Abstract
Background/Aim: A previous study indicated that amentoflavone inhibits tumor growth of breast cancer. However, the anti-cancer effects and mechanism of amentoflavone in hepatocellular carcinoma (HCC) have not been elucidated. The aim of the present study was to verify the effect of amentoflavone on tumor progression in HCC. Materials and Methods: HCC SK-Hep1 cells were treated with different concentrations of amentoflavone or 10 μM PD98059 (extracellular signal-regulated kinases (ERK) inhibitor) for 48 h, respectively, and then cell viability, NF-ĸB activation, levels of tumor progression-associated proteins, and cell invasion were evaluated with 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), NF-ĸB reporter gene assay, western blotting, and cell invasion assay. Results: The results demonstrated that both amentoflavone and PD98059 not only significantly reduced cell viability, NF-ĸB activation, and cell invasion, but also inhibited the expression of tumor progression-associated proteins. In addition, we found that amentoflavone suppresses ERK phosphorylation. Conclusion: The results of the present study suggest that amentoflavone down-regulates ERK-modulated tumor progression in HCC.
Keywords: Amentoflavone, hepatocellular carcinoma, NF-ĸB, ERK.
Extracellular molecules, such as growth factors and hormones, trigger mitogen-activated protein kinase (MAPK) cascade-modulated normal cell proliferation, survival, and differentiation. Aberrant MAPK cascade is linked to cancer and other diseases. MAPK extracellular signal-regulated kinase (ERK) is phosphorylated by Raf/MAPK/ERK kinase (MEK)1/2 dual-specificity protein kinase and is associated with overexpression of oncogenic proteins that result in tumor progression (1-2). In hepatocellular carcinoma (HCC) tissues, increased expression of phospho-ERK (pERK) was correlated with poor disease-specific overall survival (3). Development of new anti-HCC agents that block ERK activation may provide benefits in treatment of HCC.
Nuclear factor-kappaB (NF-ĸB), a critical transcription factor, regulates the expression of many oncogenes in tumorigenesis. Active NF-ĸB promotes tumor growth, anti-apoptosis, angiogenesis, and metastasis of HCC through overexpression of tumor progression-associated proteins encoded by NF-ĸB-targeted genes (4-5). The MAPK cascade has been shown to activate NF-ĸB-modulated tumor progression in various cancer cells. Blockage of ERK activation may downregulate NF-ĸB-modulated tumor progression in HCC (6-8).
Amentoflavone, a polyphenolic compound that exists in many plants, has been indicated to induce anti-inflammation, anti-cancer effects, and protection of cardiovascular and central nervous system (9). In a previous study, we found amentoflavone, as a sorafenib sensitizer, which enhances sorafenib-induced cytotoxicity and apoptosis in sorafenib-resistant HCC (10). However, the anti-cancer effects and mechanism of amentoflavone in HCC are ambiguous. Therefore, the aim of the present study was to verify the effect of amentoflavone on tumor progression in HCC. The role of ERK inactivation on amentoflavone-induced inhibition of tumor progression was also investigated.
Materials and Methods
Chemicals and reagents. Amentoflavone, MTT, and Dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), L-glutamine and penicillin-streptomycin (PS) were purchased from Gibco/Life Technologies (Carlsbad, CA, USA). Hygromycin was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). jetPEI-DNA transfection reagent was from Polyplus Transfection (Sélestat, Bas-Rhin, France). D-luciferin was obtained from Promega (Madison, WI, USA). Extracellular signal-regulated kinase (ERK) inhibitor PD98059 was boughtfrom Selleckchem (Houston, TX, USA). Primary antibodies of cellular FADD-like IL-1β-converting enzyme (FLICE)-inhibitory protein (C-FLIP), and Cyclin-D1 purchased from Cell Signaling Technology (Beverly, MA, USA). A primary antibody of X-linked inhibitor of apoptosis protein (XIAP) was obtained from Thermo Fisher Scientific (Fremont, CA, USA). Primary antibodies for matrix metallopeptidase (MMP 9) and vascular endothelial growth factor (VEGF) were obtained from EMD Millipore (Billerica, MA, USA). Primary antibodies of phosphorylated extracellular signal-regulated kinase (pERK) and ERK were obtained from Merck Millipore (Billerica, MA, USA) and Santa Cruz Biotechnology (Santa Cruz, CA, USA), respectively. Matrigel was obtained from Corning (Tewksbury, MA, USA).
