Abstract
BACKGROUND
Serum/glucocorticoid-regulated kinase 1 (SGK1), a known target of the androgen receptor (AR) and glucocorticoid receptor (GR), is reported to enhance cell survival. This study sought to better define the role of SGK1 and GR in prostate cancer.
METHODS
Immunohistochemistry was performed for AR, GR, and SGK1 on primary prostate cancers (n = 138) and 18 prostate cancers from patients treated with androgen deprivation therapy. Relative staining intensity was compared utilizing a Fisher’s exact test. Univariate analyses were performed using log-rank and chi-squared tests to evaluate prostate cancer recurrence with respect to SGK1 expression.
RESULTS
SGK1 expression was strong (3+) in 79% of untreated cancers versus 44% in androgen-deprived cancers (P = 0.003). Conversely, GR expression was present in a higher proportion of androgen-deprived versus untreated cancers (78% vs. 38%, P = 0.002). High-grade cancers were nearly twice as likely to have relatively low (0 to 2+) SGK1 staining compared to low-grade cancers (13.8% vs. 26.5%, P = 0.08). Low SGK1 expression in untreated tumors was associated with increased risk of cancer recurrence (adjusted log-rank test P = 0.077), 5-year progression-free survival 47.8% versus 72.6% (P = 0.034).
CONCLUSIONS
SGK1 expression is high in most untreated prostate cancers and declines with androgen deprivation. However, these data suggest that relatively low expression of SGK1 is associated with higher tumor grade and increased cancer recurrence, and is a potential indicator of aberrant AR signaling in these tumors. GR expression increased with androgen deprivation, potentially providing a mechanism for the maintenance of androgen pathway signaling in these tumors. Further study of the AR/GR/SGK1 network in castration resistance.
Keywords: SGK1, androgen receptor, glucocorticoid receptor, prostate cancer
INTRODUCTION
Localized prostate cancers are treated with curative intent by surgery or radiation, however, as many as 40% of patients will develop recurrent disease over time, and it remains the second leading cause of cancer related death in men [1,2]. There are established predictors of prostate cancer recurrence or progression and widely used prognostic nomograms, which in large part utilize common pathologic criteria [3–6]. Furthermore, there are multiple biomarkers in development to help further hone prostate cancer prognostication [7,8]. Nonetheless, the biologic factors modulating prostate cancer behavior remain an important area of research.
The majority of prostate cancers rely on the androgen receptor (AR) for cell survival and proliferation, and the pathway remains important in the progression of prostate cancer even in patients whose disease progresses despite androgen deprivation therapy [9,10]. Recently, serum/glucocorticoid-regulated kinase1 (SGK1) was found to be upregulated by AR activation in prostate cancer cell lines resulting in enhanced prostate cancer cell survival in vitro [11–13]. SGK1 is a serine/threonine protein kinase with 54% homology in its catalytic domain to Akt and is involved in a multitude of metabolic and survival functions [14]. SGK1 is transcriptionally induced and its protein product plays an important role in cellular responses to stressors such as oxidation, heat, and ultraviolet radiation [14]. SGK1 has been shown to be overexpressed in a proportion of human breast cancers [15] and to be important in protection from stress-induced apoptosis in breast cancer cell lines [16,17]. Similarly, androgen-sensitive prostate cancer cell lines that ectopically express SGK1 demonstrate increased survival following androgen-deprivation compared to those that do not overexpress the SGK1 [11]. In addition, in vitro studies using small interfering RNAs targeting SGK1 or small molecule pharmacologic inhibitors of SGK1 demonstrate that inhibition of SGK1 activity leads to decreased androgen-mediated prostate cancer cell growth [11,18]. To our knowledge, there has been only one study examining SGK1 expression in primary human prostate tumors; somewhat surprisingly, SGK1 expression was significantly decreased in prostate cancers as compared to benign prostatic hypertrophy [19]. However, from the recent findings suggesting that SGK1 expression is regulated by the AR and important in prostate cancer cell survival, we hypothesized that SGK1 expression may be increased in a subset of prostate cancers. Furthermore, because increased SGK1 expression can enhance epithelial cell survival, we investigated whether SGK1 expression in primary prostate cancers correlates with an increased risk of prostate cancer relapse.
