Highlights
-
•
We present a singular case of secondary lymphedema is the most frequent long-term complication of axillary lymphadenectomy. It can result in complication as erysipelas, warts, Papilloma Cutis Lymphostatica (PCL), or angiosarcomas. Moreover, in women affected by breast cancer an accurate differential diagnosis among these conditions or complication related to radiation dermatitis or cutaneous metastasis is essential. We report the case of a 60-year-old postmenopausal Caucasian woman affected by secondary lymphedema following complete mastectomy for breast cancer. These lesions had clinical typical features of PCL, but histopathological analysis revealed dermo-hypodermic metastasis of breast carcinoma.
-
•
The presence of skin lesions in secondary lymphedema after oncological lymphadenectomy requires an accurate differential diagnosis. In fact, these lesions can emulate degenerative or infective skin diseases; anyway, in patients affected by secondary lymphedema other less common conditions - as PLC, nodular-type lichen myxedematosus or Gottron’s carcinoid papillomatosis - should be taken into account.
-
•
Our case reports the possibility that metastases of breast cancer might also mimic these conditions.
Keywords: Breast cancer, Lymphedema, Papilloma Cutis Lymphostatica, Metastases
Abstract
Introduction
Secondary lymphedema is the most frequent long-term complication of axillary lymphadenectomy. It can result in complication as erysipelas, warts, Papilloma Cutis Lymphostatica (PCL), or angiosarcomas. Moreover, in women affected by breast cancer an accurate differential diagnosis among these conditions or complication related to radiation dermatitis or cutaneous metastasis is essential.
Presentation of case
We report the case of a 60-year-old postmenopausal Caucasian woman affected by secondary lymphedema following complete mastectomy for breast cancer. The patient after surgery was treated with radiotherapy, chemotherapy and hormone therapy, developing a lympedema of left arm after few months. These lesions had clinical typical features of PCL, but histopathological analysis revealed dermo-hypodermic metastasis of breast carcinoma.
Discussion
The presence of skin lesions in secondary lymphedema after oncological lymphadenectomy requires an accurate differential diagnosis. In fact, these lesions can emulate degenerative or infective skin diseases; anyway, in patients affected by secondary lymphedema other less common conditions – as PLC, nodular-type lichen myxedematosus or Gottron’s carcinoid papillomatosis – should be taken into account.
Conclusion
Our case reports the possibility that metastases of breast cancer might also mimic these conditions.
1. Introduction
Secondary lymphedema of upper limbs is the most frequent long-term complication of axillary lymphatic tissue resections, with an incidence ranging between 6% and 30% [1]. Most frequent sequela of lymphedema are erysipelas and warts, but in very rare cases it can result in more severe complications, such as Papillomatosis Cutis Lymphostatica (PCL) or angiosarcomas. PCL is a benign, usually asymptomatic and underreported condition resulting from chronic lymphedema [2]. It was more frequently reported in primary lymphedema or as complication of a secondary damage of lymphatic vessels due to diabetes. To date, few cases of PCL have been documented after a iatrogenic secondary lymphedema [[3], [4]]. Also carcinomas or angiosarcomas arising in limbs affected by chronic lymphedema have been documented [[5], [6]]. Moreover, lesions arising in upper limb in women affected by breast carcinoma could be related to cutaneous metastases or radiation dermatitis following radiation therapy [[7], [8]]. For this reason, the presence of skin lesions of the upper limbs in patients affected by breast cancer requires an accurate differential diagnosis among cancer recurrences or metastasis, therapeutical complications, and dermatological conditions. The work has been reported in line with the SCARE criteria [9].
