Abstract
Background/Aim: There exist few research articles regarding the anticancer activity of azulene-related compounds. We investigated here the relative cytotoxicity of 10 azulene amide derivatives against cancer and normal cells. Materials and Methods: Cytotoxicity against four human oral squamous cell carcinoma (OSCC) cell lines and three human oral normal cells (gingival fibroblasts, periodontal ligament fibroblasts and pulp cells) was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide method. Antitumor activity was evaluated by tumor-specificity (TS) (ratio of mean 50% cytotoxic concentration (CC50) against normal cells to that against OSCC cell lines) and potency-selectivity expression (PSE) (ratio of TS to CC50 against tumor cells). Apoptosis-inducing activity was evaluated by cleavage of poly ADP-ribose polymerase and caspase-3 with western blot analysis. Results. N-Propylguaiazulenecarboxamide [1] showed the highest TS and PSE values, compared to that of doxorubicin, and induced apoptosis in two OSCC cell lines. QSAR analysis demonstrated that their tumor-specificity of azulene amide derivatives was correlated with hydrophobicity and molecular shape. Conclusion: Compound [1] can be considered as a lead compound for manufacturing new anticancer drug candidates.
Keywords: Azulene amides, cytotoxicity, tumor specificity, hydrophobicity, molecular shape, apoptosis
Azulene, an isomer of naphthalene, has a dipole moment and a resonance energy with intermediate values between that of benzene and naphthalene, and is considerably more reactive, when compared with two arenes. Azulene gargle has been reported to reduce the incidence of postoperative sore caused by general anesthesia (1). Azulene sulfonate inhibited the capsaicin-induced plasma exudation in the pharyngeal mucosa of the rat (2). 6-Isopropyl-3-[4-(4-chloro-phenylsulfonylamino)butyl]azulene-1-sulfonic acid sodium salt (KT2-962), thromboxane A receptor antagonist, significantly reduced the incidence of ventricular fibrillation during the ischemic period and also myocardial infarct size, possibly by its direct free radical scavenging properties (3). Guaiazulene, a lipophilic azulene derivative, protected rats from paracetamol hepatotoxicity via its antioxidant activity (4) and its inhibitory effect on some cytochrome P450 activities (5). However, there is a limited number of studies that investigated the cytotoxicity of guaiazulene against human leukemic cell lines (HL-60, K562) (6,7), freshly prepared rat neuron cells, neuroblastoma cell lines (8) and human gingival fibroblasts (9).
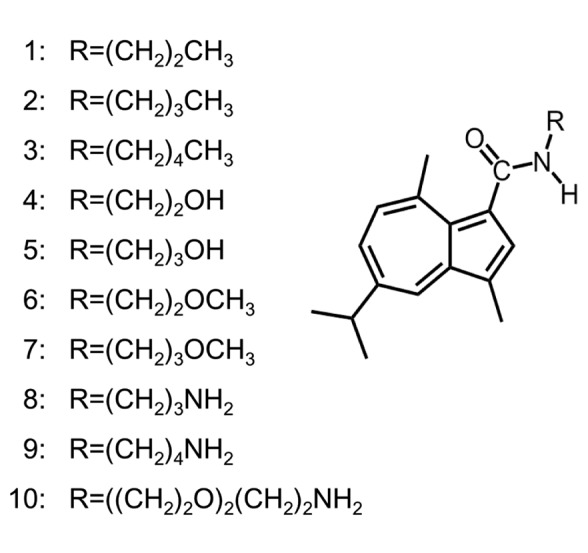
In order to search for tumor-selective guaiazulene derivatives, we have synthesized ten azulene amide derivatives (Figure 1) and investigated their anticancer activity. Since previous reports have shown that anticancer agents induce apoptosis in clinical cancer tissues (10), we also investigated the induction of apoptosis by these compounds.
Figure 1. Structure of azulene amide derivatives .
