Abstract
Background/Aim: Using drains after breast surgery is a preventive, but invasive measure to reduce seroma formation. A polyurethane-based tissue adhesive (TissuGlu®) might facilitate drainage-free wound healing after mastectomy in a non-invasive manner. Patients and Methods: Retrospectively, data from 84 patients (42 receiving TissuGlu®, 42 receiving a drainage) who underwent mastectomy, were collected (90 days postoperative follow-up). Study endpoints were defined as the number of fluid-related postoperative clinical interventions, cumulative volume of postoperative wound fluid, duration of hospitalization and postoperative complications. Results: In the entirety of postoperative interventions, no significant difference could be demonstrated (p=0.298). The drainage arm showed significantly less seroma aspirations (p=0.024) and complications (p=0.012). A significantly reduced length of hospitalization (p<0.001) and less cumulative wound secretion volume (p<0.001) appeared in the TissuGlu® group. Conclusion: The polyurethane-based tissue adhesive is a less invasive alternative to drain use in mastectomy
Keywords: Breast cancer therapy, mastectomy, seroma formation, postoperative complications, dead space reduction, surgical adhesive, TissuGlu
With a reported incidence ranging from 3% to over 93%, seromas are among the most frequent postoperative complications after breast surgery (1-4). A universally accepted definition of seroma does not exist in the current literature nor is there a consensus on diagnosis or therapy recommendations (4). Many hypotheses exist regarding the pathophysiology of postoperatively formed seromas. They appear to be the result of an inflammatory process induced by surgery combined with leakage from intraoperatively damaged lymphatics (5-8). The accumulation of fluids may lead to a separation of the skin flap from the pectoral muscle or its fascia (9,10), filling the intraoperatively created dead space. A large proportion of postoperatively formed seromas resorb within one month without any intervention (2,11). However, approximately 15% of all seromas become a clinically relevant problem due to pain, infections, necrosis or dehiscence (4,12). These complications often result in recurrent aspirations, re-insertion of drains or surgical wound revisions (4,13). In addition, they may lead to a delay in the healing of the wound, extend the duration of the postoperative inpatient stay and may also delay the initiation of adjuvant therapies (14-17). This leads, not only, to increased wound care expenditure and reduced patient well-being but is also unfavorable from a health-economic viewpoint (2,7,16). Many patient characteristics (age, BMI, nicotine consumption, comorbidities such as diabetes or arterial hypertension), disease-specific (tumor grading or staging, possible nodal involvement, type of tumor) or therapeutic factors (surgical intervention - mastectomy vs. breast conserving surgery or immediate reconstruction after mastectomy, axillary dissection, neoadjuvant chemotherapy, use of anticoagulant drugs such as heparin or antihormonal therapy using tamoxifen) have been investigated regarding their association with seroma formation in numerous studies (10,15,17-20). However, only a few of these factors seem to have a significant effect on seroma production (10). Current therapeutic approaches to decrease postoperatively formed seromas focus on the reduction of the dead space between skin flaps and pectoral muscles resulting after removal of the mammary gland. The methods evaluated for reduction of the dead space are numerous and range from the application of various substrates such as tranexamic acid (21) or fibrin sealants (22,23) to the fixation of the skin flap to the underlying muscle using special surgical suture procedures (24). A more commonly used method, which has been established for decades but is insufficiently supported by evidence, is the application of various drainage systems (10,25,26). The lack of clarity regarding the drainage system, the removal criteria and the number of drains to be used persists in spite of many studies (10). In addition, there is no demonstrated superiority in the use of drains with respect to postoperative seroma formation compared to other methods used to minimize the dead space. Furthermore, the use of drains is associated with pain and discomfort, longer hospital stays and often results in postoperative complications (9). In many studies the effect of use of various surgical instruments (scalpel, electrocautery, ultrasound dissector) (27,28) to open the wound cavity and remove the gland body, the efficacy of postoperatively applied pressure bandages (29) and postoperative immobilization (30) to reduce postoperative seroma formation have been explored. A new possibility of dead space reduction appears to be the use of a polyurethane-based tissue adhesive (TissuGlu® Surgical Adhesive, Cohera Medical, Inc., Pittsburgh, PA, USA). Promising results have been reported in the application after abdominal wall plastic surgery (31,32). In addition, retrospective studies and case reports show a potential of the adhesive for effective dead space reduction and reduced seroma production after mastectomy (33-35).