In addition to the AR, SGK1 is also a direct target of glucocorticoid receptor (GR) activation in epithelial cells. Interestingly, standard chemotherapy regimens for castrate-resistant prostate cancer include glucocorticoids [20,21]. Although a proportion of patients show responses by a reduction in the tumor marker PSA and obtain palliative benefits from glucocorticoid treatment, there are no phase III data demonstrating that glucocorticoids provide a survival benefit [22]. Therefore, it is unclear how glucocorticoids function in metastatic prostate cancer. Because SGK1 is a known effector of the glucocorticoid pathway, coupled with the fact that glucocorticoids are a component of therapy in castrate-resistant prostate cancer, we also investigated GR expression in both treatment-naive and androgen-deprived/castrate-resistant prostate cancers. To date, there were few reports examining GR expression in human prostate cancer [23,24] and no information on GR status in prostate cancer from androgen-deprived patients. As GR and AR share some common genomic targets (e.g., SGK1), we hypothesized that GR expression would increase in the setting of androgen deprivation to compensate for decreased AR signaling.
MATERIALS AND METHODS
Human Tissue Procurement
Tissue microarrays were constructed by the Human Tissue Research Center at the University of Chicago Medical Center with Institutional Review Board approval. As described previously, a nested case control was used to select 138 prostate cancer samples from over 500 consecutive radical prostatectomies performed at the University of Chicago between 1995 and 2002 for the tissue microarray used in this study [25]. Areas involved by prostate cancer and adjacent non-neoplastic prostatic tissue were punched (2 mm cores) from the formalin-fixed, paraffin embedded samples and arrayed with 72–108 cores per slide. These tissues are referred to as ‘‘treatment-naive prostate cancer’’ (TN-PC). Of this total (excluding duplicates), 126 samples had a reported Gleason grade, 125 had available staging data, and 122 had an associated preoperative PSA. In addition, 18 full tissue sections of prostate cancer following hormonal treatment (androgen deprivation ± antiandrogen) were cut into 4-μm sections, mounted on slides for immunohistochemistry staining. These samples are referred to as ‘‘androgen-deprived prostate cancer’’ (AD-PC). These 18 samples included prostate cancer tissue samples from 10 radical prostatectomies after preoperative androgen deprivation, 6 transurethral prostatectomies, 1 pelvic exenteration, and 1 femoral head biopsy with metastatic prostate cancer. Five of these 18 full sections were samples from bona fide ‘‘castrate-resistant’’ prostate cancers in that the samples were from patients with progression of disease despite pharmacologic or surgical castration. Patient age ranged between 42 and 93 years (42–81 for treatment-naive and 48–93 for androgen-deprived patients), with a median age of 63 years, and a mean age of 62.4 years. Patient disease characteristics including grade, stage and PSA are detailed in Table I. The majority of both treatment-naive and androgen-deprived samples had a Gleason grade between 6 and 7 (75% and 67%, respectively). The majority of prostatectomy samples were organ-confined as pathologic stage T2 (56%). Twenty-eight patients (20%) had extra-capsular extension, and another 23 patients (17%) were not organ-confined because of seminal vesicle invasion, invasion of bladder/rectum, nodal involvement, or bone metastasis at the time of surgery.
TABLE I.
