Abstract
Aim of the study
Hepatic encephalopathy and hyperammonemia is a clinical complication associated with liver cirrhosis. The brain is the target organ for ammonia toxicity. Ammonia-induced brain injury is related to oxidative stress, locomotor activity dysfunction, and cognitive deficit, which could lead to permanent brain injury, coma and death if not appropriately managed. There is no promising pharmacological intervention against cirrhosis-associated brain injury. Taurine (TAU) is one of the most abundant amino acids in the human body. Several physiological and pharmacological roles have been attributed to TAU. TAU may act as an antioxidant and is an excellent neuroprotective agent. This study aimed to evaluate the effect of TAU supplementation on cirrhosis-associated locomotor activity disturbances and oxidative stress in the brain.
Material and methods
Rats underwent bile duct ligation (BDL) surgery, and plasma and brain ammonia level, plasma biochemical parameters, and rats’ locomotor function were monitored. Furthermore, brain tissue markers of oxidative stress were assessed.
Results
It was found that plasma and brain ammonia was increased, and markers of liver injury were significantly elevated in the cirrhotic group. Impaired locomotor activity was also evident in BDL rats. Moreover, an increase in brain tissue markers of oxidative stress was detected in the brain of cirrhotic animals. It was found that TAU supplementation (50, 100, and 200 mg/kg, gavage) alleviated brain tissue markers of oxidative stress and improved animals’ locomotor activity.
Conclusions
These data suggest that TAU is a potential protective agent against cirrhosis-associated brain injury.
Keywords: amino acid, hepatic encephalopathy, hyperammonemia, neurotoxicity, oxidative stress
Introduction
Hepatic encephalopathy (HE) and hyperammonemia is a clinical feature of chronic liver injury and cirrhosis [1]. Although the exact mechanism(s) of HE-associated complications is not known, there is agreement on the predominant role of ammonia [2]. Typically, ammonia is metabolized to urea by the liver. When the liver is damaged (e.g., by diseases or xenobiotics), this organ is not able to detoxify ammonia. The brain is a crucial target organ for ammonia toxicity. Several mechanisms have been proposed for ammonia-induced neurotoxicity [3, 4]. It has been found that ammonia has direct toxic effects on neurons and astrocytes [4]. Ammonia causes brain edema, neuroinflammation, and oxidative stress when its level rises during HE [5]. Consequently, suppression of the brain function, coma, permanent brain injury, or death might occur in patients with HE [5]. Impaired locomotor activity and cognitive dysfunction are well-established symptoms of ammonia neurotoxicity [6, 7]. Suppression of brain function during HE and hyperammonemia could lead to coma and death if not appropriately managed [6, 7]. Altered motor function in patients with chronic HE and hyperammonemia could reduce the quality of life of cirrhotic patients.
Oxidative stress and its associated complications are known to be implicated in ammonia-induced brain injury [3, 8, 9]. It has been reported that ammonia caused severe oxidative/nitrosative stress, biomembrane disruption, lipid peroxidation, and defects in cellular antioxidant systems in the brain tissue [10-15]. Hence, antioxidants and protective agents might have therapeutic value in the management of hyperammonemia-associated brain injury and its associated complications.
Taurine (2-aminoethane sulfonic acid; TAU) is one of the most abundant amino acids in the human body [16]. Although TAU is not incorporated in protein structures, several physiological and pharmacological properties are attributed to this amino acid [17-21]. The cytoprotective properties of this chemical have been widely investigated [22-27]. The therapeutic effect of TAU against several diseases has also been mentioned [28-30]. It has been found that TAU provides protection against several neurological disorders as well as xenobiotic-induced neurotoxicity [31-36].
Although the protective properties of taurine have been widely investigated, the effect of this chemical against hyperammonemia-associated complications such as impairment of locomotor activity and brain injury has not been entirely revealed. In the current study, bile duct ligation (BDL) was used as a reliable animal model of cirrhosis [37, 38]. Then, plasma biomarkers of liver injury were assessed. Moreover, brain tissue oxidative stress markers were measured, and animals’ locomotor activity was monitored to investigate the effect of TAU supplementation on cirrhosis-associated brain injury and impairment of locomotor activity.
Material and methods
Chemicals
Fatty acid-free bovine serum albumin (BSA) fraction V, dithiobis-2-nitrobenzoic acid (DTNB), 6-hydroxy-2,5,7,8-tetramethyl chroman-2-carboxylic acid (Trolox), 4,2hydroxyethyl,1-piperazine ethane sulfonic acid (HEPES), thiobarbituric acid (TBA), 2′,7′dichlorofluorescein diacetate (DCFH-DA), taurine (TAU), malondialdehyde (MDA), glutathione (GSH), sodium phosphate dibasic (Na2HPO4), sucrose, potassium chloride (KCl), Coomassie brilliant blue, dithiothreitol, ethylene glycol-bis (2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), and ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Hydroxymethyl aminomethane hydrochloride (Tris-HCl), and trichloroacetic acid (TCA) were obtained from Merck (Darmstadt, Germany). All salts (analytical grade) for preparing buffer solutions were obtained from Merck (Darmstadt, Germany).
Animals
Male Sprague Dawley rats (n = 48; 200-250 g weight) were obtained from the Animal Breeding Center, Shiraz University of Medical Sciences, Shiraz, Iran. Rats were housed in plastic cages over wood-chip bedding (ambient temperature of 23 ± 1ºC, 12L: 12D photo schedule, ≈40% of relative humidity). Animals were allowed free access to a standard chow diet (Behparvar, Tehran, Iran) and tap water. All the experiments were conducted in conformity with the guidelines for care and use of experimental animals approved by the local ethics committee of Shiraz University of Medical Sciences, Shiraz, Iran (#14822).
Animal model of cirrhosis
Bile duct ligation (BDL) in rats is an animal model of cirrhosis with all complications of chronic HE including a rise in blood ammonia and its associated neurobiological complications [14, 39]. For BDL surgery, animals were anesthetized (10 mg/kg of xylazine and 70 mg/kg of ketamine, i.p.), a midline incision was made, and the common bile duct was identified, doubly ligated, and cut between these two ligatures [40]. The sham operation comprised laparotomy and bile duct identification and manipulation without ligation.