Patients and Methods
Eighty-four patients who were undergoing treatment in the period from April 2008 to July 2014 due to breast carcinoma in the Breast Center of the University Clinic of Greifswald were included in this retrospective, single-center study. All patients included in the study underwent mastectomy with or without axillary involvement (Sentinel lymph node biopsy (SLNB) or Axillary lymph node dissection (ALND)). Forty-two patients were provided with drainage (control group) as part of the operational procedure (data collection from April 2008 to March 2013). In 42 other women, the intraoperative application of TissuGlu adhesive (test group) (data collection from April 2013 to July 2014) took place instead of drain placement. Patients did not have to meet any special inclusion criteria like demographics or risk factors. Any patient receiving mastectomy with or without axillary involvement was automatically included in this retrospective trial. Perioperatively, all patients received a single - shot prophylactic antibiotic using 1.5 g of unacid (ampicillin/sulbactam).
Operating procedure and postoperative follow-up. The surgical procedure was carried out as per standard of care by experienced surgeons of the breast center of the University Women’s Clinic. After removal of the mammary gland, a redon suction drainage (10CH) was used in the control group. To the extent that lymph nodes were additionally resected within the framework of axillary lymph node dissection (ALND), a further redon drain was placed in the axillary cavity. The use of a second redon drain (axillary) due to extensive resection was also deemed necessary in eight of the 21 patients who received an SLNE in addition to mastectomy and in one of the 18 patients who received a mastectomy only. In the test group, the tissue adhesive was applied with a specially developed applicator in linear droplets. The application was carried out, according to the manufacturer’s instructions, after mastectomy and careful hemostasis on the fascia of the pectoralis major. When axillary procedures were also indicated in patients of the test group, the tissue adhesive was also inserted into the axillary wound cavity. Subsequent wound closure was performed with subcutaneous (2-0 PCL, resorbable) and continuous cutaneous (4-0 monocryl, resorbable) sutures or by surgical staple. A compression dressing was applied and kept for 24 hours in both groups, as per the institution’s standard of care. Postoperatively, wound monitoring and measurements of the drained wound secretion volume were performed within the framework of daily inpatient visits. When the 24 h drainage volume was ≤30 ml, drains were removed. Patients were generally discharged after drain removal. Post-discharge follow-up was performed either in the University of Greifswald, Germany, or in practices of established gynecologists. In addition to the local wound care and assessment of the healing process, a sonographic assessment of the surgical area was also performed. The frequency of outpatient follow-up was determined by frequency and extent of complications such as persistent hematomas or seromas. Indications for seroma aspirations were clinically documented symptoms (pain, redness, swelling) as well as a sonographically measurable distance between skin flap and pectoral fascia of ≥1 cm, caused by the seroma, measured with the patient in a prone position. A renewed inpatient stay as well as surgical wound revisions with subsequent placement of drains were necessary when hematomas or seroma formations were not able to be resolved with conservative measures.
Outcome assessment. The aim of the study was to compare the number and frequency of postoperative wound healing related clinical interventions of both study groups. The most important interventions included the removal or re-insertion of a drain, seroma aspirations, wound revisions and hematoma clearance. Drain removal was considered an “invasive procedure„ in that it was deemed to represent the entire cycle of drain use, including intraoperative insertion and attachment with cutaneous sutures, permanence of the open drain wound and finally removal. In addition, the study included the recording of the volume of collected, drained and/or aspirated wound secretions. The duration of hospitalization, the frequency and type of postoperative complications (hematomas, seromas) and the correlation between the incidence of seromas and patient-specific risk factors (age, BMI, hyptertension, diabetes mellitus, nicotine use) were investigated for both study populations. The retrospective analysis included a 90-day postoperative follow-up. Statistical evaluation was performed using SSPS. Significance measurements for continuous variables were performed using the Mann-Whitney U-test. For discrete variables, significance was evaluated using a chi-square test.
