Abstract
The endocrine control mechanisms for female mammalian aggression have been largely unstudied. Although it has been proposed that androgens may modulate female aggressive behavior in a similar manner to males, very little conclusive evidence exists. Previous work in male marmosets found that post-encounter increases in testosterone (T) were dependent on the intensity of aggression displayed during the aggressive encounter. We exposed female marmosets (Callithrix kuhlii), a monogamous and biparental primate, to aggressive interactions with unfamiliar intruders. Individual female marmosets exhibited changes in T and estradiol (E2) that are associated with aggressiveness dependent on the intensity of aggression displayed as well as their role during the encounter. Resident females exhibited increased E2 immediately following an encounter in which they displayed high rates of aggression. If resident females received high rates of aggression from the intruder, the resident displayed increased T 24 hr following the encounter. Interestingly, if the female was an intruder in the encounter, the intensity of her aggression was associated with increased cortisol immediately following the trials, whereas received aggression was associated with increased T and E2 immediately following the trial. Female primates do exhibit situation-dependent changes in gonadal steroids in association with aggression that may serve to prime them for future aggressive interactions.
Keywords: challenge hypothesis, neuroendocrine, aggression, intruder challenge
INTRODUCTION
In many species of birds, reptiles, and mammals, both males and females engage in aggressive behavior to protect territory, resources, mates, or offspring [see review Stockley & Bro-Jorgensen, 2011]. The examination of the neuroendocrine modulations associated with aggression has focused on males and testosterone (T), whereas females have been left understudied. The impetus for this focus has been the development and testing of the Challenge Hypothesis proposed by Wingfield et al. [1990]. The Challenge Hypothesis predicts variation in circulating concentrations of T in association with aggressive behaviors of male animals relative to their social and parental status, in light of the higher costs associated with maintaining T on immune function and parental care behaviors [Hasselquist et al., 1999; Peters, 2000; Wingfield et al., 1987]. Specifically, males from polygynous species that are highly engaged in territorial protection and mate acquisition, and spend very little time engaged in parental care, are predicted to consistently show high concentrations of circulating androgens, including T throughout the breeding season [Johnsen, 1998; Wingfield et al., 1990; see review in Gleason et al., 2009]. On the other hand, males in monogamous social systems, which are more invested in paternal care, are expected to only show increases in T when engaged in aggressive behaviors, with T quickly returning to baseline shortly after the interaction, thus lessening the impact on paternal care. Evidence in support of the Challenge Hypothesis has been described for many species of lizards, birds, and mammals, including nonhuman primates [Cavigella & Pereira, 2000; Creel et al., 1993; Johnsen, 1998; Ross et al., 2004; Wingfield et al., 1987, 1990].
In some species, females can be just as aggressive as males during intraspecific encounters [Elekonich & Wingfield, 2000; Mayer & Rosenblatt, 1987; Stockley & Bro-Jorgensen, 2011; Vom Saal et al., 1995]. As is the case in males, there are a number of negative impacts associated with increased concentrations of androgens over time for females, including decreased maternal care behavior, reduced fecundity, and lowered immunity [Gerber et al., 2010; O’Neal et al., 2008; Rutkowska et al., 2005; Zysling et al., 2006]. In males, T is the main focus of study as the dominant acting gonadal steroid, whereas in females a number of hormones may play a role in behavioral actions including T, estradiol (E2), progesterone (P4), cortisol (CORT), and oxytocin (OT).
Female aggression, specifically associated with late pregnancy status and postpartum protection of pups (i.e. maternal aggression), has been associated with abrupt increases in E2 and decreases in P4 for rats [Leopoldo de Sousa et al., 2010; Mayer & Rosenblatt, 1987]. The endocrine changes in rats are in direct contrast to the evidence from mice in which maternal aggression is associated with increased P4 and decreased E2 [Svare et al., 1986]. Maternal aggression has repeatedly been associated with increased circulating and brain concentrations of OT in a number of mammals [see review, Campbell, 2007]. Finally, the HPA axis and circulating concentrations of CORT and its correlates have been associated with maternal aggressive interactions, as both a response to stress [Castro & Matt, 1997; Mendoza & Mason, 1986; Smith & French, 1997a] and a priming agent for the actions of OT [Trainor et al., 2010].
The few studies that have been published examining female aggression and hormonal responses not associated with a maternal role of the female in lizards, birds, and mammals have had contradictory results. Studies on birds have found that steroid hormones, such as T and E2, are not associated with aggressive interactions of females [Canoine & Gwinner, 2005; Elekonich & Wingfield, 2000; Zysling et al., 2006]. Female–male aggression in iguanas was associated with increased T during the mating season and female–female aggression in the iguana was associated with increased E2 and P4 [Rubenstein & Wikelski, 2005]. Increased E2 was also associated with female–female aggression in mountain spiny lizards [Woodley et al., 2000]. Work in Siberian hamsters found no association between gonadal steroid hormones and female aggression [Scotti et al., 2007], neither did a study of California mice [Davis & Marler, 2003]. However, female bank voles treated with exogenous T and E2 showed elevated levels of aggression [Kapusta, 1998]. Increased T and aggression have been associated with dominance rankings in wild female baboons [Beehner et al., 2005], but no further studies have examined nonhuman primate responses to aggressive encounters and the neuroendocrinological correlates.
Callitrichines are small New World primates that are cooperative breeders and tend to be socially monogamous. Infant care and survival are highly dependent on paternal care, with males being the primary carrier of the infants from 2 weeks of age to weaning [Digby, 1995a; Goldizen, 1987; Price, 1992]. T levels in male Callithrix kuhlii are lowest in males during the period of maximal infant care, higher in males without parental experience, and higher in males that have lower rates of infant care [Nunes et al., 2000, 2001, 2002]. Previous behavioral studies have revealed varying responses to intruders associated with both the gender and the age of the intruder. Male black tufted ear marmosets (C. kuhlii) respond more aggressively to adult male intruders than either juvenile male or female intruders. Additionally, resident males have increased T 2–6 hr and 24 hr following encounters with adult male intruders during which the resident male exhibited aggressive behavior [Ross et al., 2004]. The magnitude of the hormonal response was positively associated with the intensity of the aggression during the male–male encounter.