Animal treatments
TAU (dissolved in tap water) was administered orally (Gavage) for 28 consecutive days. The treatments were as follows: 1) Control (vehicle-treated; tap water 2 ml/kg); 2) BDL; 3) BDL + TAU 50 mg/kg/day, oral; 4) BDL + TAU 100 mg/kg/day, oral; 5) BDL + TAU 200 mg/kg/day, oral; 6) TAU 200 mg/kg/day, oral. On day 29 after the BDL operation, animals’ locomotor activity was assessed. Then, animals were anesthetized (thiopental, 80 mg/kg, i.p.) and samples were collected. The sole TAU (200 mg/kg, oral) was administered to ensure its safety.
Motor coordination and activity tests
All motor coordination and activity tests were conducted on day 29 after BDL surgery.
Open field test behavior
Open field behavior is applied as an index of animals’ locomotor activity in animal models of HE [41, 42]. In the current investigation, the open field apparatus was made of a white Plexiglas box (100 cm L × 100 cm W × 30 cm H, and the box floor was divided into squares of 10 × 10 cm) [43]. The open field arena was equipped with a webcam (2.0 Megapixel, Gigaware, UK) and animals activity was monitored and recorded from a separate room. Rats’ behavior was recorded for fifteen minutes, and the total number of crossed squares was counted (total locomotion) [44].
Rotarod test
Based on a previously reported procedure, each rat underwent five sessions of rotarod performance [45]. The speed of the rotarod was 5 and 15 rpm with a cut-off point 300 s. The time up to which the rat stayed on the rotating rod was automatically recorded [45, 46].
Gait test
Animals’ hind paws were wetted with ink. Afterward, using a runway procedure, rats were allowed to walk down on a paper strip (60 cm long, 10 cm wide) from the brightly lit corridor toward a dark side. The distance between the points of the left and right hind paws was measured and recorded [45].
Beam walk
Animals had to cross a beam (15 mm diameter; 80 cm long; elevated 50 cm over the ground). The beam communicated with a box at one end. Animals were first trained with a series of three approximate trials. Then, the time of beam cross and the number of foot slips were recorded [47].
Adhesive-removal test
The adhesive removal test was performed to evaluate animal’ sensorimotor impairment, [48, 49]. A small adhesive-back paper dot (8-mm diameter) was placed on the rat forepaw to cover the hairless part of the paw. The animal was placed in a box (40 cm L × 40 cm W × 15 cm H) and the time to remove the strip (with a cut-off point of 180 s) was recorded [48, 49].
Negative geotaxis test
Based on a previously reported procedure, rats were placed on an inclined surface (30°) with their heads facing downward [50]. The time for each animal to turn 180° was measured with a cut-off point of 90 s [50].
Blood biochemistry
A Mindray BS-200 autoanalyzer (Mindray chemistry analyzers for low-volume laboratories, Guangzhou, China) and standard commercial kits (Pars Azmun, Tehran, Iran) were used to measure serum albumin, bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) [51]. Plasma ammonia level was measured with standard kits based on the absorbance photometry method of phenate-hypochlorate reaction [52]. Brain ammonia level of cirrhotic animals was determined according to a previously reported method [44] (Table 1). Briefly, forebrain (cerebral cortex) samples (100 mg) were dissected, homogenized, and deproteinized in 3 ml of an ice-cooled (4°C) lysis solution (trichloroacetic acid, 6% w/v in double distilled water). After centrifugation (12,000 g, 10 minutes, 4°C), the supernatant was collected and neutralized (KHCO3; 2 mol/l, pH = 7). Afterward, the ammonia content of the supernatant was measured using standard kits [52].
Table 1.
Serum biochemical measurements
| Biomarkers assessed | Treatments | ||||
|---|---|---|---|---|---|
| Control (vehicle-treated) | BDL | BDL + TAU 50 mg/kg | BDL + TAU 100 mg/kg | BDL + TAU 200 mg/kg | |
| ALT (U/l) | 51 ± 4 | 430 ± 25* | 259 ± 60a | 183 ± 61a | 164 ± 65a |
| AST (U/l) | 83 ± 15 | 302 ± 21* | 250 ± 27a | 116 ± 47a | 102 ± 35a |
| LDH (U/l) | 402 ± 79 | 917 ± 96* | 688 ± 43 | 512 ± 30a | 593 ± 24a |
| Total bilirubin (mg/dl) | 0.054 ± 0.005 | 11 ± 2* | 7 ± 3 | 6.5 ± 1.8 | 6.4 ± 3.47 |
| Albumin (mg/dl) | 3.66 ± 0.22 | 2.95 ± 0.41* | 3.00 ± 0.22 | 3.19 ± 0.12a | 3.30 ± 0.11a |
| Plasma ammonia (mg/dl) | 237 ± 66 | 1262 ± 214* | 969 ± 160 | 898 ± 239 | 827 ± 217a |
| Brain ammonia (mg/g tissue) | 11 ± 2 | 41 ± 9* | 32 ± 7 | 27 ± 10 | 23 ± 7a |
Data are shown as mean ± SD (n = 8).
Indicates significantly different as compared with control group (p < 0.001).
Indicates significantly different as compared with BDL group (p < 0.05).
BDL – bile duct ligated, Tau – taurine
Statistical analysis
Data are shown as mean ± SD. The comparison of data sets was performed by the one-way analysis of variance (ANOVA) and Tukey’s post hoc test. Differences between groups were considered statistically significant when p < 0.05.
Results
Liver cirrhosis in BDL rats was accompanied with severe changes in blood biochemistry as compared with the sham-operated group. On the other hand, it was found that TAU treatment (50, 100, and 200 mg/kg/day, oral) decreased serum biomarkers of liver injury in cirrhotic animals. A higher level of ammonia was detected in the plasma of BDL rats. Brain tissue ammonia level was also significantly higher in cirrhotic animals in comparison with the sham-operated group. It was found that both plasma and brain ammonia level was lower in TAU-supplemented animals (200 mg/ kg/day, oral).