Results
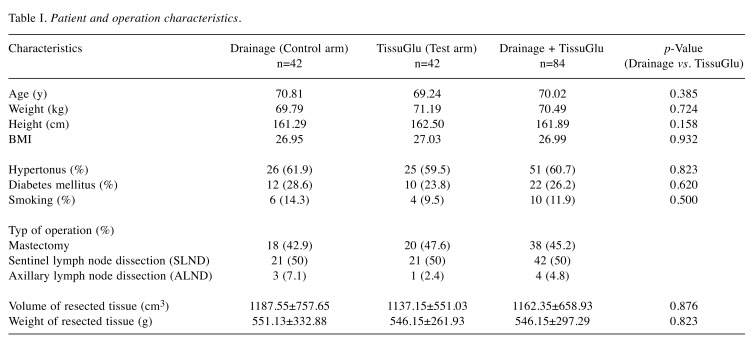
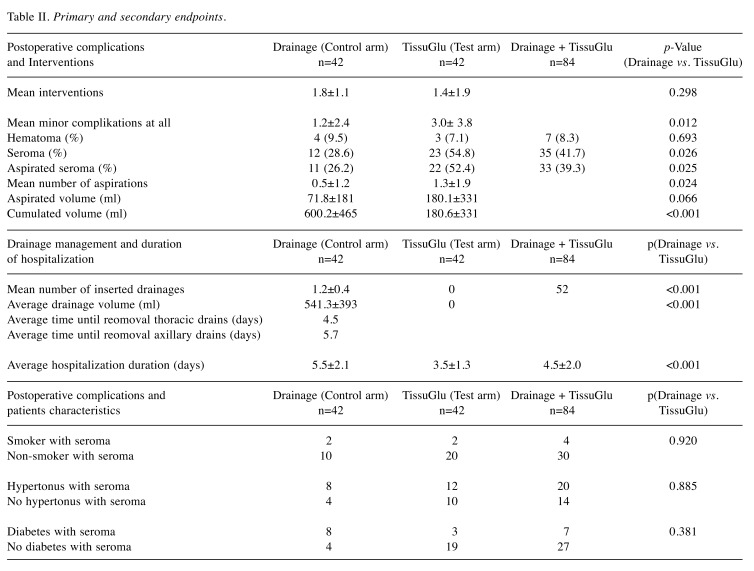
Eighty-four (84) patients were included in the study. Forty-two (42) patients were treated with one or two (with SLNE or ALND) drains (control group), and in 42 other women intraoperative reduction of dead space was performed by application of the TissuGlu® adhesive (test group). The average age of the participating patients for the control group was 70 years (range=46-93 years) and the test group was 69 years (range=46-93 years). No statistically significant difference in age distribution (p=0.385) was found. Similarly, the study participants of both groups did not differ in height (control group 161.2 cm, test group 162.5 cm, p=0.158), weight (control group 69.7 kg, test group 71.1 kg, p=0.724) or BMI (control group 26.9, test group 27.0, p=0.932). In addition, 61.9% of the control group was affected by arterial hypertension vs. 59.5% of the test group (p=0.823). The proportion of diabetics (28.6% vs. 23.8%, p=0.620) and smokers (14.3% vs. 9.5%, p=0.500) was somewhat higher in the control group compared to the test group, but without a significant difference. A simple mastectomy without lymph node dissection was performed in 42.9% (n=18) of women of the control group and 47.6% (n=20) of the women in the test group. In 50% (n=21) of patients in both study groups, a sentinel lymph node biopsy (SLNE) was performed. In the control group, 7.1% (n=3) of the subjects, in the test group 2.4% (n=1) of the women received a complete axillary lymph node clearance (ALNE) in addition to the mastectomy. Statistically significant differences between groups were found neither for the volume of resected tissue (control group 1,187.5 cm3, test group 1,137.1 cm3, p=0.876) nor for its weight (control group 551.1 g, test group 546.1 g, p=0.823). All the patient-specific characteristics recorded are listed in Table I. The number of clinical interventions for fluid management was compared between groups. Fewer overall clinical interventions related to fluid management per patient were observed in the test group compared to the control group (1.4±1.9 vs. 1.8±1.1, p=0.298). While in the control group all patients required a clinical intervention (n=42, 100%) in the test group about half of the patients needed an intervention due to fluid accumulation (n=22, 52.4%). In the control group 12 patients (28.6%) had a clinically relevant fluid accumulation after drain removal, with 11 of these (26.2%) requiring aspirations. In the test group where drains were not used, 23 patients (54.8%) developed a seroma, with 22 of these (52.4%) beeing clinically relevant and therefore receiving