Aggression of female callitrichids during intruder encounters varies by species. Female–female encounters in golden lion tamarins (Leontopithecus rosalia) were characterized by highly aggressive interactions, including high rates of physical attacks and chases [French & Inglett, 1989]. Cotton-top tamarins (Saguinus oedipus), common marmosets (Callithrix jacchus), saddleback tamarins (Saguinus fuscicollis), red bellied tamarins (Saguinus labiatus), and black tufted ear marmosets (C. kuhlii) all show higher rates of aggression during female–female encounters than female–male encounters; but this aggression is often limited to low intensity noncontact displays, including scent marking, genital displays, and tongue flicking. This variability in response has been attributed to species differences in the mechanisms of reproductive inhibition, with those species that exhibit low intensity agonism often being species in which subordinate females are reproductively suppressed [French et al., 1995].
We used an intruder paradigm to test the behavioral and endocrine responses of female C. kuhlii to encounters with unfamiliar conspecifics. Resident females were exposed to encounters with adult and juvenile intruders of both sexes. We expected the intensity of agonistic displays to be greatest in resident female–adult female intruder encounters. If the intruder elicits an aggressive response from the resident female, and female marmoset aggression is governed by similar mechanisms as male marmoset aggression, then we predicted that exposure to a female intruder would elicit high rates of aggressive and agonistic displays that are associated with increases in T immediately following the encounter. Additionally, as T is quickly aromatized to E2 in females of many mammalian species [Callard et al., 1978], we predicted that circulating T and E2 would be increased 24 hr following the encounter. Because the reaction to the encounter may differ for the resident and the intruder female, we also monitored the intruder’s behavioral and endocrine responses to these trials. Finally, because agonistic encounters may serve as potent stressors, we monitored changes in CORT following the encounter for both the resident and intruder females.
METHODS
Subjects
Five pairs of Wied’s black tufted ear marmosets (C. kuhlii), consisting of an adult breeding male and an adult breeding female, served as resident subjects for the study. The animals were housed at the University of Nebraska at Omaha Callitrichid Research Facility. The animals were maintained in pairs and small family groups in cages measuring at least 1.2 × 0.9 × 2.4 m. The cages contained natural branches, a nest tube, a feeding platform, and enrichment devices. Visual access between groups was limited, but olfactory and auditory contact was available with two to three other family groups. All protocols were approved by the University of Nebraska at Omaha Institutional Animal Care Committee and adhered to the American Society of Primatologists principles for the ethical treatment of nonhuman primates. For further details of animal husbandry and housing, see Schaffner et al. [1995].
Twenty marmosets were used as intruder subjects in our observations. Thirteen marmosets (six males and seven females) were adult intruders (424 months) and were breeding adults in their own social groups. Seven intruders (four males and three females) served as juvenile intruders. Juveniles were between the ages of 6 months and 2 years of age, and resided in a social group that contained older adult parents.
Procedure
To protect the intruder from physical injury during the encounters, they were placed in an intruder cage (60 × 40 × 40 cm) constructed of wire mesh. The intruder cage eliminated the possibility of physical contact between residents and intruders while still providing good visual contact between interacting marmosets. All residents were exposed and habituated to the intruder cage before any testing began, by placing the empty intruder cage in each home cage for three 8 hr time periods before the first test. We did not test groups in which the female was in the third trimester of pregnancy or had nursing infants. Post hoc analysis of the female’s reproductive status during testing based upon inter-birth intervals found that all females were either nonpregnant or in the first and second month of pregnancy during the trials. The resident pair and intruder were first observed during a 10-min pretrial period. The intruders were removed from their home cage by coaxing them into a small transport cage attached to their home cage. From the transport cage, the animals were transferred to the intruder cage. After transfer, intruders were allowed to acclimate to the cage for 5 min in an empty room. After this period, the intruder was placed on the floor in the home cage of the resident pair to be tested.
Two observers recorded behaviors during the intruder encounter; one observer focused on the behavior of the intruder and the resident of the same sex, and one recorded the behavior of the other resident. Interobserver reliability was at least 90% for all trials, as determined by comparing the scores of the social interactions between resident partners. Observations occurred at 20 second intervals for 30 min, and were recorded using Observer 3.0s on a laptop computer. Patterns of agonistic and aggressive behavior toward the intruder, affiliative and sexual behavior within the resident pair, and general activity of both the resident and intruder were recorded during each observation, as described in Ross et al. [2004]; see Table I. Following the intruder trial, the intruder was released back into its home cage and the behavior of both residents and intruder was monitored for an additional 10 min. All intruder encounter trials started between 08:00 and 09:00 hr.
TABLE I.
Definitions of Behaviors Collected During the Intruder Trials for Both the Residents and Intruders
| Behavior | Method | Description |
|---|---|---|
| State of resident | ||
| Moving | I | Focal animal travels to a new position |
| Rest | I | Focal animal is not traveling |
| Feeding | I | Focal animal is consuming food or water |
| Grooming | I | Focal animal is scratching/cleaning body |
| Playing | I | Focal animal is in apparent play behavior alone or with others. Usually includes chase behaviors |
| Social interactions | ||
| Allogrooming | I | Grooming or being groomed |
| Contact | I | Resident pair in physical contact |
| Near | I | Focal animals is within 10 cm of mate |
| Copulating | A | Focal animal engaged in mating behavior |
| Proximity to cage | ||
| Near | I | Focal animal is within 10 cm of intruder cage |
| Aggression | ||
| Erh-erh | A | Gutteral vocalization accompanied by attack |
| Long call | A | Contact call, long in duration and high in pitch |
| A | Multisyllable vocalization | |
| Scent marking | A | Genital rub on surfaces |
| Genital display | A | Exposing genital area by lifting tail |
| Attack | A | Focal animal bites intruder cage and chases |
| Piloerection | A | Focal animal’s hair is extended from body |
| Intruder demeanor | ||
| Neutral/attentive | I | Intruder follows movement makes no response |
| Neutral/nonattentive | I | Intruder is nonresponsive |
| Submissive | I | Intruder cowering |
| Agonistic | I | Intruder actively attacks and chases resident |
Method of data collection was either instantaneous (I) or all occurrences (A) (as defined in French et al., 1995; Ross et al., 2004; Schaffner & French, 1997).
