Abstract
Background/Aim: The purpose of this study was to compare the clinical behavior of several grades of endometrioid carcinoma (EC) compared to high-grade serous carcinoma (HGSC), based on World Health Organization 2014 criteria. Materials and Methods: Clinicopathological features were compared between all grades of EC and HGSC, and between HGSC and either grade 1/2 or grade 3 EC. Results: Sixty-five patients with EC and 214 with HGSC were identified. Among patients with EC, 56 displayed 1/2 EC and nine had grade 3 EC. The progression-free (PFS) and overall (OS) survival of patients with grade 1/2 EC were better than of those of patients with HGSC; however, PFS and OS did not statistically differ between patients with grade 3 EC and those with HGSC. Grade 1/2 EC, but not grade 3, was a better prognostic factor compared with HGSC. Conclusion: A grading system for EC would be beneficial for the accurate prognosis of ovarian cancer.
Keywords: Ovarian endometrioid carcinoma, ovarian high-grade serous carcinoma, prognosis, World Health Organization 2014 criteria
Epithelial ovarian cancer is the fourth most common cause of death of female patients with cancer in the developed world (1). One of the predictive factors for the prognosis of patients with ovarian cancer is the histological subtype (2). Endometrioid carcinoma (EC) encompasses approximately 10% of all histological ovarian cancer subtypes and is the second most commonly encountered primary ovarian malignancy in developing countries (3,4). EC is associated with high chemosensitivity and a relatively better prognosis than other types of ovarian cancer (5-8).
World Health Organization (WHO) criteria for ovarian carcinomas changed in 2014 (9), and serous ovarian carcinomas were further categorized into low-grade and high-grade serous carcinomas (HGSC). However, the grading system for EC, which is divided into three conventional grades based on morphological features, did not change. To date, no studies have thoroughly examined differences in clinical behavior between EC and HGSC.
Molecular genetic studies have revealed that ovarian cancer can be divided into two categories: Type 1, which generally behaves in an indolent fashion and lacks mutations in the TP53 tumor suppressor, and type 2, which is more aggressive than type I and more than 80% of such cases present TP53 mutations (10). Furthermore, the prognosis of patients with type I is generally better than those with type II ovarian carcinomas. Based on the genetic backgrounds of EC, grade 1 and 2 EC were categorized as type 1, while grade 3 EC was assigned to type 2 tumors (6,11). Although grade 1/2 EC displays genetic backgrounds that differ from those of grade 3, there have been few reports that have investigated the clinical prognosis of EC with respect to each grade. Furthermore, there are no studies that have examined the differences in clinical course between grade 1/2 or 3 EC and HGSC as far as we are aware.
The purpose of our study was to compare the prognosis of patients with EC and HGSC according to the 2014 WHO criteria. We specifically compared the prognosis of patients with grade 1/2 EC, grade 3 EC, and HGSC.
Materials and Methods
This study was approved by the Institutional Review Board of the National Defense Medical College (No. 2530). Among patients with epithelial ovarian cancer treated at our hospital between 1983 and 2013, patients diagnosed with EC and HGSC according to the 2014 WHO criteria were identified and included in this study. Histological subtypes were re-evaluated by two independent observers (M.M. and H.T.), according to the 2014 WHO histologic criteria (9). When discrepancies occurred between observers, immunochemical analysis of the first diagnosis at operation was used as supportive information. Patients with pathologies other than EC and HGSC, those who were diagnosed with other cancers, and those who could not be evaluated due to lack of available surgical specimens were excluded. Clinical characteristics were retrospectively analyzed from the medical charts of the patients.
The patients were classified based on the International Federation of Gynecology and Obstetrics (FIGO) 2014 stage classification (11). Primary therapy included primary debulking surgery alone, primary debulking surgery and adjuvant chemotherapy, neoadjuvant chemotherapy and interval debulking surgery, and chemotherapy alone. Among the patients who only received chemotherapy, those diagnosed with tissue biopsy before chemotherapy were included in our study. Chemotherapy was classified into two categories: taxane- and platinum-based chemotherapy, and conventional platinum-based chemotherapy. The regimen of taxane- and platinum-based chemotherapy comprised of paclitaxel or docetaxel plus carboplatin. Conventional platinum-based chemotherapy included the combination of platinum including cisplatin or carboplatin and other cytotoxic agents such as cyclophosphamide, doxorubicin, and epirubicin.