an aspiration. The difference of seroma formation (p=0.026) and fluid aspirations (p=0.025) in both groups was statistically significant. Patients in the control group required an average of 0.5 aspirations per patient after drain removal vs. 1.3 in the test group (p=0.024). Further on, aspirated fluid volume was lower in the control group (71.8 ml vs. 180.1 ml, p=0.066). Although, in the test group, a significantly lower mean cumulative wound fluid volume (drainage + aspiration volume) was observed (180±331 ml vs. 600±465 ml, p<0.001).
Table I. Patient and operation characteristics .
The study also investigated relationships between patient-specific risk factors and therapeutic measures. No statistically significant correlations were found between age (p=0.429), body height (p=0.813) or BMI (p=0.056) with regard to seroma formation. However, a significant difference in seroma formation as a function of the body weight could be shown for both groups (p=0.030). The higher the body weight, the more likely the postoperative seroma formation. There was no significant correlation with regard to postoperative seroma formation in the other investigated comorbidities (nicotine abuse (p=0.920), hypertonus (p=0.381) or diabetes mellitus (p=0.885)). The resected tissue of patients who postoperatively developed a seroma was in average significantly higher in weight (651.21±324.12 g vs. 470.10±261.99 g, p=0.008) and in volume (1370.24±703.85 cm3 vs. 1010.56±604.40 cm3, p=0.017) than the tissue of patients without seroma formation. The average weight of resected tissue in this study was 546.15±297.29 g and the average volume was 1162.35±658.93 g.
Other endpoints related to drainage management and hospitalization. An average drainage volume of 541.3±393 ml was determined for the control group vs. 0 ml for the test group. Axillary drains were removed on average after 5.7 days (range=3-12 days). Thoracic (breast) drains were removed on average after 4.5 days (range=2-8 days). A statistically significant reduction in the duration of hospitalization was observed after application of the TissuGlu® tissue adhesive. Patients of the test arm were discharged from hospital after an average of 3.5±1.3 days whereas the mean patient stay for the control group was 5.5±2.1 days, (p<0.001). A second inpatient stay related to persistent hematoma, requiring surgical wound revision and a subsequent drain placement, was necessary in one patient of the test group. None of the patients participating in the study showed signs of local wound infection in the postoperative course. Recorded clinical endpoints are summarized in Table II.
Table II. Primary and secondary endpoints.