2. Case report
We present the case of a 60-year-old postmenopausal Caucasian woman affected by secondary lymphedema following complete mastectomy and axillary dissection for a Luminal B invasive lobular carcinoma of left breast. Immunohistochemistry testifies negativity for HER-2 mutation. Patient underwent mastectomy and complete lymphadenectomy of left axillary region in November 2015. The patient refused the reconstructive surgical treatment of the breast. After surgery, the case was presented to the multidisciplinary breast cancer board of our institution and the patient underwent local radiotherapy and adjuvant chemotherapy based on anthracycline/taxane regimen followed by hormone therapy with letrozole. In October 2016, the patient presented with a stage III left upper limb lymphedema [10] with the appearance of isochromic nodular lesions of the skin (Fig. 1, Fig. 2). Segmental lymphoscintigraphy of upper limbs documented a severe lymphedema of the left upper limb with a high value of Transport Index (28), while lymphoscintigrafic examination was normal in the contralateral upper limb. The patient underwent therapy with physiotherapy, bandages and plastic devices; in December 2016, however, the patient reported further diffusion and reddening of the skin lesions affecting the left upper limb (Fig. 3, Fig. 4). The physical examination revealed the presence of painless non-confluent oval nodular lesions, distributed on the volar surface of left arm and forearm. Clinically, these lesions had the typical features of lymphostatic papillomatosis; nonetheless, due to the patient’s history, we performed an excisional biopsy of one of the lesions. Histopathologic analysis revealed the presence of 0.7 cm mass characterized by presence of pleomorphic spindle cells with large hyper-chromatic nuclei and dense eosinophilic cytoplasm. Immunohistochemistry was positive for protein Ki67 and estrogen receptor, confirming the diagnosis of dermo-hypodermic metastasis of invasive breast carcinoma. The patient was considered hormone-refractory and she underwent to first line chemotherapy with anti-VEGF in combination to taxane. To date, she has undergone clinical and imaging (chest X-ray, abdominal ultrasound, and whole-body SPECT/CT scan) follow-up with no evidence of lymphatic or visceral metastases.
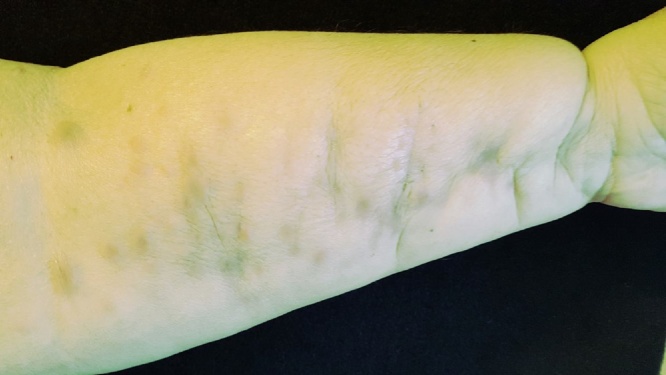
Fig. 1.
Initial clinical presentation of lesions.
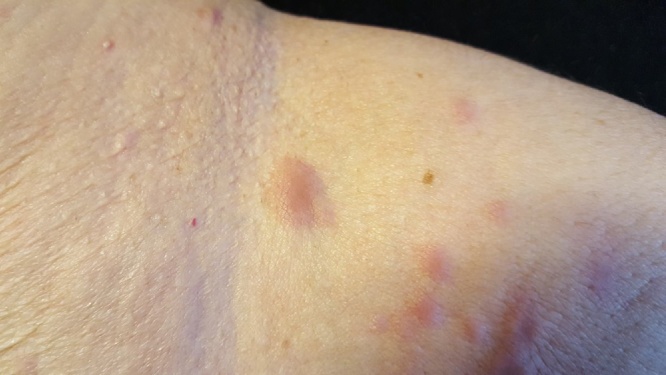
Fig. 2.
Detail of initial nodular lesion.