Each resident pair was presented with two different adult males, two adult females, and one female and one male juvenile in a randomized order. Three weeks separated all trials with an animal, whether the animal served as a resident or an intruder. Additionally, at least 2 days separated all trials in the colony in order to prevent a general disturbance to the colony. Control trials were randomly scheduled among experimental trials while leaving 3 weeks between each use of an animal. A control resident trial was conducted by placing an empty intruder cage in the resident cage and observations were performed in the same manner as described above. After the 30 min trial period, the intruder cage was removed from the room and a 10- min post-observation was conducted. A control intruder trial was conducted by placing the intruder animal in the intruder cage and then placing the intruder cage in a novel empty cage the size of a standard family housing. This trial was used to control for nonencounter-related handling effects. Observations were made as described for the experimental trial. The animal was then released into its home cage and observed. These trials served as control for both behavioral responses to novel situations, as well as a control for the daily circadian rhythms of hormones to be assayed.
Before testing (06:00–08:00 hr), urine was collected noninvasively from all resident and intruder subjects, using the procedures previously outlined in French et al. [1996]. Urine was collected every 2 hr following the trial from each of the animals until 17:00 hr and then again the following morning. All samples were centrifuged at 7,000 rpm for 2 min to remove debris and the supernatant was transferred to a clean minivial. The samples were then stored at −201C until the assays were performed.
Hormone Analyses
CORT concentrations were measured in all urine samples using an enzyme immunoassay developed and validated for use in C. kuhlii, as previously described in Smith and French [1997a,b]. Recovery of all standards (range 1.95–1,000 pg) added to quality control pools was 10172%. The intra-assay coefficients of variation for medium and low concentration pools were 4.46 and 3.47%, respectively (n 5 20). The interassay coefficients of variation for the medium and low concentration pools were 14.29 and 17.75%, respectively (n 5 20).
T and E2 concentrations were measured in samples using an enzyme immunoassay adapted for C. kuhlii, as outlined in Nunes et al. [2000] and Fite and French [2000], respectively. Briefly, samples were hydrolyzed with 20 ml b-glucuronidase, and then extracted with 5 ml diethyl ether. The ether was evaporated and samples reconstituted in phosphate-buffered saline. Recovery of the T standards was 96.973% and E2 was 93.272%. Intra-assay coefficients of variation for the high and low control pools were 5.16 and 3.85%, respectively (n 5 19), for T and 2.37 and 3.06%, respectively (n 5 18), for E2. The interassay coefficients of variation for the high and low control pools were 9.68 and 8.34%, respectively (n 5 19), for T and 9.51 and 17.68%, respectively (n 5 18), for E2.
Data Analyses
Since residents experienced two separate intruder encounters with adult intruders, the replicate trials with adult intruders were collapsed into a composite score for each intruder sex. All behavioral data were standardized to a frequency per 10 min to allow direct comparisons between pre-/trial-/post-values. Several aggressive displays and behavioral patterns were summed to produce a composite measure of aggression. Previous research with this species has found that the behaviors, including attack, chase, erh-erh vocalizations, cage mark, genital display, and piloerection, correlate strongly with each other and have previously been collapsed into a single category, the high-level aggression category [Ross et al., 2004; Schaffner & French, 1997]. This composite score will be simply referred to as aggressive displays throughout [Ross et al., 2004]. A four-factor analysis of variance was used to determine differences among trial conditions, and Bonferroni adjustments were used for post hoc tests to evaluate all between-subjects main effects. Specifically, for resident behaviors the design was a 2 (resident sex) × 2 (intruder sex) × 2 (intruder age) × 3 (time of the observation: pretrial, during, post-trial). Control trials were not included in these analyses, because no aggressive behaviors were displayed during any of the trials in the presence of the empty cage. All results are reported as means7standard error.
The hormonal data for both residents and intruders were analyzed using the percent change relative to the baseline morning samples, for the samples collected 2–6 hr after the trial (samples averaged together) and samples 24 hr after the trial, accounting for both shorter and longer term changes in steroid production and excretion. These times were chosen based on previous evidence that excretion of steroids may commence as early as 2–6 hr following a change in the plasma concentrations [Smith & French, 1997a,b]. Changes from baseline values in CORT, T, and E2 concentrations of female residents were analyzed using an analysis of variance, specifically 5 (exposure conditions: adult male intruder, juvenile male intruder, adult female intruder, juvenile female intruder, control) × 2 (time after the trial: 2–6 hr, 24 hr). The change in CORT, T, and E2 for intruder females was tested with a 2 (age of the intruder: adult, juvenile) × 2 (condition: intruder trial, control) × 2 (time after the trial: 2–6 hr, 24 hr). The controls were included in the ANOVA in order to determine whether changes in hormone concentrations throughout the day after an intruder trial were distinct from changes that are associated with normal circadian rhythms [Smith & French, 1997b].
To compare the relationships between aggression and hormone changes, as well as examining the relationship between changes in CORT, T, and E2, partial correlations were conducted controlling the ID of both the resident and intruder. For these analyses, the samples were not collapsed or averaged between trials. Each resident was analyzed using every trial in which they participated; i.e. two trials with adult male intruders and two trials with adult female intruders. Hormone samples were averaged for the 2–6 hr time period and analyzed as changes from the baseline values. Analyses first examined the relationship between female resident aggression and CORT, T, or E2 for all intruders; further analysis restricted the comparison to exposure to intruder females only. Similar tests were used to compare changes in CORT, T, and E2 over time, as well as relationships between aggression, CORT, T, and E2 for intruders. All analyses were done using SPSS 13.0 and an a level of 0.05 was set for all analyses.