Discussion
The results of this retrospective study show the potential of using TissuGlu® for flap adhesion. The implementation of the adhesive does not only lead to an overall less invasive procedure of wound fluid management for mastectomy patients with improved patient comfort but also to earlier discharge. Nevertheless, higher rates of postoperative fluid accumulation with higher seroma volume and the consecutive need for fluid aspiration could be seen in the adhesive group compared to the drain group so that the invasiveness of drain usage is partially compensated by an increased frequency of fluid aspirations in the adhesive group. These results are consistent with and build on previous evidence indicating the potential of flap adhesion with TissuGlu® (32,36). Eichler et al. were the first to publish a report on the use of the adhesive in mastectomy to successfully resolve a recurring seroma in a high-risk patient (34). They then compared a series of 32 mastectomy patients with TissuGlu® use and drains performed in 2013 with a retrospective control group of 173 patients who had standard wound closure. They did not find a significant difference in seroma formation but were able to show a significantly reduced time to drain removal (4.2 days control group vs. 3.5 in the test group, p<0.05), a significantly lower rate of superficial hematoma (17% of the control group versus 3% in the test group, p<0.05) and lower rate of major complications requiring rehospitalization and/or revision (6.9% to 0%, ns), all suggesting that the adhesive had a useful effect in closing the dead space (35). The same group followed up with a small series report exploring the possibility/feasibility of eliminating drain use in mastectomy patients (33). Interest in drain-free plastic surgery procedures has increased in recent years. Hunstad et al. demonstrated that drain-free abdominoplasty is feasible in select patients using TissuGlu® for flap adhesion (31), while others have reported similar results with quilting sutures (24). Use of the adhesive does require attention to intraoperative as well as postoperative factors. Insufficient immobilization of the operative area and inadvertently renewed lifting of the skin flap, for example, during the closure of the wound can result in disruption of the adhesive droplets during the approximately 45 min curing time. In order to ensure adequate effectiveness of the adhesive, light pressure should be applied to the area after application and any movement of the flap during the rest of the closure should be avoided. The application of a compression bandage is recommended before the patient is moved from the operating table. A significantly reduced cumulative volume of wound fluid formed in the test group shows the potential of the adhesive in terms of reduction of dead space. In addition, the increased cumulative volume detected in the control group can be interpreted as a possible consequence of a stimulus which promotes the secretion of the wound by the drain itself. The fact that elimination of drain use can cause up to 40% reduction in the seroma volume formed during the postoperative course, is demonstrated by a study carried out by Taylor et al. (37). Stehbens also pointed out that suction drains may actually suck air into the wound, potentially contributing to seroma formation and delayed wound healing (38). In the present study, an association between reduced postoperative hematoma formation and the use of TissuGlu® was suggested. Similar results were presented by Eichler et al. in their previously cited study (35). While no hemostatic properties for the adhesive are suggested, it could be hypothesized that fixation of the flap and the resulting reduced movement or friction between the tissue planes could explain these observations. Our results indicate that the length of the hospital stay can be significantly reduced by using the tissue adhesive. While earlier return to daily routine such as showering promotes the patient’s sense of well-being, an important but difficult to quantify benefit, an earlier hospital discharge is of measurable relevance to the health economy, possibly leading to an overall reduction of patient-related treatment costs. Regarding possible factors influencing postoperative seroma production, we were able to identify an association between body weight and increasing seroma rates. Other authors have made similar observations (2,15). We also showed that both volume and mass of the removed tissue are predictive factors for postoperative seroma formation. This seems to be due to the direct causality between resected volume and dead space. Similar findings were obtained by Zielinski et al. in a prospective study (20). Baker et al. could not establish a correlation between weight of the resected tissue and seroma formation (9). In contrast to Sforza et al. (39) we could not demonstrate any clear evidence for a correlation between seroma formation and the BMI or the nicotine consumption of the patients. Our results are in agreement with those described by Baker et al. (9). Nor did we find any indications for a correlation between age or height and postoperative seroma formation. Similar conclusions were drawn by Sforza et al. (39). Other studies have shown a correlation (1,20). According to our analyses, comorbidities such as diabetes mellitus or arterial hypertension appear to be unrelated to an increased rate of postoperative seroma formation. Other studies have shown opposite results (20). Like Say et al., we did not find that a pronounced nicotine consumption increases the incidence of postoperative seroma formation but we assume that this relationship was not detectable in our study due to the low number of smokers (40). In addition, we noted that patients with a shorter duration of hospital stay were more likely to require aspirations than is the case with longer hospital stays. This association may be related to the fact that in the context of this study, patients without drains were on average released earlier and also required more aspirations.