Materials and Methods
Materials. The following chemicals and reagents were obtained from the indicated companies: Dulbecco’s modified Eagle’s medium (DMEM) from GIBCO BRL, Grand Island, NY, USA; fetal bovine serum (FBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), doxorubicin-HCl (DXR) from Sigma-Aldrich Inc. (St. Louis, MO, USA); dimethyl sulfoxide (DMSO) from Wako Pure Chem. Ind., Osaka, Japan; Culture plastic dishes and plates (96-well) were purchased from Becton Dickinson (Franklin Lakes, NJ, USA). Protease and phosphatase inhibitors were purchased from Roche Diagnostics (Tokyo, Japan). Antibodies against cleaved caspase-3 (Cell Signaling Technology Inc., Beverly, MD, USA), PARP (Cell Signaling Technology Inc., Beverly, MD, USA) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), Trevigen, Gaithersburg, MD, USA) were used as primary antibodies. As secondary antibodies, α-rabbit IgG (DAKO, Tokyo, Japan) antibodies, which were conjugated with horseradish peroxidase, were used.
Synthesis of alklyaminogroups. N-propylguaiazulenecarboxamide [1], N-butylguaiazulenecarboxamide [2], N-pentylguaiazulenecarboxamide [3], N-(2-hydroxyethyl)guaiazulenecarboxamide [4], N-(3-hydroxy-propyl)guaiazulenecarboxamide [5], N-(2-methoxyethyl)guaiazulene-carboxamide [6], N-(3-methoxypropyl)guaiazulenecarboxamide [7], N-(2-aminopropyl)guaiazulenecarboxamide [8], N-(3-aminobutyl)guai- azulenecarboxamide [9], N-(2-(2-aminoethoxy)ethoxyethyl)guai-azulenecarboxamide [10] were synthesized, according to previous reports (11-14). All compounds were dissolved in DMSO at 40 mM and stored at –20˚C before use.
Cell culture. HGF, HPLF and HPC cells, established from the first premolar tooth extracted from the lower jaw of a 12-year-old girl (15) and OSCC cell lines (Ca9-22, HSC-2, HSC-3, HSC-4), purchased from Riken Cell Bank, Tsukuba, Japan, were cultured at 37˚C in DMEM supplemented with 10% heat-inactivated FBS, 100 units/ml, penicillin G and 100 μg/ml streptomycin sulfate under a humidified 5% CO2 atmosphere. HGF, HPLF and HPC at 12~20 population doubling level (PDL) were used in the present study.
Assay for cytotoxic activity. Cells were inoculated at 2×103 cells/0.1 ml in a 96-microwell plate (Becton Dickinson Labware, Franklin Lakes, NJ, USA). After 48 h, the medium was removed by suction with an aspirator and replaced with 0.1 ml of fresh medium containing different concentrations of the test compounds. Control cells were treated with the same amounts of DMSO present in each diluent solution. Cells were incubated for 48 h and the relative viable cell number was then determined by the MTT method. In brief, the treated cells were incubated for another 2 h in fresh culture medium containing 0.2 mg/ml MTT. Cells were then lysed with 0.1 ml of DMSO and the absorbance at 560 nm of the cell lysate was determined using a microplate reader (Infinite F 50 R, TECAN, Kawasaki, Japan). The CC50 was determined from the dose–response curve of triplicate samples.
Calculation of tumor-selectivity index (TS). TS was calculated using the following equation: TS=mean CC50 against three normal oral cells/mean CC50 against OSCC cell lines [(D/B) in Table I] (16). Since both Ca9-22 and HGF cells were derived from the gingival tissue (17), the relative sensitivity of these cells was also compared [(C/A) in Table I]. We did not use human normal oral keratinocytes for tumor-specificity assay, since many anticancer drugs showed potent cytotoxicity against normal keratinocytes by inducing apoptosis (18).