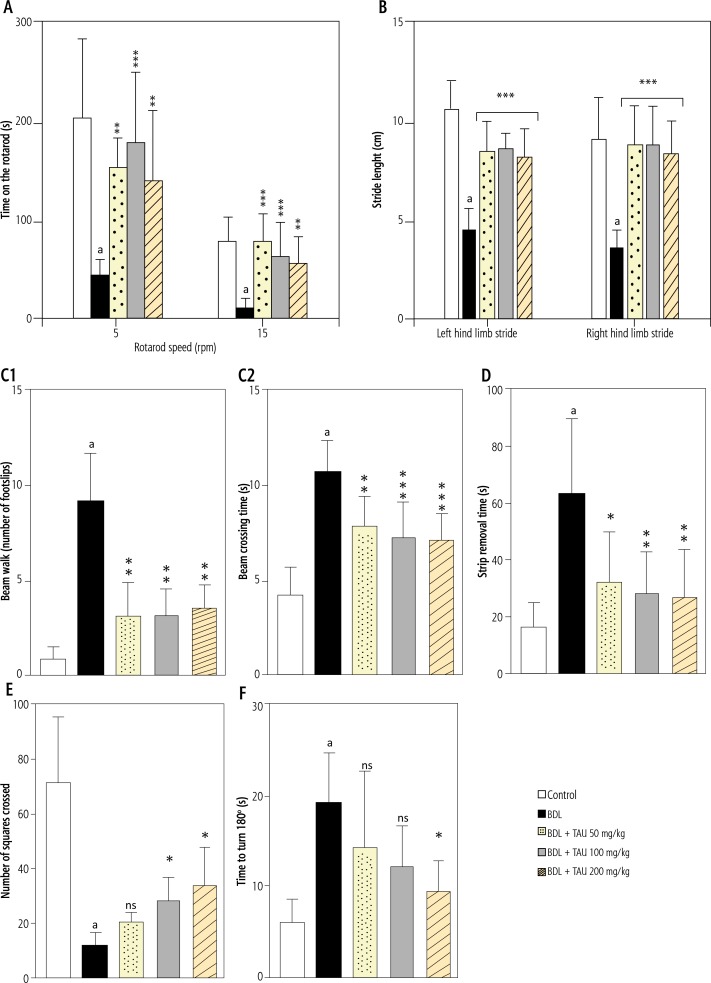
Evaluation of animals’ motor coordination revealed a significant decrease of locomotor activity in BDL rats. Lower open field activity, and impaired rotarod, beam walk, and gait test were evident in cirrhotic animals. The adhesive removal test as an index of sensorimotor activity was also impaired in the BDL group. It was found that TAU supplementation (50, 100, and 200 mg/kg/day, oral) significantly decreased the impairment of animals’ locomotor activity in BDL rats (Fig. 1).
Fig. 1.
Effect of taurine (Tau ) administration on the animals’ locomotor activity in bile duct ligated (BDL) rat model of cirrhosis. A) rotarod test, B) gait test, C1 and C2) beam walk activity, D) adhesive-removal test, E) open field behavior; and F) negative geotaxis test
BDL – bile duct ligated, Tau – taurine
Data are given as mean ± SD (n = 8).
aIndicates significantly different as compared with control group (p < 0.001).
Asterisks indicate significantly different as compared with BDL group (*p < 0.05; ***p < 0.001).
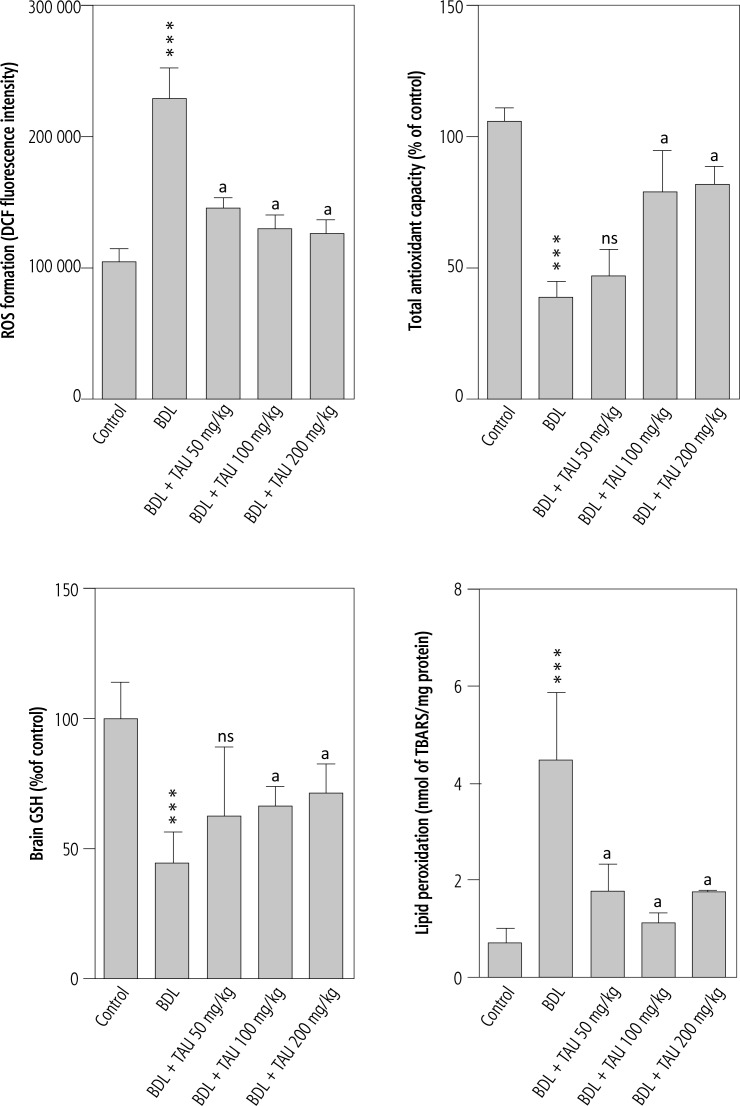
It was found that markers of oxidative stress were significantly higher in the brain tissue of cirrhotic rats. A high level of reactive oxygen species (ROS), along with tissue glutathione depletion, and severe lipid peroxidation, were detected in the brain tissue of the BDL group in comparison with sham-operated animals. Moreover, the antioxidant capacity of the brain tissue was significantly decreased in cirrhotic rats. It was found that TAU treatment (50, 100, and 200 mg/kg, i.p.) significantly mitigated brain tissue biomarkers of oxidative stress in cirrhotic animals. Lower levels of ROS and lipid peroxidation were detected in TAU-supplemented groups. TAU (50, 100, and 200 mg/kg, i.p.) also preserved tissue antioxidant capacity and prevented brain glutathione depletion (Fig. 2).
Fig. 2.
Effect of taurine supplementation on brain tissue markers of oxidative stress in cirrhotic animals
***Indicates significantly different as compared with control group (p < 0.001).
aIndicates significantly different as compared with BDL group (p < 0.001).
ns – not significant as compared with BDL group (p > 0.05).
BDL – bile duct ligated, Tau – taurine
It is noteworthy that the sole TAU administration caused no significant changes in animals’ locomotor activity in comparison with the control (vehicle-treated) group (data not shown). On the other hand, markers of oxidative stress remained unchanged (except for glutathione content, which was higher; p < 0.05) in the brain tissue of TAU-treated animals in comparison with the control group (data not shown). On the other hand, the effect of different doses of TAU (50, 100, and 200 mg/kg/day, oral) on animals’ locomotor activity and brain markers of oxidative stress was not dose-dependent.