Residual tumor was evaluated by operational record following primary therapy.The Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (12) was used to evaluate treatment effectiveness. Serum levels of tumor markers, including CA-125, were not used to evaluate response to chemotherapy. Progression-free survival (PFS) was defined as the time from initial diagnosis of the disease to diagnosis of clinical relapse, progression, or death. Overall survival (OS) was defined as the time from initial diagnosis of the disease to death or the date of last follow-up contact.
Statistical analysis was performed using the JMP 12.0.0 software package (SAS Institute, Inc., Tokyo, Japan). Kaplan–Meier survival curves of PFS and OS were compared using the log-rank test. The chi-square test and Mann–Whitney U-test were used to evaluate differences in patient characteristics. Multivariate analyses were conducted using the Cox proportional hazards model. Statistical significance was defined as p<0.05.
Results
After pathological review, 65 patients with EC and 214 patients with HGSC were included in our study. The characteristics of patients with all grades of EC and HGSC are shown in Table I. Patients with EC were younger than those with HGSC (median ages of 52 and 57 years, respectively, p<0.01). Patients with EC in stage I were more frequently observed than those with HGSC (52% and 11%, respectively, p<0.01), and more patients with EC achieved no residual tumor after primary therapy than patients with HGSC (60% and 45%, respectively, p<0.01). During the period of this study, one patient with EC and 15 patients with HGSC rejected primary chemotherapy. Patients with EC less frequently underwent taxane- and platinum-based chemotherapy, in comparison to patients with HGSC (28% and 50%, respectively, p<0.01). There were no statistical differences of best response rates between patients with EC and those with HGSC (84% and 74%, respectively p=0.26).
