Abstract
Background/Aim: The aim of this study was to develop a method for sequentially collecting cerebrospinal fluid (CSF) from an unanesthetized microminipig, which shares many physiological and anatomical similarities with humans, such as diurnality, and investigate the diurnal variation of melatonin concentration in the CSF. Materials and Methods: A catheter was placed percutaneously into the subarachnoid space of an anesthetized animal, and the tip of the catheter was placed into the cisterna magna under X-ray. We then sequentially collected CSF at light-on and -off times from the unanesthetized animal for several weeks. After catheter placement, a period of one week or more was necessary to relieve the contamination of RBCs in the CSF. Results: A higher melatonin level in the CSF was noted during lights-off time, and the level was higher than that in the serum. Conclusion: This model of sequential collection of CSF will contribute to research in brain functions.
Keywords: Intrathecal catheter, cerebrospinal fluid, melatonin, swine, minipig
Studies of cerebrospinal fluid (CSF) have been conducted in physiological and biochemical areas such as sleep and dementia (1). CSF collection methods have been reported for various animals. In recent years, there has been an increase in the number of studies on miniature pigs, which have many similarities to humans such as diurnal, omnivorous, physiology, lipid metabolism and anatomy (2-6); however, there are few reports on porcine CSF collection methods. Development of a specific technique for sequential collection of CSF using transdermal intrathecal catheter in minipigs could represent a significant advance in both veterinary clinical anesthesia and analgesia, as well as in translational medicine in various areas of brain research such as sleep (7).
Intrathecal injection techniques using a single puncture of the spinal space have already been described in laboratory animals (8), including pigs (9); however, studies concerning the placement of a spinal catheter are limited to only a few animal models (10,11). To the best of our knowledge, there are few studies describing the insertion of a spinal catheter in pigs (6,12). To date, a less invasive insertion technique of CSF samples for porcine animal experimentation has not been developed (6).
In 2011, the microminipig was developed as the smallest miniature pig in the world (13) and this strain has similar profiles of general biochemistry and hematology (14-17) to domestic swine and humans. The utility of microminipigs as experimental animals has been demonstrated in research on atherosclerosis (18-21), pharmacological cardiovascular research (22,23), sleep conditions (24,25), coagulation activity (17), toxicological studies (26,27), genomic sequencing (28), and developmental engineering (29). As well as other miniature pigs, microminipig represents a potentially appropriate experimental model, since their lipoprotein metabolism (21,30), as well as their anatomy, physiology and feeding and sleep habits are similar to those of humans.
The aim of this study was to evaluate and validate the technique for sequential collection of CSF from cisterna magna using transdermal intrathecal catheter by lumbosacral puncture and to confirm a diurnal variation of melatonin in CSF of the microminipig under an unanesthetized condition.
Materials and Methods
Animals and diet. Five microminipigs were obtained from a breeder (Fuji Micra Inc., Shizuoka, Japan) and maintained in a dedicated room with filtered air laminar flow at Kagoshima University. The room was maintained at a temperature of 24±3˚C and a relative humidity of 50±20%, with a 12-h light/dark cycle. Tap water was available ad libitum, and the animals were provided with a commercial porcine diet (Horeborekobuta; Marubeni Nisshin Feed Inc., Tokyo, Japan). All protocols were approved by the Ethics Committees of Animal Care and Experimentation, Kagoshima University (MD16073) and Shin Nippon Biomedical Laboratories, Ltd., Drug Safety Research Laboratories (IACUC 703-065), which is fully accredited by AAALAC International. Finally, the research was performed according to the Institutional Guidelines for Animal Experiments and in compliance with the Japanese Act on Welfare and Management of Animals (Act No. 105 and Notification No. 6).
Preliminary computed tomography (CT) and anatomical study confirming locations for needle and catheter insertion. Using another two 2-year old intact animals, one male and one female, (body weight: 17.0 and 19.0 kg, respectively), the spinal needle was inserted at the lumbar 6 (L6) and L7 intervertebral space in the prone position under deep anesthesia with isoflurane inhalation by guiding with the X-ray fluoroscopy (VPX-200, Canon Medical Systems Corporation, Tochigi, Japan). After confirming the outflow of CSF, contrast medium (OYPALOMIN 370, Konica Minolta Japan Inc. Tokyo, Japan) was injected into the intrathecal space. For visualizing the contrast medium, Whole body CT (Aquilion TSX-201A, Canon Medical Systems Corporation) scanning was performed. Additionally, three male animals two of which were approximately 10 months old (body weight: 5.4 and 6.4 kg), and one that was approximately 4-year old (body weight: 24.6 kg) were used. These animals were anesthetized and then sacrificed by bilateral axillary artery exsanguination for anatomical study.