Discussion
Chronic hepatic encephalopathy (HE) and hyperammonemia is a common event associated with cirrhosis [53, 54]. HE is a neuropsychiatric syndrome which can lead to permanent brain injury, coma, and death if not appropriately managed [1, 5, 6]. On the other hand, chronic and frequently subclinical hyperammonemia is associated with different degrees of cirrhosis and could affect patients’ CNS function and quality of life [55, 56]. A wide range of impaired psychomotor performance including tremor, rigidity, akinesia, athetosis, as well as cognitive dysfunction, is associated with cirrhosis and chronic HE [1, 57-59].
Ammonia is the most suspected molecule involved in the pathogenesis of HE-induced brain injury [2]. Oxidative stress and its consequences are established to play a significant role in the pathogenesis of hyperammonemia-induced brain injury [9-12, 60-62]. In the current study, it was revealed that TAU supplementation (50, 100, and 200 mg/kg, i.p.) to cirrhotic rats recovered animals’ regular locomotor activity and alleviated brain tissue markers of oxidative stress.
The neuroprotective properties of TAU have been widely investigated [63]. It is well established that taurine treatment efficiently encounters oxidative stress and its consequences in brain tissue [64-66]. Several neurological disorders have also been shown to benefit from TAU supplementation [31-36, 67]. The effects of this amino acid against xenobiotic-induced CNS injury have also been widely investigated [68, 69].
Oxidative stress and its associated events play a central role in ammonia-induced neurotoxicity [12, 70]. It has been found that markers of oxidative stress were increased in the brain tissue of cirrhotic animals [71-73]. Increased ROS level, severe lipid peroxidation, and decreased brain tissue glutathione stores were detected in the brain of cirrhotic animals (Fig. 2) [74]. Brain tissue antioxidant capacity was also impaired in BDL rats (Fig. 2). In the current study, TAU (50, 100, and 200 mg/kg, i.p.) effectively alleviated oxidative stress and its consequences in the brain of cirrhotic animals. Previously we also found that TAU treatment alleviated brain tissue markers of oxidative stress in an acute liver failure animal model of hyperammonemia [75]. It has been reported that TAU could significantly mitigate oxidative stress and its related events in different experimental models including several neurological disorders [24, 76]. Hence, the antioxidant capacity of TAU might play a significant role in its neuroprotective properties during liver failure.
Brain mitochondria are among major targets of ammonia toxicity [77, 78]. It has been established that hyperammonemia leads to a brain energy crisis [77, 78]. Previously we found that TAU could preserve brain mitochondrial function in hyperammonemic conditions [79, 80]. Hence, another dominant mechanism for the neuroprotective effects of TAU in cirrhosis could be mediated through its impact on cellular mitochondria. Interestingly, some investigations have also mentioned that the anti-oxidative stress effects of TAU might be mediated through its effects on cellular mitochondria [81-84]. Hence, another important mechanism for the neuroprotective properties of TAU in cirrhotic animals could be mediated through its effects on brain mitochondria. The impact of TAU supplementation on brain mitochondrial function and energy metabolism in cirrhosis deserves further investigations.
Impaired cycling of Gln-Glu between neurons and astrocytes is documented in the brain of hyperammonemic models [85]. Consequently, the extracellular concentration of glutamate is increased. Glutamate is the primary excitatory neurotransmitter in the CNS which activates the N-methyl aspartate (NMDA) type of glutamate receptors. Hence, brain glutamatergic neurotransmission is severely affected during hyperammonemia and HE [86, 87]. It is well established that one of the leading contributors to the toxic effects of ammonia in the brain tissue is the over-activation of NMDA receptors [87, 88]. This over-activation is known as the ammonia-induced “excitotoxic” response [87, 88]. It has been found that the “excitotoxic response” plays a significant role in the pathogenesis of ammonia-induced brain injury [87, 88]. Deleterious events such as dysregulation of cytoplasmic calcium level and excessive formation of reactive oxygen/nitrogen species might lead to NMDA receptor over-activation [5, 70]. Hence, the excitotoxic response is tightly linked to ammonia-induced oxidative/nitrosative stress in the CNS. The antiexcitotoxic effect of TAU is an essential feature of this amino acid [89, 90]. It has been shown that TAU mitigated the excitotoxic response in cultured neurons [91, 92]. Hence, the anti-excitotoxic effects of this amino acid could also play a role in its neuroprotective effects during hyperammonemic episodes.
Neuroinflammation is another major complication during hyperammonemia and HE [93-95]. It has been found that neuroinflammation during hyperammonemia significantly deteriorates locomotor activity [96]. On the other hand, the anti-inflammatory effect of TAU has been mentioned in several investigations [97-100]. Hence, this amino acid might also alleviate brain tissue inflammation in hyperammonemic animals. The effect of TAU on brain inflammation in different models of hyperammonemia could be the subject of future studies.
We previously found that TAU administration to chronic and acute liver failure animal models could prevent a rise in blood and brain ammonia level [79, 101]. The effect of TAU on ammonia level could be due to the direct effects of TAU on the liver and preserved ammonia detoxification capability of this organ. Hence, the hepatoprotective effects of TAU might also play a significant role in the neuroprotection provided by this amino acid (Fig. 3). In the current study, we found that TAU supplementation efficiently mitigated blood and brain ammonia level as well as impairment in animals’ locomotor activity during cirrhosis. Furthermore, TAU treatment prevented ammonia-induced oxidative stress and its consequences in rat brain. All these data indicate TAU as a potentially safe and clinically applicable agent against HE and its associated complications in humans.
Fig. 3.
Schematic representation of the potential mechanisms of neuroprotection provided by taurine in cirrhotic rats. Taurine might protect against ammonia neurotoxicity through a series of interconnected mechanisms
Interestingly, it has been found that brain TAU level is changed during acute or chronic HE [102]. Hence, some investigations have mentioned a potential role of TAU in the pathogenesis of HE. It has also been found that TAU prevented bilirubin-induced neurotoxicity [68, 103]. As chronic liver failure and cirrhosis are associated with high bilirubin levels, part of the neuroprotection provided by taurine in BDL animals might be mediated through its effect on bilirubin-induced CNS injury. The precise effects of TAU supplementation on bilirubin-induced neurotoxicity during cirrhosis need further research.