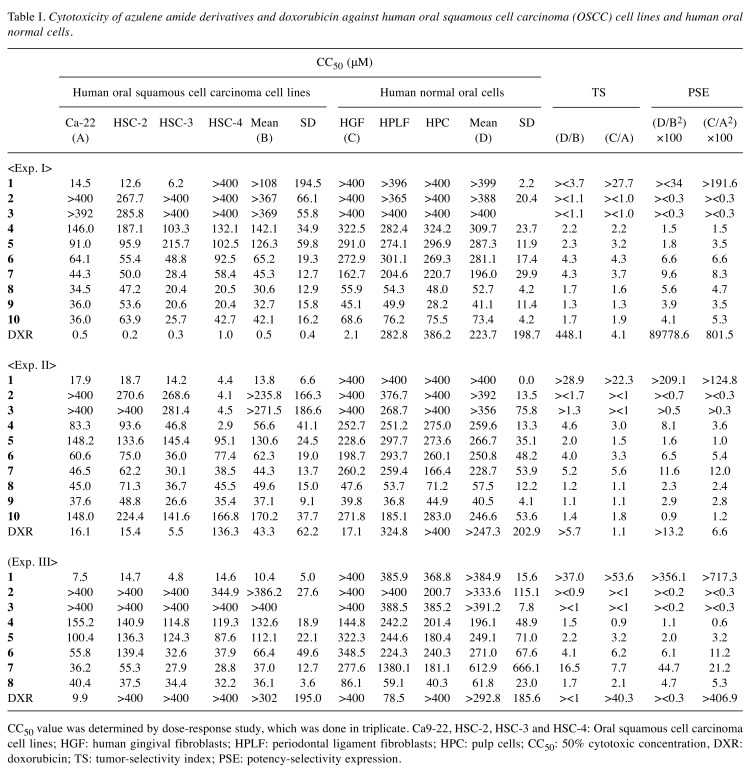
Table I. Cytotoxicity of azulene amide derivatives and doxorubicin against human oral squamous cell carcinoma (OSCC) cell lines and human oral normal cells.
CC50 value was determined by dose-response study, which was done in triplicate. Ca9-22, HSC-2, HSC-3 and HSC-4: Oral squamous cell carcinoma cell lines; HGF: human gingival fibroblasts; HPLF: periodontal ligament fibroblasts; HPC: pulp cells; CC50: 50% cytotoxic concentration, DXR: doxorubicin; TS: tumor-selectivity index; PSE: potency-selectivity expression.
Calculation of potency-selectivity expression (PSE). PSE was calculated using the following equation: PSE=TS/CC50 against tumor cells ×100 (16) [that is, (D/B2) ×100 (HGF, HPLF, HSC vs. Ca9-22, HSC-2, HSC-3, HSC-4) and (C/A2) ×100 (HGF vs. Ca9-22 in Table I).
Western blot analysis. The cells were washed with phosphate-buffered saline (PBS) and re-suspended in 50 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1 mM EDTA, 0.1% sodium dodecyl sulfate (SDS), 0.5% deoxycholic acid, 1% NP-40 and protease inhibitors (RIPA buffer). After ultrasonication using Bioruptor (UCD-250; Cosmo Bio, Tokyo, Japan) for 12.5 min (10 sec on, 20 sec off) at the middle level of output (250 W) at 4˚C, the soluble cellular extracts were recovered after centrifugation for 10 min at 16,000 × g. The protein concentration of each sample was determined using the BCA Protein Assay Reagent Kit (Thermo Fisher Scientific, Waltham, MA, USA) and cell extracts were subjected to western blot (WB) analysis. The blots were probed with the primary antibody followed by a horseradish peroxidase-conjugated secondary antibody. The immune complexes were visualized using Pierce Western Blotting Substrate Plus (Thermo Fisher Scientific). WB results were documented and quantified using ImageQuant LAS 500 (GE Healthcare, Tokyo, Japan) (19).
Calculation of chemical descriptors. Since the CC50 values had a distribution pattern close to a logarithmic normal distribution, we used the pCC50 (i.e., the −log CC50) for the comparison of the cytotoxicity between the compounds. The mean pCC50 values for normal cells and tumor cell lines were defined as N and T, respectively (19).
The 3D-structure of each chemical structure (drawn by Marvin Sketch ver 16, ChemAxon, Budapest, Hungary, http://www. chemaxon.com) was optimized by CORINA Classic (Molecular Networks GmbH, Nürnberg, Germany) with partial charge calculations (amber-10: EHT) in Molecular Operating Environment (MOE) version 2015.1001 (Chemical Computing Group Inc., Quebec, Canada). The number of structural descriptors calculated from MOE after the elimination of overlapped descriptors was 297.