Case 1 (Checking blood cells contamination in CSF). A 2-year old female animal (body weight: 18.8 kg) was used. The transdermal intrathecal catheter placement was performed under deep anesthesia induced by isoflurane inhalation. In the prone position, a 21 G (0.8×89 mm) spinal needle was inserted at the lumbosacral space in a ventro-cranial orientation forming a 60˚angle (Figure 1A and B). After pulling out the inner needle and confirming the outflow of CSF, a guide wire (Radifocus Guidewire M, 0.46 mm in diameter, length 150 cm; Terumo Corporation, Tokyo, Japan) was inserted into the spinal needle, and the top of the wire was localized at the cisterna magna under X-ray (Figure 1C). A polyethylene catheter (1.22 mm in outside diameter, Hagitec Co., Ltd., Chiba, Japan) was inserted, and the opening of the catheter was localized at the cisterna magna using the guide wire. The catheter was filled with contrast medium and was confirmed that the opening of the catheter was located in the cisterna magna using X-ray (Figure 1D). The catheter was fixed through the subcutaneous tissue of the back. Approximately 1 ml samples of CSF leakage were collected from an unanesthetized animal every week for 3 weeks for observation of red blood cell contamination in CSF. The gross color and appearance of CSF were observed, and CSF cell counts such as red blood cell (RBC) and white blood cell (WBC) were performed using a Bürker-Türk hemocytometer method. CSF measurement for total protein (TP), lactate dehydrogenase (LDH), creatine kinase (CK), glucose (Glc), sodium (Na), potassium (K), chloride (Cl), and calcium (Ca) was carried out by Clinical Pathology Laboratory, Co. Ltd. (Kagoshima, Japan).
Figure 1. Transdermal intrathecal catheter by lumbosacral puncture in Cases 1 and 2 (A-D). In the prone position, a spinal needle was inserted at the lumbosacral space in a ventro-cranial orientation forming an angle of approximately 60° (A). The top of the needle was recognized by the closed triangle while it was inserted (B). After pulling out the inner needle and confirming CSF leakage, the guide wire was inserted into the spinal needle, and the top of the wire was localized at the cisterna magna under X-ray (opened triangle, C). A polyethylene catheter was inserted using the guide wire. The opening of the catheter, which was filled with contrast medium (closed triangle, D), was recognized in the cisterna magna under X-ray (opened triangle, D).
Case 2 (Study on sequential collection of CSF and diurnal variation of melatonin concentration in CSF). A 2-year-old female animal (body weight: 19.4 kg) was used. The transdermal intrathecal catheter placement was performed in the same manner as described in Case 1. At the same time, a temperature logger (Thermochron, KN Laboratories, Tokyo Japan) for body temperature (BT) monitoring was implanted in the cervical subcutaneous region, and a harness with an actigraph (Octagonal Basic Motionlogger; Ambulatory Monitoring, Inc., Ardsley, NY, USA) for locomotor activity (LA) monitoring was attached (24,25). BT and LA were measured for 24 hours during the test period. At 3 weeks after catheter placement, approximately 1 ml CSF leakage was collected from an unanesthetized animal at 9:00, 15:00, and 21:00 on Day 1 and Day 3 for measurement of melatonin in addition to the seven parameters in Case 1 (Clinical Pathology Laboratory, Co. Ltd.). Blood was collected from the cranial vena cava at the same time as CSF collection and was analyzed in the same manner as CSF. Amino acids in the CSF and plasma including glutamine (Gln) and alanine (Ala) were also measured by high performance liquid chromatography (JLC-500/V, JEOL Ltd., Tokyo, Japan).