Collectively, the data presented in the current study suggest that TAU exhibits neuroprotective effects against impairment of locomotor activity and oxidative stress associated with cirrhosis. Hence, TAU supplementation could be not only an excellent hepatoprotective strategy but also a potential therapeutic option against hyperammonemia-associated CNS complications. Indeed, further investigations are needed for understanding the effect of TAU supplementation on other critical aspects of HE such as brain edema.
Acknowledgments
The authors gratefully thank the Pharmaceutical Sciences Research Center of Shiraz University of Medical Sciences for providing technical facilities for this investigation. The current study was financially supported by the Vice-Chancellor of Research Affairs of Shiraz University of Medical Sciences (Grant number: 01-36-15281).
Disclosure
Authors report no conflict of interest.
References
- 1.Felipo V. Hepatic encephalopathy: effects of liver failure on brain function. Nat Rev Neurosci. 2013;14:851–858. doi: 10.1038/nrn3587. [DOI] [PubMed] [Google Scholar]
- 2.Shawcross D, Jalan R. The pathophysiologic basis of hepatic encephalopathy: central role for ammonia and inflammation. Cell Mol Life Sci. 2005;62:2295–2304. doi: 10.1007/s00018-005-5089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norenberg M. Oxidative and nitrosative stress in ammonia neurotoxicity. Hepatology. 2003;37:245–248. doi: 10.1053/jhep.2003.50087. [DOI] [PubMed] [Google Scholar]
- 4.Bosoi CR, Rose CF. Identifying the direct effects of ammonia on the brain. Metab Brain Dis. 2008;24:95–102. doi: 10.1007/s11011-008-9112-7. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht J, Jones EA. Hepatic encephalopathy: molecular mechanisms underlying the clinical syndrome. J Neurol Sci. 1999;170:138–146. doi: 10.1016/s0022-510x(99)00169-0. [DOI] [PubMed] [Google Scholar]
- 6.Dams K, Meersseman W, Wilmer A. Hyperammonemia in the Adult Critical Care Setting. Yearbook of Intensive Care and Emergency Medicine. Berlin, Heidelberg: Springer; 2008. pp. 481–490. [Google Scholar]
- 7.Prakash R, Mullen KD. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2010;7:515–525. doi: 10.1038/nrgastro.2010.116. [DOI] [PubMed] [Google Scholar]
- 8.Felipo V, Butterworth RF. Mitochondrial dysfunction in acute hyperammonemia. Neurochem Int. 2002;40:487–491. doi: 10.1016/s0197-0186(01)00119-x. [DOI] [PubMed] [Google Scholar]
- 9.Ommati MM, Jamshidzadeh A, Niknahad H, et al. N-acetylcysteine treatment blunts liver failure-associated impairment of locomotor activity. PharmaNutrition. 2017;5:141–147. [Google Scholar]
- 10.Braissant O, McLin VA, Cudalbu C. Ammonia toxicity to the brain. J Inherited Metab Dis. 2013;36:595–612. doi: 10.1007/s10545-012-9546-2. [DOI] [PubMed] [Google Scholar]
- 11.Görg B, Qvartskhava N, Bidmon H-J, et al. Oxidative stress markers in the brain of patients with cirrhosis and hepatic encephalopathy. Hepatology. 2010;52:256–265. doi: 10.1002/hep.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norenberg MD, Jayakumar AR, Rao KVR. Oxidative Stress in the Pathogenesis of Hepatic Encephalopathy. Metab Brain Dis. 2004;19:313–329. doi: 10.1023/b:mebr.0000043978.91675.79. [DOI] [PubMed] [Google Scholar]
- 13.Rama Rao KV, Reddy PVB, Tong X, Norenberg MD. Brain Edema in Acute Liver Failure. Am J Pathol. 2010;176:1400–1408. doi: 10.2353/ajpath.2010.090756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shawcross DL, Shabbir SS, Taylor NJ, Hughes RD. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2010;51:1062–1069. doi: 10.1002/hep.23367. [DOI] [PubMed] [Google Scholar]
- 15.Skowrońska M, Albrecht J. Oxidative and nitrosative stress in ammonia neurotoxicity. Neurochem Int. 2013;62:731–737. doi: 10.1016/j.neuint.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Lourenco R, Camilo ME. Taurine: a conditionally essential amino acid in humans? An overview in health and disease. Nutr Hosp. 2002;17:262–270. [PubMed] [Google Scholar]
- 17.Sarkar P, Basak P, Ghosh S, et al. Prophylactic role of taurine and its derivatives against diabetes mellitus and its related complications. Food Chem Toxicol. 2017;110:109–121. doi: 10.1016/j.fct.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Mezzomo NJ, Fontana BD, Kalueff AV, et al. Understanding taurine CNS activity using alternative zebrafish models. Neurosci Biobehav Rev. 2017;83:525–539. doi: 10.1016/j.neubiorev.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Guo J, Zhang Y, Zhang J. The beneficial effects of taurine in preventing metabolic syndrome. Food Funct. 2016;7:1849–1863. doi: 10.1039/c5fo01295c. [DOI] [PubMed] [Google Scholar]
- 20.De Luca A, Pierno S, Camerino DC. Taurine: the appeal of a safe amino acid for skeletal muscle disorders. J Transl Med. 2015;13:243. doi: 10.1186/s12967-015-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oja SS, Saransaari P. Open questions concerning taurine with emphasis on the brain. Adv Exp Med Biol. 2015;803:409–413. doi: 10.1007/978-3-319-15126-7_31. [DOI] [PubMed] [Google Scholar]
- 22.Eppler B, Dawson R., Jr Cytoprotective role of taurine in a renal epithelial cell culture model. Biochem Pharmacol. 2002;63:1051–1060. doi: 10.1016/s0006-2952(02)00843-2. [DOI] [PubMed] [Google Scholar]
- 23.Heidari R, Babaei H, Eghbal MA. Cytoprotective effects of taurine against toxicity induced by isoniazid and hydrazine in isolated rat hepatocytes. Arch Indust Hyg Toxicol. 2013;64:201–210. doi: 10.2478/10004-1254-64-2013-2297. [DOI] [PubMed] [Google Scholar]
- 24.Heidari R, Babaei H, Eghbal MA. Amodiaquine-induced toxicity in isolated rat hepatocytes and the cytoprotective effects of taurine and/or N-acetyl cysteine. Res Pharm Sci. 2014;9:97–105. [PMC free article] [PubMed] [Google Scholar]