Cell culture. HCC SK-Hep1 cells were provided by Professor Jing- Gung Chung at the Department of Biological Science and Technology, China Medical University, Taichung and used to verify anti-HCC effect of amentoflavone. Cells were maintained in DMEM containing10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin in a humidified incubator at 37˚C in 5% CO2 and 95% air (11).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT) assay. SK-Hep1 cells were seeded into 96-well plates with 2×104/well and incubated overnight. Cells were treated with different concentrations of amentoflavone or PD98059 (ERK inhibitor) for 48 h, respectively, and then cell viability was evaluated with MTT assay, as described by Chen et al. (12).
Plasmid transfection. NF-ĸB-luciferase reporter plasmid (pNF-ĸB/luc2) was obtained from Promega (Madison, WI, USA). 1×106 SK-Hep1 cells were cultured overnight in 10 cm dish, and then transfected with pNF-ĸB/luc2 by using jetPEI-DNA transfection reagent as previously described (13).
NF-ĸB reporter gene assay. SK-Hep1 cells were seeded into 96-well plates (2×104/well) and incubated overnight. Cells were treated with different concentrations of amentoflavone or PD98059 for 48 h, respectively. Relative NF-ĸB activity was investigated with NF-ĸB reporter gene assay and rectified with cell viability, which was obtained by MTT assay as previously described (7).
Western blotting assay. 3×106 SK-Hep1 cells were cultured overnight in a 10 cm dish, and then treated with different concentrations of amentoflavone or 10 μM PD98059 for 48 h, respectively. After treatments, total protein from cells was isolated and collected by using lysis buffer (50 mM Tris- HCl pH 8.0, 120 mM NaCl, 0.5% NP-40, and 1 mM phenylmethanesulfonyl fluoride) and centrifugation. Protein levels of MMP-9, XIAP, VEGF, Cyclin-D1, ERK, and pERK were evaluated with Western blotting assay as described by Wang et al. (14). Quantification of protein bands was performed by using Bio-Rad Image Lab software (Bio-Rad Laboratories, Inc., CA, USA)
Invasion assay. Transwell insert with 8 μm pore size was coated with 50 μl matrigel solution (1 to 1 matrigel with DMEM) and placed overnight at 37˚C. 3×106 SK-Hep1 cells were incubated overnight in a 10 cm dish and then treated with different concentrations of amentoflavone or 10 μM PD98059 for 48 h, respectively. After treatments, 1×106 viable cells were harvested with centrifugation and resuspended in 1 ml serum free DMEM. 100 μl cell suspension was added into the apical chamber of transwell insert and maintained for 48 h. Effect of treatments on cell invasion was evaluated with cell invasion assay as described by Lai et al. (15). Light Nikon ECLIPSE Ti-U microscope was used for photography of invaded cells at ×100 and number of invaded cells was quantified by using ImageJ software version 1.50 (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis. Analysis of variance (ANOVA) was used to test significance of difference between each experimental group. Data was expressed as mean±stand error. p-Value less than 0.05 was considered significant.