Prosate Cancer Tumor Characteristics
| Treatment- naïve | Androgen-deprived | |
|---|---|---|
| Gleason grade number (%) | ||
| 5 | 23 (18.2) | 3 (16.7) |
| 6 | 35 (27.8) | 3 (16.7) |
| 7 | 60 (47.6) | 9 (50) |
| 8–9 | 8 (6.4) | 3 (16.7) |
| Pathologic stage number (%) | ||
| T2aN0 | 7 (5.6) | |
| T2bN0 | 11 (8.8) | |
| T2cN0 | 58 (46.4) | |
| T2cN1 | 1 (0.8) | |
| T3aN0 | 27 (21.6) | |
| T3aN1 | 3 (2.4) | |
| T3bN0 | 12 (9.6) | |
| T3bN1 | 3 (2.4) | |
| T4 | 3 (2.4) | |
| PSA | ||
| Mean (±standard deviation) | 9.0 (7.35) | |
| Median (range) | 6.75 (3.3–43.8) | |
Immunohistochemistry
Immunohistochemical stains for androgen (AR) and GR as well as SGK1 were performed as previously described [15,26,27]. Briefly, slides were covered with xylene for a total of 10 min, then immersed in ethanol of decreasing dilutions (100% ethanol to 70% ethanol) and washed with Tris-buffered saline (TBST) for 2 min. Antigens were unmasked with sodium citrate buffer pH 6.0 followed by heat treatment at 95°C. After cooling, slides were placed in 3% hydrogen peroxide for 20 min and then washed in TBST. Next, to block endogenous peroxi-dase and protein, slides were incubated in the dark in a milk-peroxide solution (90 parts dH2O, 5 parts skim milk, and 5 parts 3% hydrogen peroxide) and then again washed in TBST. The primary antibody, in blocking buffer, was then applied to the slide. The slides were incubated with the primary antibody for 30 min and then washed. After application of the primary antibody, the DAKO EnVision+ System-HRP [DAB (3,3′-diaminobenzidine)] was used. Omitting the primary antibody step served as a negative control for all tissues. The following antibodies were used: anti-AR (AR441 1:50, Dako), anti-GR (NCL-GCR 1:20, Novocastra), and anti-SGK1 (1:150, Affinity Bioreagents, C Terminal SGK1 antibody). All antibodies were assessed semi-quantitatively in both prostate cancer and adjacent non-neoplastic prostatic tissue. Any nuclear and/or cytoplasmic immunoreactivity was recorded. Intensity was graded on a four-point scale from 0 to 3 to represent negative, weak, moderate, and strong, respectively. This scoring was performed by two pathologists (D.S. and H.A.), who reviewed the immunoreactivity together and arrived at a consensus score that was used in the analyses.
Follow-Up and Cancer Recurrence Analysis
Follow-up data were collected using the University of Chicago Prostate Cancer Database (UCPC). All patients undergoing surgery, in this case radical prostatectomy, are captured in the electronic UCPC Database. The database contains detailed demographic, surgical, pathological, functional as well as long-term oncologic outcome data. The follow-up and outcome data were collected using surveys (mail, e-mail, and phone) at, 3, 6, 12, and 24 months after surgery and annually thereafter. Professional support by the Survey Lab at University of Chicago was utilized. Oncologic outcome was confirmed by mailed/ emailed/faxed tumor marker (PSA) results, reports for adjuvant treatments as well as queries to death registries. For prostate cancer recurrence analysis, PSA recurrence post-prostatectomy was defined as any PSA value above the lower limits of detection. Patients whose PSA failed to nadir to undetectable post-prostatectomy were considered to have met the recurrence endpoint at the date of first PSA follow-up. Patients were censored from the analysis at the time of last follow-up if they were alive and had not met criteria for PSA recurrence. Time to PSA progression was calculated for groups based on Gleason grade and stage. Gleason scores 5 and 6 were considered low grade and were combined for this analysis. Similarly the Gleason scores 7, 8, and 9 were grouped as they represent more aggressive biology. Although it has been reported that Gleason sum 4 + 3 versus 3 + 4 cancers behave differently, many of the pathology reports available from this tissue set did not differentiate between the two, but rather listed the cancer as ‘‘Gleason 7.’’ Thus, the group was analyzed together [28,29].
Statistical Analysis
Data were summarized using descriptive statistics. Fisher’s exact test was used to compare categorical variables (e.g., high vs. low staining levels) between groups. The Kaplan and Meier method was used to estimate progression-free survival (PFS). Univariate analyses comparing PFS between groups were performed using the log-rank test. In addition, the Wilcoxon–Breslow–Gehan log-rank test, which gives higher weight to the earlier data points, was used to compare PFS between SGK-1 expression groups. Univariate Cox proportional hazards regression models were used to estimate hazard ratios between groups. PFS rates at 2 and 5 years were compared using a chi-squared test with a complementary log–log transformation of the Kaplan–Meier estimator [30].
