Abstract
Background/Aim: This study aimed to create a predictive tool for estimating the remaining lifespan of patients after whole-brain irradiation (WBI) for cerebral metastases from bladder cancer. Patients and Methods: In 34 of these patients clinical parameters were analyzed for survival including age at start of WBI, gender, Karnofsky score, number of cerebral metastases and involvement of extra-cranial sites of metastasis. Results: Involvement of extra-cranial sites (14%) and Karnofsky score (9%) showed the greatest difference regarding 6-month survival and were considered for the tool. Points were assigned based on the following: no involvement of extra-cranial sites=1 point, involvement of extra-cranial sites=0 points, Karnofsky score ≥70=1 point, Karnofsky score ≤60=0 points. Patients’ scores were 0, 1 or 2 points with 6-month survival rates of 13%, 27% and 50%, respectively. Conclusion: Based on two clinical parameters, a tool was developed that may help estimate the lifespan of patients irradiated for cerebral metastases from bladder cancer.
Keywords: Cancer of the bladder, cerebral metastases, whole-brain irradiation, prognostic tool, estimation of survival
In adult cancer patients developing cerebral metastases, those with cancer of the urinary bladder account for only 1-2% (1,2). The survival prognosis of this subgroup is poor when compared to patients with cerebral metastasis from other tumor entities like breast cancer and requires significant improvement (1). Better outcomes of treatment may be achieved with new therapeutic developments, for example minimally-invasive surgical techniques, novel systemic therapies and new radiotherapy approaches including stereotactic radiosurgery for more than three lesions and whole-brain-irradiation (WBI) with hippocampal sparing (3-9). Another approach that has gained importance, when aiming to improve the outcomes of cancer patients, is the personalization of the treatment, i.e. optimally tailoring the treatment to the situation and the needs of an individual patient. Such personalized approaches should consider the patient’s remaining lifespan (1,10,11). Patients with a longer lifespan should receive a treatment that improves their prognoses in terms of local control and survival but with a low risk of late treatment-related morbidity, since many of these patients live long enough to be at risk of experiencing late toxicity (12). In contrast, patients with a short remaining lifespan would be better candidates for a less intensive, shorter treatment to avoid spending much of their remaining life receiving therapy (13). These considerations apply also to radiotherapy of cerebral metastases, in particular if the patients are assigned to receive WBI. Different dose-fractionation schedules