Results
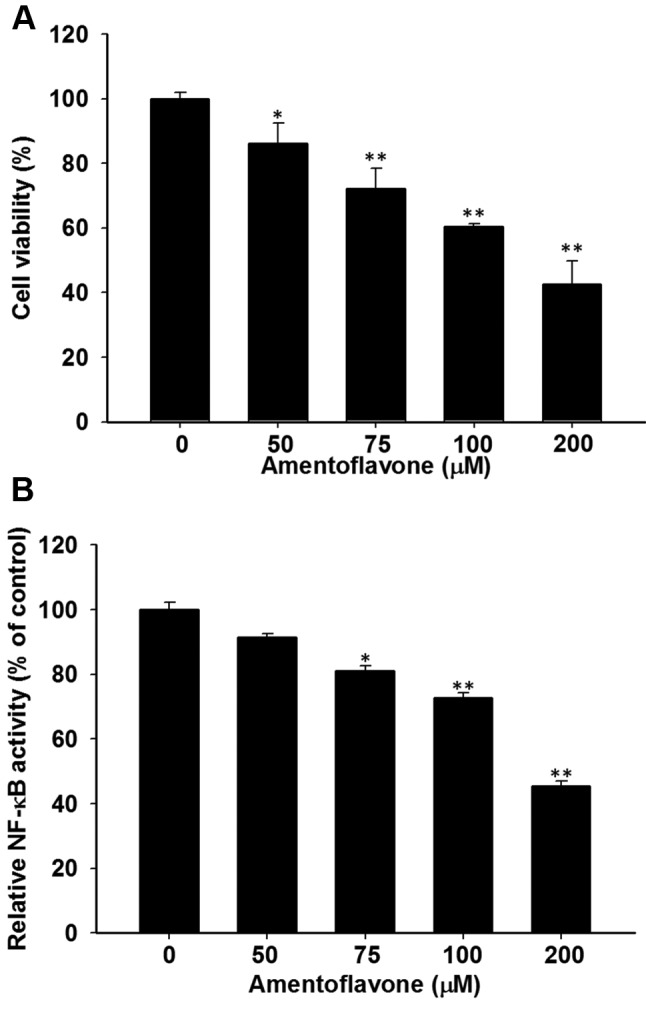
Amentoflavone induces cytotoxicity and NF-ĸB inactivation in SK-Hep1 cells. SK-Hep1 cells after exposed to various concentrations of amentoflavone were analyzed for cell viability. Cell viability was significantly reduced in a dose dependent manner, by 14%-58% compared to control (Figure 1A). Figure 1B demonstrates that increasing the amentoflavone concentration significantly inhibited NF-ĸB activation of SK-Hep1 cells by 10-55% as compared to the control.
Figure 1. Effect of amentoflavone on cell viability and NF-ĸB activation in SK-Hep1 cells. Cells were treated with different concentrations (0-200 μM in 0.1% DMSO) of amentoflavone for 48 h. (A) Change of cell viability was evaluated with MTT assay. (B) NF-ĸB activation was determined with NF-ĸB reporter gene assay and corrected by using cell viability. *p<0.05 and **p<0.01 compared to control (0.1 % DMSO treatment) .
Blockage of ERK activation reduces cell growth and NF-ĸB activation in SK-Hep1 cells. PD98059, an ERK inhibitor, was used to treat SK-Hep1 cells. Subsequently, change of cell viability and NF-ĸB activation were investigated with MTT and NF-ĸB reporter gene assay. Figure 2A shown PD98059 significantly decreases cell growth of SK-Hep1 in a dose-dependent manner, by 33-62% compared to control. Figure 2B indicates NF-ĸB activation of SK-Hep1 cells was also significantly reduced by PD98059 treatment compared to control.
Figure 2. Effect of PD98059 (ERK inhibitor) on cell viability and NF-ĸB activation in SK-Hep1 cells. Cells were treated with different concentrations (0-30 μM in 0.1% DMSO) of PD98059 for 48 h. (A) Change of cell viability was evaluated by using MTT assay, (B) NF-ĸB activation was investigated with NF-ĸB reporter gene assay and corrected by using cell viability. *p<0.05 and **p<0.01 compared to control (0.1 % DMSO treatment).