Table I. Characteristics of patients with all grades of endometrioid carcinoma and high-grade serous carcinoma.
RT, Residual tumor; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; chemotherapy: Taxane + platinum, paclitaxel/docetaxel plus carboplatin; conventional, cyclophosphamide and platinum or cyclophosphamide, doxorubicin, and platinum or epirubicin and platinum. Response was evaluated in the patients with measurable disease who underwent chemotherapy.
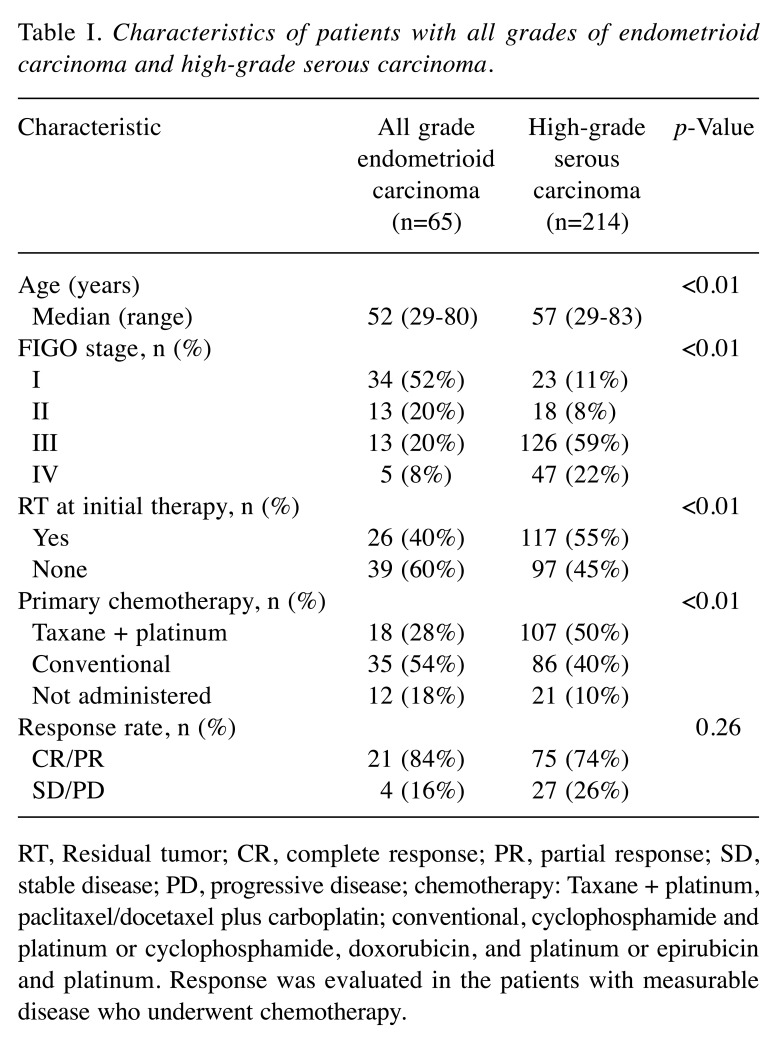
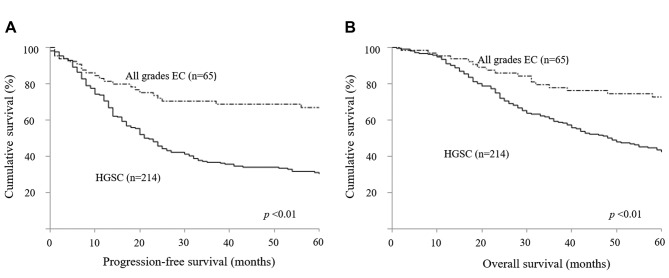
Kaplan–Meier survival curves of PFS and OS are shown in Figure 1. PFS and OS in patients with EC were significantly better than in those with HGSC (p<0.01). In multiple regression analyses between all grades of EC and HGSC, EC was a better prognostic factor for PFS [hazard ratio (hazard ratio)=0.57; p=0.01) and OS (HR=0.54; p<0.01) than HGSC, in addition to FIGO stage and residual tumor (Table II).
Figure 1. Kaplan–Meier analyses of progression-free (A) and overall (B) survival in patients with all grades of endometrioid carcinoma (EC; n=65) and high-grade serous carcinoma (HGSC; n=214).
Table II. Multivariate analysis for progression-free and overall survival in patients with all grades of endometrioid carcinoma (EC) and high-grade serous carcinoma (HGSC).
CI, Confidence interval; taxane + platinum, paclitaxel/docetaxel plus carboplatin; conventional, cyclophosphamide and platinum or cyclophosphamide, doxorubicin, and platinum or epirubicin and platinum.
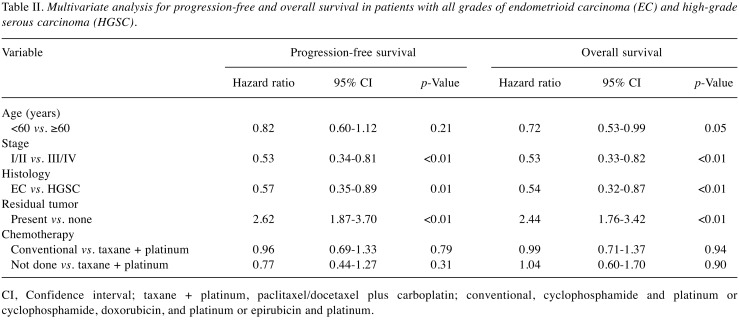
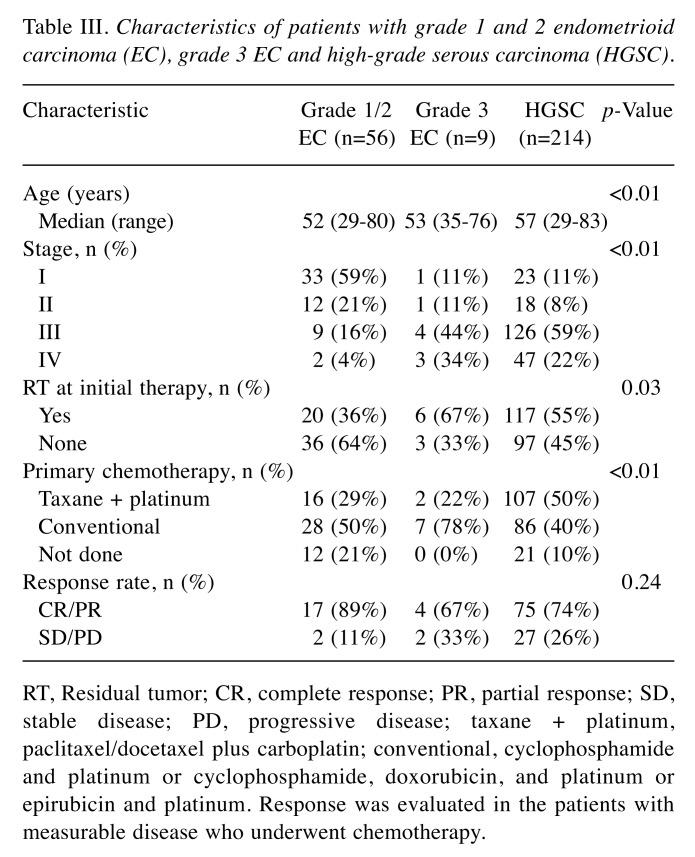
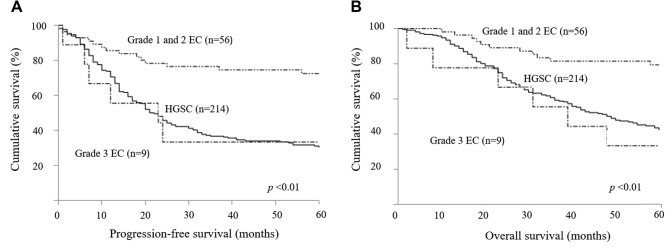
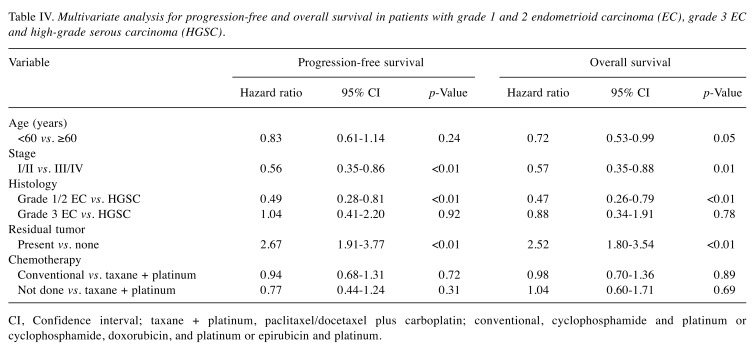
The characteristics of patients with grade 1/2 EC, grade 3 EC, and HGSC are listed in Table III. Among the patients with EC, there were 56 patients with grade 1/2 and nine patients with grade 3. Patients with HGSC were older (p<0.01) and more frequently received taxane- and platinum-based chemotherapy (p<0.01) than those with