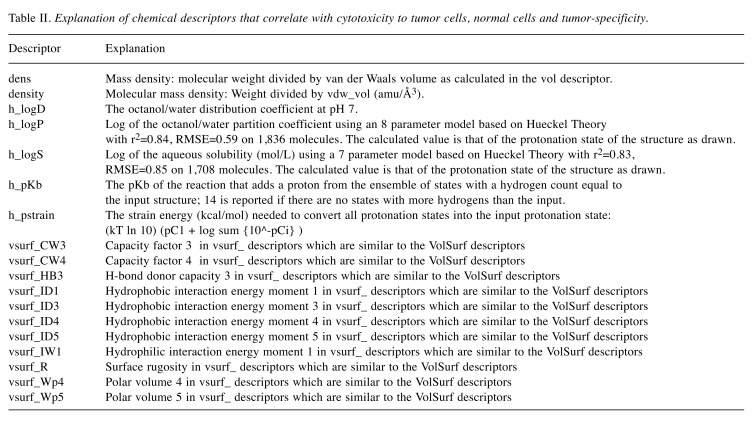
Eighteen MOE descriptors (20) listed in Table II were significantly correlated with T, N and T-N. Since the use of Dragon descriptors produced heavy overlapping of similar descriptors that showed higher correlation with T, N and T-N, and therefore correlation diagrams were plotted using MOE descriptors.
Table II. Explanation of chemical descriptors that correlate with cytotoxicity to tumor cells, normal cells and tumor-specificity.
Statistical treatment. Each experimental value is expressed as the mean±standard deviation (SD) of triplicate or quadruplicate measurements. The relation among cytotoxicity, tumor specificity index and chemical descriptors was investigated using simple regression analyses by JMP Pro version 12.2.0 (SAS Institute Inc., Cary, NC, USA).
Results
Cytotoxicity. Ten azulene amide derivatives used in this study were classified into four categories, having N-alkyl [1-3], hydroxylalkyl [4, 5], methoxyalkyl [6, 7] and aminoalkyl [8~10] groups at the end of carboxamide (Figure 1). We investigated their cytotoxic activity against four human oral squamous cell carcinoma (OSCC) cell lines (Ca9-22, HSC-2, HSC-3, HSC-4) and three normal oral cells (HGF, HPLF, HPC). The results of three independent experiments (each dose-response study was done in triplicate) are shown in Table I.
We first investigated their cytotoxicity against OSCC cell lines. Among N-propyl group, [1] having 2 methylene units showed the highest cytotoxicity (mean CC50=>108, 13.8, 10.4 μM), and with the increase of methylene units to 3 or 4, their cytotoxicity declined (>367, >236, >386 μM; >369, >272, >386 μM), possibly due to the insolubility in culture medium. Hydroxylalkyl [4, 5], methoxyalkyl [6, 7] and aminoalkyl [8~10] groups showed intermediate range of cytotoxicity.
Cytotoxicity of all compounds against normal cells was lower than that against OSCC cell lines.
Tumor-specificity (TS). TS was determined by dividing the mean CC50 value towards three normal cells by the mean CC50 value towards four OSCC cell lines (D/B, Table I). Considering that HGF is the normal cell corresponding to cancer cell Ca9-22 (both derived from gingival tissues), TS value was also generated by dividing the CC50 value towards HGF cells by the CC50 value towards Ca9-22 cells (C/ A, Table I). Among ten compounds [1-10], [1] showed the highest tumor-specificity (TS= ><3.7, >28.9, 37.0 in D/B; >27.7, >22.3, >53.6 in C/A in three independent experiments), followed by [7] (TS= 4.3, 5.2, 16.5 in D/B; 3.7, 5.6, 7.7 in C/A) and [6] (TS=4.3, 4.0, 4.1 in D/B; 4.3, 3.3, 6.2 in C/A). These values are comparable with those of doxorubicin (TS=448.1, >5.7, ><1 in D/B; 4.1, 1.1, >40.3 in C/A). TS values of other compounds were <4.6 in D/B and <3.2 in C/A (Table I).
PSE value. In order to identify the most promising compounds in terms of both good potencies and selectively cytotoxic, the potency-selectivity expression (PSE) values were calculated. [1] showed much higher PSE value [><34, >209.1, >356.1 in (D/B2) ×100; >191.6, >124.8, >717.3 in (C/A2) ×100)], followed by [7] [9.6, 11.6, 44.7 in (D/B2) ×100; 8.3, 12.0, 21.2 in (C/A2) ×100)] and [6] [6.6, 6.5, 6.1 in (D/B2) ×100; 6.6, 5.4, 11.2 in (C/A2) ×100)]. PSE values of other compounds were <8.1 in (D/B2) ×100 and <5.3 in (C/A2) ×100.