In conclusion, in this retrospective study, we were able to show that the tissue adhesive without drain placement has a potential for reducing the volume of the space with a consequent reduction in postoperatively formed wound secretion volume. Some patients without drains however will require aspirations. Clear advantages from the use of the glue result from the reduction of the length of hospital stay. An early release not only promotes the accelerated recovery of patients through more rapid integration into normal everyday life but is also of economic importance. To further evaluate the reduction of postoperative complications and interventions as well as clinical benefit of the glue, prospective studies are encouraged.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Gonzalez EA, Saltzstein EC, Riedner CS, Nelson BK. Seroma formation following breast cancer surgery. Breast J. 2003;9(5):385–388. doi: 10.1046/j.1524-4741.2003.09504.x. [DOI] [PubMed] [Google Scholar]
- 2.Kuroi K, Shimozuma K, Taguchi T, Imai H, Yamashiro H, Ohsumi S, Saito S. Pathophysiology of seroma in breast cancer. Breast Cancer. 2005;12(4):288–293. doi: 10.2325/jbcs.12.288. [DOI] [PubMed] [Google Scholar]
- 3.Loo WT, Chow LW. Factors predicting seroma formation after mastectomy for Chinese breast cancer patients. Indian J Cancer. 2007;44(3):99–103. doi: 10.4103/0019-509x.38940. [DOI] [PubMed] [Google Scholar]
- 4.Sajid MS, Hutson KH, Rapisarda IF, Bonomi R. Fibrin glue instillation under skin flaps to prevent seroma-related morbidity following breast and axillary surgery. Cochrane Database Syst Rev. 2013;5:CD009557. doi: 10.1002/14651858.CD009557.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnema J, Ligtenstein DA, Wiggers T, van Geel AN. The composition of serous fluid after axillary dissection. Eur J Surg. 1999;165(1):9–13. doi: 10.1080/110241599750007441. [DOI] [PubMed] [Google Scholar]
- 6.McCaul JA, Aslaam A, Spooner RJ, Louden I, Cavanagh T, Purushotham AD. Aetiology of seroma formation in patients undergoing surgery for breast cancer. Breast (Edinburgh, Scotland) 2000;9(3):144–148. doi: 10.1054/brst.1999.0126. [DOI] [PubMed] [Google Scholar]
- 7.Tadych K, Donegan WL. Postmastectomy seromas and wound drainage. Surg Gynecol Obstet. 1987;165(6):483–487. [PubMed] [Google Scholar]
- 8.Watt-Boolsen S, Nielsen VB, Jensen J, Bak S. Postmastectomy seroma. A study of the nature and origin of seroma after mastectomy. Dan Med Bull. 1989;36(5):487–489. [PubMed] [Google Scholar]
- 9.Baker E, Piper J. Drainless mastectomy: Is it safe and effective. Surgeon. 2017;15(5):267–271. doi: 10.1016/j.surge.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava V, Basu S, Shukla VK. Seroma formation after breast cancer surgery: what we have learned in the last two decades. J Breast Cancer. 2012;15(4):373–380. doi: 10.4048/jbc.2012.15.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeffrey SS, Goodson WH 3rd, Ikeda DM, Birdwell RL, Bogetz MS. Axillary lymphadenectomy for breast cancer without axillary drainage. Arch Surg. 1995;130(8):909–912. doi: 10.1001/archsurg.1995.01430080111018. [DOI] [PubMed] [Google Scholar]
- 12.Paepke S, Blohmer JU, Ohlinger R, Warm M, Kiechle M. Komplikationen in der Mammachirurgie; Serome. Senologie - Zeitschrift für Mammadiagnostik und -therapie. 2014;11(01):21–25. [Google Scholar]