EC. Patients with grade 1/2 EC were more often diagnosed at earlier stage (p<0.01), and underwent more surgery that resulted in no residual tumor than patients with HGSC and grade 3 EC (p<0.01). There were no statistical significances in best response rate between patients with grade 1/2 EC, grade 3 EC, or HGSC (89%, 67%, and 74%, respectively, p=0.24). PFS and OS were better in patients with grade 1/2 EC than in patients with either grade 3 EC or HGSC (p<0.01) (Figure 2). Multiple regression analyses revealed that grade 1/2 EC was a better prognostic factor of PFS (grade 1/2 EC vs. HGSC, HR=0.49; p<0.01) and OS (grade 1/2 EC vs. HGSC, HR=0.47; p<0.01) than HGSC, but the prognosis of grade 3 EC was equivalent to that of HGSC for PFS (grade 3 EC vs. HGSC, HR=1.04; p=0.92) and OS (grade 3 EC vs. HGSC, HR=0.88; p=0.78). In addition, FIGO stage and residual tumor were prognostic factors for PFS and OS (Table IV).
Table III. Characteristics of patients with grade 1 and 2 endometrioid carcinoma (EC), grade 3 EC and high-grade serous carcinoma (HGSC) .
RT, Residual tumor; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; taxane + platinum, paclitaxel/docetaxel plus carboplatin; conventional, cyclophosphamide and platinum or cyclophosphamide, doxorubicin, and platinum or epirubicin and platinum. Response was evaluated in the patients with measurable disease who underwent chemotherapy.
Figure 2. Kaplan–Meier analyses of progression-free (A) and overall (B) survival of patients with grade 1 and 2 endometrioid carcinoma (EC), grade 3 EC (n=9), and high-grade serous carcinoma (HGSC).
Table IV. Multivariate analysis for progression-free and overall survival in patients with grade 1 and 2 endometrioid carcinoma (EC), grade 3 EC and high-grade serous carcinoma (HGSC).
CI, Confidence interval; taxane + platinum, paclitaxel/docetaxel plus carboplatin; conventional, cyclophosphamide and platinum or cyclophosphamide, doxorubicin, and platinum or epirubicin and platinum.