RESULTS
Immunohistochemistry of SGK1, GR, and AR Expression
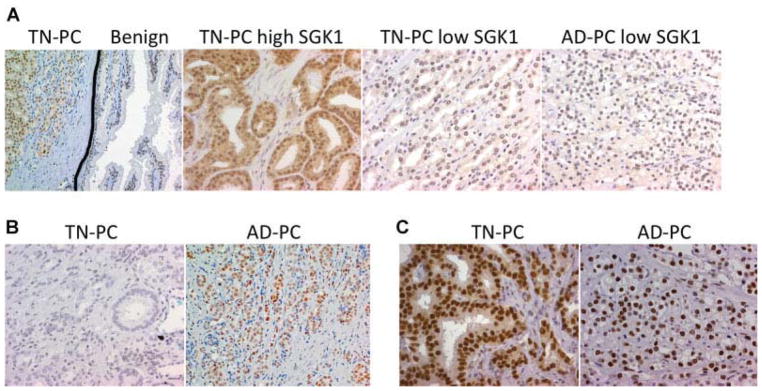
AR and SGK1 were expressed in essentially all prostate epithelium including TN-PC, AD-PC, and unaffected adjacent prostate tissue (UPT). As expected, nuclear AR expression was uniformly strong (3+) in all prostate cancer tissue samples regardless of treatment status (Table II, Fig. 1). SGK1 expression was consistently more intense in the prostate cancer samples compared to UPT (Fig. 1). Additionally, SGK1 expression was more intense in the nucleus versus the cytoplasm. As SGK1 expression by immunohistochemistry was strong in the majority of specimens, the four-point scale for SGK1 expression was grouped as high (3+) versus low (0, 1, and 2+) for further analysis. With this classification, 100 of 126 (79%) TN-PC samples were strongly positive for SGK1 compared to only 8 out of 18 (44%) AD-PC samples (P = 0.003) (Table II, Fig. 1). There was no association between SGK1 expression and tumor stage. The percentage of patients with a low Gleason score (Gleason sum 5 or 6) and low SGK1 expression was nearly half of that compared to the percentage of high Gleason grade (7–9) tumors with low SGK1 expression (13.8% vs. 26.5%, P = 0.08). This implies that SGK1 expression may inversely correlate with prostate tumor grade.
TABLE II.
IHC Expression of SGK1, AR, and GR
| SGK1 3+ (%)*(n = 126) | AR+ (%)(n = 126) | GR+ (%)**(n = 126) | |
|---|---|---|---|
| TN-PC | 100 (79) | 126 (100) | 48 (38) |
| AD-PC | 8 (44) | 18 (100) | 14 (78) |
P = 0.003,
P = 0.002 (Fisher’s exact test).
Fig. 1.
Immunohistochemical analysis of prostate cancer tissue. A: SGK1 expression. Representative pictures showing SGK1 expression is increased in prostate cancercell scompared to benign surrounding epithelium (1 stpanel-20 μ magnification, black lineis drawn to show demarcation of cancerous area).In TN-PC (middle two panels) SGK1 expression is variable. In AD-PC, high SGK1 expression was less frequent (40×). B: GR was expressed in a higher percentage of AD-PC compared to TN-PC (40×).C: AR expression was universally positive and pre dominantly nuclear in both TN-PC and AD-PC(40×).
We next examined GR expression. Unlike AR, which was universally strongly positive regardless of whether the patient was untreated or androgen-deprived, GR expression was more variable. Specifically, GR expression was present in a significantly higher proportion of AD-PC compared to TN-PC (78% vs. 38% positive staining, P = 0.002) (Table II, Fig. 1). Interestingly GR was highly expressed in four of five castrate-resistant AD-PC samples (80%), suggesting that increased GR activity may contribute to castration-resistance by bypassing AR blockade.