RESULTS
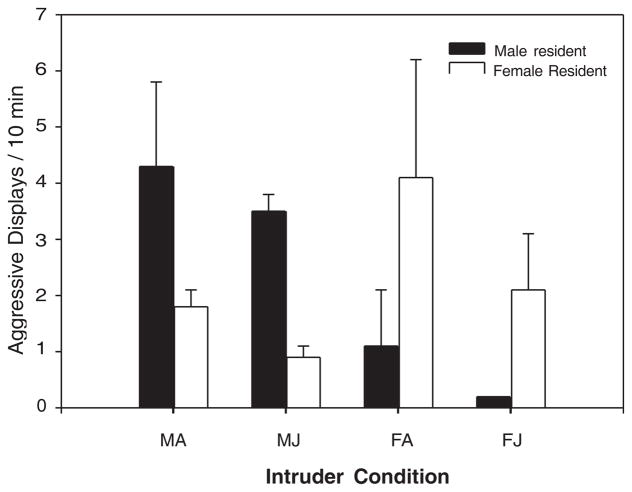
Resident marmosets responded to intruder trials in a sex specific manner with higher rates of aggressive displays in the presence of a same-sex intruder (F(1,8) 5 10.50, P 5 0.012), whereas the status of the intruder as juvenile or adult did not result in significantly different levels of aggressive displays (Fig. 1). Female residents showed increased rates of scent marking during trials with female intruders (female intruder: 0.3370.07 scent marks/10 min, male intruder: 0.1470.02 scent marks/10 min; F(1, 4) 5 9.29, P 5 0.038). We found that the number of instantaneous samples in which the females were in contact with their mate was significantly increased during trials in which they were exposed to a male intruder (male intruder: 0.3870.08 contact/10 min, female intruder: 0.267 0.09 contact/10 min; F(1,4) 5 26.66, P 5 0.007). No other behaviors scored for the female resident differed owing to the sex of the intruder.
Fig. 1.
Aggression directed toward intruders as a function of the resident sex, intruder sex, and intruder age. MA, male adult; MJ, male juvenile; FA, female adult; FJ, female juvenile.
Physical proximity to the females’ social partner varied as a function of phase of the trial (F(2,16) 5 10.04, P 5 0.007). Post hoc analysis revealed that females spent considerably more time near, but not in contact with, their male partners during the intruder phase of the trial, relative to the post-trial observation (trial: 0.8370.09, post-trial: 0.4770.08, P 5 0.01). A significant interaction was found between trial type and time for the behavioral score of move (F(8,32) 5 2.87, P 5 0.016) with marmosets in all the intruder trial phases of the experiment showing more movement than before or after the intruder trial (trial: 0.8070.85, pre: 0.4070.21, post: 0.3670.24). Females also displayed higher rates of genital displays during the intruder trial phase than during the post-trial time (F(2,8) 5 5.17, P 5 0.036, trial: 0.0570.02 genital displays/10 min, post-trial: 0.0170.01 genital displays/10 min, P 5 0.05). No other behaviors scored during the intruder trials, including allogrooming, time away from the mate, sex, self-grooming, feeding, playing, twitter calls, long calling, or time on the intruder cage, differed significantly owing to the phase of the trial or the trial condition.
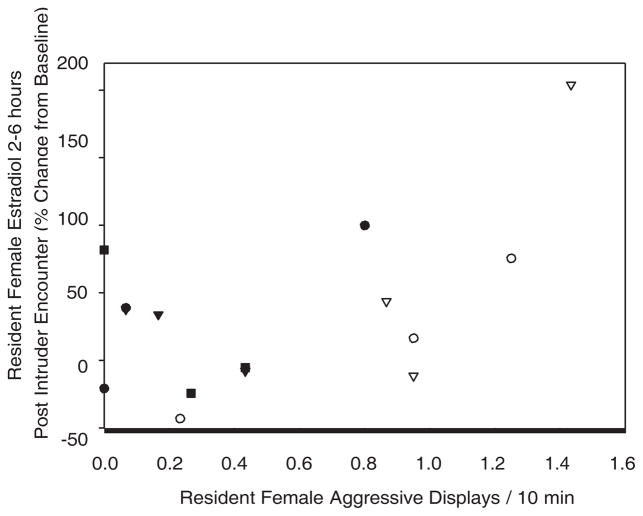
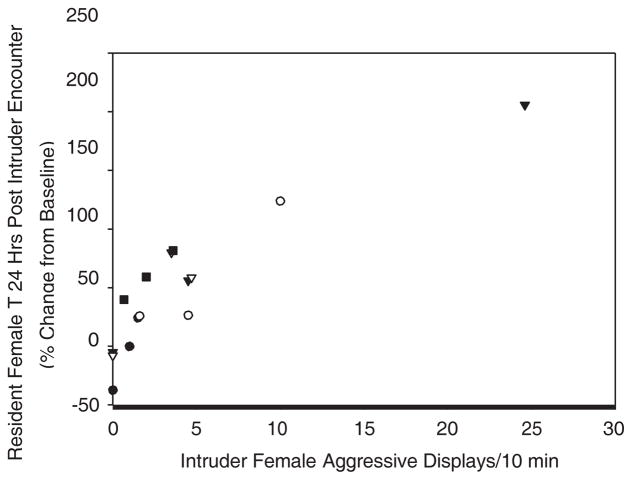
Female residents had no significant hormonal changes from baseline at either 2–6 hr post or 24 hr post for CORT, T, or E2 as a function of the gender of the intruder as tested by ANOVA. Variation in aggression displayed during a trial was not significantly associated with variation in baseline hormone concentrations of CORT, T, or E2 for the resident females. When we further examined just the trials involving female intruders, female residents displayed a significant positive relationship between sexual bouts during a trial and the number of aggressive displays during the trial (r 5 0.56, P 5 0.037, n 5 15, controlled ID n 5 5). Additionally, female residents were found to have a significant positive relationship between the change from the baseline of E2 2–6 hr post and the number of sexual bouts (sex r 5 0.815, P 5 0.000) and the number of aggressive displays during the trial (aggressive displays r 5 0.533, P 5 0.05; when the outlier is removed, the positive relationship remains but is not significant r 5 0.45, n.s.) (Fig. 2). The number of sexual bouts during the trial were also significantly positively associated with changes from the baseline in T 2–6 hr after the trial (r 5 0.57, P 5 0.033). There was also a significant positive relationship between the changes from the baseline in CORT 24 and E2 24 for females exposed to female intruders (r 5 0.625, P 5 0.04). Although there was no relationship between the number of aggressive displays performed by the resident and those done by the intruder, there was a significant positive relationship between the number of aggressive displays done by the female intruder during the trial and the female residents’ change from the baseline for T 24 following the trial (r 5 0.79, P 5 0.004; when the two outliers are removed, the relationship remains but is marginally significant r 5 0.56, P 5 0.1) (Fig. 3).
