Abstract
RATIONALE
Inhaled hypertonic saline (HS) is an effective therapy for muco-obstructive lung diseases. However, the mechanism of action and principles pertinent to HS administration remain unclear.
METHODS
An in vitro system aerosolized HS to epithelial cells at rates comparable to in vivo conditions. Airway surface liquid (ASL) volume and cell height responses were measured by confocal microscopy under normal and hyperconcentrated mucus states.
MAIN RESULTS
Aerosolized HS produced a rapid increase in ASL height and decrease in cell height. Added ASL volume was quickly reabsorbed following termination of nebulization, though cell height did not recover within the same time frame. ASL volume responses to repeated HS administrations were blunted but could be restored by a hypotonic saline bolus interposed between HS administrations. HS-induced ASL hydration was prolonged with hyperconcentrated mucus on the airway surface, with more modest reductions in cell volume.
CONCLUSIONS
Aerosolized HS produced osmotically-induced increases in ASL height that were limited by active Na+ absorption and cell volume-induced reductions in cell water permeability. Mucus on airway surfaces prolonged the effect of HS via mucus dependent osmotic forces, suggesting that the duration of action of HS is increased in patients with hyperconcentrated mucus.
Keywords: aerosolization, ASL, epithelium, hypertonic saline, cell volume
Introduction
The effectiveness of mucociliary clearance is heavily dependent on adequate hydration of mucus. Under normal circumstances, airway epithelial cells have the capacity to absorb and secrete ions via active ion transport, with movement of water governed by transepithelial osmotic gradients. The net effect of this balance between secretion and absorption is to maintain mucus layer hydration at levels adequate to promote efficient mucus clearance [1]. Hyperconcentrated mucus, with failed mucus clearance, is characteristic of many muco-obstructive diseases, including cystic fibrosis (CF) [2], primary ciliary dyskinesia [3], and non-CF bronchiectasis [4].
Aerosolized hypertonic saline (HS) is an effective therapy in adults with CF [5,6] and non-CF bronchiectasis [7,8], producing improvements in MCC, forced expiratory volume in one second (FEV1), and quality of life.. Despite its clinical utility, the mechanism of action of HS remains speculative. Accelerating MCC via electrostatic interactions with mucins [9], expanding ASL hydration [10], or inhibiting epithelial sodium channels (ENaC) (9) have been proposed as mechanisms. Most mechanistic studies deposited large volumes of HS onto HBE surfaces and have not mimicked in vivo aerosol deliveries. Further, most studies using cultured HBE were performed without endogenous normal (2% solids) or hyperconcentrated (~8% solids) mucus on HBE surfaces [5,10].
As prescribed for clinical use, HS is aerosolized to the lower airway surfaces in small (nanoliter/cm2/min) volumes over protracted intervals (~15 minutes). In this study, an in vitro aerosolization system was developed to mimic in vivo aerosol delivery rates to airway epithelia that exhibited a range of mucus concentrations, spanning “normal” to muco-obstructive conditions. The primary goal was to characterize the kinetics of HS effects on airway surface hydration. The ancillary goal was to identify strategies to improve the efficacy of HS in the treatment of lung disease. Because of the great diversity of nebulizers and HS strengths used in clinical practice, and the fact that the osmotic effectiveness of HS depends on the rate of NaCl delivery to airway surfaces, our studies are described in µg NaCl deposited per cm2 per min, rather than % HS at a given deposition rate. Some of these data have been previously reported in the form of an abstract [11–15].
Methods
A full detail of the methods is provided in the Supplement.
HBE Cultures and Apical Mucus Content
Primary HBE cells generated from cells isolated from explanted lungs were maintained at an air-liquid interface (ALI) until fully differentiated. Culture surfaces were washed daily to produce HBE cultures with small amounts of residual mucus that mimicked normal epithelia, whereas HBE preparations with the mucus layer left unwashed for two weeks were generated to produce higher mucus compositions.
Confocal Measurements of ASL
Primary HBE cells were labeled with 2.0 µM calcein-AM (Invitrogen) basolaterally. ASL was visualized by adding a 5µl of Texas Red Dextran (Invitrogen, 70,000 MW, 5mg/ml) luminally. HS (7%) solution was aerosolized utilizing a vibrating mesh nebulizer (Aeroneb Lab; Aerogen), modified to deliver small volumes (nanoliters per minute). Nebulizers were cleaned daily in accordance with Cystic Fibrosis Foundation guidelines and tested periodically for functionality. The system was mounted in an environmental chamber that controlled air currents and regulated temperature to 37°C, humidity at >50%, and CO2 at 5%, interfaced to a scanning confocal microscope (SP5, Leica). Cell and ASL heights were measured by high-speed X-Z confocal scanning. Following baseline imaging, 7% HS was nebulized to apical surfaces of HBE cells at the prescribed rate and effects were measured at 30-second intervals.
ELISA determination IL-8 Secretion
Production of IL-8 in HBE was measured 24 hours after nebulizing either 3, 8, or 18 µg NaCl/cm2/min or sham nebulization of PBS (control). For comparison, parallel cultures were treated with a bulk addition of hypertonic saline (100 µl of a 7% solution). Concentrations of IL-8 in the culture medium were measured by using a human IL-8 ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacture’s protocol. [Briefly, after incubation, the culture medium was removed. Samples and standards were added to wells of microplates precoated with an anti-human IL-8 monoclonal antibody and incubated for 2 hours. Each well was washed and incubated with the enzyme-linked polyclonal antibody specific for human IL-8 for 2 hours. The wells were washed to remove unbound antibody-enzyme reagent, substrate solution added to each well for 20 min at room temperature, the enzyme reaction was stopped, and IL-8 concentrations determined by comparison of the optical density results with the standard curve.