Results
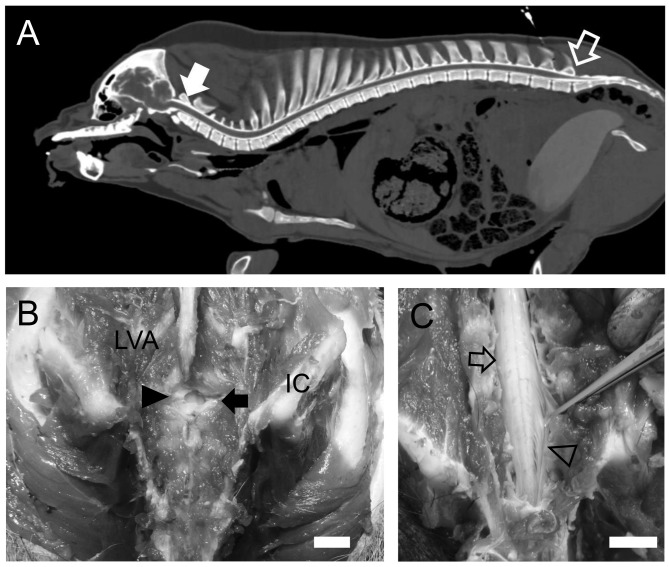
In a preliminary study of CT imaging of the intrathecal space with contrast medium, we confirmed that the cisterna magna was wider than other parts at the intrathecal space of the cervical spine, and the rich contrast medium in the intrathecal space was recognized at the lumbosacral space while the relatively thin spinal cord appeared (Figure 2A). The last lumbar and first sacral (S1) intervertebral space are most reliable for the introduction of a needle since the cauda equina is anatomically located at the last lumber-S1 (Figure 2B and C).
Figure 2. The preliminary study: Computed tomography image of the intrathecal space of cisterna magna (arrow) with intrathecal contrast medium was wider than the other areas of the intrathecal space of the cervical spine (A). The rich contrast medium in the intrathecal space was recognized at the lumbosacral space while the relatively thin spinal cord appeared (opened arrow), which was near the point of needle insertion. Lumbosacral space (closed arrow) is covered by ligamentum flavum (closed arrow head) and has enough space to insert the spinal needle (B). Lumbar enlargement (open arrow) is located on the head side than at the last lumber vertebra and the spinal cord at the lumbosacral space reveals cauda equina (open arrow head) (C). LVA: Lumbar vertebral arch; IC: iliac crest. Bar=1 cm .
The technique applied in Cases 1 and 2, allowed transdermal intrathecal catheter placement since the top of the catheter reached the cisterna magna in both animals, as confirmed by radiographic examinations. Neither animal showed abnormal conditions such as brief ataxia, and trouble with CSF leakage such as stenosis of the spinal canal or looping of the device itself were not noted during several weeks after recovery from anesthesia.
In Case 1, we confirmed the offset period after transdermal intrathecal catheter placement (Table I). The CSF just after catheter insertion was slightly reddish and slightly semitransparent, and the gross color changes of the CSF continued after 3 days. After 1 week, the CSF became colorless and transparent, but RBC count and LDH activity in the CSF were still high. These values decreased in 2-3 weeks. Neither a high or low level was observed in the other 6 biochemical parameters from 1 to 3 weeks after transdermal intrathecal catheter placement.
Table I. Gross color and appearance, red blood cell count and chemistry of CSF in Case 1.
NE: Not examined; TP: total protein; LDH: lactate dehydrogenase; CK: creatine kinase; Glc: glucose; Na: sodium; K: potassium; Cl: chloride; Ca: calcium.
In Case 2, we confirmed a technique for sequential collection of CSF to measure diurnal variation of melatonin and amino acids including Gln and Ala in CSF for confirming whether physiological variation of substances in CSF can be evaluated with this collection method (Table II). Melatonin in CSF showed a higher value at night than during the day time. However, no diurnal variation was observed in Gln or Ala. We also observed a decrease in BT and LA at night, representing the diurnality of microminipigs (Figure 3). Increases in BT and LA even at night were accordant with the collection of CSF and blood. In the same biochemical test as Case 1, LDH elevation was not observed (Table III).
Table II. Diurnal variation of melatonin and amino acids concentration in CSF and blood in Case 2.