- 25.Schaffer S, Azuma J, Takahashi K, Mozaffari M. Why is taurine cytoprotective? Taurine. 2003;5:307–312. doi: 10.1007/978-1-4615-0077-3_39. [DOI] [PubMed] [Google Scholar]
- 26.Sinha M, Manna P, Sil PC. Taurine, a conditionally essential amino acid, ameliorates arsenic-induced cytotoxicity in murine hepatocytes. Toxicol In Vitro. 2007;21:1419–1428. doi: 10.1016/j.tiv.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Waterfield CJ, Mesquita M, Parnham P, Timbrell JA. Cytoprotective effects of taurine in isolated rat hepatocytes. Toxicol In Vitro. 1994;8:573–575. doi: 10.1016/0887-2333(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 28.Yamori Y, Taguchi T, Hamada A, et al. Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J Biomed Sci. 2010;17:S6. doi: 10.1186/1423-0127-17-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y-J, Arneja AS, Tappia PS, Dhalla NS. The potential health benefits of taurine in cardiovascular disease. Exp Clin Cardiol. 2008;13:57–65. [PMC free article] [PubMed] [Google Scholar]
- 30.Huxtable RJ, Michalk D. Taurine in Health and Disease. Springer Science & Business Media; 2013. [Google Scholar]
- 31.Kong WX, Chen SW, Li YL, et al. Effects of taurine on rat behaviors in three anxiety models. Pharmacol Biochem Behav. 2006;83:271–276. doi: 10.1016/j.pbb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Barbeau A, Huxtable RJ. Taurine and Neurological Disorders. J Neuropathol Exp Neurol. 1978:37. [Google Scholar]
- 33.Huxtable RJ. Taurine in nutrition and neurology. Vol. 139. Springer Science & Business Media; 2013. [Google Scholar]
- 34.Zhou J, Li Y, Yan G, et al. Protective Role of Taurine Against Morphine-Induced Neurotoxicity in C6 Cells via Inhibition of Oxidative Stress. Neurotox Res. 2011;20:334. doi: 10.1007/s12640-011-9247-x. [DOI] [PubMed] [Google Scholar]
- 35.Xu S, He M, Zhong M, et al. The neuroprotective effects of taurine against nickel by reducing oxidative stress and maintaining mitochondrial function in cortical neurons. Neurosci Lett. 2015;590:52–57. doi: 10.1016/j.neulet.2015.01.065. [DOI] [PubMed] [Google Scholar]
- 36.Sun M, Gu Y, Zhao Y, Xu C. Protective functions of taurine against experimental stroke through depressing mitochondria-mediated cell death in rats. Amino Acids. 2011;40:1419–1429. doi: 10.1007/s00726-010-0751-8. [DOI] [PubMed] [Google Scholar]
- 37.Jamshidzadeh A, Heidari R, Latifpour Z, et al. Carnosine ameliorates liver fibrosis and hyperammonemia in cirrhotic rats. Clin Res Hepatol Gastroenterol. 2017;41:424–434. doi: 10.1016/j.clinre.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Heidari R, Moezi L, Asadi B, et al. Hepatoprotective effect of boldine in a bile duct ligated rat model of cholestasis/cirrhosis. PharmaNutrition. 2017;5:109–117. [Google Scholar]
- 39.Butterworth RF, Norenberg MD, Felipo V, et al. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. 2009;29:783–788. doi: 10.1111/j.1478-3231.2009.02034.x. [DOI] [PubMed] [Google Scholar]
- 40.Moezi L, Heidari R, Amirghofran Z, et al. Enhanced anti-ulcer effect of pioglitazone on gastric ulcers in cirrhotic rats: The role of nitric oxide and IL-1b. Pharmacol Rep. 2013;65:134–143. doi: 10.1016/s1734-1140(13)70971-x. [DOI] [PubMed] [Google Scholar]
- 41.Apelqvist G, Wikell C, Hindfelt B, et al. Altered open-field behavior in experimental chronic hepatic encephalopathy after single venlafaxine and citalopram challenges. Psychopharmacology. 1999;143:408–416. doi: 10.1007/s002130050966. [DOI] [PubMed] [Google Scholar]
- 42.Kugelberg FC, Apelqvist G, Wikell C, Bengtsson F. Open-Field Behavioural Alterations in Liver-Impaired and Sham-Operated Rats after Acute Exposure to the Antidepressant Venlafaxine. Basic Clin Pharmacol Toxicol. 2005;97:155–161. doi: 10.1111/j.1742-7843.2005.pto_97385.x. [DOI] [PubMed] [Google Scholar]
- 43.Avraham Y, Grigoriadis NC, Poutahidis T, et al. Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br J Pharmacol. 2011;162:1650–1658. doi: 10.1111/j.1476-5381.2010.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heidari R, Ghanbarinejad V, Mohammadi H, et al. Mitochondria protection as a mechanism underlying the hepatoprotective effects of glycine in cholestatic mice. Biomed Pharmacother. 2018;97:1086–1095. doi: 10.1016/j.biopha.2017.10.166. [DOI] [PubMed] [Google Scholar]
- 45.Carter RJ, Morton J, Dunnett SB. Motor Coordination and Balance in Rodents. Current Protocols in Neuroscience. John Wiley & Sons, Inc; 2001. [DOI] [PubMed] [Google Scholar]
- 46.Metz GAS, Merkler D, Dietz V, et al. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000;883:165–177. doi: 10.1016/s0006-8993(00)02778-5. [DOI] [PubMed] [Google Scholar]
- 47.Luong TN, Carlisle HJ, Southwell A, Patterson PH. Assessment of Motor Balance and Coordination in Mice using the Balance Beam. J Vis Exp. 2011;10:2376. doi: 10.3791/2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chander K, Vaibhav K, Ejaz Ahmed M, et al. Quercetin mitigates lead acetate-induced behavioral and histological alterations via suppression of oxidative stress, Hsp-70, Bak and upregulation of Bcl-2. Food Chem Toxicol. 2014;68:297–306. doi: 10.1016/j.fct.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Bouet V, Boulouard M, Toutain J, et al. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat Protoc. 2009;4:1560–1564. doi: 10.1038/nprot.2009.125. [DOI] [PubMed] [Google Scholar]
