Abstract
Background/Aim: During recent years, a survival advantage was reported for first-line treatment of advanced pancreatic cancer with two new regimens, FOLFIRINOX and gemcitabine/nab-paclitaxel, over gemcitabine monotherapy. Gemcitabine/nab-paclitaxel administration on days 1, 8 and 15 of a 4-week cycle is associated with some practical disadvantages. We adopted a biweekly regimen with the same dose density. Patients and Methods: Patients with Eastern Cooperative Oncology Group performance status 0-2 diagnosed with advanced histologically or cytologically confirmed pancreatic cancer and no prior treatment were included in the study. Study combination included 1.5 g/m2 gemcitabine and 175 mg/m2 nab-paclitaxel given every 2 weeks. Survival analysis was performed using the Kaplan–Meier method. Results: Forty-six patients were treated with this regimen. Adverse events were similar to those of the original regimen. Median progression-free and overall survival were 5 and 10 months, respectively. Conclusion: Biweekly gemcitabine/nab-paclitaxel seems to have a similar safety and efficacy profile as the original regimen.
Keywords: Pancreatic cancer, modified regimen, convenient, nabpaclitaxel, gemcitabine, biweekly
Pancreatic cancer is the fourth leading cause of cancer-related death worldwide. Despite progress in the field of oncology, the outcome of patients with metastatic disease remains poor (1,2). The standard chemotherapy regimen was based on gemcitabine (3). This changed recently when a randomized trial demonstrated a survival advantage of FOLFIRINOX (85 mg/m2 oxaliplatin, 180 mg/m2 irinotecan, 400 mg/m2 leucovorin and 400 mg/m2 fluorouracil bolus followed by 2,400 mg/m2 as a 46-hour continuous infusion, every 2 weeks) over gemcitabine monotherapy (4). FOLFIRINOX almost doubled progression-free (PFS) and overall (OS) survival but toxicity was increased. In addition, another randomized phase III trial (MPACT) reported the superiority of the combination of gemcitabine (1,000 mg/m2) and nab-paclitaxel (125 mg/m2) given on days 1, 8 and 15 of a 4-week cycle over gemcitabine monotherapy. The median OS was 8.5 months in the combination group versus 6.7 months in the monotherapy group (5).
However, there are some practical disadvantages associated with a weekly schedule, particularly in patients with poor performance status such as those with pancreatic cancer. On this basis, we have adopted a biweekly regimen of the combination of gemcitabine and nab-paclitaxel with the same dose intensity in the first-line setting. Here, we present the results of our observational study.
Patients and Methods
Patients and study design. The study was approved by the Ethics Committee of the Saint Savvas Anticancer Hospital, Athens, Greece. We performed a retrospective analysis of a prospectively collected database including all new patients with pancreatic cancer admitted to our clinic treated with biweekly gemcitabine in combination with nab-paclitaxel.
Eligibility. Patients with locally advanced/unresectable or metastatic histologically or cytologically proven pancreatic adenocarcinoma were eligible. All patients had to have Eastern Cooperative Oncology Group performance status ≤2, while no previous treatment for pancreatic cancer was allowed.
Treatment schedule. Patients received 175 mg/m2 nab-paclitaxel followed by 1.5 g/m2 gemcitabine on days 1 and 15, repeated every 28 days. The treatment was continued until unacceptable toxicity or evidence of disease progression. Premedication consisted of standard antiemetic drugs. We did not administer primary prophylactic granulocyte-colony stimulating factor.
Assessments. Evaluation was performed every 2 to 4 cycles, according to the treating physician’s plan. Computed tomography of the chest and abdomen was used mainly and in some cases magnetic resonance imaging of the abdomen. Radiologists of the hospital or private practice interpreted the different imaging examinations and compared them to the previous ones.
In addition, blood tests including complete blood cell count, and biochemical tests were performed 1 day before chemotherapy administration. Tumor markers carcinoembryonic antigen and cancer antigen 19.9 (CA-19.9) were tested according to the treating physician’s instructions, approximately every two to three cycles. Patients were seen on the day of chemotherapy, when adverse events were recorded, according to Common Terminology Criteria for Adverse Events, version 4.0.
Statistical considerations. Study endpoints were PFS, OS and toxicity profile. Descriptive statistics were used to evaluate patients’ characteristics and toxicities.