Mathematical Modeling
The airway fluid transport model of Warren et al. [16] was utilized in the construction of an integrative model of human airway ion and fluid transport [17,18]. This model solves an ordinary differential equation system for membrane potentials (Va, Vb), intracellular ion concentrations ([Na+]i, [Cl−]i, [K+]i), extracellular ion concentrations ([Na+]e, [Cl−]e, [K+]e), and extracellular and intracellular fluid volumes (We, Wi) [18]. For the HS simulations, water with a high concentration of NaCl was “added” to the apical surface for designated volumes.
Statistical Analysis
All data analyses and graphing were performed in SigmaPlot (Systat). Mean values were compared via one-way ANOVA, with Holm-Sidak method for pairwise multiple comparisons in statistically significant findings. For non-normal data, the Kruskal-Wallis one-way ANOVA was used, with multiple groups compared via the Dunn Method. Paired t-tests or Mann-Whitney Rank Sum test were used where appropriate.
Results
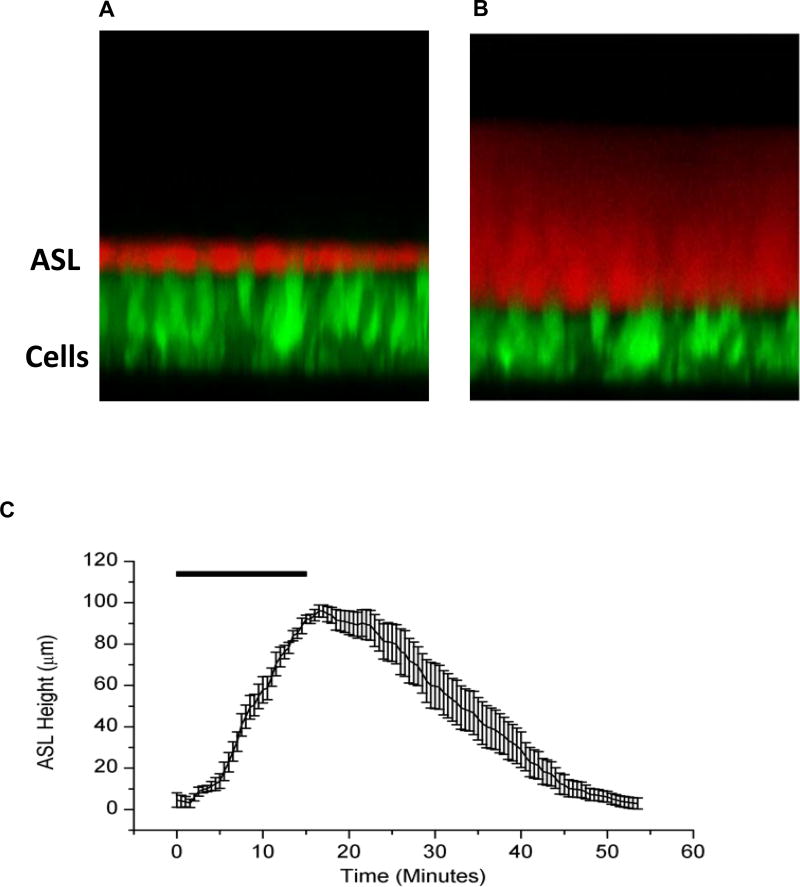
The first series of studies focused on the effect of HS on HBE cultures with “normal” mucus concentrations (1.9 ± 1.7% solids) on apical surfaces. HS was delivered to HBE cultures for 15 minutes at a rate of 8µg NaCl/cm2/min (7% HS delivered at 110 nl/cm2/min) to mimic a standard jet nebulizer delivery rate for human subjects (see Supplement). A rapid and significant increase in ASL height was observed, reflecting the osmotic-driven transepithelial fluid flow in response to deposited HS [19] (Figure 1). Net reabsorption of the osmotically expanded ASL began immediately after nebulization was terminated, and ASL height returned to baseline height within 60 minutes after aerosol initiation.
Figure 1.
(A) XZ-confocal image of a cross section of airway epithelial cells stained with Calcein, a fluorescent green dye, and ASL stained with Texas Red Dextran, a cell impermeable red dye. (B) After administration of an osmotic stimulus (HS), there is an increase in ASL height along with a decline in cell height. (C) HS aerosolized to the surface of HBE cultures results in a significant but transient increase in ASL height, lasting under an hour. n=8 for each experiment. Rectangle represents duration of HS administration (8µg NaCl/cm2/min for 15 min).
