randomized control study must be conducted to analyze the effect of steroids.
In conclusion, the annual incidence is higher in Korea than other countries. The annual incidence in Korea is 55.9 per 100,000 children, and most cases involve children younger than 9 years, with a peak age of 5 years. HSP commonly occurred during the spring and winter seasons. Younger children can have a more severe clinical course. Physicians are more likely to use steroids in complicated cases. This nationwide survey provides valuable information to better understand HSP in children and to inspire for further studies on HSP.
ACKNOWLEDGMENTS
We would like to thank the Korean National Health Insurance Sharing Service for providing access to the claims database (No. NHIS-2017-1-118).
Footnotes
Funding: This study was funded by the grant of the Korean Society for Pediatric Gastroenterology, Hepatology, and Nutrition (grant 2016).
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Shim JO, Chung JY. Data curation: Shim JO, Han K, Kim GH, Ko JS, Chung JY. Formal analysis: Shim JO, Han K, Park S, Kim GH, Ko JS, Chung JY. Methodology: Shim JO, Han K. Investigation: Shim JO, Park S, Ko JS, Chung JY. Writing - original draft: Shim JO. Writing - review & editing: Shim JO, Han K, Kim GH, Ko JS, Chung JY.
SUPPLEMENTARY MATERIALS
The eligible population of children in Korea between 2006 and 2015
Variations of annual incidences of HSP in Korea by age from 2006 to 2015.
Variations in the monthly distribution of children with HSP in Korea from 2006 to 2015.
Annual incidences of children with HSP according to the regions in Korea.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Mills JA, Michel BA, Bloch DA, Calabrese LH, Hunder GG, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Henoch-Schönlein purpura. Arthritis Rheum. 1990;33(8):1114–1121. doi: 10.1002/art.1780330809. [DOI] [PubMed] [Google Scholar]
- 3.Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: final classification criteria. Ann Rheum Dis. 2010;69(5):798–806. doi: 10.1136/ard.2009.116657. [DOI] [PubMed] [Google Scholar]
- 4.Rosenblum ND, Winter HS. Steroid effects on the course of abdominal pain in children with Henoch-Schӧnlein purpura. Pediatrics. 1987;79(6):1018–1021. [PubMed] [Google Scholar]
- 5.Aalberse J, Dolman K, Ramnath G, Pereira RR, Davin JC. Henoch-Schönlein purpura in children: an epidemiological study among Dutch paediatricians on incidence and diagnostic criteria. Ann Rheum Dis. 2007;66(12):1648–1650. doi: 10.1136/ard.2006.069187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Al YK, Hejazi Z, Majeed HA. Henoch Schönlein purpura in Arab children. Analysis of 52 cases. Trop Geogr Med. 1990;42(1):52–57. [PubMed] [Google Scholar]
- 7.Mossberg M, Segelmark M, Kahn R, Englund M, Mohammad AJ. Epidemiology of primary systemic vasculitis in children: a population-based study from southern Sweden. Scand J Rheumatol. 2018;7:1–8. doi: 10.1080/03009742.2017.1412497. [DOI] [PubMed] [Google Scholar]
- 8.Penny K, Fleming M, Kazmierczak D, Thomas A. An epidemiological study of Henoch-Schönlein purpura. Paediatr Nurs. 2010;22(10):30–35. doi: 10.7748/paed2010.12.22.10.30.c8135. [DOI] [PubMed] [Google Scholar]
- 9.Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet. 2002;360(9341):1197–1202. doi: 10.1016/S0140-6736(02)11279-7. [DOI] [PubMed] [Google Scholar]
- 10.Piram M, Maldini C, Biscardi S, De Suremain N, Orzechowski C, Georget E, et al. Incidence of IgA vasculitis in children estimated by four-source capture-recapture analysis: a population-based study. Rheumatology (Oxford) 2017;56(8):1358–1366. doi: 10.1093/rheumatology/kex158. [DOI] [PubMed] [Google Scholar]
- 11.Yang YH, Hung CF, Hsu CR, Wang LC, Chuang YH, Lin YT, et al. A nationwide survey on epidemiological characteristics of childhood Henoch-Schönlein purpura in Taiwan. Rheumatology (Oxford) 2005;44(5):618–622. doi: 10.1093/rheumatology/keh544. [DOI] [PubMed] [Google Scholar]
- 12.Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch-Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25(2):171–178. doi: 10.1097/BOR.0b013e32835d8e2a. [DOI] [PubMed] [Google Scholar]
- 13.Lee YH, Kim YB, Koo JW, Chung JY. Henoch-Schӧnlein purpura in children hospitalized at a tertiary hospital during 2004–2015 in Korea: epidemiology and clinical management. Pediatr Gastroenterol Hepatol Nutr. 2016;19(3):175–185. doi: 10.5223/pghn.2016.19.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanco R, Martínez-Taboada VM, Rodríguez-Valverde V, García-Fuentes M, González-Gay MA. Henoch-Schönlein purpura in adulthood and childhood: two different expressions of the same syndrome. Arthritis Rheum. 1997;40(5):859–864. doi: 10.1002/art.1780400513. [DOI] [PubMed] [Google Scholar]
- 15.Nakaseko H, Uemura O, Nagai T, Yamakawa S, Hibi Y, Yamasaki Y, et al. High prevalence of sinusitis in children with Henoch-Schönlein purpura. Int J Pediatr. 2011;2011:562638. doi: 10.1155/2011/562638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haroon M. Should children with Henoch-Schӧnlein purpura and abdominal pain be treated with steroids? Arch Dis Child. 2005;90(11):1196–1198. doi: 10.1136/adc.2005.077743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss PF, Feinstein JA, Luan X, Burnham JM, Feudtner C. Effects of corticosteroid on Henoch-Schönlein purpura: a systematic review. Pediatrics. 2007;120(5):1079–1087. doi: 10.1542/peds.2007-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niaudet P, Habib R. Methylprednisolone pulse therapy in the treatment of severe forms of Schönlein-Henoch purpura nephritis. Pediatr Nephrol. 1998;12(3):238–243. doi: 10.1007/s004670050446. [DOI] [PubMed] [Google Scholar]
- 19.Mollica F, Li Volti S, Garozzo R, Russo G. Effectiveness of early prednisone treatment in preventing the development of nephropathy in anaphylactoid purpura. Eur J Pediatr. 1992;151(2):140–144. doi: 10.1007/BF01958961. [DOI] [PubMed] [Google Scholar]
- 20.Hahn D, Hodson EM, Willis NS, Craig JC. Interventions for preventing and treating kidney disease in Henoch-Schönlein Purpura (HSP) Cochrane Database Syst Rev. 2015;(8):CD005128. doi: 10.1002/14651858.CD005128.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The eligible population of children in Korea between 2006 and 2015
Variations of annual incidences of HSP in Korea by age from 2006 to 2015.
Variations in the monthly distribution of children with HSP in Korea from 2006 to 2015.
Annual incidences of children with HSP according to the regions in Korea.