Fig. 2.
Percent change from baseline for resident females’ E2 2–6 hr following an encounter with a female intruder and the aggressive displays exhibited by the resident female during the intruder encounter (partial correlation; r 5 0.533, P 5 0.05), individuals are represented by each symbol.
Fig. 3.
Percent change from baseline for resident females’ T 24 hr following an encounter with a female intruder and the number of aggressive displays received by the resident female during the intruder encounter (partial correlation; r 5 0.79; P 5 0.004), individuals are represented by each symbol.
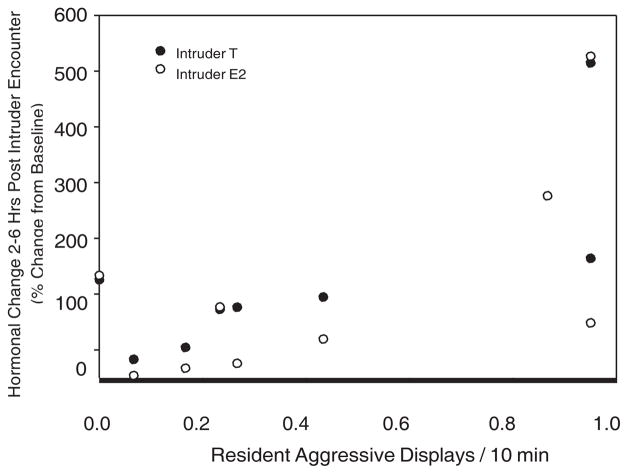
Female intruders responded quite differently to the encounter than did the residents. Female intruders showed a significant positive relationship between the number of aggressive displays done by the female resident during the trial and the change from the baseline in T 2–6 as well as E2 2–6 (T 2–6 r 5 0.79, P 5 0.033; E2 2–6 r 5 0.78, P 5 0.049; n 5 7) (Fig. 4). Female intruders also showed a significant positive relationship between the number of aggressive displays that they exhibited during the trial and their change from the baseline for CORT 2–6 (r 5 0.76, P 5 0.017).
Fig. 4.
Percent change from baseline for intruder females’ T 2–6 and E2 2–6 hr following an intruder challenge and the number of aggressive displays received by the intruder during the encounter (partial correlations; T2–6 r 5 0.794, P 5 0.033; E2 2–6, r 5 0.78, P 5 0.049).
DISCUSSION
Female marmosets exhibited sex-specific behavioral and hormonal responses to nonfamiliar intruders. Specifically, female residents displayed more aggressive behaviors toward adult female intruders than to any other type of social intruder. Females displayed higher rates of agonistic displays and scent marking than has been previously reported for this species [French et al., 1995; Schaffner & French, 1997]. Female marmosets also specifically displayed high rates of aggression toward adult female intruders. These aggressive behaviors were positively associated with changes in hormonal concentrations dependent on whether the female was a resident giving or receiving aggression. Although the sample size for this study was small and it may be difficult to extrapolate our results to female aggression in general, we find it very interesting that rather than hormonal shifts being associated simply with the female’s presence in an aggressive encounter, the hormonal changes reported were specifically associated with the female’s role in the aggressive encounter. For female residents, high rates of displayed aggression was associated with increases in E2 immediately following the encounter, whereas high rates of aggression received was associated with increased T 24 hr following the encounter. Interestingly, if the female was an intruder, high rates of aggression displayed was associated with increased CORT immediately following the trial, whereas increased aggression received was associated with increased T and E2.
Unlike other studies that have found little association between steroid hormone concentrations and aggression in females, we found situationally dependent relationships. Previous work on female vertebrate aggression has focused on seasonal breeders with strict nest building, mate acquisition, egg laying, egg care, and offspring care seasons. These females often have high levels of T or E2 during the mating and nest building season as a baseline, and more closely resemble the aggressive interactions and hormonal responses of males of polygynous species [Canoine & Gwinner, 2005; Elekonich & Wingfield, 2000; Rubenstein & Wikelski, 2005]. Very little work has been done with females from monogamous species for which breeding, mate guarding, and infant care may occur simultaneously. Recent studies of California mice have demonstrated that female residents exhibit an immediate increase in plasma corticosterone and OT following a 10 min exposure to an unfamiliar same-sex intruder [Trainor et al., 2010]. The changes in corticosterone and OT were not correlated with the aggression of the encounter and were associated with the length of the photoperiod that the females were maintained in. Artificial manipulation of hormones through implants in California mice has found decreases in P4 and the P4/T ratio owing to the presence of an intruder that was not associated with the amount of aggression displayed by the resident [Davis & Marler, 2003]. This suggested that decreasing the P4/T ratio serves to prime the female for the next intruder interaction; however, animals were sacrificed 30 min following the trial for blood collection, which did not allow enough time to verify priming effects. These rodent studies have controlled the reproductive cycle of the female either by using nulliparous [Davis & Marler, 2003] or naïve diestrus mice [Trainor et al., 2010]. Work in hamsters suggests that lactating females display higher rates of aggression than cycling females [Siegal et al., 1983], and many rodent studies have suggested that nipple stimulation is directly linked to increased female aggression [Gandelman & Simon, 1980; Svare & Mann, 1983].