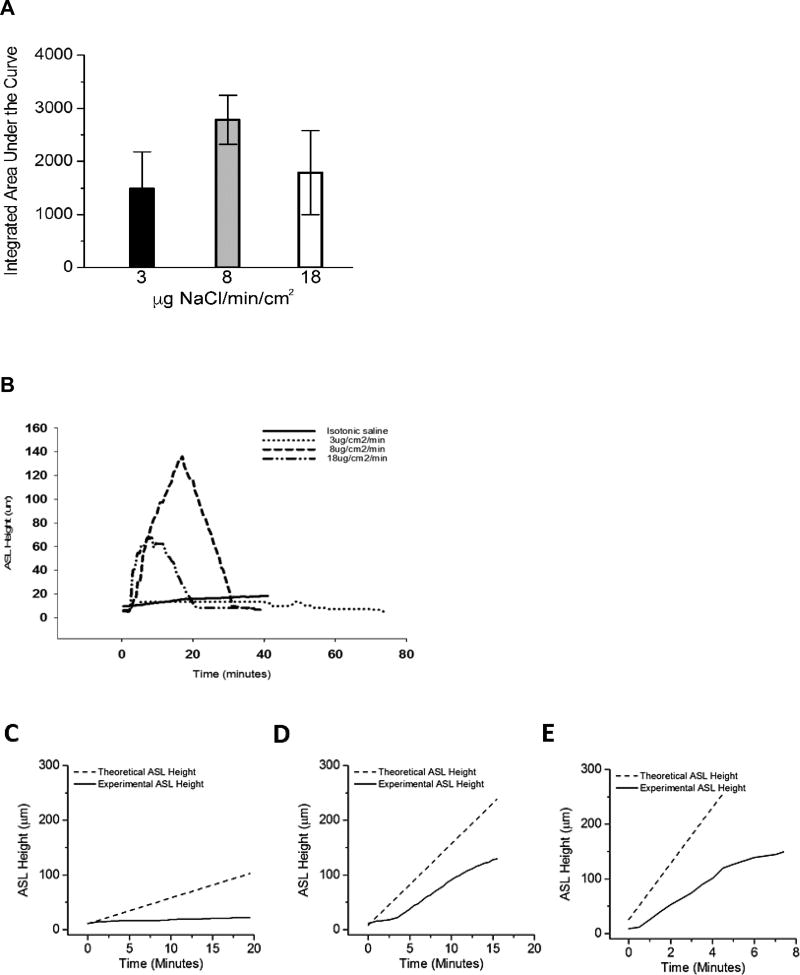
In clinical practice, jet nebulizers deliver HS in typically a 4 ml dose over approximately 15 minutes, whereas vibrating mesh nebulizers deliver the same volume in about 5 minutes. To address whether HS delivery rates affect ASL volume responses, a constant total mass of salt was nebulized onto the HBE surface at varying nebulization rates and, hence, durations of nebulization. The rates selected for study were designed to a mimic delivery of 7% HS via jet nebulizer (8 µg NaCl/cm2/min), a vibrating mesh nebulizer (18 µg NaCl /cm2/min), and an arbitrarily defined “slow” nebulizer (3 µg/cm2/min) (Figure 2A). Both the 8 and 18 µg NaCl/cm2/min rates produced rapid and significant increases in ASL height. The response of cultures to the 3 µg NaCl/cm2/min rate was considerably smaller with respect to ASL volume expansion, but longer due to the extended nebulization duration. All cultures, irrespective of nebulization rate, rapidly absorbed the added volume from the ASL once nebulization was terminated.
Figure 2.
Effects of variation in HS delivery rates on ASL volumes responses. (A) Comparison of ASL heights achieved with three rates of nebulized HS (low, 3µg NaCl/cm2/min; moderate, 8 µg NaCl/cm2/min; and high, 18 µg NaCl/cm2/min) with duration varied to hold constant total delivered mass of salt (demonstrated by length of bar at top). Isotonic saline showed for comparison. p<0.05 for all groups compared to isotonic saline control. (B) Both absolute height change and total hydration (integrated area over time) favored the mid-range dosing regimen but did not reach statistical significance (A; p=0.373). (C,D,E) Dashed lines indicates the expected rise in ASL height if all salt deposited resulted in an equimolar flux of water into the ASL. Solid black lines indicate the actual change in ASL height observed during experiments. The failure of these two lines to overlay indicates an inhibition of the transport of water from the epithelial cells into the ASL. (C) Low dose nebulization (3 µg NaCl/cm2/min). Theoretical slope 4.72 µm/min versus experimental slope of 0.45 µm/min, p<0.001. (D) Medium dose nebulization (8 µg NaCl/cm2/min). Theoretical slope 14.92 µm/min versus experimental slope of 8.66 µm/min, p<0.001. (E) High dose nebulization (18 µg NaCl/cm2/min). Theoretical slope 51.49 µm/min versus experimental slope of 20.78 µm/min, p<0.001. N=4 per experimental condition.
Area under the curves (AUCs) of the ASL height data were calculated as an index of “hydration activity” of the nebulized solution. Interestingly, faster salt deposition (18 µg/cm2/min) produced a lower, though not statistically different, total ASL hydration compared to standard jet nebulizer rates (8 µg/cm2/min). Slower aerosol deposition (3 µg/cm2/min) produced trends towards lower peak and AUC values compared to the faster rates (Figure 2B).
Assuming no active sodium-mediated volume absorption or change in apical membrane water permeability during aerosolization, deposition of a constantly accumulating mass of salt to the surface of airway epithelia is predicted to produce a linear increase in ASL volume. For all rates studied, the measured ASL height deviated consistently from the predicted height (Figures 2C–E). The deviation of measured ASL height from predicted values could reflect active Na+ absorption, reductions in HBE water permeabilities that govern rates of osmotically driven water flow to the HBE surface, or both.
With respect to the role of Na+ transport on ASL volume responses to HS administration, we speculated that Na+ transport would modify the magnitude of HS-induced ASL volume expansion immediately after initiation of HS administration. To experimentally investigate this possibility, ASL volume responses to the low rate of HS administration (3 µg NaCl/cm2/min) were measured in the presence and absence of a selective ENaC blocker (VX-371, Vertex Pharmaceuticals). As compared to HS alone, the co-administration of VX-371 (50 µg/mL) produced a more rapid and sustained ASL response during nebulization (Figure 3A). A mathematical model of airway epithelial ion and water transport quantitatively analyzed the relationship between HS-mediated changes to ASL height and active Na+ absorption in the presence and absence of ENaC blockade (Figure 3B). The fit of the model to experimental data describing ASL response for HS administration in the absence of ENaC inhibition was achieved by maintaining Na+ transport at basal rates during HS aerosolization and increasing Na+ transport roughly five-fold during the re-absorptive phase. The mathematical model replicated the effect of ASL kinetics with ENaC inhibition, with a more rapid rate of accumulation and overshoot of ASL height, consistent with inhibition of active Na+ absorption during HS administration.
Figure 3.
Effect of sodium reabsorption on HS-mediated ASL height. (A) Experimental data of hypertonic saline (at 8 µg NaCl/cm2/min) in the absence and presence of a potent sodium channel blocker (VX-371 at 50 µg/ml). (B) Mathematical model predictions of the HS effect with normal ENaC conductance (solid line, consistent with experimental data) and 15% of ENaC channel normal conductance (dashed line).