Gln: Glutamine; Ala: alanine.
Figure 3. Locomotor activity (LA) and body temperature (BT) in Case 2: White and gray horizontal bands indicate the times that lights were on (7:00 → 19:00) and off (19:00 → 7:00). Open and closed arrows indicate meal times and CSF and blood collection points. The left longitudinal axis indicates activity counts per hour by LA. LA and BT rise at feeding and collection, while the LA and BT during the night indicate comparatively low activity count and temperature.
Table III. Diurnal variation of CSF chemistry in Case 2.
LDH: Lactate dehydrogenase; CK: creatine kinase; Glc: glucose; Na: sodium; K: potassium; Cl: chloride; Ca: calcium.
Discussion
The technique described in this study allowed spinal catheter placement in all animals. The catheter slid in easily, proving the size of the device to be appropriate for such minipigs.
The preliminary study helped in finding adequate locations for needle and catheter insertion. In domestic piglets, the L2 and L3 intervertebral space was chosen as the most reliable and easily punctured, and a spinal needle was introduced between the L2 and L3 spinous processes in a ventro-cranial orientation until CSF leakage (6). The CT imaging with contrast medium of the intrathecal space revealed that the thick spinal cord disappeared at the intrathecal space of the last lumbar. We also confirmed the anatomical location of the cauda equina at the last lumber-S1 in Microminipigs. Therefore, we considered the last lumbar and S1 intervertebral space to be most reliable for the introduction of the needle in prevention of trauma and hematoma in the spinal cord, because the spinal nerve of the cauda equina is thinner and minimally invasive. Moreover, since the sacrum has no spinous processes, it is easier to reach the intervertebral space in the prone position by approaching in the midline. The intrathecal space in the cisterna magna was wider than in other areas, suggesting that there is more volume of CSF in this area. Moreover, neurotransmitter metabolite concentrations in CSF from the cisterna magna were higher than those in lumbar spinal fluid (31,32). We decided to introduce the tip of the catheter into the cisterna magna.
During Cases 1 and 2, the catheter placement method using a guide wire was considered to be less invasive during puncture because the spinal needle was thin, and the inside diameter of the catheter could conversely be increased, thus a long-term patency period can be obtained. Based on the results in Table I, the method in this study does not affect the general condition of catheter indwelling for several weeks, and we also succeeded in performing sequential collection of CSF from an unanesthetized animal. Sequential collections of approximately 1 ml of CSF leakage for several weeks was thought to be possible since the tip of the catheter was localized at the cisterna magna, which holds relatively rich CSF.
In Case 1, the offset period after transdermal intrathecal catheter placement required over 1 week to reduce the effects of erythrocyte contamination, because the colorless and transparent CSF at 1 week after the catheter placement revealed high RBC and LDH.
In Case 2, the cisterna magna had relatively abundant CSF, and CSF collection was shown to be possible even more than once a day with no problems in the general condition of the animals. In addition, the concentrations of melatonin in the blood and CSF were high at light-off time like in humans (33), while no remarkable diurnal variation was observed in Gln or Ala. Moreover, the concentrations of melatonin in CSF at night were higher than those in blood, suggesting that melatonin is produced by the posterior pituitary, and there is a route directly from the posterior pituitary to CSF in addition to the route through blood circulation (34). We also confirmed that this animal was in a sleep-like state at night due to decreases in LA and BT at light-off time as shown in our previous report (24,25). The increases in LA and BT even at night were accordant with the collection of CSF and blood, suggesting that the treatment disrupts transiently physiological sleep. Therefore, the collection of CSF at night should be conducted quickly.
In conclusion, we established a transdermal intrathecal catheter placement technique using microminipigs. This transdermal intrathecal catheter placement makes it possible to sequentially collect CSF, which shows the diurnal variation of melatonin, from the cisterna magna in an unanesthetized animal for several weeks. This microminipig model of sequential CSF collection contributes to physiological and biochemical research. Moreover, this technique may be useful in pharmacological research to examine CSF using sequential collections after administration of medicine.
Conflicts of Interest
There exist no conflicts of interest in regard to this study.