SGK1 Expression and Prostate Cancer Recurrence
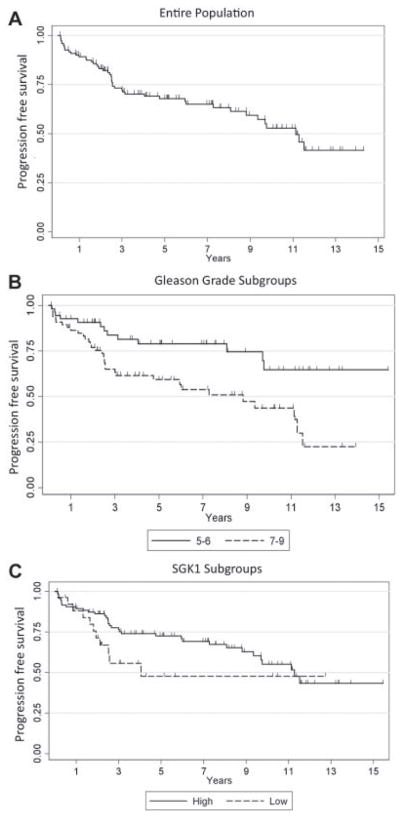
To date, of the 122 TN-PC patients for whom there is follow-up data available, PSA recurrence post-pros-tatectomy has occurred in 37.7% (n = 46). Of the 76 patients who have not met PSA recurrence criteria, the median follow-up is 5.5 years (range 0.11–15.4 years). The median progression-free survival [(PFS) = time from prostatectomy to PSA recurrence] for the entire cohort is 11.13 years (95% CI: 8.8-not reached) (Fig. 2A). Time to PSA progression was also calculated for patient subgroups based on tumor Gleason grade and stage. As expected, there was a statistically significant difference in PFS between the ‘‘low’’ and ‘‘high’’ Gleason grade subgroups (P = 0.004) (Fig. 2B), and patients with high Gleason scores were at a higher risk of progression (HR = 2.51) on univariate analysis. Higher tumor stage was similarly associated with an increased risk of relapse (P < 0.0001). Overall, there was a non-statistically significant trend towards decreased PFS (worse outcome) associated with low (0 to 2+) SGK1 expression compared to high SGK1 expression (log-rank P = 0.116; Wilcoxon–Gehan–Breslow log-rank test P = 0.077) (Fig. 2C). At 2 years, 86.2% (95% CI: 77.3–91.7%) of patients were alive without progression in the high SGK1 group versus only 71.4% (95% CI: 49.1–85.2%) alive without progression in the low SGK1-expression group (P = 0.083). At 5 years of follow-up, the percentage of patients without progression in the high SGK1 group was 72.6% (95%CI: 61.8–80.8%) versus only 47.8% (24.1–68.2%) in the low SGK1 group (P = 0.034). These data support the hypothesis that low SGK1 expression in primary prostate tumors is associated with a worse clinical outcome compared to high SGK1 expression.
Fig. 2.
PSA progression free survival estimates. Kaplan–Meier log-rank survival estimates of progression-free survival for (A). All patients (B). Stratified by Gleason grade low (5–6) versus intermediate/high (7–9). C: Stratified by SGK1 expression high (3+) versus low (0 to 2+).
DISCUSSION
SGK1 has recently been identified as an AR-regulated target gene that encodes a protein kinase important in prostate cancer cell survival. Currently, there is a paucity of knowledge regarding SGK1 expression and its clinical significance in primary prostate cancers. We examined the expression of SGK1 along with its nuclear receptor regulators AR and GR in both untreated and androgen-deprived human prostate cancer. We found that SGK1 is expressed in virtually all prostate cancers, but that the level of SGK1 expression is variable. SGK1 expression was consistently more intense in tumor epithelial cells compared to unaffected surrounding prostate tissue, supporting the notion that increased AR activity induces SGK1 expression. This observation contradicts a previous study examining SGK1 expression, which demonstrated a decrease in expression in tumor tissue samples when compared to benign prostatic hypertrophy [19]. This difference may be due to the comparison of cancer to benign prostatic hypertrophy in the previous study, rather than a comparison to unaffected adjacent normal prostate tissue as in our study. Furthermore, our finding that SGK1 expression decreases following androgen-deprivation therapy supports the finding that SGK1 expression is AR-mediated.
Although only statistically significant at 5 years, it is none-the-less interesting that we found an increased risk of prostate cancer recurrence in patients with lower SGK1 expression. This finding contradicted our initial hypothesis that high SGK1 expression in untreated primary prostate cancers would predict an increased risk of recurrence secondary to enhanced cancer cell survival. However, because SGK1 is an AR target gene, lower expression of SGK1 despite strong AR expression may reflect aberrant androgen pathway signaling associated with a less differentiated tumor phenotype. This hypothesis is consistent with the association between a higher Gleason grade and lower SGK1 expression found in our study. In support of this hypothesis, another study examining the expression of the AR target gene PSA/HK3 in prostate cancer found a similar inverse correlation between this AR-regulated gene and biochemical recurrence [31]. On the other hand, a recent publication by Donovan et al. [32] using a novel qualitative immunofluorescence scoring system for nuclear AR expression found an association of higher nuclear AR expression with increased prostate cancer specific mortality. Other studies examining AR(NR3C3) mRNA levels in primary tumor tissue also found an increase in biochemical relapse in patients with higher AR(NR3C3) mRNA compared to benign surrounding tissues [33,34]. These findings likely reflect a difference between measurable AR expression and actual AR pathway activity reflecting the complexity of AR pathway signaling in prostate cancer biology. There are multiple possible mechanisms underlying potentially decreased AR signaling, even in the setting of intact testosterone. These are only now coming to light, and include AR splice variants and mutations. [35–37].