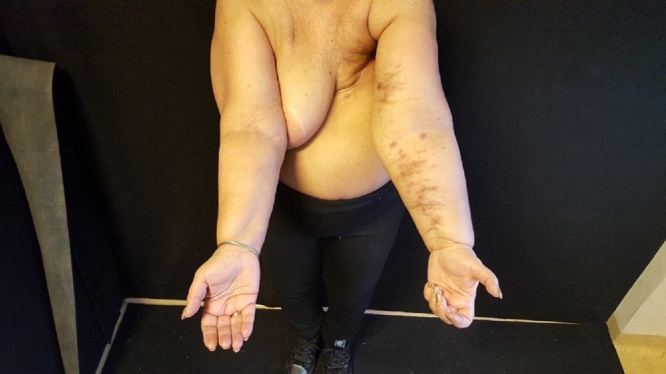
Fig. 3.
Clinical presentation of multiple nodular reddened lesions.
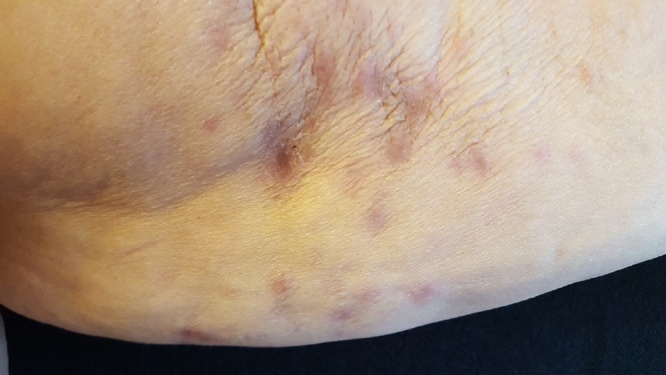
Fig. 4.
Detail of reddened lesions.
3. Discussion
Presence of oval exophitic non-pigmented lesion in the homolateral upper limb of woman who underwent mastectomy and complete lymphadenectomy of axillary region for a breast cancer need an accurate differential diagnosis, even more in presence of lymphedema.
First of all, differential diagnosis includes degenerative skin conditions such as keratoacanthoma, pilomatricoma, pyoderma vegetans, and non-pigmented skin cancers; however the presence of multiple exophitic lesions makes these unlikely diagnoses. Infective complications of lymphedema are more plausible in this patient; in fact, lymphedema is the most common long-term complication of axillary lymph node dissection [1] and association of reduced immune surveillance and skin damage in the lymphedematous regions [11] may lead to skin infections. In more severe cases, lymphedema can result in papillomatosis cutis lymphostatica (PCL), characterized by the presence of multiple skin-colored, confluent, partly hyperkeratotic, verrucous papules on limbs [6]. In such cases, skin infections, as erysipelas, may result from skin loss of continuity. Warts and angiosarcomas were also reported in patients affected by PCL [[5], [6]]. Finally, nodular-type lichen myxedematosus and Gottron’s carcinoid papillomatosis [12] skin should also be considered in case of multiple exophytic lesions on limbs, but the absence of hypothyroidism and the negativity for HPV serology assay exclude these diagnoses.
All of the above conditions are compatibles with the skin lesions affecting the left upper limb of our patient; cutaneous metastases of breast cancer, on the other hand, have been documented in several studies, as evidenced by our research at one of the most important databases in the literature[13]. These can be polymorphous, mimicking wheal rash [14], erythema annullare centrifugum [15], nodules [16] or exophytic lesions [7].
In any case, excisional biopsy is indispensable for a correct and precocious diagnosis of skin metastasis of breast cancer mimicking PCL in a limb affected by lymphedema. Furthermore, as in our case, skin metastases may be the first clinical evidence of recurrence and their detection implies the adjustment of chemotherapy regimen and thus holds prognostic and therapeutic significance.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding
Authors have not received funding or sponsor for paper production.
Ethical approval
To carry out this scientific work, there was no need to resort to the ethics committee. Ethical approval has been exempted from our institution.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on reques.
Author contribution
All the authors contributed equally to the writing of the paper.
Research registration number
NA.
Guarantor
Francesco Ciancio MD.