Kaplan–Meier method was applied for PFS and OS calculation. Analysis was performed using SPSS v.24 (IBM Corp., Armonk, NY, USA). PFS was calculated from the starting date of chemotherapy to disease progression or death, whichever occurred first. If the patient did not have an event, they were censored on the date of the last known progression assessment. OS was determined from the starting date of chemotherapy to death. Patients who were still alive were censored on the date of their last known follow-up visit. Data were analyzed by intention-to-treat.
Results
Patients characteristics and treatment. Between 30 May 2014 and 31 August 2017, 46 patients were treated, of whom 26 were male. The median age at diagnosis was 67 years. Table I outlines baseline patient characteristics, including CA-19.9 value, number of metastatic sites and location of the primary tumor. The majority of the patients had metastatic disease. Fifty-six percent of the patients for whom information was available had at least one metastatic site and 19 % of them had at least two. The liver was the most common metastatic site; 44% had only liver metastases.
Table I. Patient baseline characteristics.
CA-19.9: Cancer antigen 19.9; normal range 0-35 U/ml.
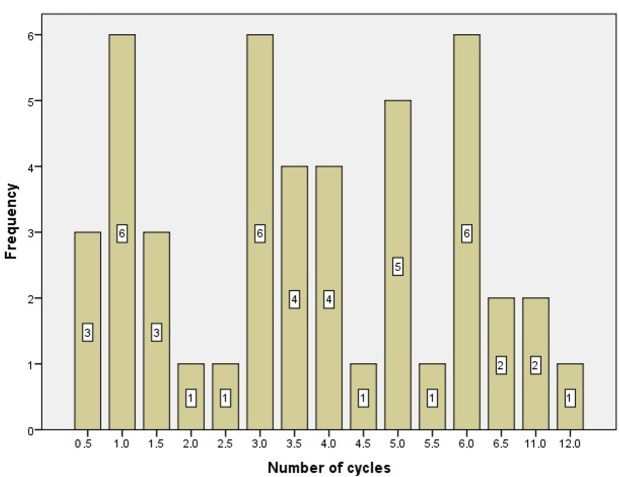
Patients received 0.5 to 12 cycles of gemcitabine/nab-paclitaxel (median of 3.5 cycles). The number of cycles distributed is depicted in Figure 1. A total of 182.5 cycles were administered during the study. Dose modification (reduction or delay) was required for 60% patients due to hematological or other toxicity.
Figure 1. Distribution of the number of cycles of the study treatment administered.
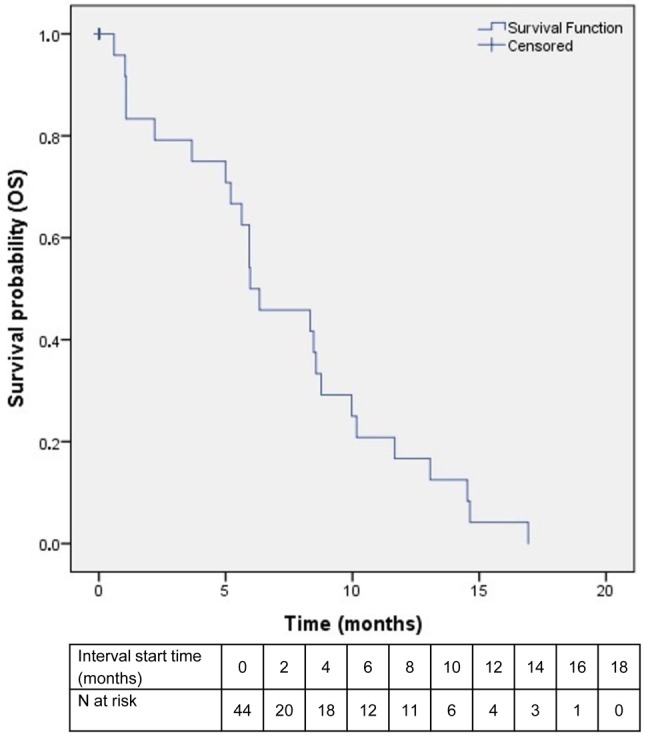
Efficacy. With a median follow-up of 6 (range=1-24) months, 33 events were noted, including 24 deaths and nine cases of disease progression. Of the remaining patients, six were still on treatment at the time of submission and another six had been lost-to-follow-up. Patients who received one cycle or fewer were not included in the survival analysis (N=9) but were analyzed for toxicity. PFS ranged between 1 and 18 months, with a median value of 5 months (Figure 2). OS ranged from 1 to 24 months, with a median value of 10 (Figure 3).