of WBI are available for this situation, mainly 20 Gy in 5 fractions over 1 week, 30 Gy in 10 fractions over 2 weeks and 40 Gy in 20 fractions over 4 weeks (1). In order to select the appropriate WBI-schedule for an individual, it would be critical to know his or her expected lifespan. This could be estimated with the use of predictive tools (10,11). Since in patients with cerebral metastases survival prognoses vary between different tumors types, the availability of a specific predictive tool for each tumor is desirable. This study was particularly focused on patients treated with WBI for cerebral metastases from cancer of the bladder.
Patients and Methods
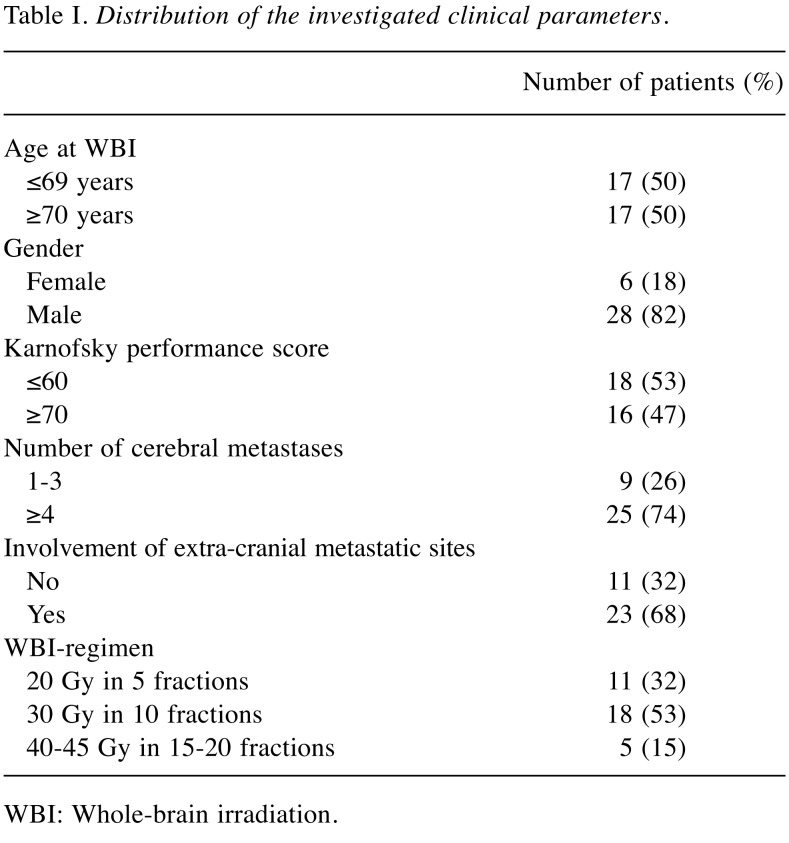
A cohort of 34 patients with cerebral metastasis who were treated with WBI alone was retrospectively analyzed for five clinical parameters regarding potential associations with survival. Some patients were already part of a previous study (14). The factors investigated included age at start of WBI (≤69 vs. ≥70 years, median=69.5 years), gender, general condition given by the Karnofsky performance score of ≤60 vs. ≥70, median=60%), number of cerebral metastases (1-3 vs. ≥4, median=4) and involvement of extra-cranial sites by metastatic disease (no vs. yes). In addition, the impact of the dose-fractionation schedule of WBI (20 Gy in 5 fractions over 1 week vs. 30 Gy in 10 fractions over 2 weeks vs. 40-45 Gy in 15-20 fractions over 3-4 weeks) was investigated. The distributions of these parameters are summarized in Table I. The analyses of survival were done with the Kaplan-Meier method plus the log-rank test (15). The two clinical parameters that showed the greatest difference with respect to 6-month survival rate were selected to be included in the prognostic tool.
Table I. Distribution of the investigated clinical parameters.
WBI: Whole-brain irradiation.
Results
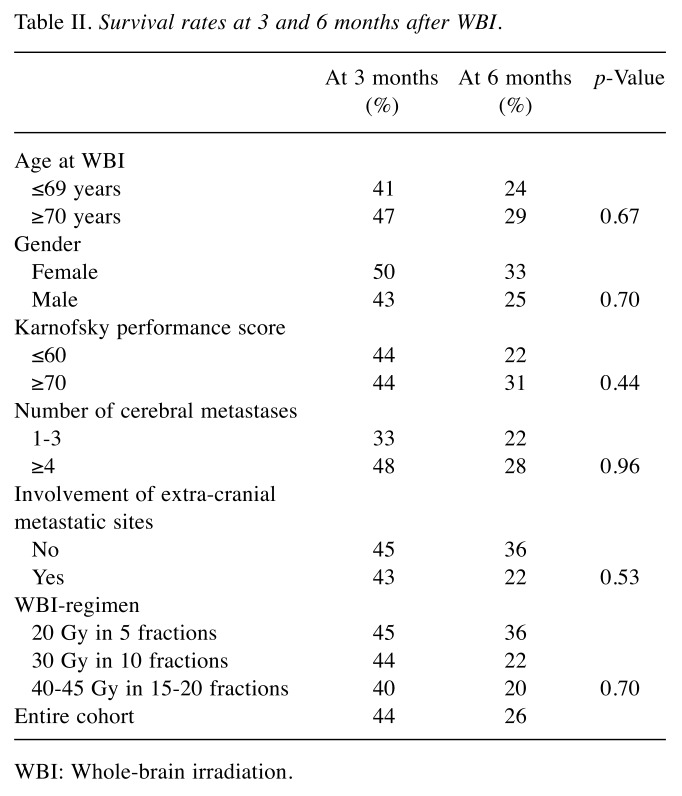
In the entire cohort, the 3-month and 6-month survival rates were 44% and 26%, respectively. On analysis of survival, involvement of extra-cranial sites by metastatic disease (14%) and the Karnofsky performance score (9%) had the greatest differences in the 6-month survival rate and were included in the scoring tool (Table II). The following points were assigned to these parameters: no involvement of extra-cranial sites=1 point, involvement of extra-cranial sites=0 points, Karnofsky performance score of ≥70=1 point, Karnofsky performance score of ≤60=0 points. Thus, the scores for individual patients were 0, 1 or 2 points. The corresponding survival rates were 40%, 45% and 50%, respectively, at 3 months, and 13%, 27% and 50%, respectively, at 6 months following WBI (p=0.30). The median survival times were 3 months, 3 months and 5 months, respectively.