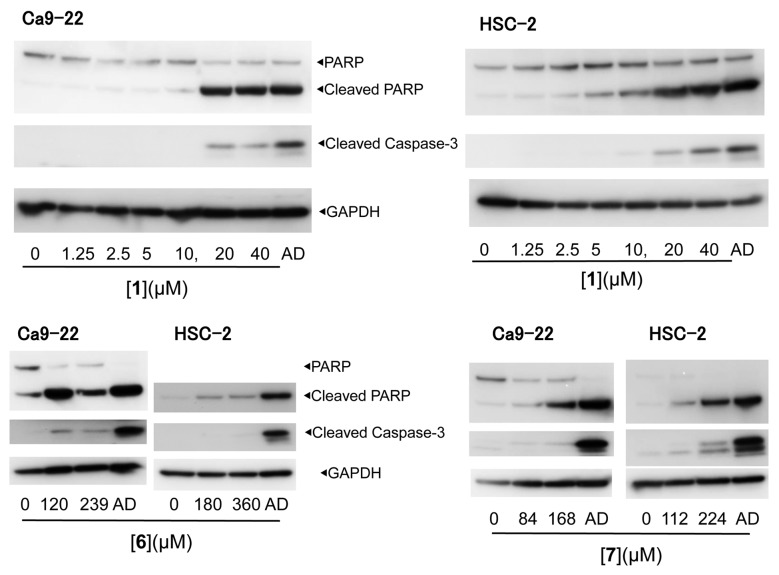
Western blot analysis demonstrated that [1, 6, 7] induced the cleavage of poly ADP-ribose polymerase and caspase-3, suggesting the induction of apoptosis (21) (Figure 2).
Figure 2. Apoptosis induction by [1, 6, 7] in Ca9-22 and HSC-2 human oral squamous cell carcinoma cell lines. AD: Actinomycin D (1 μM).
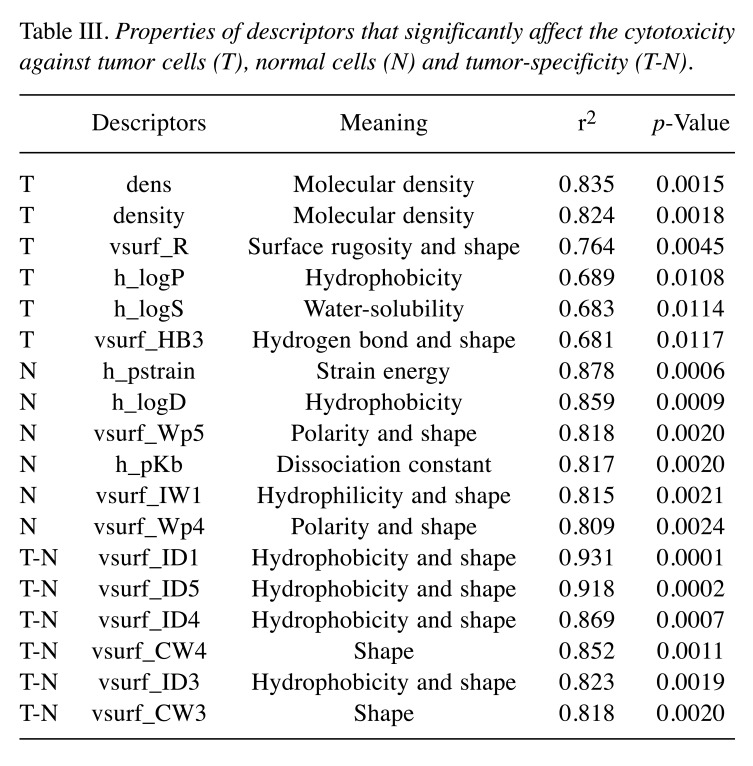
Computational analysis. Since [2] and [3] showed very low cytotoxicity (CC50>400 μM) in most cells, producing noise in QSAR analysis, we performed the QSAR analysis of eight azulene amide derivatives omitting [2] and [3], in regards to their cytotoxicity against tumor cells and normal cells. Among a total of 297 MOE descriptors), 18 descriptors correlated well with cytotoxicity and tumor specificity (Table III).