Acknowledgements
The Authors thank all the staff members of the Division of Laboratory Animal Sciences, Natural Science Center for Research and Education, Kagoshima University, who kept the animals in good condition. The Authors would also like to thank Dr. Kaichiro Takeishi for technical assistance and Ms. Jenn Fukuyama, Mr. Ben Hollingum and Ms. Connie Sinks from Shin Nippon Biomedical Laboratories, Ltd. for editing in this study. This research was partly supported by the Japan Society for the Promotion of Science (JSPS) Kakenhi [grant number 16K08023, HK] and the Kodama Memorial Fund for Medical Research (HK).
References
- 1.Slats D, Claassen JA, Lammers GJ, Melis RJ, Verbeek MM, Overeem S. Association between hypocretin-1 and amyloid-beta42 cerebrospinal fluid levels in Alzheimer’s disease and healthy controls. Curr Alzheimer Res. 2012;9:1119–1125. doi: 10.2174/156720512804142840. [DOI] [PubMed] [Google Scholar]
- 2.Bollen PJA, Hansen AK, Alstrup AKO. Important biological features. In: The Laboratory SWINE, 2nd edition. Bollen PJA, Hansen AK and Alstrup AKO (eds.). 2010:pp. 1–13. [Google Scholar]
- 3.Swanson KS, Mazur MJ, Vashisht K, Rund LA, Beever JE, Counter CM, Schook LB. Genomics and clinical medicine: rationale for creating and effectively evaluating animal models. Exp Biol Med (Maywood) 2004;229:866–875. doi: 10.1177/153537020422900902. [DOI] [PubMed] [Google Scholar]
- 4.Karali M, Manfredi A, Puppo A, Marrocco E, Gargiulo A, Allocca M, Corte MD, Rossi S, Giunti M, Bacci ML, Simonelli F, Surace EM, Banfi S, Auricchio A. MicroRNA-restricted transgene expression in the retina. PLoS One. 2011;6:e22166. doi: 10.1371/journal.pone.0022166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testa F, Surace EM, Rossi S, Marrocco E, Gargiulo A, Di lorio V, Ziviello C, Nesti A, Fecarotta S, Bacci ML, Giunti M, Della Corte M, Banfi S, Auricchio A, Simonelli F. Evaluation of Italian patients with leber congenital amaurosis due to AIPL1 mutations highlights the potential applicability of gene therapy. Invest Ophthalmol Vis Sci. 2011;52:5618–5624. doi: 10.1167/iovs.10-6543. [DOI] [PubMed] [Google Scholar]
- 6.Lambertini C, Ventrella D, Barone F, Sorrentino NC, Dondi F, Fraldi A, Giunti M, Surace EM, Bacci ML, Romagnoli N. Transdermal spinal catheter placement in piglets: Description and validation of the technique. J Neurosci Methods. 2015;255:17–21. doi: 10.1016/j.jneumeth.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Lucey BP, Gonzales C, Das U, Li J, Siemers ER, Slemmon JR, Bateman RJ, Huang Y, Fox GB, Claassen JA, Slats D, Verbeek MM, Tong G, Soares H, Savage MJ, Kennedy M, Forman M, Sjogren M, Margolin R, Chen X, Farlow MR, Dean RA, Waring JF. An integrated multi-study analysis of intra-subject variability in cerebrospinal fluid amyloid-beta concentrations collected by lumbar puncture and indwelling lumbar catheter. Alzheimers Res Ther. 2015;7:53. doi: 10.1186/s13195-015-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bevan AK, Duque S, Foust KD, Morales PR, Braun L, Schmelzer L, Chan CM, McCrate M, Chicoine LG, Coley BD, Porensky PN, Kolb SJ, Mendell JR, Burghes AH, Kaspar BK. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol Ther. 2011;19:1971–1980. doi: 10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romagnoli N, Ventrella D, Giunti M, Dondi F, Sorrentino NC, Fraldi A, Surace EM, Bacci ML. Access to cerebrospinal fluid in piglets via the cisterna magna: optimization and description of the technique. Lab Anim. 2014;48:345–348. doi: 10.1177/0023677214540881. [DOI] [PubMed] [Google Scholar]