It is clear that this study has several limitations. Foremost is that the percentage of tumors with low SGK1 expression was only ~25% of the total sample size; analysis of this population is therefore limited. The second major limitation is that many patients were lost to follow-up over time. The wide range of follow-up from 6 weeks to over 15 years may confound the PFS analyses. In addition, several of the statistical associations between SGK1 expression and clinical parameters were suggestive of an association while not meeting the P = 0.05 cut-off, although the association with relapse at 5 years was statistically significant. A larger sample size, potentially enriched for patients with higher grade disease, would likely strengthen our findings. Furthermore, more consistent long-term follow-up would also potentially allow more robust statistical analyses to be made. Such studies, including multivariate analyses, receiver operating curves, and correlation coefficients would clearly be necessary to justify the use of SGK1 staining as a prognostic biomarker.
Although SGK1 is a known effector of the glucocorticoid pathway [16] and glucocorticoids are utilized in systemic therapy for castrate-resistant prostate cancer, little is known about either GR expression in prostate cancer or how glucocorticoids may be exerting a therapeutic effect. To our knowledge, there have only been two prior studies investigating GR expression in prostate cancer [23,24]. In line with Yemelyanov’s GR expression data, we have also found that GR was expressed in approximately a third of PC samples when compared to adjacent normal prostate tissue. Interestingly, our study demonstrates that GR is expressed in a higher proportion of AD-PC compared to TN-PC samples, which neither of the prior studies examined. Furthermore, of the five castrate-resistant samples from the AD-PC group, four overexpressed GR (80%). Although the sample size is small, this finding is interesting and could be explained by the fact that in an androgen-depleted environment, GR expression increases to compensate for decreased AR activity. AR and GR share similar DNA binding domain sequences as well as some of the same downstream effector genes, including SGK1 [38–40]. It is well known that the AR remains relevant in the progression to castrate-resistant prostate cancer [9,10]. It is also possible that in an androgen-deplete, castrate environment, GR may retain a role in transcriptional regulation of androgen-regulated genes such as SGK1, and could serve as a survival pathway for castrate-resistant prostate cancer. Our observation that SGK1 expression remained high in nearly half of androgen-deprived cancers supports this hypothesis. Further work studying the role of SGK1 and GR in the survival of prostate cancer cells following androgen deprivation could lead to the development of SGK1 and GR modulators in castrate-resistant disease.
CONCLUSIONS
This study provides strong supporting translational evidence from patient-derived prostate tissue that AR signaling regulates SGK1 expression in prostate cancer. Interestingly, there is a suggestion that SGK1 expression is inversely associated with grade and cancer recurrence. It is possible that aberrant AR signaling is associated with a poorly differentiated phenotype and unfavorable outcome. Following androgen deprivation, GR expression increases and SGK1 expression remains high in a subset of patients. Further study is needed to clarify the role of the AR/GR/SGK1 network in castration resistance. This study highlights an interesting interaction between AR signaling, SGK1, and GR in prostate cancer.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ward JF, Moul JW. Rising prostate-specific antigen after primary prostate cancer therapy. Nat Clin Pract Urol. 2005;2(4):174–182. doi: 10.1038/ncpuro0145. [DOI] [PubMed] [Google Scholar]