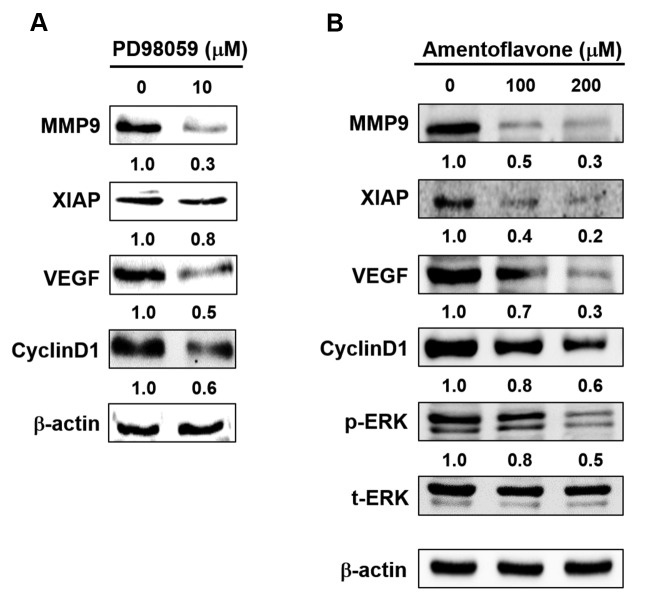
Amentoflavone suppresses expression of tumor progression-associated proteins through inhibition of ERK phosphorylation in SK-Hep1 cells. Western blotting assay was used to evaluate protein levels of MMP-9, XIAP, VEGF, Cyclin-D1, and pERK of SK-Hep1 cells after PD98059 or amentoflavone treatment. Figure 3A shows that PD98059 inhibits protein levels of MMP-9, XIAP, VEGF and cyclin-D1 by 0.4-0.8-fold as compared to those of control. In addition to the inhibition of tumor progression-associated protein expression, amentoflavone also decreased protein levels of pERK in Figure 3B.
Figure 3. 3. Effect of PD98059 and amentoflavone on the expression of tumor progression-associated proteins in SK-Hep1 cells. Cells were treated with 10 μM PD98059, or different concentrations (0, 100, 200 μM) of amentoflavone for 48 h, respectively. Protein levels of MMP-9, XIAP, VEGF, cyclin-D1, and pERK were evaluated with western blotting assay. (A) PD98059 treatment, (B) Amentoflavone treatment.
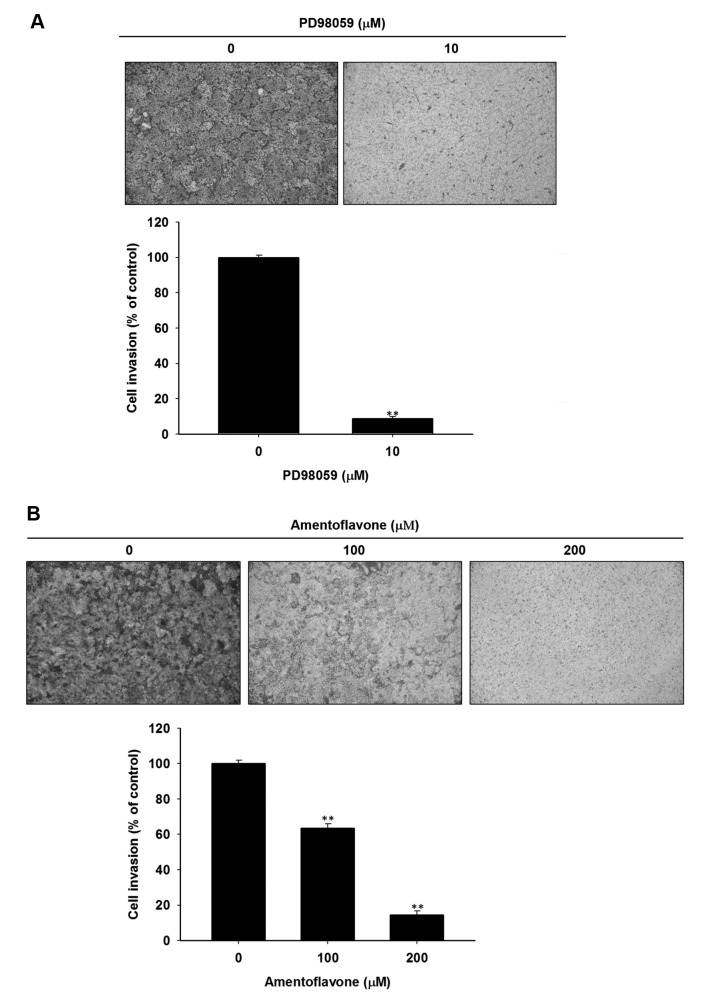
Both amentoflavone and PD98059 inhibit the invasive ability of SK-Hep1 cells. Figure 4A indicates that amentoflavone significantly decreased the number of invaded cells by 40-80% as compared to control. We also found that 10 μM PD98059 significantly reduces cell invasion by 90% as compared to control (Figure 4B).