References
- 1.Petrek J.A., Heelan M.C. Incidence of breast carcinoma-related lymphedema. Cancer. 1998;83:2776–2781. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2776::aid-cncr25>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Kasper R.S., Nobbe S. Images in clinical medicine. Papillomatosis cutis lymphostatica. N. Engl. J. Med. 2014;370(January (1)):69. doi: 10.1056/NEJMicm1307463. [DOI] [PubMed] [Google Scholar]
- 3.Aydin D., Heidenheim M. Papillomatosis cutis lymphostatica. Clin. Case Rep. 2016;4(September (10)):1012. doi: 10.1002/ccr3.671. (eCollection 2016 Oct.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guida M., Cramarossa A., Fistola E., Porcelli M., Giudice G., Lubello K., Colucci G. High activity of sequential low dose chemo-modulating Temozolomide in combination with Fotemustine in metastatic melanoma. A feasibility study. J. Transl. Med. 2010;8(November):115. doi: 10.1186/1479-5876-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niederauer H.H., Schultz-Ehrenburg U., Tiedjen K.U. Tumor form of lymphostatic papillomatosis of the skin. Hautarzt. 1991;42(August (8)):518–522. (Article in German) [PubMed] [Google Scholar]
- 6.Epstein J.I., Mendelsohn G. Squamous carcinoma of the foot arising in association with long-standing verrucous hyperplasia in a patient with congenital lymphedema. Cancer. 1984;54(September (5)):943–947. doi: 10.1002/1097-0142(19840901)54:5<943::aid-cncr2820540534>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Handa U., Kundu R., Dimri K. Cutaneous metastasis a study of 138 cases diagnosed by fine-needle aspiration cytology. Acta Cytol. 2016;(December) doi: 10.1159/000453252. [DOI] [PubMed] [Google Scholar]
- 8.Bray F.N., Simmons B.J., Wolfson A.H., Nouri K. Acute and chronic cutaneouss reactions to ionizing radiation therapy. Deematol. Ther. (Heidelb.) 2016;6(June (2)):185–206. doi: 10.1007/s13555-016-0120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Rajmohan D.P., The SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34(October):180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 10.International Society of Lymphology The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology. 2013;46(March (1)):1–11. [PubMed] [Google Scholar]
- 11.Stewart-Treves W.J. Syndrome: a lethal complication of postmastectomy lymphedema and regional immune deficiency. Arch. Surg. 1979:11482. doi: 10.1001/archsurg.1979.01370250084018. [DOI] [PubMed] [Google Scholar]
- 12.Niederauer H.H., Schultz-Ehrenburg U., Tiedjen K.U. Tumor form of lymphostatic papillomatosis of the skin. Hautarzt. 1991;42(August (8)):518–522. [PubMed] [Google Scholar]
- 13.Russo Lo, Spolveri G., Ciancio F., Mori F.A. Mendeley: an easy way to manage, share, and synchronize papers and citations. Plast. Reconstr. Surg. 2013;131(6):946e–947e. doi: 10.1097/PRS.0b013e31828bd400. [DOI] [PubMed] [Google Scholar]
- 14.Damaskos C., Dimitroulis D., Pergialiotis V., Doula C., Koulermou G., Antoniou E.A., Frangoulis M., Stergios K., Kontzoglou K. An unexpected metastasis of breast cancer mimicking wheal rush. G. Chir. 2016;37(May–June):136–138. doi: 10.11138/gchir/2016.37.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabater V., Ferrando F., Morera A., Palomar L. Cutaneous metastasis of inflammatory breast carcinoma mimicking an erythema annulare centrifugum: a sign of locally recurrent cancer. Clin. Exp. Dermatol. 2016;41(December (8)):906–910. doi: 10.1111/ced.12953. [DOI] [PubMed] [Google Scholar]
- 16.Kamaraju S., Depke J., Povletich J., Currey A., Weil E. Cutaneous metastasis due to Breast cancer in a patient with primary biliary cirrhosis: a case report. Case Rep. Oncol. 2016;9(November (3)):718–725. doi: 10.1159/000452145. [DOI] [PMC free article] [PubMed] [Google Scholar]