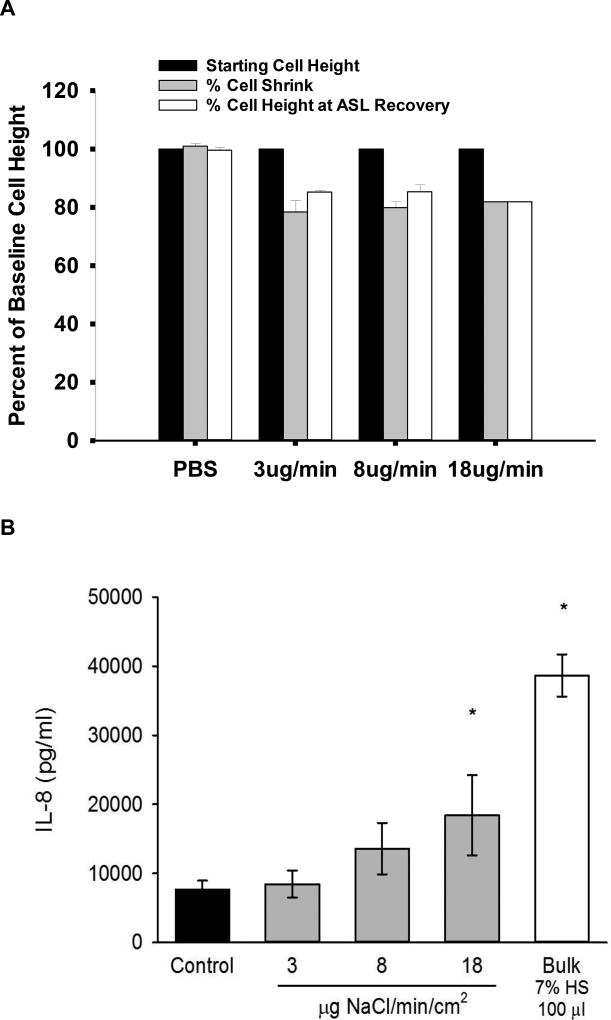
Previous studies have demonstrated that HS can produce a reduction in cell volume [20,21], which can then inhibit cell water permeabilities [21–23]. Additionally, studies suggest that administration of hypertonic saline also will result in inflammatory changes within the airway epithelia, particularly interleukin-8 (IL-8), a known neutrophil chemoattractant [24–26]. Accordingly, we measured HBE cell volume responses and IL-8 production to HS aerosol administration to test whether reductions in cell water permeabilities blunted ASL responses to aerosolized HS and/or induced pro-inflammatory cytokines. Figure 4A shows that cell height (a surrogate for cell volume) was reduced during and after HS nebulization by ~ 20% from initial cell heights, (p<0.01). In contrast, cell volume was not affected by nebulization of isotonic saline. These data suggest that deposition of luminal HS osmotically draws water from cells onto the surface via apical membrane water channels. Figure 4B demonstrates cumulative IL-8 production by HBE 24 hours after nebulizing HS or PBS control to airway surfaces. A stepwise increase in IL-8 production was noted with increasing doses of HS compared with PBS control (statistically different from control at 18µg dose and bulk dosing.
Figure 4.
(A) Cell volume changes in response to osmotic stimulus of HS at varying HS deposition rates. A 20% decrease in cell height in response to the hypertonic stimulus was observed at all rates, which was significantly different from baseline and isotonic (PBS) saline administration (p<0.01 by Holm-Sidak method). Cell height did not recover during the time frame of the experimentation (60 minutes). Cell volume reduction was a consistent finding regardless of rate of HS administration. (n=4 for each condition). (B) Production of IL-8 in response to increasing doses of nebulized or bulk HS. Statistically different from control (PBS) at the 18 µg dose (p<0.002 by Holm-Sidak method) and bulk dosing (100µl of 7% HS) (p<0.001).
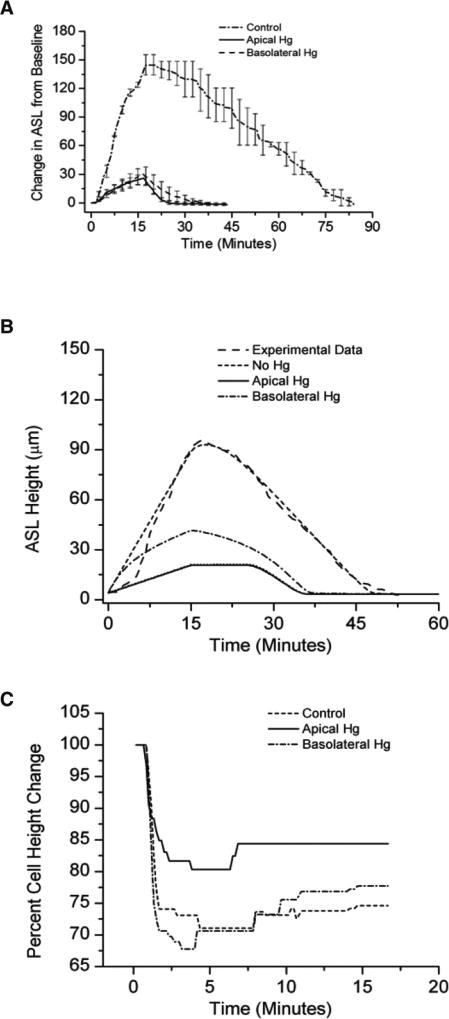
The contribution of cellular water permeability to ASL volume responses was investigated further using a pharmacologic approach. Because mercury-sensitive water channels (aquaporins; AQPs) have been identified in the apical and basolateral membranes of HBE epithelia [27,28], the role of these channels on HS-mediated changes in ASL volume responses was evaluated experimentally. Because mercury has off-target toxic effects with long exposure, the protocols involved acute exposures to mercury and HS [29]. As shown in Figure 5A, both apical and basolateral HgCl2 administration significantly reduced ASL volume responses to aerosolized HS. Inhibition of apical AQPs resulted in a smaller decrease in cell height compared to basolateral AQP inhibition. These data suggest that in the setting of aerosolized HS, cell volume homeostasis is dominated by the apical membrane water permeability (Figure 5B). Modeling studies mimicked the experimental data (Figure 5C).