Unfortunately, as a result of colony constraints, we were unable to control the reproductive status of the marmoset females. However, we avoided testing groups with females in the third trimester of pregnancy or with nursing infants, as these time periods would be most likely associated with significant pregnancy-induced hormonal shifts and likely maternal aggression. The post hoc analysis of the interbirth intervals found that all females were either nonpregnant or in the first and second month of pregnancy during all experimental trials. Although it is possible that early pregnancy may affect female aggression and hormonal responses to an intruder, we do not suspect that this is the case. Marmosets are socially monogamous and are often referred to as pair-bonded primates [Gerber et al., 2002], and the pairs tested in this experiment were long-term pair mates, not new pairs. Pair-bonded mammals typically display high levels of aggression toward unfamiliar intruders, including sexually receptive females, rather than soliciting them as future mates. This maintenance of the pair bond occurs whether the female of the bond is currently pregnant or nonpregnant, as the male represents a resource not only as a mate but also as a care provider for future infants [Fernandez-Duque et al., 1997; Young et al., 2011]. Furthermore, marmoset pregnancies are unusual in that they display a brief pause for the first month of pregnancy in which the embryo does not begin growth and development [Merker et al., 1988]. Therefore, we do not believe that the early pregnancy status is likely to play a large role in the aggressive responses of female marmoset residents to intruders, but future research should attempt to control this variable.
Female marmosets displayed a unique hormonal response in which the changes in steroid hormone concentrations were associated not simply with the level of aggression during the encounter, but also with the role the female played in the encounter. The marmosets’ responses most closely resemble those reported for lizards, in which E2 directly correlated with aggression, whereas T seemed indirectly associated [Rubenstein & Wikelski, 2005]. The authors concluded that in lizards rapid aromatization of T to E2 occurred during a fighting encounter. In male Peromyscus, winners of an encounter have significantly different behavioral and hormonal responses than do the losers of the encounters [Fuxjager et al., 2010; Gleason et al., 2009]. Winners of an encounter are significantly more likely to exhibit increased T, increased activity of androgen receptor in several brain regions, and to display aggressive behaviors in future encounters. Female Syrian hamsters have been found to be less likely to show repeated submissive behaviors following the loss of an encounter if they are implanted with E2 and T capsules [Faruzzi et al., 2005]. Resident female marmosets showed dramatic increases in urinary E2 immediately following an aggressive encounter, suggesting rapid aromatization and usage of T to facilitate aggressive actions during the encounter. Although urinary assays of hormones might not account for small transient changes in plasma concentrations, we were able to detect the longer term changes in the steroids. Furthermore, resident females that were not aggressive during an encounter and yet encountered high rates of aggression from the female intruder showed increased concentrations of T 24 hr following a trial, which may reflect hormonal priming for future aggressive interactions. In the wild, high rates of intergroup encounters have been recorded for several species of callitrichids [Digby, 1995b]. Thus, hormonal priming may be particularly necessary for females to protect their mating status and family cohesion following an encounter with a particularly aggressive female intruder.
In male C. kuhlii, it was the intensity of the aggression displayed by the individual that was associated with increases in T both 2–6 and 24 hr following an encounter [Ross et al., 2004]. Although the females displayed alterations in steroid concentrations associated with aggression, the changes were not identical to those expressed by the males. The neuroendocrine changes associated with aggression for females seem to be more sensitive to the intricacies of the interaction than were changes in the males. In many ways, this is to be expected in light of both the social system of the marmoset and the reproductive pressures upon the females. High concentrations of T in males have been linked to negative outcomes on immune function and decreased levels of parental care; thus, there are predicted trade-offs to increasing T in favor of aggression [Hasselquist et al., 1999; Peters, 2000; Wingfield et al., 1990]. For females, these trade-offs may be even more delicate [O’Neal et al., 2008]. Female marmosets are not seasonal breeders, and changes in T and E2 may have deleterious impacts on early and mid-pregnancies. Additionally, as female marmosets may spend at least part of their lifetime reproductively suppressed by a dominant female [Smith & French, 1997b], females may be particularly sensitive to receiving aggression and their roles in these social encounters. Interestingly, the role of the HPA axis may be particularly important for intruder females, with immediate increases in CORT being found only in these females. No changes in CORT were found in the male marmosets at all, regardless of status of the individual, aggression received, or aggression displayed [Ross et al., 2004]. So, although females display an androgen-associated response to aggression, it is a context-specific pathway that is much different from male marmosets.
In conclusion, we found that, although females show marked changes in the hormonal concentrations following an intruder encounter, the modulations were specific to not only the aggression of the encounter but also the role the female played in the encounter. The female marmoset offers a unique model to examine the impact of social status and the role of aggression in maintenance of social groups and the underlying neuroendocrine mechanisms associated with these behaviors.
Acknowledgments
Contract grant sponsor: National Science Foundation; Contract grant numbers: IBN: 97-23842; 00-91030.
We thank the following people for help in data collection and animal management: Kim Patera, Denise Hightower, Danny Revers, Dan Jorgensen, Chad Hansen, Scott Nunes, and Jeffrey Fite. Support for this project came from grants to Jeffrey French from the National Science Foundation (IBN: 97-23842 and 00-91030).