- 10.Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 2003;55:1007–1041. doi: 10.1016/s0169-409x(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 11.Poon YY, Chang AY, Ko SF, Chan SH. Catheterization of the thoracic spinal subarachnoid space in mice. J Neurosci Methods. 2011;200:36–40. doi: 10.1016/j.jneumeth.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Federici T, Taub JS, Baum GR, Gray SJ, Grieger JC, Matthews KA, Handy CR, Passini MA, Samulski RJ, Boulis NM. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther. 2012;19:852–859. doi: 10.1038/gt.2011.130. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko N, Itoh K, Sugiyama A, Izumi Y. Microminipig, a non-rodent experimental animal optimized for life science research: preface. J Pharmacol Sci. 2011;115:112–114. doi: 10.1254/jphs.10R16FM. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi H, Yamada T, Miura N, Takahashi Y, Yoshikawa T, Izumi H, Kawarasaki T, Miyoshi N, Tanimoto A. Reference values of hematological and biochemical parameters for the world smallest microminipigs. J Vet Med Sci. 2012;74:933–936. doi: 10.1292/jvms.11-0571. [DOI] [PubMed] [Google Scholar]
- 15.Kawaguchi H, Yamada T, Miura N, Noguchi M, Izumi H, Miyoshi N, Tanimoto A. Sex differences of serum lipid profile in novel microminipigs. In Vivo. 2013;27:617–621. [PubMed] [Google Scholar]
- 16.Kakimoto T, Otsuka A, Kawaguchi H, Ogata K, Tanimoto A, Kanouchi H. Plasma homocysteine concentrations in novel microminipigs. In Vivo. 2014;28:579–82. [PubMed] [Google Scholar]
- 17.Miura N, Kucho K, Noguchi M, Miyoshi N, Uchiumi T, Kawaguchi H, Tanimoto A. Comparison of the genomic sequence of the microminipig, a novel breed of swine, with the genomic database for conventional pig. In Vivo. 2014;28:1107–1111. [PubMed] [Google Scholar]
- 18.Miyoshi N, Horiuchi M, Inokuchi Y, Miyamoto Y, Miura N, Tokunaga S, Fujiki M, Izumi Y, Miyajima H, Nagata R, Misumi K, Takeuchi T, Tanimoto A, Yasuda N, Yoshida H, Kawaguchi H. Novel microminipig model of atherosclerosis by high fat and high cholesterol diet, established in Japan. In Vivo. 2010;24:671–680. [PubMed] [Google Scholar]
- 19.Akioka K, Kawaguchi H, Kitajima S, Miura N, Noguchi M, Horiuchi M, Miyoshi N, Tanimoto A. Investigation of necessity of sodium cholate and minimal required amount of cholesterol for dietary induction of atherosclerosis in microminipigs. In Vivo. 2014;28:81–90. [PubMed] [Google Scholar]
- 20.Kawaguchi H, Miyoshi N, Miura N, Fujiki M, Horiuchi M, Izumi Y, Miyajima H, Nagata R, Misumi K, Takeuchi T, Tanimoto A, Yoshida H. Microminipig, a non-rodent experimental animal optimized for life science research: novel atherosclerosis model induced by high fat and cholesterol diet. J Pharmacol Sci. 2011;115:115–121. doi: 10.1254/jphs.10R17FM. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi H, Yamada T, Miura N, Ayaori M, Uto-Kondo H, Ikegawa M, Noguchi M, Wang KY, Izumi H, Tanimoto A. Rapid development of atherosclerosis in the world’s smallest Microminipig fed a high-fat/high-cholesterol diet. J Atheroscler Thromb. 2014;21:186–203. doi: 10.5551/jat.21246. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama H, Nakamura Y, Saito H, Nagayama Y, Hoshiai K, Wada T, Izumi-Nakaseko H, Ando K, Akie Y, Sugiyama A. Pharmacological characterization of microminipig as a model to assess the drug-induced cardiovascular responses for non-clinical toxicity and/or safety pharmacology studies. J Toxicol Sci. 2017;42:93–101. doi: 10.2131/jts.42.93. [DOI] [PubMed] [Google Scholar]