Figure 4. Effect of PD98059 and amentoflavone on cell invasion in SK-Hep1 cells. Cells were treated with 10 μM PD98059, or different concertation (0, 100, 200 μM) of amentoflavone for 48 h, respectively. Invasive ability of SK-Hep1 cells was determined by using cell invasion assay. (A) PD98059 treatment, (B) Amentoflavone treatment. **p<0.01 as compared to control (0.1 % DMSO treatment).
Discussion
In our previous study amentoflavone induced cell cycle arrest and apoptosis leading to inhibition of breast cancer cell growth (16). However, the anti-cancer effect and the mechanism of action of amentoflavone in HCC is unknown. In this study, we demonstrated anti-cancer effect of amentoflavone and the role of ERK inactivation on amentoflavone-induced inhibition of tumor progression in HCC. In previous studies on HCC cell lines, such as SK-Hep1 and Huh7, we found that inhibition of NF-ĸB activation impairs tumor progression including anti-apoptosis, angiogenesis, proliferation, and metastasis. In addition, blockage of ERK activation may also downregulate NF-ĸB-modulated tumor progression (5,7).
Sorafenib, the oral multi-kinase inhibitor used for the treatment of HCC, has been shown to diminish HCC growth through inhibition of Raf/MEK/ERK signaling transduction (17). The effect of sorafenib on NF-ĸB-modulated tumor progression in HCC was demonstrated from our previous studies. Sorafenib inhibits NF-ĸB-modulated tumor progression by triggering ERK dephosphorylation in HCC both in vitro and in vivo (7,18). In this study, we showed that ERK dephosphorylation is a critical factor in amentoflavone-induced inhibition of tumor progression in SK-Hep1 cells. Pan et al. also indicated that amentoflavone inhibits metastatic potential through suppression of ERK/NF-ĸB activation in osteosarcoma cells (8).
In conclusion, this study demonstrated that amentoflavone has the potential to inhibit HCC progression through suppression of ERK activation.
Acknowledgements
This study was supported by grants RD2017-016 and CTU106-P-16 from the National Yang-Ming University Hospital, Yilan and Central-Taiwan University of Science and Technology, Taichung, Taiwan, respectively. This study was supported by Taipei Medical University/Taipei Medical University Hospital (Grant no. TMU105-AE1-B49). The Authors acknowledge the technical services provided by Clinical Medicine Research Laboratory of National Yang-Ming University Hospital and Translational Laboratory, Department of Medical Research, Taipei Medical University Hospital.
References
- 1.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 2.Yang S, Liu G. Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol Lett. 2017;13:1041–1047. doi: 10.3892/ol.2017.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz KJ, Wohlschlaeger J, Lang H, Sotiropoulos GC, Malago M, Steveling K, Reis H, Cicinnati VR, Schmid KW, Baba HA. Activation of the ERK and AKT signalling pathway predicts poor prognosis in hepatocellular carcinoma and ERK activation in cancer tissue is associated with hepatitis C virus infection. J Hepatol. 2008;48:83–90. doi: 10.1016/j.jhep.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Liu YC, Wu RH, Wang WS. Regorafenib diminishes the expression and secretion of angiogenesis and metastasis associated proteins and inhibits cell invasion via NF-ĸB inactivation in SK-Hep1 cells. Oncol Lett. 2017;14:461–467. doi: 10.3892/ol.2017.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai JJ, Pan PJ, Hsu FT. Regorafenib induces extrinsic and intrinsic apoptosis through inhibition of ERK/NF-ĸB activation in hepatocellular carcinoma cells. Oncol Rep. 2017;37:1036–1044. doi: 10.3892/or.2016.5328. [DOI] [PubMed] [Google Scholar]