Figure 5.
Incubation with mercury chloride apically or basolaterally resulted in a diminished ASL response to aerosolized HS (at 8µg NaCl/cm2/min) compared with native cultures. (A) Experimental data. p<0.001 via Holm-Sidak method for comparisons to control. (B) Mathematical model of experimental data. N=8 for each condition. (C) Cell height change to aerosolized HS in the absence (control) or presence of selective block of the apical or basolateral cell membrane with mercury chloride (p<0.001 for all comparisons).
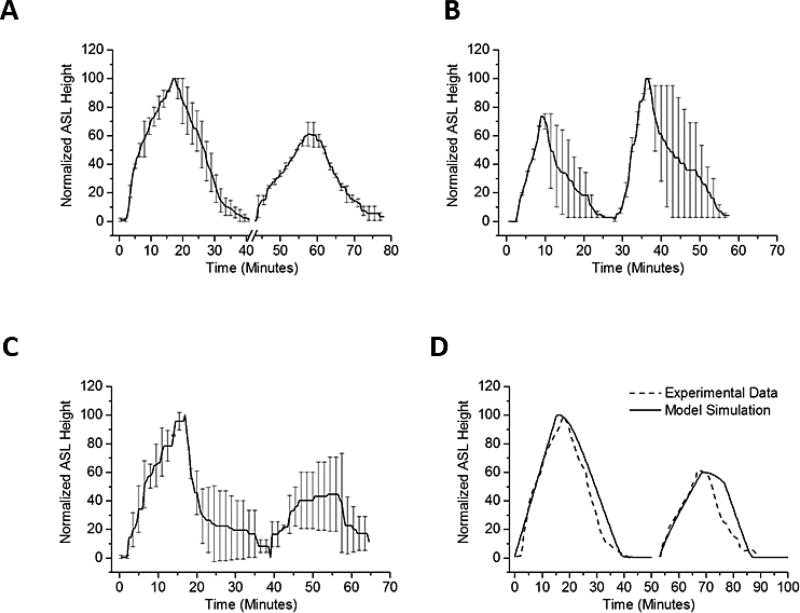
We hypothesized that persistent reduction in cell volumes observed after HS administration would limit the ability of HBEs to respond to repeated administrations of HS. This hypothesis was tested by exposing HBE cultures to sequential HS administrations (8 µg NaCl/cm2/min), with the second administration delivered 15 minutes after ASL height had returned to baseline following the first HS dose. ASL height and AUC of the second administration was significantly reduced compared to initial HS administration (Figure 6A).
Figure 6.
Sequential ASL volume responses to aerosolized 7% HS for 15 min (8µg NaCl/cm2/min). (A) Sequential ASL volume responses to aerosolized HS. A smaller ASL response to a second administration of aerosolized HS was observed when two identical HS doses were separated by 15 minutes. Break in x-axis indicate time between two doses. For each dose, HS was nebulized at 8 µg NaCl/cm2/min, administered continuously for 15 minutes. The ASL height of the second peak was about 60% of the first peak. P<0.001 by Mann-Whitney Rank Sum test. (B) A hypotonic saline rinse was interposed between HS administration. The hypotonic solution administration produced cellular swelling associated with improved ASL volume responses to a subsequent administration of HS (~140% of first dose). The difference in response was statistically significant (p=0.002 via Mann-Whitney Rank Sum test) in favor of the post-hypotonic peak. (C) Interposition of an isotonic rinse between the first and second HS administration. The same second dose effect is not seen when doses of HS were separated by an isotonic saline bolus. (p=0.78 by Mann-Whitney Rank Sum Test). ASL height of second peak persisted ~50% of the first peak. (D) In model simulation, the water permeability of the HBE apical membrane during the second dose was reduced to 10% of the basal water permeability. N=4 for each condition.
We next directly tested the hypothesis that the reduction in the effectiveness of the second HS administration reflected reduced HBE cell water permeabilities consequent with cell volume reduction, rather than persistent acceleration of Na+ transport. To test this notion, a small volume of hypotonic saline (0.63% NaCl, 10 µl) was transiently added (3 minutes) to the apical surface of HBE’s following the first aerosolized HS administration. Application of the hypotonic saline bolus resulted in cell swelling (increase in height 32 ± 9 µm) that restored cell height to baseline levels. The interposition of the hypotonic bolus was associated with a statistically larger response in ASL and AUC to the second HS administration (Figure 6B). In contrast, applying a bolus dose of isotonic saline (0.9% NaCl, 10 µl) produced neither cell swelling nor a recovery in responsiveness to the subsequent HS challenge (Figure 6C). Modeling this phenomenon predicted that reduction in apical water permeability to about 10% of baseline levels would produce the AUC decrement with the second HS administration observed experimentally (Figure 6D).