- 23.Zahorul Islam M, Kawaguchi H, Miura N, Miyoshi N, Yamazaki-Himeno E, Shiraishi M, Miyamoto A, Tanimoto A. Hypertension alters the endothelial-dependent biphasic response of bradykinin in isolated Microminipig basilar artery. Microvasc Res. 2017;114:52–57. doi: 10.1016/j.mvr.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Takeishi K, Horiuchi M, Kawaguchi H, Deguchi Y, Izumi H, Arimura E, Kuchiiwa S, Tanimoto A, Takeuchi T. acupuncture improves sleep conditions of minipigs representing diurnal animals through an anatomically similar point to the acupoint (GV20) effective for humans. Evid Based Complement Alternat Med. 2012;2012:472982. doi: 10.1155/2012/472982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeishi K, Kawaguchi H, Akioka K, Noguchi M, Arimura E, Abe M, Ushikai M, Okita S, Tanimoto A, Horiuchi M. Effects of dietary and lighting conditions on diurnal locomotor activity and body temperature in microminipigs. In Vivo. 2018;32:55–62. doi: 10.21873/invivo.11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshikawa T, Takahashi Y, Kawaguchi H, Utsunomiya S, Miura N, Izumi H, Miyoshi N, Tanimoto A. A dermal phototoxicity study following intravenous infusion administration of ciprofloxacin hydrochloride in the novel microminipigs. Toxicol Pathol. 2013;41:109–113. doi: 10.1177/0192623312452489. [DOI] [PubMed] [Google Scholar]
- 27.Guruge KS, Noguchi M, Yoshioka K, Yamazaki E, Taniyasu S, Yoshioka M, Yamanaka N, Ikezawa M, Tanimura N, Sato M, Yamashita N, Kawaguchi H. Microminipigs as a new experimental animal model for toxicological studies: comparative pharmacokinetics of perfluoroalkyl acids. J Appl Toxicol. 2016;36:68–75. doi: 10.1002/jat.3145. [DOI] [PubMed] [Google Scholar]
- 28.Miura N, Kawaguchi H, Nagasato T, Yamada T, Ito T, Izumi H, Shameshima H, Miyoshi N, Tanimoto A, Maruyama I. Coagulation activity and white thrombus formation in the microminipig. In Vivo. 2013;27:357–361. [PubMed] [Google Scholar]
- 29.Miyoshi K, Kawaguchi H, Maeda K, Sato M, Akioka K, Noguchi M, Horiuchi M, Tanimoto A. Birth of cloned microminipigs derived from somatic cell nuclear transfer embryos that have been transiently treated with valproic acid. Cell Reprogram. 2016;18:390–400. doi: 10.1089/cell.2016.0025. [DOI] [PubMed] [Google Scholar]
- 30.Yamada S, Kawaguchi H, Yamada T, Guo X, Matsuo K, Hamada T, Miura N, Tasaki T, Tanimoto A. Cholic acid enhances visceral adiposity, atherosclerosis and nonalcoholic fatty liver disease in microminipigs. J Atheroscler Thromb. 2017;24:1150–1166. doi: 10.5551/jat.39909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaughn DM, Coleman E, Simpson ST, Whitmer B, Satjawatcharaphong C. A rostrocaudal gradient for neuro-transmitter metabolites and a caudorostral gradient for protein in canine cerebrospinal fluid. Am J Vet Res. 1988;49:2134–2137. [PubMed] [Google Scholar]
- 32.Djukic M, Spreer A, Lange P, Bunkowski S, Wiltfang J, Nau R. Small cisterno-lumbar gradient of phosphorylated Tau protein in geriatric patients with suspected normal pressure hydrocephalus. Fluids Barriers CNS. 2016;13:15. doi: 10.1186/s12987-016-0039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruce J, Tamarkin L, Riedel C, Markey S, Oldfield E. Sequential cerebrospinal fluid and plasma sampling in humans: 24-hour melatonin measurements in normal subjects and after peripheral sympathectomy. J Clin Endocrinol Metab. 1991;72:819–823. doi: 10.1210/jcem-72-4-819. [DOI] [PubMed] [Google Scholar]
- 34.Tan D-X, Manchester LC, Reiter RJ. CSF generation by pineal gland results in a robust melatonin circadian rhythm in the third ventricle as a unique light/dark signal. Med Hypotheses. 2016;86:3–9. doi: 10.1016/j.mehy.2015.11.018. [DOI] [PubMed] [Google Scholar]