Discussion
There have been several evaluations of EC prognosis; however, these studies included several different histological grading systems, such as FIGO, Shimizu-Silverberg, and WHO (6,15-18). In 2014, the WHO criteria for EC diagnosis were modified, which divided the classification of serous carcinomas into low-grade serous carcinoma and HGSC. Until now, there have been no studies comparing prognoses between different tumor grades of EC, or between each grade of EC and HGSC, by the 2014 WHO criteria. To our knowledge, this is the first examination of the clinical outcome of EC and HGSC based on the 2014 WHO criteria.
In previous reports, the majority of EC were low-grade tumors and early-staged disease with favorable prognoses (14,15). Similarly, in our study, more cases of EC were low-grade tumors and observed in early stages, and the prognosis of patients with EC was better than of those with HGSC. Thus, EC was a better prognostic factor, even when the diagnosis of EC and HGSC was performed using the 2014 WHO criteria.
Many reports have demonstrated that performance status, FIGO stage, histological subtype, and the volume of residual disease after initial debulking surgery are prognostic factors of PFS and OS (1,2). However, whether Silverberg or FIGO tumor grade are truly prognostic factors remains controversial (19). Our results suggest that tumor grade, defined by the 2014 WHO criteria, has an important impact on the survival of patients with EC. Grade 3 EC presents aggressive tumor activity that is similar to HGSC, while grade 1/2 EC displays milder tumor activity than HGSC.
Such phenotypic observations correlate with the genetic background of the tumor. Recent DNA microarray analyses have shown that high-grade EC and HGSC display similar gene-expression profiles, with significant correlation observed for TP53 (4,7,19,20). This suggests that the histogenetic relationship between high-grade EC and HGSC may derive from a similar histological subtype (4,7,21). Furthermore, the majority of high-grade EC lack β-catenin and KRAS mutations, which are usually detected in low-grade EC. This further suggests that high-grade EC and low-grade EC are biologically distinct tumors (4,7).
Despite this evidence, the differential diagnosis of high-grade EC and HGSC has remained challenging because they frequently present common architectures within their respective solid components, and some pathologists classify high-grade EC as HGSC (4,6). Thus, in our study, when diagnosis differed between observers, immunochemical analysis was used as supportive information, as recommended by the 2014 WHO guidelines. However, the results of our study do not deny the inclusion of misdiagnosis, and in the future, a more accurate diagnosis will need to include a genetic analysis.
Our study is limited by the scope of the analysis, which was performed at a single institution. We investigated a small number of patients, and in particular, few patients presented grade 3 EC. Furthermore, the differential diagnoses between grade 3 EC and HGSC has not been completely resolved; however, because the prognosis of grade 3 EC is as poor as that of HGSC, we suggest that the development of a new strategy for grade 3 EC diagnosis is needed.
In conclusion, patients with EC have a better prognosis than those with HGSC. However, the PFS and OS of patients with grade 3 EC are significantly worse than of those with grade 1/2 EC and is similar to those with HGSC. Thus, the accurate diagnosis of grade 3 EC is of paramount importance, and grade 3 EC is a histogenetically distinct form of EC.
Conflicts of Interest
The Authors declare that they have no conflicts of interest in regard to this study.
Acknowledgements
None.