- 13.Stanczyk M, Grala B, Zwierowicz T, Maruszynski M. Surgical resection for persistent seroma, following modified radical mastectomy. World J Surg Oncol. 2007;5:104. doi: 10.1186/1477-7819-5-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khater A, Elnahas W, Roshdy S, Farouk O, Senbel A, Fathi A, Hamed E, Abdelkhalek M, Ghazy H. Evaluation of the quilting technique for reduction of postmastectomy seroma: a randomized controlled study. Int J Breast Cancer. 2015;2015:287398. doi: 10.1155/2015/287398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Lal B, Misra MC. Post-mastectomy seroma: a new look into the aetiology of an old problem. J R Coll Surg Edinb. 1995;40(5):292–294. [PubMed] [Google Scholar]
- 16.Sanders RP, Goodman NC, Amiss LR Jr., Pierce RA, Moore MM, Marx G, Morgan RF, Spotnitz WD. Effect of fibrinogen and thrombin concentrations on mastectomy seroma prevention. J Surg Res. 1996;61(1):65–70. doi: 10.1006/jsre.1996.0082. [DOI] [PubMed] [Google Scholar]
- 17.Woodworth PA, McBoyle MF, Helmer SD, Beamer RL. Seroma formation after breast cancer surgery: incidence and predicting factors. Am Surg. 2000;66(5):444–450. [PubMed] [Google Scholar]
- 18.Aitken DR, Hunsaker R, James AG. Prevention of seromas following mastectomy and axillary dissection. Surg Gynecol Obstet. 1984;158(4):327–330. [PubMed] [Google Scholar]
- 19.Bryant M, Baum M. Postoperative seroma following mastectomy and axillary dissection. Br J Surg. 1987;74(12):1187. doi: 10.1002/bjs.1800741239. [DOI] [PubMed] [Google Scholar]
- 20.Zielinski J, Jaworski R, Irga N, Kruszewski JW, Jaskiewicz J. Analysis of selected factors influencing seroma formation in breast cancer patients undergoing mastectomy. Arch Med Sci. 2013;9(1):86–92. doi: 10.5114/aoms.2012.29219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oertli D, Laffer U, Haberthuer F, Kreuter U, Harder F. Perioperative and postoperative tranexamic acid reduces the local wound complication rate after surgery for breast cancer. Br J Surg. 1994;81(6):856–859. doi: 10.1002/bjs.1800810621. [DOI] [PubMed] [Google Scholar]
- 22.Jain PK, Sowdi R, Anderson AD, MacFie J. Randomized clinical trial investigating the use of drains and fibrin sealant following surgery for breast cancer. Br J Surg. 2004;91(1):54–60. doi: 10.1002/bjs.4435. [DOI] [PubMed] [Google Scholar]
- 23.van Bastelaar J, Theunissen LL, Snoeijs MG, Beets GL, Vissers YL. Flap fixation using tissue glue or sutures appears to reduce seroma aspiration after mastectomy for breast cancer. Clin Breast Cancer. 2017;17(4):316–321. doi: 10.1016/j.clbc.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Trefoux-Bourdet A, Body G, Jacquet A, Hebert T, Kellal I, Marret H, Ouldamer L. Quilting suture after mastectomy in prevention of postoperative seroma: a prospective observational study. Gynecol Obstet Fertil. 2015;43(3):205–212. doi: 10.1016/j.gyobfe.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal A, Ayantunde AA, Cheung KL. Concepts of seroma formation and prevention in breast cancer surgery. ANZ J Surg. 2006;76(12):1088–1095. doi: 10.1111/j.1445-2197.2006.03949.x. [DOI] [PubMed] [Google Scholar]
- 26.Puttawibul P, Sangthong B, Maipang T, Sampao S, Uttamakul P, Apakupakul N. Mastectomy without drain at pectoral area: a randomized controlled trial. J Med Assoc Thai. 2003;86(4):325–331. [PubMed] [Google Scholar]