- 3.Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169(2):517–523. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 4.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 5.Makarov DV, Trock BJ, Humphreys EB, Mangold LA, Walsh PC, Epstein JI, Partin AW. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007;69(6):1095–1101. doi: 10.1016/j.urology.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr, Dotan ZA, Fearn PA, Kattan MW. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98(10):715–717. doi: 10.1093/jnci/djj190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fradet Y. Biomarkers in prostate cancer diagnosis and prognosis: Beyond prostate-specific antigen. Curr Opin Urol. 2009;19(3):243–246. doi: 10.1097/MOU.0b013e32832a08b5. [DOI] [PubMed] [Google Scholar]
- 8.Fiorentino M, Capizzi E, Loda M. Blood and tissue biomarkers in prostate cancer: State of the art. Urol Clin North Am. 2010;37(1):131–141. doi: 10.1016/j.ucl.2009.11.006. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62(4):1008–1013. [PubMed] [Google Scholar]
- 10.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23(32):8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 11.Shanmugam I, Cheng G, Terranova PF, Thrasher JB, Thomas CP, Li B. Serum/glucocorticoid-induced protein kinase-1 facilitates androgen receptor-dependent cell survival. Cell Death Differ. 2007;14(12):2085–2094. doi: 10.1038/sj.cdd.4402227. [DOI] [PubMed] [Google Scholar]
- 12.Zou JX, Guo L, Revenko AS, Tepper CG, Gemo AT, Kung HJ, Chen HW. Androgen-induced coactivator ANCCA mediates specific androgen receptor signaling in prostate cancer. Cancer Res. 2009;69(8):3339–3346. doi: 10.1158/0008-5472.CAN-08-3440. [DOI] [PubMed] [Google Scholar]
- 13.Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21(16):2005–2017. doi: 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tessier M, Woodgett JR. Serum and glucocorticoid-regulated protein kinases: Variations on a theme. J Cell Biochem. 2006;98(6):1391–1407. doi: 10.1002/jcb.20894. [DOI] [PubMed] [Google Scholar]
- 15.Sahoo S, Brickley DR, Kocherginsky M, Conzen SD. Coordinate expression of the PI3-kinase downstream effectors serum and glucocorticoid-induced kinase (SGK-1) and Akt-1 in human breast cancer. Eur J Cancer. 2005;41(17):2754–2759. doi: 10.1016/j.ejca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Mikosz CA, Brickley DR, Sharkey MS, Moran TW, Conzen SD. Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J Biol Chem. 2001;276(20):16649–16654. doi: 10.1074/jbc.M010842200. [DOI] [PubMed] [Google Scholar]
- 17.Wu W, Chaudhuri S, Brickley DR, Pang D, Karrison T, Conzen SD. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64(5):1757–1764. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- 18.Sherk AB, Frigo DE, Schnackenberg CG, Bray JD, Laping NJ, Trizna W, Hammond M, Patterson JR, Thompson SK, Kazmin D, Norris JD, McDonnell DP. Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res. 2008;68(18):7475–7483. doi: 10.1158/0008-5472.CAN-08-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rauhala HE, Porkka KP, Tolonen TT, Martikainen PM, Tammela TL, Visakorpi T. Dual-specificity phosphatase 1 and serum/glucocorticoid-regulated kinase are downregulated in prostate cancer. Int J Cancer. 2005;117(5):738–745. doi: 10.1002/ijc.21270. [DOI] [PubMed] [Google Scholar]
- 20.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 21.Petrylak DP, Ankerst DP, Jiang CS, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99-16. J Natl Cancer Inst. 2006;98(8):516–521. doi: 10.1093/jnci/djj129. [DOI] [PubMed] [Google Scholar]
- 22.Fakih M, Johnson CS, Trump DL. Glucocorticoids and treatment of prostate cancer: A preclinical and clinical review. Urology. 2002;60(4):553–561. doi: 10.1016/s0090-4295(02)01741-7. [DOI] [PubMed] [Google Scholar]
- 23.Yemelyanov A, Czwornog J, Chebotaev D, Karseladze A, Kulevitch E, Yang X, Budunova I. Tumor suppressor activity of glucocorticoid receptor in the prostate. Oncogene. 2007;26(13):1885–1896. doi: 10.1038/sj.onc.1209991. [DOI] [PubMed] [Google Scholar]
- 24.Mohler JL, Chen Y, Hamil K, Hall SH, Cidlowski JA, Wilson EM, French FS, Sar M. Androgen and glucocorticoid receptors in the stroma and epithelium of prostatic hyperplasia and carcinoma. Clin Cancer Res. 1996;2(5):889–895. [PubMed] [Google Scholar]
- 25.Lotan TL, Lyon M, Huo D, Taxy JB, Brendler C, Foster BA, Stadler W, Rinker-Schaeffer CW. Up-regulation of MKK4, MKK6 and MKK7 during prostate cancer progression: An important role for SAPK signalling in prostatic neoplasia. J Pathol. 2007;212(4):386–394. doi: 10.1002/path.2194. [DOI] [PubMed] [Google Scholar]