References
- 1.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 2.Miyamoto M, Takano M, Goto T, Kato M, Sasaki N, Tsuda H, Furuya K. Clear cell histology as a poor prognostic factor for advanced epithelial ovarian cancer: a single institutional case series through central pathologic review. J Gynecol Oncol. 2013;24:37–43. doi: 10.3802/jgo.2013.24.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kline RC, Wharton JT, Atkinson EN, Burke TW, Gershenson DM, Edwards CL. Endometrioid carcinoma of the ovary: retrospective review of 145 cases. Gynecol Oncol. 1990;39:337–346. doi: 10.1016/0090-8258(90)90263-k. [DOI] [PubMed] [Google Scholar]
- 4.Lim D, Murali R, Murray MP, Veras E, Park KJ, Soslow RA. Morphological and immunohistochemical reevaluation of tumors initially diagnosed as ovarian endometrioid carcinoma with emphasis on high-grade tumors. Am J Surg Pathol. 2016;40:302–312. doi: 10.1097/PAS.0000000000000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terada KY, Ahn HJ, Kessel B. Differences in risk for type 1 and type 2 ovarian cancer in a large cancer screening trial. J Gynecol Oncol. 2016;27:e25. doi: 10.3802/jgo.2016.27.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology. 2011;43:420–432. doi: 10.1097/PAT.0b013e328348a6e7. [DOI] [PubMed] [Google Scholar]
- 7.Geyer JT, López-García MA, Sánchez-Estevez C, Sarrió D, Moreno-Bueno G, Franceschetti I, Palacios J, Oliva E. Pathogenetic pathways in ovarian endometrioid adenocarcinoma: a molecular study of 29 cases. Am J Surg Pathol. 2009;33:1157–1163. doi: 10.1097/PAS.0b013e3181a902e1. [DOI] [PubMed] [Google Scholar]
- 8.Nasioudis D, Chapman-Davis E, Witkin SS, Holcomb K. Prognostic significance of lymphadenectomy and prevalence of lymph node metastasis in clinically-apparent stage I endometrioid and mucinous ovarian carcinoma. Gynecol Oncol. 2017;144:414–419. doi: 10.1016/j.ygyno.2016.11.038. [DOI] [PubMed] [Google Scholar]
- 9.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO Classification of Tumours of Female Reproductive Organs, Fourth Edition. Lyon,IACR. 2014 [Google Scholar]
- 10.Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mutch DG, Prat J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol. 2014;133:401–404. doi: 10.1016/j.ygyno.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz LH, Bogaerts J, Ford R, Shankar L, Therasse P, Gwyther S, Eisenhauer EA. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009;45:261–267. doi: 10.1016/j.ejca.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Gurung A, Hung T, Morin J, Gilks CB. Molecular abnormalities in ovarian carcinoma: clinical, morphological and therapeutic correlates. Histopathology. 2013;62:59–70. doi: 10.1111/his.12033. [DOI] [PubMed] [Google Scholar]
- 14.Garzetti GG, Ciavattini A, Goteri G, De Nictolis M, Cignitti M, Tranquilli AL, Romanini C. Endometrioid carcinoma of the ovary. Retrospective study. Eur J Gynaecol Oncol. 1993;14:51–55. [PubMed] [Google Scholar]
- 15.Zwart J, Geisler JP, Geisler HE. Five-year survival in patients with endometrioid carcinoma of the ovary versus those with serous carcinoma. Eur J Gynaecol Oncol. 1998;19:225–228. [PubMed] [Google Scholar]
- 16.Shimizu Y, Kamoi S, Amada S, Hasumi K, Akiyama F, Silverberg SG. Toward the development of a universal grading system for ovarian epithelial carcinoma. I. Prognostic significance of histopathologic features – problems involved in the architectural grading system. Gynecol Oncol. 1998;70:2–12. doi: 10.1006/gyno.1998.5051. [DOI] [PubMed] [Google Scholar]
- 17.Silverberg SG. Histopathologic grading of ovarian carcinoma: a review and proposal. Int J Gynecol Pathol. 2000;19:7–15. doi: 10.1097/00004347-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Zeppernick F, Meinhold-Heerlein I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Arch Gynecol Obstet. 2014;290:839–842. doi: 10.1007/s00404-014-3364-8. [DOI] [PubMed] [Google Scholar]
- 19.Gilks CB, Ionescu DN, Kalloger SE, Köbel M, Irving J, Clarke B, Santos J, Le N, Moravan V, Swenerton K, Cheryl Brown Ovarian Cancer Outcomes Unit of the British Columbia Cancer Agency Tumor cell type can be reproducibly diagnosed and is of independent prognostic significance in patients with maximally debulked ovarian carcinoma. Hum Pathol. 2008;39:1239–1251. doi: 10.1016/j.humpath.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz DR1, Kardia SL, Shedden KA, Kuick R, Michailidis G, Taylor JM, Misek DE, Wu R, Zhai Y, Darrah DM, Reed H, Ellenson LH, Giordano TJ, Fearon ER, Hanash SM, Cho KR. Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Cancer Res. 2002;62:4722–4729. [PubMed] [Google Scholar]
- 21.Madore J, Ren F, Filali-Mouhim A, Sanchez L, Köbel M, Tonin PN, Huntsman D, Provencher DM, Mes-Masson AM. Characterization of the molecular differences between ovarian endometrioid carcinoma and ovarian serous carcinoma. J Pathol. 2010;220:392–400. doi: 10.1002/path.2659. [DOI] [PubMed] [Google Scholar]