We next investigated whether the presence of hyperconcentrated mucus associated with muco-obstructive diseases [30] alters the kinetics of HS-induced hydration, utilizing HBE cultures exhibiting hyperconcentrated mucus (12 ± 4.3% solids). Cystic fibrosis (CF) is the disorder best characterized by hyperconcentrated mucus. However, because other muco-obstructive diseases, e.g., primary ciliary dyskinesia [3] and non-CF bronchiectasis [4] exhibit hyperconcentrated mucus, and we have previously shown that CF cells and normal HBE in the presence of hyperconcentrated mucus behave similarly in response to nebulized hypertonic saline [13]. Accordingly, we focused our studies of muco-obstructive disease mucus on normal HBE with hyperconcentrated mucus to make the data relevant to other possible muco-obstrcutive lung diseases. Pre-HS administration, ASL height in the hyperconcentrated mucus cultures was ~ 3-fold higher than the normal mucus cultures. Notably, the changes in ASL height with HS administration were substantially increased in hyperconcentrated mucus cultures as compared to normal mucus cultures (Figure 1, 7A). Although both normal and hyperconcentrated mucus cultures absorbed the increased ASL volume immediately after cessation of nebulization, the rate of reabsorption was slower in hyperconcentrated versus normal mucus cultures (5.5 vs. 2.8 µm/min, p=0.052, Figure 7B). In the hyperconcentrated mucus cultures, the total duration of HS-induced ASL volume expansion was approximately double that of normal mucus cultures. Further, in repetitive HS administration protocols, the second administration of HS produced an ASL response similar to the first administration, Figure 7C. Pertinent to this observation, cell heights in HBEs covered by hyperconcentrated mucus decreased less in response to HS (15 ± 4.7% vs. 23 ± 5.7%) than in normal cultures, though this difference did not achieve statistical significance.
Figure 7.
The effects of hyperconcentrated mucus on HBE surfaces on nebulized HS (at 8µg NaCl/cm2/min) in inducing ASL volume responses. (A) In the normal (2% mucus) state (solid line), there was a 3-fold increase in ASL volume during nebulization. HBE cultures with hyperconcentrated mucus (12%) (Dashed line) exhibited a 7-fold increase in ASL above baseline ASL. The duration of ASL being increased above basal levels was also increased in hyperconcentrated mucus HBE cultures. (B) Rates of reabsorption (time to baseline ASL, in µm/min) were significantly different between the normal and the hyperconcentrated mucus cultures. P=0.052 by Mann-Whitney Rank Sum Testing. (C) Two sequential doses of HS administered to hyperconcentrated cultures. ASL height difference favors the second dose (p<0.001). (D) Dashed line indicates the expected rise in ASL height if all salt deposited remained on the surface and resulted in an equimolar flux of water into the ASL. Solid line indicates the actual change in ASL height observed during experiments. Compare to Figures 2C–E: In the presence of an intact mucus layer (12% solids), actual and expected ASL height more closely approximated each other early in the HS delivery interval.
The larger peak ASL response and slower absorption of NaCl and water in the hyperconcentrated versus normal mucus cultures suggested an additional force was governing water flux in the hyperconcentrated mucus system. To generate an index of the magnitude of this effect, the predicted versus measured ASL heights during HS administration were compared (Figure 7D). The measured early administration values were significantly closer to the predicted value for hyperconcentrated compared with normal mucus cultures (see Figures 2C–E), suggesting that the presence of concentrated mucus generated additional mucus-related osmotic forces that retarded fluid absorption [30].
Discussion
Our in vitro studies of clinically-relevant rates of aerosolized HS delivered to HBE cultures with 2% mucus solids revealed a rapid HS-induced expansion of ASL volume. This ASL expansion in response to HS aerosols is similar to that reported in vivo in mice measured by synchrotron-based tomography and in HBE cultures by optical coherence tomography [31,32]. Further, acute aerosolization of HS has been reported to reduce airway mucus concentrations in COPD subjects [33]. Thus, the ASL expansion findings, juxtaposed to our findings that the osmolarity of ASL during HS nebulization does not likely exceed 370 mOsm/L (see below), argues that the major effect of HS on mucus clearance is via ASL volume expansion and mucus dilution.
The HS-induced ASL volume response was mediated by water flux in response to the deposition of osmotically active NaCI on HBE surfaces. Two observations suggest that aquaporin-mediated transepithelial water fluxes dominated the ASL response to aerosolized HS. First, the flux of water to the lumen in response to HS was accompanied by a reduction in cell height/volume, suggesting intracellular water moved into ASL. This notion is consistent with previous studies [21] that reported the aquaporin-dominated apical membrane water permeability of HBE was ~ 10 fold higher than the basolateral membrane. Note, this configuration permits the use of changes in cell volume as an “osmometer” to detect ASL osmolality. The cell volume responses to HS aerosols suggest that ASL achieved osmolalities of ~370 mOsm during HS administration (7% HS given at 7.7 µg/NaCl/cm2/min), far less than achieved by direct HS additions. The second observation was that the ASL responses to aerosolized HS were blocked by mercury chloride, an inhibitor of AQP 3–5 known to be expressed in HBEs. These data, along with the cell modeling data presented, strongly suggest that aquaporin-mediated cellular water permeabilities participate in the ASL volume responses to aerosolized HS (Figure 4).
The effects of aerosolized HS on cell volume reduction and transepithelial Na+ transport likely explain the discrepancy between the predicted ASL responses to deposition of HS on HBE surfaces and measured responses. First, reductions in cell volume were associated with reductions in cell water permeabilities, limiting water fluxes toward a hypertonic lumen. Second, as evidenced by a greater maximal response to HS administration in the presence of an ENaC blocker, active Na+ transport removed a component of the deposited NaCI during aerosol administration. An interesting observation that emerged from the modeling of active Na+ transport responses to HS was that an increase in Na+ transport rates was required to mimic the rate of ASL volume absorption post cessation of aerosol. This effect likely reflects dilution of local extra-cellular inhibitors of ENaC, e.g. ATP, that accelerated the rate of absorption during HS administration [34].
A key issue with respect to HS therapeutic responses relates to the concentration of mucus on airway surfaces. The presence of hyperconcentrated mucus on HBE surfaces was associated with increased ASL heights, a longer duration of ASL hydration to a single HS administration, and larger responses to repetitive doses of HS. We postulate that mucus acts as a “sponge”, providing a concentration-dependent polymer gel-mediated osmotic driving force, added to that of HS-induced osmotic gradients, which modulates maximal mucus/ASL heights on the HBE surface. The increased durability of the ASL expansion in response to aerosolized HS in hyperconcentrated mucus cultures parallels the longer duration of action of HS in CF as compared to normal subjects observed in in vivo MCC studies [5,35].