- 26.Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: Expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23(2):205–212. doi: 10.1038/modpathol.2009.159. [DOI] [PubMed] [Google Scholar]
- 27.Belova L, Delgado B, Kocherginsky M, Melhem A, Olopade OI, Conzen SD. Glucocorticoid receptor expression in breast cancer associates with older patient age. Breast Cancer Res Treat. 2009;116(3):441–447. doi: 10.1007/s10549-008-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan TY, Partin AW, Walsh PC, Epstein JI. Prognostic significance of Gleason score 3 + 4 versus Gleason score 4 + 3 tumor at radical prostatectomy. Urology. 2000;56(5):823–827. doi: 10.1016/s0090-4295(00)00753-6. [DOI] [PubMed] [Google Scholar]
- 29.Wright JL, Salinas CA, Lin DW, Kolb S, Koopmeiners J, Feng Z, Stanford JL. Prostate cancer specific mortality and Gleason 7 disease differences in prostate cancer outcomes between cases with Gleason 4 + 3 and Gleason 3 + 4 tumors in a population based cohort. J Urol. 2009;182(6):2702–2707. doi: 10.1016/j.juro.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein JP, Logan B, Harhoff M, Andersen PK. Analyzing survival curves at a fixed point in time. Stat Med. 2007;26(24):4505–4519. doi: 10.1002/sim.2864. [DOI] [PubMed] [Google Scholar]
- 31.Sterbis JR, Gao C, Furusato B, Chen Y, Shaheduzzaman S, Rav-indranath L, Osborn DJ, Rosner IL, Dobi A, McLeod DG, Sesterhenn IA, Srivastava S, Cullen J, Petrovics G. Higher expression of the androgen-regulated gene PSA/HK3 mRNA in prostate cancer tissues predicts biochemical recurrence-free survival. Clin Cancer Res. 2008;14(3):758–763. doi: 10.1158/1078-0432.CCR-07-1356. [DOI] [PubMed] [Google Scholar]
- 32.Donovan MJ, Osman I, Khan FM, Vengrenyuk Y, Capodieci P, Koscuiszka M, Anand A, Cordon-Cardo C, Costa J, Scher HI. Androgen receptor expression is associated with prostate cancer-specific survival in castrate patients with metastatic disease. BJU Int. 105(4):462–467. doi: 10.1111/j.1464-410X.2009.08747.x. [DOI] [PubMed] [Google Scholar]
- 33.Rosner IL, Ravindranath L, Furusato B, Chen Y, Gao C, Cullen J, Sesterhenn IA, McLeod DG, Srivastava S, Petrovics G. Higher tumor to benign ratio of the androgen receptor mRNA expression associates with prostate cancer progression after radical prostatectomy. Urology. 2007;70(6):1225–1229. doi: 10.1016/j.urology.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Li R, Wheeler T, Dai H, Frolov A, Thompson T, Ayala G. High level of androgen receptor is associated with aggressive clinicopathologic features and decreased biochemical recurrence-free survival in prostate: Cancer patients treated with radical prostatectomy. Am J Surg Pathol. 2004;28(7):928–934. doi: 10.1097/00000478-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koochekpour S. Androgen receptor signaling and mutations in prostate cancer. Asian J Androl. 2010;12(5):639–657. doi: 10.1038/aja.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, Coleman IM, Nguyen HM, Sun H, Nelson PS, Plymate SR. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120(8):2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cleutjens CB, Steketee K, van Eekelen CC, van der Korput JA, Brinkmann AO, Trapman J. Both androgen receptor and glucocorticoid receptor are able to induce prostate-specific antigen expression, but differ in their growth-stimulating properties of LNCaP cells. Endocrinology. 1997;138(12):5293–5300. doi: 10.1210/endo.138.12.5564. [DOI] [PubMed] [Google Scholar]
- 39.Ho KC, Marschke KB, Tan J, Power SG, Wilson EM, French FS. A complex response element in intron 1 of the androgen-regulated 20-kDa protein gene displays cell type-dependent androgen receptor specificity. J Biol Chem. 1993;268(36):27226–27235. [PubMed] [Google Scholar]
- 40.Chen S, Wang J, Yu G, Liu W, Pearce D. Androgen and gluco-corticoid receptor heterodimer formation. A possible mechanism for mutual inhibition of transcriptional activity. J Biol Chem. 1997;272(22):14087–14092. doi: 10.1074/jbc.272.22.14087. [DOI] [PubMed] [Google Scholar]