- 6.Hoesel B, Schmid JA. The complexity of NF-ĸB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu FT, Liu YC, Chiang IT, Liu RS, Wang HE, Lin WJ, Hwang JJ. Sorafenib increases efficacy of vorinostat against human hepatocellular carcinoma through transduction inhibition of vorinostat-induced ERK/NF-ĸB signaling. Int J Oncol. 2014;45:177–188. doi: 10.3892/ijo.2014.2423. [DOI] [PubMed] [Google Scholar]
- 8.Pan PJ, Tsai JJ, Liu YC. Amentoflavone inhibits metastatic potential through suppression of ERK/NF-ĸB activation in osteosarcoma U2OS cells. Anticancer Res. 2017;37:4911–4918. doi: 10.21873/anticanres.11900. [DOI] [PubMed] [Google Scholar]
- 9.Yu S, Yan H, Zhang L, Shan M, Chen P, Ding A, Li SF. A review on the phytochemistry, pharmacology, and pharmacokinetics of amentoflavone, a naturally-occurring biflavonoid. Molecules. 2017;22:pii E299. doi: 10.3390/molecules22020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen WL, Hsieh CL, Chen JH, Huang CS, Chen WT, Kuo YC, Chen CY, Hsu FT. Amentoflavone enhances sorafenib-induced apoptosis through extrinsic and intrinsic pathways in sorafenib-resistant hepatocellular carcinoma SK-Hep1 cells in vitro. Oncol Lett. 2017;14:3229–3234. doi: 10.3892/ol.2017.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma CY, Ji WT, Chueh FS, Yang JS, Chen PY, Yu CC, Chung JG. Butein inhibits the migration and invasion of SK-HEP-1 human hepatocarcinoma cells through suppressing the ERK, JNK, p38, and uPA signaling multiple pathways. J Agric Food Chem. 2011;59:9032–9038. doi: 10.1021/jf202027n. [DOI] [PubMed] [Google Scholar]
- 12.Chen JH, Chen WL, Liu YC. Amentoflavone induces anti-angiogenic and anti-metastatic effects through suppression of NF-ĸB activation in MCF-7 cells. Anticancer Res. 2015;35:6685–6693. [PubMed] [Google Scholar]
- 13.Chiang IT, Liu YC, Hsu FT, Chien YC, Kao CH, Lin WJ, Chung JG, Hwang JJ. Curcumin synergistically enhances the radiosensitivity of human oral squamous cell carcinoma via suppression of radiation-induced NF-ĸB activity. Oncol Rep. 2014;31:1729–1737. doi: 10.3892/or.2014.3009. [DOI] [PubMed] [Google Scholar]
- 14.Wang WH, Chiang IT, Ding K, Chung JG, Lin WJ, Lin SS, Hwang JJ. Curcumin-induced apoptosis in human hepatocellular carcinoma j5 cells: critical role of ca(+2)-dependent pathway. Evid Based Complement Alternat Med. 2012;2012:512907. doi: 10.1155/2012/512907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai KC, Hsu SC, Kuo CL, Ip SW, Yang JS, Hsu YM, Huang HY, Wu SH, Chung JG. Phenethyl isothiocyanate inhibited tumor migration and invasion via suppressing multiple signal transduction pathways in human colon cancer HT29 cells. J Agric Food Chem. 2010;58:11148–11155. doi: 10.1021/jf102384n. [DOI] [PubMed] [Google Scholar]
- 16.Pei JS, Liu CC, Hsu YN, Lin LL, Wang SC, Chung JG, Bau DT, Lin SS. Amentoflavone induces cell-cycle arrest and apoptosis in MCF-7 human breast cancer cells via mitochondria-dependent pathway. In Vivo. 2012;26:963–970. [PubMed] [Google Scholar]
- 17.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 18.Chiang IT, Liu YC, Wang WH, Hsu FT, Chen HW, Lin WJ, Chang WY, Hwang JJ. Sorafenib inhibits TPA-induced MMP-9 and VEGF expression via suppression of ERK/NF-ĸB pathway in hepatocellular carcinoma cells. In Vivo. 2012;26:671–681. [PubMed] [Google Scholar]