Figure 2. Kaplan–Meier curve of progression-free survival (PFS) among patients in the intention-to-treat population. Only patients who received more than one cycle of study combination were included in the study .
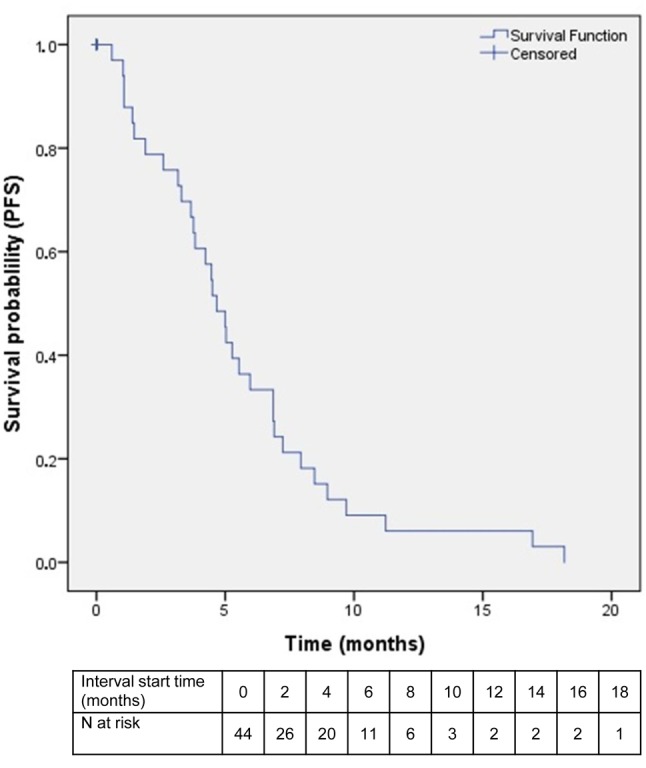
Figure 3. Kaplan–Meier curve of overall survival (OS) among patients in the intention-to-treat population. Only patients who received more than one cycle of study combination were included in the study.
Safety. No toxic death was observed during the study. Adverse events occurred as expected from the drug combination. Grade 3/4 hematological toxicity was observed in 40% of patients, whereas only a very small percentage experienced febrile neutropenia. Ninety percent of patients reported non-hematological toxicity, mainly fatigue, peripheral neuropathy and nausea. Grade 3/4 adverse events in general were observed in 45% of our patients. No case of grade 4 neuropathy was noted.
Discussion
This was a single-center observational trial of an alternative regimen of nab-paclitaxel/gemcitabine combination in patients with advanced pancreatic cancer. The study schedule was initiated by the need for a more convenient and resource-sparing schedule while maintaining the efficacy of the established one. To achieve this, we used a regimen based on the same dose intensity as the weekly protocol and we hypothesized that efficacy was similar. In our study, the median PFS was 5 months, versus 5.5 months in the pivotal study (5). Despite the small number of patients, our results indicate a similar efficacy and safety to the original schedule. Another alternative regimen of the same drug combination was also published recently, with a lower dose density (1,000 mg/m2 gemcitabine and 125 mg/m2 nab-paclitaxel on days 1 and 15), reporting similar efficacy (6). Median PFS and OS were 5.4 and 10 months respectively. The study was criticized however for its retrospective, single-center and less rigorous nature, as well as for differences in patient populations and methodology, not allowing a reliable comparison to the MPACT study (7).
In the phase I/II study of gemcitabine/nab-paclitaxel, dose-limiting toxicities included mainly sepsis and neutropenia (8). Maximum tolerated dose of gemcitabine on days 1, 8 and 15 was established at 1,000 mg/m2, with a 48% response rate. This dose is equivalent to the dose of 1,500 mg/m2 every 2 weeks that we administered. Peripheral neuropathy associated with nab-paclitaxel is another common adverse event that leads mainly to dose reductions. In the MPACT study, 54% of the patients developed peripheral neuropathy, 17% of grade III. It was reported more often after long treatment duration and this is probably why it was associated with a longer survival (9). In our study, we recorded similar rates of this adverse event.