- 50.Tchekalarova J, Kubova H, Mareš P. Postnatal caffeine exposure: effects on motor skills and locomotor activity during ontogenesis. Behav Brain Res. 2005;160:99–106. doi: 10.1016/j.bbr.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 51.Heidari R, Jamshidzadeh A, Keshavarz N, Azarpira N. Mitigation of Methimazole-Induced Hepatic Injury by Taurine in Mice. Sci Pharm. 2014;83:143–158. doi: 10.3797/scipharm.1408-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chatauret N, Desjardins P, Zwingmann C, et al. Direct molecular and spectroscopic evidence for increased ammonia removal capacity of skeletal muscle in acute liver failure. J Hepatol. 2006;44:1083–1088. doi: 10.1016/j.jhep.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 53.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 54.Cordoba J, Ventura-Cots M, Simón-Talero M, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF) J Hepatol. 2014;60:275–281. doi: 10.1016/j.jhep.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62:437–447. doi: 10.1016/j.jhep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Ahluwalia V, Heuman DM, Feldman G, et al. Correction of hyponatraemia improves cognition, quality of life, and brain oedema in cirrhosis. J Hepatol. 2015;62:75–82. doi: 10.1016/j.jhep.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banaclocha MM. Therapeutic potential of N-acetylcysteine in age-related mitochondrial neurodegenerative diseases. Med Hypotheses. 2001;56:472–477. doi: 10.1054/mehy.2000.1194. [DOI] [PubMed] [Google Scholar]
- 58.Kamboj SS, Sandhir R. Protective effect of N-acetylcysteine supplementation on mitochondrial oxidative stress and mitochondrial enzymes in cerebral cortex of streptozotocin-treated diabetic rats. Mitochondrion. 2011;11:214–222. doi: 10.1016/j.mito.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 59.Thong-Ngam D, Samuhasaneeto S, Kulaputana O, Klaikeaw N. N-acetylcysteine attenuates oxidative stress and liver pathology in rats with non-alcoholic steatohepatitis. World J Gastroenterol. 2007;13:5127–5132. doi: 10.3748/wjg.v13.i38.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tñnez I, Muńoz MC, Medina FJ, et al. Comparison of melatonin, vitamin E and L-carnitine in the treatment of neuro- and hepatotoxicity induced by thioacetamide. Cell Biochem Funct. 2007;25:119–127. doi: 10.1002/cbf.1276. [DOI] [PubMed] [Google Scholar]
- 61.Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut. 2008;57:1156–1165. doi: 10.1136/gut.2007.122176. [DOI] [PubMed] [Google Scholar]
- 62.Kosenko E, Kaminsky M, Kaminsky A, et al. Superoxide Production and Antioxidant Enzymes in Ammonia Intoxication in Rats. Free Radical Res. 1997;27:637–644. doi: 10.3109/10715769709097867. [DOI] [PubMed] [Google Scholar]
- 63.Menzie J, Pan C, Prentice H, et al. Taurine and central nervous system disorders. Amino Acids. 2014;46:31–46. doi: 10.1007/s00726-012-1382-z. [DOI] [PubMed] [Google Scholar]
- 64.Parildar-Karpuzoğlu H, Mehmetçik G, Ozdemirler-Erata G, et al. Effect of taurine treatment on pro-oxidant-antioxidant balance in livers and brains of old rats. Pharmacol Report. 2008;60:673–678. [PubMed] [Google Scholar]
- 65.Pushpakiran G, Mahalakshmi K, Anuradha CV. Taurine restores ethanol-induced depletion of antioxidants and attenuates oxidative stress in rat tissues. Amino Acids. 2004;27:91–96. doi: 10.1007/s00726-004-0066-8. [DOI] [PubMed] [Google Scholar]
- 66.Saransaari P, Oja SS. Taurine and neural cell damage. Amino Acids. 2000;19:509–526. doi: 10.1007/s007260070003. [DOI] [PubMed] [Google Scholar]
- 67.Wang G-H, Jiang Z-L, Fan X-J, et al. Neuroprotective effect of taurine against focal cerebral ischemia in rats possibly mediated by activation of both GABAA and glycine receptors. Neuropharmacology. 2007;52:1199–1209. doi: 10.1016/j.neuropharm.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 68.Gao X, Yang X, Zhang B. Neuroprotection of taurine against bilirubin-induced elevation of apoptosis and intracellular free calcium ion in vivo. Toxicol Mech Methods. 2011;21:383–387. doi: 10.3109/15376516.2010.546815. [DOI] [PubMed] [Google Scholar]
- 69.O’Byrne MB, Tipton KF. Taurine-induced attenuation of MPP+ neurotoxicity in vitro. J Neurochem. 2000;74:2087–2093. doi: 10.1046/j.1471-4159.2000.0742087.x. [DOI] [PubMed] [Google Scholar]
- 70.Lemberg A, Fernández MA. Hepatic encephalopathy, ammonia, glutamate, glutamine and oxidative stress. Ann Hepatol. 2009;8:95–102. [PubMed] [Google Scholar]
- 71.Häussinger D, Görg B. Interaction of oxidative stress, astrocyte swelling and cerebral ammonia toxicity. Curr Opin Clin Nutr Metab Care. 2010;13:87–92. doi: 10.1097/MCO.0b013e328333b829. [DOI] [PubMed] [Google Scholar]
- 72.Bosoi CR, Rose CF. Oxidative stress: a systemic factor implicated in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2013;28:175–178. doi: 10.1007/s11011-012-9351-5. [DOI] [PubMed] [Google Scholar]
- 73.Görg B, Qvartskhava N, Bidmon H-J, et al. Oxidative stress markers in the brain of patients with cirrhosis and hepatic encephalopathy. Hepatology. 2010;52:256–265. doi: 10.1002/hep.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.da Silva MH, da Rosa EJ, de Carvalho NR, et al. Acute Brain Damage Induced by Acetaminophen in Mice: Effect of Diphenyl Diselenide on Oxidative Stress and Mitochondrial Dysfunction. Neurotox Res. 2012;21:334–344. doi: 10.1007/s12640-011-9288-1. [DOI] [PubMed] [Google Scholar]
- 75.Jamshidzadeh A, Abdoli N, Niknahad H, et al. Taurine Alleviates Brain Tissue Markers of Oxidative Stress in a Rat Model of Hepatic Encephalopathy. Trend Pharm Sci. 2017;3:181–192. [Google Scholar]
- 76.Heidari R, Esmailie N, Azarpira N, et al. Effect of Thiol-reducing Agents and Antioxidants on Sulfasalazine-induced Hepatic Injury in Normotermic Recirculating Isolated Perfused Rat Liver. Toxicol Res. 2016;32:133–140. doi: 10.5487/TR.2016.32.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rao KVR, Norenberg MD. Cerebral Energy Metabolism in Hepatic Encephalopathy and Hyperammonemia. Metab Brain Dis. 2001;16:67–78. doi: 10.1023/a:1011666612822. [DOI] [PubMed] [Google Scholar]