Table II. Survival rates at 3 and 6 months after WBI .
WBI: Whole-brain irradiation.
Discussion
Advanced cancer of the bladder is a very aggressive malignant disease, associated with a poor prognosis. This applies particularly to patients who have developed distant metastases including metastases to the brain. In order to improve the outcomes of these patients, multi-disciplinary approaches including novel radiotherapy or surgical techniques and novel drugs or drug combinations are often used. In addition, personalization of the anticancer treatment has become more popular, which generally takes into account patient’s remaining lifespan. Therefore, treatment personalization may be facilitated by applying prognostic factors associated with survival or even better by using predictive tools, i.e. survival scores. Such scores are already available for bladder cancer patients for treatment of the primary tumor, a loco-regional recurrence and for metastatic disease in general (14,16,17). The present study was conducted to create a survival score for patients receiving WBI alone for cerebral metastases from cancer of the bladder. Based on two clinical parameters, namely the patient’s general condition given by the Karnofsky performance score and involvement of extra-cranial sites by metastatic disease, a predictive tool was developed. Three prognostic groups were formed, 0 points, 1 point and 2 points. Patients achieving only 0 points had a very poor prognosis with a 6-month survival rate of only 13%. Therefore, these patients appear good candidates for a short course of WBI such as 20 Gy in 5 fractions over 1 week. In a previous study of 442 patients, the comparison of this WBI-regimen (N=232) to 30 Gy in 10 fractions over 2 weeks (N=210) did not show a significant difference between the 6-month survival rates that were 24% and 27%, respectively (p=0.29) (12). Grade 3 WBI-related acute toxicity rates were 9% with 20 Gy in 5 fractions and 6% with 30 Gy in 10 fractions, respectively (p=0.35). Required median dexamethasone doses were 24 mg/day and 20 mg/day, respectively. Those patients who achieved 1 point on the newly developed score presented here had a 6-month survival rate of 27%, which is still relatively unfavorable and similar to the 24% and 27% observed in the previous study. Therefore, these patients may also appear best treated with 20 Gy in 5 fractions.
Patients achieving 2 points had the most favorable survival prognosis of the present cohort with a 6-month survival rate of 50% and a median survival time of 5 months. However, when compared to the 6-month survival rates of patients with cerebral metastases from other primary tumors, a 6-month survival probability of 50% and a median survival time of 5 months may be considered an intermediate prognosis. Previous studies have shown that patients with intermediate prognoses appear most suitable candidates for longer-course WBI with 30 Gy in 10 fractions (10,11). However, when following the recommendations regarding the appropriate WBI-regimen, one should be aware of possible selection biases inherent to the retrospective nature of the data used to create the predictive tool. Nevertheless, this tool can serve physicians who would like to personalize the treatment for each case.
In conclusion, based on the patient’s general condition and extra-cranial metastatic involvement, a new predictive tool was created to estimate the lifespan of patients receiving WBI for cerebral metastases from bladder cancer and to support personalizing their care.
Conflicts of Interest
On behalf of all Authors, the corresponding Author states that there is no conflict of interest related to this study.