We did not perform any subgroup analysis due to the small number of patients included in our study. In the MPACT study, PS, number and location of metastatic sites and age at diagnosis were associated with survival (10,11). The level of CA-19.9 was not found to be an independent prognostic factor in any of the analyses. It should be noted that in our study there were more older patients compared to the other studies. More than half of our patients were more than 65 years old, with a median age of 67 years, versus 63 years in the MPACT study and 61 years in the FOLFIRINOX study. In addition, we also included many patients with PS 2, approximately 35% of the patients versus only 8% in the MPACT study and none in the FOLFIRINOX study.
We decided to administer the study combination to all eligible patients diagnosed with advanced pancreatic cancer as first-line treatment. Gemcitabine/nab-paclitaxel is an active regimen for pancreatic cancer, as also shown in the neoadjuvant setting (12). However, attempts to combine it with other agents, such as immunotherapy (13) or apatorsen, a heat-shock protein 27 targeting antisense oligonucleotide (14), did not lead to a better response.
A comparison of gemcitabine/nab-paclitaxel to FOLFIRINOX regimen in the first-line setting was attempted in a small retrospective study (15). They concluded that the gemcitabine/nab-paclitaxel combination led to a better efficacy and safety profile. In another retrospective analysis based on a national electronic database in the USA, the two regimens were found to be equally effective, but gemcitabine/nab-paclitaxel was associated with less toxicity. Moreover, the above combination was reported to be more cost-effective (16,17). According to the current guidelines (18), both regimens can be used as first-line treatment for advanced pancreatic carcinoma.
We chose mainly FOLFIRINOX as second-line chemotherapy after progressive disease, if the patient’s PS was compatible, or gemcitabine monotherapy. The use of second-line chemotherapy based on fluoropyrimidines was also supported by an exploratory analysis of the MPACT study, as it conferred a survival benefit versus no second-line treatment (19). Alternatively, gemcitabine/nab-paclitaxel can be used as second-line treatment after FOLFIRINOX, although only a few data are available (20,21).
In conclusion, combination of gemcitabine/nab-paclitaxel every 2 weeks seems to be safe and efficient for advanced pancreatic cancer, leading to reduced resource utilization and facilitation of patients comfort.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Malvezzi M, Carioli G, Bertuccio P, Boffetta P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2017, with focus on lung cancer. Ann Oncol. 2017;28:1117–1123. doi: 10.1093/annonc/mdx033. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul J-L, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet J-B, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, Groupe Tumeurs Digestives of Unicancer , PRODIGE Intergroup FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn DH, Krishna K, Blazer M, Reardon J, Wei L, Wu C, Ciombor KK, Noonan AM, Mikhail S, Bekaii-Saab T. A modified regimen of biweekly gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer is both tolerable and effective: a retrospective analysis. Ther Adv Med Oncol. 2017;9:75–82. doi: 10.1177/1758834016676011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Von Hoff DD, Renschler MF. Letter to the Editor Re: Ahn DH, Krishna K, Blazer M, et al. “A modified regimen of biweekly gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer is both tolerable and effective: a retrospective analysis. Adv Med Oncol. 2017;9:441–443. doi: 10.1177/1758834017699772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, Zhang H, Soon-Shiong P, Shi T, Rajeshkumar NV, Maitra A, Hidalgo M. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein D, Von Hoff DD, Moore M, Greeno E, Tortora G, Ramanathan RK, Macarulla T, Liu H, Pilot R, Ferrara S, Lu B. Development of peripheral neuropathy and its association with survival during treatment with nab-paclitaxel plus gemcitabine for patients with metastatic adenocarcinoma of the pancreas: A subset analysis from a randomised phase III trial (MPACT) Eur J Cancer. 2016;52:85–91. doi: 10.1016/j.ejca.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L, Tortora G, Van Laethem J-L, Young R, Penenberg DN, Lu B, Romano A, Von Hoff DD. Nab-paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107(2):10.1093. doi: 10.1093/jnci/dju413. [DOI] [PubMed] [Google Scholar]