Our studies were also designed to identify strategies to increase the effectiveness of aerosolized HS to expand ASL hydration, including studies of HS delivery rates and repetitive dosing. We found very slow rates of HS delivery were relatively ineffective. We speculate that this finding reflects the fact that NaCl deposition rates were similar to rates of endogenous active transepithelial Na+ absorption [36]. Delivery of HS at rates approximating a jet nebulizer were effective in increasing ASL hydration but a further increase in efficacy was not observed with faster rates mimicking vibrating mesh nebulizers. The absolute decrease in AUC and the slowing of ASL volume expansion observed towards the end of rapid HS administration (18 µg NaCl/cm2/min) are consistent with large reductions in transepithelial water permeabilities. Based on our IL-8 measurements and the pro-inflammatory nature of this cytokine, it is possible that faster rates of nebulization could actually be detrimental.
An important observation pertinent to HS dosing frequency was that HBE cell heights did not return to baseline levels for 4 hours following HS administration. We speculate that the absence of cell volume regulation mechanisms reflected decreased water permeabilities. The delay in return of apical water permeability after HS likely accounted for the blunted response to second HS administrations on HBE cultures with normal mucus (Figure 5). Importantly, epithelial cell swelling induced by hypotonic saline restored second HS responses. These data suggest that novel strategies may be required to optimize the effectiveness of repetitive HS dosing in patients with milder lung disease. In contrast, repetitive HS dosing in subjects with severe (12% mucus solids) disease may be an effective strategy.
In conclusion, ASL volume of HBE cultures with normal mucus concentrations increased in response to nebulized HS delivered at rates similar to those delivered clinically, but reabsorption began immediately with termination of nebulization. Neither nebulizing faster nor slower improved ASL hydration. However, the effects of aerosolized HS were more pronounced and prolonged in HBE cultures with hyper-concentrated mucus, likely reflecting the increased osmotic forces generated by concentrated mucus. These data predict a prolonged duration of HS action in patients with muco-obstructive diseases.
Supplementary Material
Take Home Message.
This study provides insight into the magnitude of effect of hypertonic saline on airway surface hydration in muco-obstructed diseases.
Acknowledgments
Funding support: CFF GORALS12LO, BUTTON07XX0, NIH 1R01HL125280-01A1, P30DK065988-11
The authors would like to acknowledge the UNC Tissue Culture Core (NIH P30 DK065988 and CFF RDP BOUCHE15R0), as well as Eric Roe for his editorial assistance. This project was funded by the CFF Leroy Matthews Physician Scientist Award (CFF GORALS12LO) as well as BUTTON07XX0, NIH R01HL125280-01A1, and NIH P30DK065988-11.
Abbreviation List
- ALI
air-liquid interface
- AQP
aquaporin
- ASL
airway surface liquid
- AUC
area under the ASL height curve
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- CO2
carbon dioxide
- ENaC
epithelial sodium channel
- FEV1
forced expiratory volume in 1 second
- HBE
human bronchial epithelium
- HS
hypertonic saline
- IL-8
interleukin 8
- mOsm/L
milli-osmoles per liter
- MCC
mucociliary clearance
- NaCl
sodium chloride
- PBS
phosphate buffered saline
- TRD
Texas Red Dextran
- UNC
University of North Carolina at Chapel Hill
Footnotes
Author contributions: Concept and design: JLG and BB; data acquisition: JLG, BB, WRT; Data Modeling: DW; Data interpretation, drafting and critically revising the work: JLG, RCB and BB
Uncategorized References
- 1.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007;261(1):5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 2.Button B, Cai LH, Ehre C, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337(6097):937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush A, Payne D, Pike S, Jenkins G, Henke MO, Rubin BK. Mucus properties in children with primary ciliary dyskinesia: comparison with cystic fibrosis. Chest. 2006;129(1):118–123. doi: 10.1378/chest.129.1.118. [DOI] [PubMed] [Google Scholar]
- 4.Redding GJ, Kishioka C, Martinez P, Rubin BK. Physical and transport properties of sputum from children with idiopathic bronchiectasis. Chest. 2008;134(6):1129–1134. doi: 10.1378/chest.08-0296. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354(3):241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 6.Elkins MR, Robinson M, Rose BR, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354(3):229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 7.Tarrant BJ, Le Maitre C, Romero L, et al. Mucoactive agents for chronic, non-cystic fibrosis lung disease: A systematic review and meta-analysis. Respirology. 2017;22(6):1084–1092. doi: 10.1111/resp.13047. [DOI] [PubMed] [Google Scholar]
- 8.Kellett F, Robert NM. Nebulised 7% hypertonic saline improves lung function and quality of life in bronchiectasis. Respir Med. 2011;105(12):1831–1835. doi: 10.1016/j.rmed.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Tang XX, Ostedgaard LS, Hoegger MJ, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest. 2016;126(3):879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarran R, Grubb BR, Parsons D, et al. The CF salt controversy: in vivo observations and therapeutic approaches. Mol Cell. 2001;8(1):149–158. doi: 10.1016/s1097-2765(01)00286-6. [DOI] [PubMed] [Google Scholar]
- 11.Goralski JL, Button B. Mucus Concentration Affects HBE Response to Nebulized Hypertonic Saline. Pediatric Pulmonology. 2014;38:182. [Google Scholar]
- 12.Goralski JL, Button B. Nebulized Hypertonic Saline Yields Disparate ASL Heights with Sequential Doses. Pediatric Pulmonology. 2013;36:107. [Google Scholar]