Table III. Properties of descriptors that significantly affect the cytotoxicity against tumor cells (T), normal cells (N) and tumor-specificity (T-N).
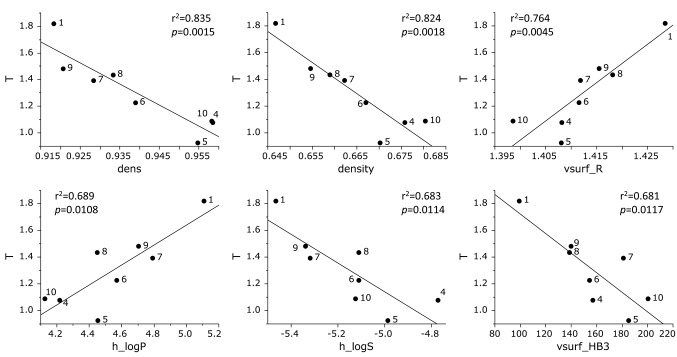
Cytotoxicity against human OSCC cell lines was correlated with dens (Molecular density) (r2=0.835, p=0.0015), density (Molecular density) (r2=0.824, p=0.0018), vsurf_R (Surface rugosity and shape) (r2=0.764, p=0.0045), h_logP (Hydrophobicity) (r2=0.689, p=0.0108), h_logS (Water-solubility) (r2=0.683, p=0.0114), vsurf_HB3 (Hydrogen bond and shape) (r2=0.681, p=0.0117) (Figure 3).
Figure 3. Determination of coefficient between chemical descriptors and cytotoxicity of eight azulene amide derivatives against tumor cells (defined as T). The mean (pCC50 i.e., the −log CC50) values for tumor cell lines were defined as T.
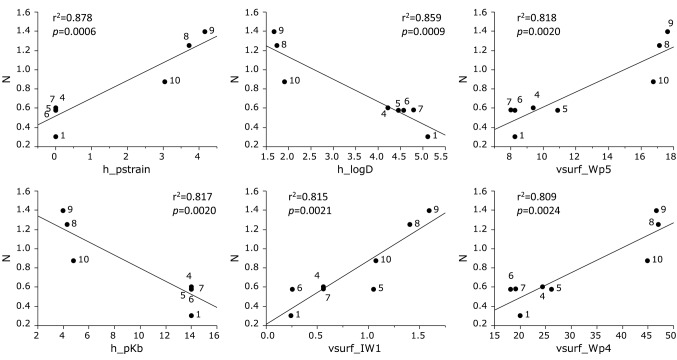
Cytotoxicity against human normal oral mesenchymal cells was correlated with h_pstrain (Strain energy) (r2=0.878, p=0.0006), h_logD (Hydrophobicity) (r2=0.859, p=0.0009), vsurf_Wp5 (Polarity and shape) (r2=0.818, p=0.0020), h_pKb (Dissociation constant) (r2=0.817, p=0.0020), vsurf_IW1 (Hydrophilicity and shape) (r2=0.815, p=0.0021) and vsurf_Wp4 (Polarity and shape) (r2=0.809, R=0.0024) (Figure 4).
Figure 4. Determination of coefficient between chemical descriptors and cytotoxicity of eight azulene amide derivatives against normal cells (defined as N). The mean (pCC50 i.e., the −log CC50) values for normal cells were defined as N.