- 11.Tabernero J, Chiorean EG, Infante JR, Hingorani SR, Ganju V, Weekes C, Scheithauer W, Ramanathan RK, Goldstein D, Penenberg DN, Romano A, Ferrara S, Von Hoff DD. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist. 2015;20:143–150. doi: 10.1634/theoncologist.2014-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ielpo B, Caruso R, Duran H, Diaz E, Fabra I, Malavé L, Ferri V, Alvarez R, Cubillo A, Plaza C, Lazzaro S, Kalivaci D, Quijano Y, Vicente E. A comparative study of neoadjuvant treatment with gemcitabine plus nab-paclitaxel versus surgery first for pancreatic adenocarcinoma. Surg Oncol. 2017;26:402–410. doi: 10.1016/j.suronc.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Weiss GJ, Blaydorn L, Beck J, Bornemann-Kolatzki K, Urnovitz H, Schütz E, Khemka V. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs. 2018;36(1):96–102. doi: 10.1007/s10637-017-0525-1. [DOI] [PubMed] [Google Scholar]
- 14.Ko AH, Murphy PB, Peyton JD, Shipley DL, Al-Hazzouri A, Rodriguez FA, Womack MS, Xiong HQ, Waterhouse DM, Tempero MA, Guo S, Lane CM, Earwood C, DeBusk LM, Bendell JC. A randomized, double-blinded, phase II trial of gemcitabine and nab-paclitaxel plus apatorsen or placebo in patients with metastatic pancreatic cancer: The RAINIER trial. Oncologist. 2017;22:1427–e129. doi: 10.1634/theoncologist.2017-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muranaka T, Kuwatani M, Komatsu Y, Sawada K, Nakatsumi H, Kawamoto Y, Yuki S, Kubota Y, Kubo K, Kawahata S, Kawakubo K, Kawakami H, Sakamoto N. Comparison of efficacy and toxicity of FOLFIRINOX and gemcitabine with nab-paclitaxel in unresectable pancreatic cancer. J Gastrointest Oncol. 2017;8:566–571. doi: 10.21037/jgo.2017.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braiteh F, Patel MB, Parisi M, Ni Q, Park S, Faria C. Comparative effectiveness and resource utilization of nab-paclitaxel plus gemcitabine vs. FOLFIRINOX or gemcitabine for the first-line treatment of metastatic pancreatic adenocarcinoma in a US community setting. Cancer Manag Res. 2017;9:141–148. doi: 10.2147/CMAR.S126073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Zhao R, Wen F, Zhang P, Wu Y, Tang R, Chen H, Zhang J, Li Q. Cost-effectiveness analysis of treatments for metastatic pancreatic cancer based on PRODIGE and MPACT trials. Tumori. 2016;2016:294–300. doi: 10.5301/tj.5000499. [DOI] [PubMed] [Google Scholar]
- 18.Ducreux M, Sa Cuhna A, Caramella C, Hollebecque A, Burtin P, Goeré D, Seufferlein T, Haustermans k, Van Laethem JL, Conroy T, Aenold D. Cancer of the Pancreas: ESMO clinical practise guidelines. Ann Oncol Suppl. 2015;5:v56–v68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 19.Chiorean EG, Von Hoff DD, Tabernero J, El-Maraghi R, Ma WW, Reni M, Harris M, Whorf R, Liu H, Li JS, Manax V, Romano A, Lu B, Goldstein D. Second-line therapy after nab-paclitaxel plus gemcitabine or after gemcitabine for patients with metastatic pancreatic cancer. Br J Cancer. 2016;115:188–194. doi: 10.1038/bjc.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen KT, Kalyan A, Beasley HS, Singhi AD, Sun W, Zeh HJ, Normolle D, Bahary N. Gemcitabine/nab-paclitaxel as second-line therapy following FOLFIRINOX in metastatic/ advanced pancreatic cancer-retrospective analysis of response. J Gastrointest Oncol. 2017;8:556–565. doi: 10.21037/jgo.2017.01.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dadi N, Stanley M, Shahda S, O’Neil BH, Sehdev A. Impact of nab-paclitaxel-based second-line chemotherapy in metastatic pancreatic cancer. Anticancer Res. 2017;37:5533–5539. doi: 10.21873/anticanres.11985. [DOI] [PubMed] [Google Scholar]