- 13.Goralski JL, Button B. CF Cells do not Hyperabsorb Sodium in the Setting of Nebulized Hypertonic Saline. Pediatric Pulmonology. 2012;34:105. [Google Scholar]
- 14.Goralski JL, Button B. An In Vitro Study of the Kinetics of Hypertonic Saline on ASL Height. Pediatric Pulmonology. 2010;33:205. [Google Scholar]
- 15.Thelin W, Donn K, Ansede J, Johnson M. The ENaC Inhibitor P-1037 is a CFTR-independent Therapeutic Agent That Promotes Sustained Airways Hydration and Mucociliary Transport. Pediatric Pulmonology. 2015;50(12):201. [Google Scholar]
- 16.Warren NJ, Tawhai MH, Crampin EJ. A mathematical model of calcium-induced fluid secretion in airway epithelium. J Theor Biol. 2009;259(4):837–849. doi: 10.1016/j.jtbi.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Wu D, Miyawaki S, Tawhai MH, Hoffman EA, Lin CL. A Numerical Study of Water Loss Rate Distributions in MDCT-Based Human Airway Models. Ann Biomed Eng. 2015;43(11):2708–2721. doi: 10.1007/s10439-015-1318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu D, Boucher RC, Button B, Elston T, Lin CL. An integrated mathematical epithelial cell model for airway surface liquid regulation by mechanical forces. J Theor Biol. 2017;438:34–45. doi: 10.1016/j.jtbi.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackmon RL, Kreda SM, Sears PR, et al. Direct monitoring of pulmonary disease treatment biomarkers using plasmonic gold nanorods with diffusion-sensitive OCT. Nanoscale. 2017;9(15):4907–4917. doi: 10.1039/c7nr00376e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischbarg J. Fluid transport across leaky epithelia: central role of the tight junction and supporting role of aquaporins. Physiol Rev. 2010;90(4):1271–1290. doi: 10.1152/physrev.00025.2009. [DOI] [PubMed] [Google Scholar]
- 21.Willumsen NJ, Davis CW, Boucher RC. Selective response of human airway epithelia to luminal but not serosal solution hypertonicity. Possible role for proximal airway epithelia as an osmolality transducer. J Clin Invest. 1994;94(2):779–787. doi: 10.1172/JCI117397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasgado-Flores H, Krishna Mandava V, Siman H, et al. Effect of apical hyperosmotic sodium challenge and amiloride on sodium transport in human bronchial epithelial cells from cystic fibrosis donors. Am J Physiol Cell Physiol. 2013;305(11):C1114–1122. doi: 10.1152/ajpcell.00166.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herschlag G, Garcia GJ, Button B, et al. A mechanochemical model for auto-regulation of lung airway surface layer volume. J Theor Biol. 2013;325:42–51. doi: 10.1016/j.jtbi.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graeber SY, Zhou-Suckow Z, Schatterny J, Hirtz S, Boucher RC, Mall MA. Hypertonic saline is effective in the prevention and treatment of mucus obstruction, but not airway inflammation, in mice with chronic obstructive lung disease. Am J Respir Cell Mol Biol. 2013;49(3):410–417. doi: 10.1165/rcmb.2013-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabary O, Escotte S, Couetil JP, et al. High susceptibility for cystic fibrosis human airway gland cells to produce IL-8 through the I kappa B kinase alpha pathway in response to extracellular NaCl content. J Immunol. 2000;164(6):3377–3384. doi: 10.4049/jimmunol.164.6.3377. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto S, Matsumoto K, Gon Y, Nakayama T, Takeshita I, Horie T. Hyperosmolarity-induced interleukin-8 expression in human bronchial epithelial cells through p38 mitogen-activated protein kinase. Am J Respir Crit Care Med. 1999;159(2):634–640. doi: 10.1164/ajrccm.159.2.9712090. [DOI] [PubMed] [Google Scholar]
- 27.Yukutake Y, Tsuji S, Hirano Y, et al. Mercury chloride decreases the water permeability of aquaporin-4-reconstituted proteoliposomes. Biol Cell. 2008;100(6):355–363. doi: 10.1042/BC20070132. [DOI] [PubMed] [Google Scholar]
- 28.Kuwahara M, Gu Y, Ishibashi K, Marumo F, Sasaki S. Mercury-sensitive residues and pore site in AQP3 water channel. Biochemistry. 1997;36(46):13973–13978. doi: 10.1021/bi9711442. [DOI] [PubMed] [Google Scholar]
- 29.Vergilio CS, Carvalho CE, Melo EJ. Mercury-induced dysfunctions in multiple organelles leading to cell death. Toxicol In Vitro. 2015;29(1):63–71. doi: 10.1016/j.tiv.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Henderson AG, Ehre C, Button B, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124(7):3047–3060. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan KS, Donnelley M, Farrow N, et al. In vivo X-ray imaging reveals improved airway surface hydration after a therapy designed for cystic fibrosis. American journal of respiratory and critical care medicine. 2014;190(4):469–471. doi: 10.1164/rccm.201405-0855LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blackmon RL, Kreda SM, Sears PR, et al. Diffusion-sensitive optical coherence tomography for real-time monitoring of mucus thinning treatments. Proc SPIE Int Soc Opt Eng. 2016;9697 doi: 10.1117/12.2208805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henderson AG, Bennett WD, Zeman KL, et al. Effects Of Inhaled Hypertonic Saline On Mucociliary Clearance And Clinical Outcomes In Patients With Chronic Bronchitis. Am J Resp Crit Care. 2017;195:A6455. [Google Scholar]
- 34.Sandefur CI, Boucher RC, Elston TC. Mathematical model reveals role of nucleotide signaling in airway surface liquid homeostasis and its dysregulation in cystic fibrosis. Proc Natl Acad Sci U S A. 2017;114(35):E7272–E7281. doi: 10.1073/pnas.1617383114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett WD, Wu J, Fuller F, et al. Duration of action of hypertonic saline on mucociliary clearance in the normal lung. J Appl Physiol (1985) 2015;118(12):1483–1490. doi: 10.1152/japplphysiol.00404.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Locke LW, Myerburg MM, Markovetz MR, et al. Quantitative imaging of airway liquid absorption in cystic fibrosis. Eur Respir J. 2014;44(3):675–684. doi: 10.1183/09031936.00220513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.