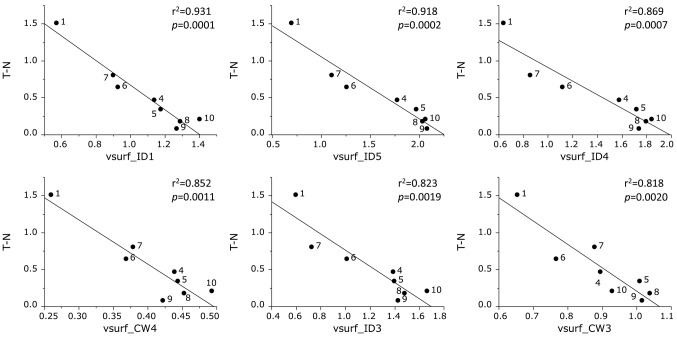
Tumor specificity was correlated with vsurf_ID1 (Hydrophobicity and shape) (r2=0.931, p=0.0001), vsurf_ID5 (Hydrophobicity and shape) (r2=0.918, p=0.0002), vsurf_ID4 (Hydrophobicity and shape) (r2=0.869, p=0.0007), vsurf_CW4 (shape) (r2=0.852, p=0.0011), vsurf_ID3 (Hydrophobicity and shape) (r2=0.823, p=0.0019) and vsurf_CW3 (shape) (r2=0.818, p=0.0020) (Figure 5).
Figure 5. Determination of coefficient between chemical descriptors and tumor specificity of eight azulene amide derivatives (defined as T-N).
Discussion
The present study demonstrated that among ten azulene amide derivatives, N-propylguaiazulenecarboxamide [1] showed the highest in vitro antitumor activity, based on its greatest TS and PSE values and apoptotic-induced activity against two of OSCC cell lines. It was unexpected that [2] and [3], which have longer methylene units, showed much lower cytotoxicity against OSCC cell lines. This may be due to the insolubility of these compounds in the culture medium. By omitting these two compounds, we found a good association of TS value and hydrophobicity. If the solubility of [2] and [3] is improved, TS value of these compounds may be much improved.
QSAR analysis demonstrated that [1] exhibited the highest value of 6 chemical descriptors (hydrophobicity and molecular shape) that correlated with TS value (Figure 5). We also confirmed that [6, 7], that ranked at the second and third position of TS values, also had high scores in these parameters (Figure 5).
On the other hand, [8, 9, 10], having an amino group at the terminal and intermediate score of hydrophobicity (Figure 3), showed the highest cytotoxicity against normal cells (Figure 4) and lowest tumor specificity (Figure 5).
Taken together, the present study suggests that compound [1] can be considered as a lead compound for manufacturing new anticancer drug candidates.
Conflicts of Interest
The Authors confirm that there are no known conflicts of interest associated with this publication and there was no significant financial support for this work that could have influenced its outcome.
Acknowledgements
This work was partially supported by KAKENHI from the Japan Society for the Promotion of Science (JSPS) (16K11519).
References
- 1.Kalil DM, Silvestro LS, Austin PN. Novel preoperative pharmacologic methods of preventing postoperative sore throat due to tracheal intubation. AANA J. 2014;82(3):188–197. [PubMed] [Google Scholar]
- 2.Sakai H, Misawa M. Effect of sodium azulene sulfonate on capsaicin-induced pharyngitis in rats. Basic Clin Pharmacol Toxicol. 2005;96(1):54–59. doi: 10.1111/j.1742-7843.2005.pto960108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge ZD, Auchampach JA, Piper GM, Gross GJ. Comparison of cardioprotective efficacy of two thromboxane A2 receptor antagonists. J Cardiovasc Pharmacol. 2003;41(3):481–488. doi: 10.1097/00005344-200303000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Kourounakis AP, Rekka EA, Kourounakis PN. Antioxidant activity of guaiazulene and protection against paracetamol hepatotoxicity in rats. J Pharm Pharmacol. 1997;49(9):938–942. doi: 10.1111/j.2042-7158.1997.tb06140.x. [DOI] [PubMed] [Google Scholar]
- 5.Kourounakis AP, Rekka EA, Kourounakis PN. Effect of guaiazulene on some cytochrome P450 activities. Implication in the metabolic activation and hepatotoxicity of paracetamol. Arch Pharm (Weinheim) 1997;330(1-2):7–11. doi: 10.1002/ardp.19973300103. [DOI] [PubMed] [Google Scholar]