- 27.Hashemi E, Kaviani A, Najafi M, Ebrahimi M, Hooshmand H, Montazeri A. Seroma formation after surgery for breast cancer. World J Surg Oncol. 2004;2:44. doi: 10.1186/1477-7819-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter KA, O’Connor S, Rimm E, Lopez M. Electrocautery as a factor in seroma formation following mastectomy. Am J Surg. 1998;176(1):8–11. doi: 10.1016/s0002-9610(98)00093-2. [DOI] [PubMed] [Google Scholar]
- 29.Chen CY, Hoe AL, Wong CY. The effect of a pressure garment on post-surgical drainage and seroma formation in breast cancer patients. Singapore Med J. 1998;39(9):412–415. [PubMed] [Google Scholar]
- 30.Shamley DR, Barker K, Simonite V, Beardshaw A. Delayed versus immediate exercises following surgery for breast cancer: a systematic review. Breast Cancer Res Treat. 2005;90(3):263–271. doi: 10.1007/s10549-004-4727-9. [DOI] [PubMed] [Google Scholar]
- 31.Hunstad JP, Michaels J, Burns AJ, Slezak S, Stevens WG, Clower DM, Rubin JP. A prospective, randomized, multicenter trial assessing a novel lysine-derived urethane adhesive in a large flap surgical procedure without drains. Aesthetic Plast Surg. 2015;39(4):616–624. doi: 10.1007/s00266-015-0498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walgenbach KJ, Bannasch H, Kalthoff S, Rubin JP. Randomized, prospective study of TissuGlu(R) surgical adhesive in the management of wound drainage following abdominoplasty. Aesthetic Plast Surg. 2012;36(3):491–496. doi: 10.1007/s00266-011-9844-3. [DOI] [PubMed] [Google Scholar]
- 33.Eichler C, Dahdouh F, Fischer P, Warm M. No-drain mastectomy - Preventing seroma using TissuGlu((R)): A small case series. Ann Med Surg (Lond) 2014;3(3):82–84. doi: 10.1016/j.amsu.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eichler C, Dahdouh F, Sauerwald A, Warm M. Seroma suppression using TissuGlu(R) in a high-risk patient post-mastectomy: a case report. J Med Case Rep. 2013;7:138. doi: 10.1186/1752-1947-7-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eichler C, Fischer P, Sauerwald A, Dahdouh F, Warm M. Flap adhesion and effect on postoperative complication rates using Tissuglu(R) in mastectomy patients. Breast Cancer. 2016;23(3):486–490. doi: 10.1007/s12282-015-0591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilbert TW, Badylak SF, Beckman EJ, Clower DM, Rubin JP. Prevention of seroma formation with TissuGlu(R) surgical adhesive in a canine abdominoplasty model: long term clinical and histologic studies. J Plast Reconstr Aesthet Surg. 2013;66(3):414–422. doi: 10.1016/j.bjps.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 37.Taylor JC, Rai S, Hoar F, Brown H, Vishwanath L. Breast cancer surgery without suction drainage: the impact of adopting a ‘no drains’ policy on symptomatic seroma formation rates. Eur J Surg Oncol. 2013;39(4):334–338. doi: 10.1016/j.ejso.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Stehbens WE. Postmastectomy serous drainage and seroma: probable pathogenesis and prevention. ANZ J Surg. 2003;73(11):877–880. doi: 10.1046/j.1445-2197.2003.02832.x. [DOI] [PubMed] [Google Scholar]
- 39.Sforza M, Husein R, Atkinson C, Zaccheddu R. Unraveling factors influencing early seroma formation in breast augmentation surgery. Aesthet Surg J. 2017;37(3):301–307. doi: 10.1093/asj/sjw196. [DOI] [PubMed] [Google Scholar]
- 40.Say CC, Donegan W. A biostatistical evaluation of complications from mastectomy. Surg Gynecol Obstet. 1974;138(3):370–376. [PubMed] [Google Scholar]