References
- Beehner JC, Phillips-Conroy JE, Whitten PL. Female testosterone, dominance rank, aggression in an Ethiopian population of hybrid baboons. American Journal of Primatology. 2005;67:101–119. doi: 10.1002/ajp.20172. [DOI] [PubMed] [Google Scholar]
- Callard GV, Petro Z, Ryan K. Conversion of androgen to estrogen and other steroids in the vertebrate brain. American Zoologist. 1978;18:511–523. [Google Scholar]
- Campbell A. Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biological Psychology. 2007;77:1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Canoine V, Gwinner E. The hormonal response of female European Stonechats to a territorial intrusion: the role of the male partner. Hormones and Behavior. 2005;47:563–568. doi: 10.1016/j.yhbeh.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Castro WLR, Matt KS. The importance of social condition in the hormonal and behavioral responses to an acute social stressor in the male siberian dwarf hamster (Phodopus sungorus) Hormones and Behavior. 1997;32:209–216. doi: 10.1006/hbeh.1997.1423. [DOI] [PubMed] [Google Scholar]
- Cavigella SA, Pereira MA. Mating season aggression and fecal T levels in male ring tailed lemurs. Hormones and Behavior. 2000;37:246–255. doi: 10.1006/hbeh.2000.1585. [DOI] [PubMed] [Google Scholar]
- Creel S, Wildt DE, Monfort SL. Aggression, reproduction, and androgens in wild dwarf mongooses: a test of the challenge hypothesis. American Naturalist. 1993;141:816–825. doi: 10.1086/285509. [DOI] [PubMed] [Google Scholar]
- Davis ES, Marler CA. The progesterone challenge: steroid hormone changes following a simulated territorial intrusion in female Peromyscus californicus. Hormones and Behavior. 2003;44:185–198. doi: 10.1016/s0018-506x(03)00128-4. [DOI] [PubMed] [Google Scholar]
- Digby L. Infant care, infanticide, and female reproductive strategies in polygynous groups of common marmosets (Callithrix jacchus) Behavioral Ecology and Sociobiology. 1995a;37:51–61. [Google Scholar]
- Digby L. Social organization in a wild population of Callithrix jacchus: II. Intragroup social behavior. Primates. 1995b;36:361–375. [Google Scholar]
- Elekonich MM, Wingfield JC. Seasonality and hormonal control of territorial aggression in female song sparrows (Passeriformes: Emberizidae: Melospiza melodia) Ethology. 2000;106:492–510. [Google Scholar]
- Faruzzi AN, Solomon MB, Demas GE, Huhman KL. Gonadal hormones modulate the display of submissive behavior in socially defeated female Syrian hamsters. Hormones and Behavior. 2005;47:569–575. doi: 10.1016/j.yhbeh.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, Mason WA, Mendoza SP. Effects of duration of separation on responses to mates and strangers in the monogamous titi monkey (Callicebus moloch) American Journal of Primatology. 1997;43:225–237. doi: 10.1002/(SICI)1098-2345(1997)43:3<225::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Fite JE, French JA. Pre- and postpartum sex steroids in female marmosets (Callithrix kuhlii): is there a link with infant survivorship and maternal behavior? Hormones and Behavior. 2000;38:1–12. doi: 10.1006/hbeh.2000.1607. [DOI] [PubMed] [Google Scholar]
- French JA, Inglett BJ. Female-female aggression and male indifference in response to unfamiliar intruders in lion tamarins. Animal Behavior. 1989;37:487–497. [Google Scholar]
- French JA, Schaffner CM, Shepherd RE, Miller ME. Familiarity with intruders modulates agonism toward out- group conspecifics in Wied’s black tufted-ear marmoset (Callithrix kuhli) Ethology. 1995;99:24–38. [Google Scholar]
- French JA, Brewer KJ, Schaffner CM, Schalley J, Hightower-Merritt TE, Smith TE. Urinary steroid and gonadotropin excretion across the reproductive cycle in female Wied’s black-tufted ear marmosets (Callithrix kuhli) American Journal of Primatology. 1996;40:231–246. doi: 10.1002/(SICI)1098-2345(1996)40:3<231::AID-AJP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Fuxjager MJ, Forbes-Lorman RM, Cross DJ, Auger CJ, Auger AP, Marler CA. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proceedings of the National Academy of Sciences. 2010;107:12393–12398. doi: 10.1073/pnas.1001394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandelman R, Simon NG. Postpartum fighting in the rat: nipple development and the presence of young. Behavioral Neurobiology. 1980;28:350–360. doi: 10.1016/s0163-1047(80)92357-2. [DOI] [PubMed] [Google Scholar]
- Gerber P, Schnell CR, Anzenberger G. Behavioral and cardiophysiological responses of common marmosets (Callithrix jacchus) to social and environmental changes. Primates. 2002;43:201–216. doi: 10.1007/BF02629648. [DOI] [PubMed] [Google Scholar]
- Gerber LR, Gonzalez-Suarez M, Hernandez-Camacho CJ, Young JK, Sabo JL. The cost of male aggression and polygyny in California sea lions (Zalophus californianus) PLoS ONE. 2010;5:e12230. doi: 10.1371/journal.pone.0012230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason ED, Fuxjager MJ, Oyegbile TO, Marler CA. Testosterone release and social context: when it occurs and why. Frontiers in Neuroendocrinology. 2009;30:460–469. doi: 10.1016/j.yfrne.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Goldizen AW. Tamarins and marmosets: communal care of offspring. In: Smuts BB, Cheney DC, Seyfarth RM, Wrangham RW, Struhsaher TT, editors. Primate societies. Chicago: University of Chicago Press; 1987. pp. 34–43. [Google Scholar]
- Hasselquist D, Marsh JA, Sherman PW, Wingfield JC. Is avian humoral immunocompetence suppressed by testosterone? Behavioral Ecology and Sociobiology. 1999;45:161–175. [Google Scholar]
- Johnsen TS. Behavioral correlates of testosterone and seasonal changes of steroids in Red-winged blackbirds. Animal Behavior. 1998;55:957–965. doi: 10.1006/anbe.1997.0642. [DOI] [PubMed] [Google Scholar]
- Kapusta J. Gonadal hormones and intrasexual aggressive behavior in female bank voles (Clethrionomys glareolus) Aggressive Behavior. 1998;24:63–70. [Google Scholar]
- Leopoldo de Sousa F, Lazzari V, Scherem deAzevedo M, deAlmeida S, Sanvitto GL, Lucion AB, Giovenardi M. Progesterone and maternal aggressive behavior in rats. Behavioral Brain Research. 2010;212:84–89. doi: 10.1016/j.bbr.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. Hormonal factors influence the onset of maternal aggression in laboratory rats. Hormones and Behavior. 1987;21:253–267. doi: 10.1016/0018-506x(87)90050-x. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason W. Contrasting responses to intruders and involuntary separation by monogamous and polygynous new world monkeys. Physiology and Behavior. 1986;38:795–801. doi: 10.1016/0031-9384(86)90045-4. [DOI] [PubMed] [Google Scholar]