References
- 1.Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, Sperduto PW, Vogelbaum MA, Radawski JD, Wang JZ, Gillin MT, Mohideen N, Hahn CA, Chang EL. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2:210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Schinzari G, Monterisi S, Pierconti F, Nazzicone G, Marandino L, Orlandi A, Racioppi M, Cassano A, Bassi P, Barone C, Rossi E. Neoadjuvant chemotherapy for patients with muscle-invasive urothelial bladder cancer candidates for curative surgery: A prospective clinical trial based on cisplatin feasibility. Anticancer Res. 2017;37:6453–6458. doi: 10.21873/anticanres.12100. [DOI] [PubMed] [Google Scholar]
- 4.Hsu FT, Sun CC, Wu CH, Lee YJ, Chiang CH, Wang WS. Regorafenib induces apoptosis and inhibits metastatic potential of human bladder carcinoma cells. Anticancer Res. 2017;37:4919–4926. doi: 10.21873/anticanres.11901. [DOI] [PubMed] [Google Scholar]
- 5.Shi CS, Li JM, Chin CC, Kuo YH, Lee YR, Huang YC. Evodiamine induces cell growth arrest, apoptosis and suppresses tumorigenesis in human urothelial cell carcinoma cells. Anticancer Res. 2017;37:1149–1159. doi: 10.21873/anticanres.11428. [DOI] [PubMed] [Google Scholar]
- 6.Silva J, Arantes-Rodrigues R, Pinto-Leite R, Faustino-Rocha AI, Fidalgo-Gonçalves L, Santos L, Oliveira PA. Synergistic effect of carboplatin and piroxicam on two bladder cancer cell lines. Anticancer Res. 2017;37:1737–1745. doi: 10.21873/anticanres.11506. [DOI] [PubMed] [Google Scholar]
- 7.Limon D, McSherry F, Herndon J, Sampson J, Fecci P, Adamson J, Wang Z, Yin FF, Floyd S, Kirkpatrick J, Kim GJ. Single fraction stereotactic radiosurgery for multiple brain metastases. Adv Radiat Oncol. 2017;2:555–563. doi: 10.1016/j.adro.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, Konski AA, Bauman GS, Shah S, Shi W, Wendland M, Kachnic L, Mehta MP. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aizawa R, Sakamoto M, Orito N, Kono M, Ogura M, Negoro Y, Sagoh T, Tsukahara K, Komatsu K, Noguchi M. The Use of external-beam radiotherapy for muscle-invasive bladder cancer in elderly or medically-fragile patients. Anticancer Res. 2017;37:5761–5766. doi: 10.21873/anticanres.12016. [DOI] [PubMed] [Google Scholar]
- 10.Rades D, Dziggel L, Haatanen T, Veninga T, Lohynska R, Dunst J, Schild SE. Scoring systems to estimate intracerebral control and survival rates of patients irradiated for brain metastases. Int J Radiat Oncol Biol Phys. 2011;80:1122–1127. doi: 10.1016/j.ijrobp.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 11.Dziggel L, Segedin B, Podvrsnik NH, Oblak I, Schild SE, Rades D. Validation of a survival score for patients treated with whole-brain radiotherapy for brain metastases. Strahlenther Onkol. 2013;189:364–366. doi: 10.1007/s00066-013-0308-3. [DOI] [PubMed] [Google Scholar]
- 12.Rades D, Kieckebusch S, Lohynska R, Veninga T, Stalpers LJ, Dunst J, Schild SE. Reduction of overall treatment time in patients irradiated for more than three brain metastases. Int J Radiat Oncol Biol Phys. 2007;69:1509–1513. doi: 10.1016/j.ijrobp.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Rades D, Panzner A, Dziggel L, Haatanen T, Lohynska R, Schild SE. Dose-escalation of whole-brain radiotherapy for brain metastasis in patients with a favorable survival prognosis. Cancer. 2012;118:3853–3859. doi: 10.1002/cncr.26680. [DOI] [PubMed] [Google Scholar]
- 14.Rades D, Manig L, Janssen S, Schild SE. A survival score for patients assigned to palliative radiotherapy for metastatic bladder cancer. Anticancer Res. 2017;37:1481–1484. doi: 10.21873/anticanres.11473. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Manig L, Janssen S, Schild SE, Rades D. A new prognostic instrument specifically designed for patients irradiated for recurrent carcinoma of the bladder. In Vivo. 2017;31:435–438. doi: 10.21873/invivo.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manig L, Janssen S, Schild SE, Rades D. A new prognostic tool for patients undergoing tadiotherapy plus upfront transurethral resection for bladder cancer. In Vivo. 2017;31:745–748. doi: 10.21873/invivo.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]