- 6.Li P, Liu X, Zhu H, Tang X, Shi X, Liu Y, Li G. Unusual Inner-salt guaiazulene alkaloids and bis-sesquiterpene from the South China Sea Gorgonian Muriceides collaris. Sci Rep. 2017;7(1):7697. doi: 10.1038/s41598-017-08100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakabayashi H, Hashiba K, Yokoyama K, Hashimoto K, Kikuchi H, Nishikawa H, Kurihara T, Satoh K, Shioda S, Saito S, Kusano S, Nakashima H, Motohashi N, Sakagami H. Cytotoxic activity of azulenes against human oral tumor cell lines. Anticancer Res. 2003;23(6C):4747–4755. [PubMed] [Google Scholar]
- 8.Togar B, Turkez H, Hacimuftuoglu A, Tatar A, Geyikoglu F. Guaiazulene biochemical activity and cytotoxic and genotoxic effects on rat neuron and N2a neuroblastom cells. J Intercult Ethnopharmacol. 2015;4(1):29–33. doi: 10.5455/jice.20141124062203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiori J, Teti G, Gotti R, Mazzotti G, Falconi M. Cytotoxic activity of guaiazulene on gingival fibroblasts and the influence of light exposure on guaiazulene-induced cell death. Toxicol In Vitro. 2011;25(1):64–72. doi: 10.1016/j.tiv.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Hannun YA. Apoptosis and the dilemma of cancer chemotherapy. Blood. 1997;89(6):1845–1853. [PubMed] [Google Scholar]
- 11.Doukas PH, Speaker TJ. Azulene analogs of pharmacologic agents I: amides. J Pharm Sci. 1971;60:184–189. doi: 10.1002/jps.2600600204. [DOI] [PubMed] [Google Scholar]
- 12.Toyama Y, Miyazawa S, Yokota M. Azulenes, their preparation, and antibacterial agents containing them. Jpn Kokai Tokkyo Koho. 2004 [Google Scholar]
- 13.Anderson AG, Anderson RG, Fujita TS. Displacement reactions on 1-azulylmethyltrimethylammonium iodide. J Org Chem. 1962;27:4535–4539. [Google Scholar]
- 14.Mathias LJ, Overberger CG. Simple syntheses of 1,3-bis(perfluoroacethyl)azulene and 1,3-azulenedicarboxylic acid. J Org Chem. 1980;45:1702–1703. [Google Scholar]
- 15.Kantoh K, Ono M, Nakamura Y, Nakamura Y, Hashimoto K, Sakagami H, Wakabayashi H. Hormetic and anti-radiation effects of tropolone-related compounds. In Vivo. 2010;24:843–852. [PubMed] [Google Scholar]
- 16.Uesawa Y, Sakagami H, Ishihara M, Kagaya H, Kanamoto T, Terakubo S, Nakashima H, Yahagi H, Takao K, Sugita Y. Quantitative structure–cytotoxicity relationship of 3-styryl-2H-chromenes. Anticancer Res. 2015;35:5299–5308. [PubMed] [Google Scholar]
- 17.Horikoshi M, Kimura Y, Nagura H, Ono T, Ito H. A new human cell line derived from human carcinoma of the gingiva. I. Its establishment and morphological studies. Jpn J Oral Maxillofac Surg. 1974;20:100–106. doi: 10.5794/jjoms.20.100. [DOI] [PubMed] [Google Scholar]
- 18.Sakagami H, Okudaira N, Masuda Y, Amano O, Yokose S, Kanda Y, Suguro M, Natori T, Oizumi H, Oizumi T. Induction of apoptosis in human oral keratinocyte by doxorubicin. Anticancer Res. 2017;37(3):1023–1029. doi: 10.21873/anticanres.11412. [DOI] [PubMed] [Google Scholar]
- 19.Tomikoshi Y, Nomura M, Okudaira N, Sakagami H, Wakabayashi H. Enhancement of cytotoxicity of three apoptosis-inducing agents against human oral squamous cell carcinoma cell line by benzoxazinotropone. In Vivo. 2016;30(5):645–650. [PubMed] [Google Scholar]
- 20.Cruciani G, Crivori P, Carrupt P-A, Testa B. Molecular fields in quantitative structure-permeation relationships: the VolSurf Approach. Mol Struct (Theochem) 2000;503:17–30. [Google Scholar]
- 21.Bressenot A, Marchal S, Bezdetnaya L, Garrier J, Guillemin F, Plénat F. Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma. J Histochem Cytochem. 2009;57(4):289–300. doi: 10.1369/jhc.2008.952044. [DOI] [PMC free article] [PubMed] [Google Scholar]