- Merker HJ, Csatow SK, Neubert HW. The embryology of Callithrix jacchus. In: Merker HJ, Hendrickx AG, editors. Nonhuman primates: developmental biology and toxicology. Berlin: Ueberreuta Wissenschaft-Wein; 1988. pp. 217–239. [Google Scholar]
- Nunes S, Fite JE, French JA. Variation in steroid hormones associated with infant care behaviour and experience in male marmosets (Callithrix kuhlii) Animal Behavior. 2000;60:857–865. doi: 10.1006/anbe.2000.1524. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera K, French JA. Endocrine variation associated with the phases of paternal behavior in black tufted-ear marmosets (Callithrix kuhlii) Hormones and Behavior. 2001;39:70–82. doi: 10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Nunes S, Brown C, French JA. Variation in circulating and excreted estradiol associated with testicular activity in male marmosets. American Journal of Primatology. 2002;56:27–42. doi: 10.1002/ajp.1061. [DOI] [PubMed] [Google Scholar]
- O’Neal DM, Reichard DG, Pavilis K, Ketterson ED. Experimentally-elevated testosterone, female parental care, and reproductive success in a songbird, the Dark-eyed Junco (Junco hyemalis) Hormones and Behavior. 2008;54:571–578. doi: 10.1016/j.yhbeh.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Peters A. Testosterone treatment is immunosuppressive in superb fairy-wrens, yet free-living males with high testosterone are more immunocompetent. Proceedings of the Royal Society of London B. 2000;267:883–889. doi: 10.1098/rspb.2000.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price EC. The costs of infant carrying in captive cotton-top tamarins. American Journal of Primatology. 1992;26:23–33. doi: 10.1002/ajp.1350260106. [DOI] [PubMed] [Google Scholar]
- Ross CN, French JA, Patera KJ. Intensity of aggressive interactions modulates testosterone in male marmosets. Physiology and Behavior. 2004;83:437–445. doi: 10.1016/j.physbeh.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Rubenstein DR, Wikelski M. Steroid hormones and aggression in female Galapagos marine iguanas. Hormones and Behavior. 2005;48:329–341. doi: 10.1016/j.yhbeh.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Rutkowska J, Cichon M, Puerta M, Gil D. Negative effects of elevated testosterone on female fecundity in zebra finches. Hormones and Behavior. 2005;47:585–591. doi: 10.1016/j.yhbeh.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Schaffner CM, French JA. Group size and aggression: recruitment incentives in a cooperatively breeding primate. Animal Behavior. 1997;54:171–180. doi: 10.1006/anbe.1996.0413. [DOI] [PubMed] [Google Scholar]
- Schaffner CM, Shepherd RE, Santos CV, French JA. Development of heterosexual relationships in Wied’s black tufted-ear marmosets. American Journal of Primatology. 1995;36:185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Scotti MAL, Place NJ, Demas GE. Short-day increases in aggression are independent of circulating gonadal steroids in female Siberian hamsters (Phodopus sungorus) Hormones and Behavior. 2007;52:183–190. doi: 10.1016/j.yhbeh.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Siegal HI, Giordano AL, Mallafre CM, Rosenblatt JS. Maternal aggression in hamsters: effects of stage of lactation, presence of pups, and repeated testing. Hormones and Behavior. 1983;17:86–93. doi: 10.1016/0018-506x(83)90018-1. [DOI] [PubMed] [Google Scholar]
- Smith TE, French JA. Psychosocial stress and urinary cortisol excretion in marmoset monkeys (Callithrix kuhli) Physiology and Behavior. 1997a;62:225–232. doi: 10.1016/s0031-9384(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Smith TE, French JA. Social and reproductive conditions modulate urinary cortisol excretion in black tufted-ear marmosets. American Journal of Primatology. 1997b;42:253–267. doi: 10.1002/(SICI)1098-2345(1997)42:4<253::AID-AJP1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Stockley P, Bro-Jorgensen J. Female competition and its evolutionary consequences in mammals. Biological Reviews. 2011;86:341–366. doi: 10.1111/j.1469-185X.2010.00149.x. [DOI] [PubMed] [Google Scholar]
- Svare BB, Mann MA. Hormonal influences on maternal aggression. In: Svare BB, editor. Hormones and Aggressive Behavior. Plenum; New York: 1983. pp. 91–104. [Google Scholar]
- Svare BB, Miele J, Kinsley C. Mice: progesterone stimulates aggression in pregnancy-terminated females. Hormones and Behavior. 1986;20:194–200. doi: 10.1016/0018-506x(86)90017-6. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Silva AL, Crean KK, Hostetler C. Sex differences in hormonal responses to social conflict in the monogamous California mouse. Hormones and Behavior. 2010;58:506–512. doi: 10.1016/j.yhbeh.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Saal FS, Franks P, Boechler M, Palanza P, Parmigiani S. Nest defense and survival of offspring in highly aggressive wild Canadian female house mice. Physiology and Behavior. 1995;58:669–678. doi: 10.1016/0031-9384(95)00121-x. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Ball GF, Dufty AM, Hegner RE, Ramenofsky M. Testosterone and aggression in birds: tests of the challenge hypothesis. American Scientist. 1987;75:602–608. [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Ball GF. The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. American Naturalist. 1990;136:829–846. [Google Scholar]
- Woodley SK, Matt KS, Moore MC. Estradiol modulation of central monoamine activity in female mountain spiny lizards. Brain Behavior and Evolution. 2000;56:175–183. doi: 10.1159/000047202. [DOI] [PubMed] [Google Scholar]
- Zysling DA, Greives TJ, Breuner CW, Casto JM, Demas GE, Ketterson ED. Behavioral and physiological responses to experimentally elevated testosterone in female dark-eyed juncos (Junco hymealis carolinensis) Hormones and Behavior. 2006;50:200–207. doi: 10.1016/j.yhbeh.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Frontiers of Neuroendocrinology. 2011;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]