- 78.Rama Rao KV, Norenberg MD. Brain energy metabolism and mitochondrial dysfunction in acute and chronic hepatic encephalopathy. Neurochem Int. 2012;60:697–706. doi: 10.1016/j.neuint.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jamshidzadeh A, Heidari R, Abasvali M, et al. Taurine treatment preserves brain and liver mitochondrial function in a rat model of fulminant hepatic failure and hyperammonemia. Biomed Pharmacother. 2017;86:514–520. doi: 10.1016/j.biopha.2016.11.095. [DOI] [PubMed] [Google Scholar]
- 80.Niknahad H, Jamshidzadeh A, Heidari R, et al. Ammonia-induced mitochondrial dysfunction and energy metabolism disturbances in isolated brain and liver mitochondria, and the effect of taurine administration: relevance to hepatic encephalopathy treatment. Clin Exp Hepatol. 2017;3:141–151. doi: 10.5114/ceh.2017.68833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jong CJ, Azuma J, Schaffer S. Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids. 2011;42:2223–2232. doi: 10.1007/s00726-011-0962-7. [DOI] [PubMed] [Google Scholar]
- 82.Hansen SH, Andersen ML, Cornett C, et al. A role for taurine in mitochondrial function. J Biomed Sci. 2010;17:1–8. doi: 10.1186/1423-0127-17-S1-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Idrissi AE. Taurine increases mitochondrial buffering of calcium: role in neuroprotection. Amino Acids. 2006;34:321–328. doi: 10.1007/s00726-006-0396-9. [DOI] [PubMed] [Google Scholar]
- 84.Hansen SH, Grunnet N. Taurine, Glutathione and Bioenergetics. In: Idrissi AE, L’Amoreaux WJ, editors. Taurine 8. New York: Springer; 2013. pp. 3–12. [DOI] [PubMed] [Google Scholar]
- 85.Verkhratsky A, Nedergaard M, Hertz L. Why are astrocytes important? Neurochem Res. 2015;40:389–401. doi: 10.1007/s11064-014-1403-2. [DOI] [PubMed] [Google Scholar]
- 86.Rao VLR, Murthy CRK, Butterworth RF. Glutamatergic synaptic dysfunction in hyperammonemic syndromes. Metab Brain Dis. 1992;7:1–20. doi: 10.1007/BF01000437. [DOI] [PubMed] [Google Scholar]
- 87.Zielińska M, Law RO, Albrecht J. Excitotoxic mechanism of cell swelling in rat cerebral cortical slices treated acutely with ammonia. Neurochem Int. 2003;43:299–303. doi: 10.1016/s0197-0186(03)00015-9. [DOI] [PubMed] [Google Scholar]
- 88.Klejman A, Węgrzynowicz Ml, Szatmari EM, et al. Mechanisms of ammonia-induced cell death in rat cortical neurons: roles of NMDA receptors and glutathione. Neurochem Int. 2005;47:51–57. doi: 10.1016/j.neuint.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Idrissi AE, Trenkner E. Growth Factors and Taurine Protect against Excitotoxicity by Stabilizing Calcium Homeostasis and Energy Metabolism. J Neurosci. 1999;19:9459–9468. doi: 10.1523/JNEUROSCI.19-21-09459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.French ED, Vezzani A, Whetsell WO, Jr, et al. Excitatory Amino Acids and Epilepsy. Springer; 1986. Anti-excitotoxic actions of taurine in the rat hippocampus studied in vivo and in vitro; pp. 349–362. [DOI] [PubMed] [Google Scholar]
- 91.Leon R, Wu H, Jin Y, et al. Protective function of taurine in glutamate‐induced apoptosis in cultured neurons. J Neurosci Res. 2009;87:1185–1194. doi: 10.1002/jnr.21926. [DOI] [PubMed] [Google Scholar]
- 92.Idrissi AE, Trenkner E. Taurine as a Modulator of Excitatory and Inhibitory Neurotransmission. Neurochem Res. 2004;29:189–197. doi: 10.1023/b:nere.0000010448.17740.6e. [DOI] [PubMed] [Google Scholar]
- 93.Shawcross DL, Davies NA, Williams R, et al. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol. 2004;40:247–254. doi: 10.1016/j.jhep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 94.Rodrigo R, Cauli O, Gomez–Pinedo U, et al. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology. 2010;139:675–684. doi: 10.1053/j.gastro.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 95.Butterworth RF. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology. 2011;53:1372–1376. doi: 10.1002/hep.24228. [DOI] [PubMed] [Google Scholar]
- 96.Cauli O, Rodrigo R, Piedrafita B, et al. Neuroinflammation contributes to hypokinesia in rats with hepatic encephalopathy: Ibuprofen restores its motor activity. J Neurosci Res. 2009;87:1369–1374. doi: 10.1002/jnr.21947. [DOI] [PubMed] [Google Scholar]
- 97.Marcinkiewicz J, Kontny E. Taurine and inflammatory diseases. Amino Acids. 2014;46:7–20. doi: 10.1007/s00726-012-1361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abdel-Moneim AM, Al-Kahtani MA, El-Kersh MA, et al. Free Radical-Scavenging, Anti-Inflammatory/Anti-Fibrotic and Hepatoprotective Actions of Taurine and Silymarin against CCl4 Induced Rat Liver Damage. PLoS One. 2015;10:e0144509. doi: 10.1371/journal.pone.0144509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Su Y, Fan W, Ma Z, et al. Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience. 2014;266:56–65. doi: 10.1016/j.neuroscience.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 100.Tsunekawa M, Wang S, Kato T, et al. Taurine 10. Dordrecht: 2017. Taurine Administration Mitigates Cisplatin Induced Acute Nephrotoxicity by Decreasing DNA Damage and Inflammation: An Immunocytochemical Study; pp. 703–716. [DOI] [PubMed] [Google Scholar]
- 101.Heidari R, Jamshidzadeh A, Niknahad H, et al. Effect of taurine on chronic and acute liver injury: Focus on blood and brain ammonia. Toxicol Report. 2016;3:870–879. doi: 10.1016/j.toxrep.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Butterworth RF. Taurine in Hepatic Encephalopathy. In: Huxtable RJ, Azuma J, Kuriyama K, Nakagawa M, Baba A, editors. Taurine 2. Springer US; 1996. pp. 601–606. [DOI] [PubMed] [Google Scholar]
- 103.Zhang B, Yang X, Gao X. Taurine protects against bilirubininduced neurotoxicity in vitro. Brain Res. 2010;1320:159–167. doi: 10.1016/j.brainres.2010.01.036. [DOI] [PubMed] [Google Scholar]