Abstract
In classical fear conditioning, a neutral conditioned stimulus (CS) is paired with an aversive unconditioned stimulus (US), which leads to a fear memory. If the CS is repeatedly presented without the US after fear conditioning, the formation of an extinction memory occurs, which inhibits fear memory expression. A previous study has demonstrated that selective cholinergic lesions in the medial septum and vertical limb of the diagonal bands of Broca (MS/vDBB) prior to fear and extinction learning disrupt contextual fear memory discrimination and acquisition of extinction memory. MS/vDBB cholinergic neurons project to a number of substrates that are critical for fear and extinction memory. However, it is currently unknown which of these efferent projections are critical for contextual fear memory discrimination and extinction memory. To address this, we induced cholinergic lesions in efferent targets of MS/vDBB cholinergic neurons. These included the dorsal hippocampus (dHipp), ventral hippocampus (vHipp), medial prefrontal cortex (mPFC), and in the mPFC and dHipp combined. None of these lesion groups exhibited deficits in contextual fear memory discrimination or extinction memory. However, vHipp cholinergic lesions disrupted auditory fear memory. Because MS/vDBB cholinergic neurons are the sole source of acetylcholine in the vHipp, these results suggest that MS/vDBB cholinergic input to the vHipp is critical for auditory fear memory. Taken together with previous findings, the results of this study suggest that MS/vDBB cholinergic neurons are critical for fear and extinction memory, though further research is needed to elucidate the role of MS/vDBB cholinergic neurons in these types of emotional memory.
Keywords: fear memory, basal forebrain, acetylcholine, ventral hippocampus, anxiety, fear extinction
Graphical abstract
1.1 Introduction1
When animals are exposed to a conditioned stimulus (CS), such as a tone, that is paired with an unconditioned stimulus (US), like a footshock, they exhibit fear behavior to the tone because the tone predicts the footshock. This phenomenon is referred to as fear conditioning (Pavlov 1927; Phillips and LeDoux 1992; Maren 2001; Rothbaum and Davis 2003; McGaugh 2004; Pare et al. 2004; Fanselow and Wassum 2015). Fear extinction is a phenomenon in which a fear CS is repeatedly presented without the US. As a result of this procedure, learning that the CS no longer predicts the US occurs (i.e. inhibitory extinction memory) (Estes and Skinner 1941; Rescorla 2001; Rothbaum and Davis 2003; Bouton et al. 2006; Quirk et al. 2006; Orsini and Maren 2012; Maren and Holmes 2015). Enhancements in fear memory and deficits in extinction memory have been observed in emotional disorders such as posttraumatic stress disorder (PTSD) and specific phobia (Rothbaum and Davis 2003; Milad et al. 2006; Milad et al. 2008; Bowers and Ressler 2015; Maren and Holmes 2015). Thus, examining the neurobiology of fear and extinction memory is critical to treating these emotional disorders.
A number of studies have implicated the ventral medial prefrontal cortex (vmPFC), hippocampus (Hipp), and amygdala nuclei in fear and extinction memory (Maren 2001; Pare et al. 2004; Bouton et al. 2006; Quirk et al. 2006; Likhtik et al. 2008; Orsini and Maren 2012; Maren et al. 2013; Zelikowsky et al. 2013; Fanselow and Wassum 2015; Maren and Holmes 2015). However, recent studies have identified relatively novel neural substrates that are critical for fear and extinction memory. Medial habenula input to the interpeduncular nucleus is critical for inhibition of fear memory (Zhang et al. 2016) and the paraventricular nucleus of the thalamus may be critical for long-term maintenance of fear memory retrieval (Do-Monte et al. 2015). We have previously observed that cholinergic lesions in the medial septum and vertical limb of the diagonal bands of Broca (MS/vDBB) prior to fear and extinction learning disrupt contextual fear memory discrimination and acquisition of fear extinction (Knox and Keller, 2015). MS/vDBB cholinergic neurons project to multiple areas of the rat brain, including the dorsal hippocampus (dHipp), ventral hippocampus (vHipp), and medial prefrontal cortex (mPFC) (Woolf et al. 1983; Mesulam et al. 1983a; Mesulam et al. 1983b; Woolf et al. 1984). These brain regions are also critical for fear and extinction memory (Quirk et al. 2003; Bouton et al. 2006; Quirk et al. 2006; Quirk et al. 2010; Orsini and Maren 2012). However, it is not known which specific MS/vDBB cholinergic projections are critical for contextual fear memory discrimination and/or extinction memory.
The goal of this project was to identify MS/vDBB cholinergic projection that are critical for contextual fear memory generalization and/or extinction memory. The experimental design is illustrated in Figure 1. We selectively lesioned MS/vDBB cholinergic input to the dHipp, vHipp, and mPFC, as well as combined mPFC and dHipp. These neural substrates were selected because of their roles in fear and extinction memory (Corcoran and Maren 2001; Milad and Quirk 2002; Corcoran and Maren 2004; Corcoran et al. 2005; Sierra-Mercado et al. 2006; Corcoran and Quirk 2007; Sierra-Mercado et al. 2011), as well as contextual processing (Hallock et al. 2013; Maren et al. 2013; Hallock and Griffin 2014; Morici et al. 2015). Cholinergic lesions in none of these neuronal substrates resulted in any deficit in contextual fear memory or extinction memory. However, vHipp cholinergic lesions disrupted auditory fear memory.
Figure 1.
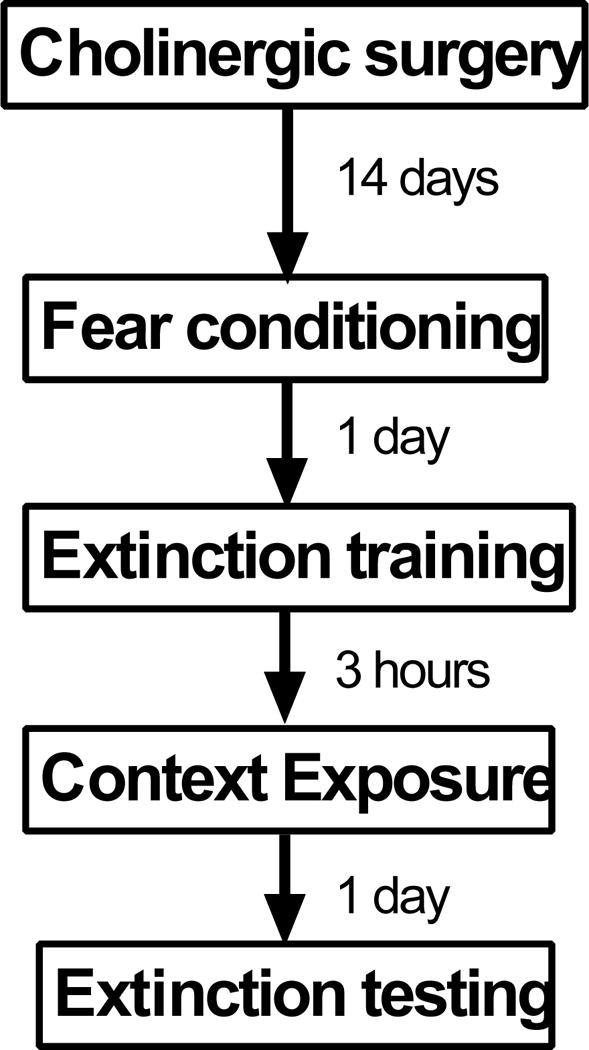
Design used in experiments in this study.
2.1 Methods
Animals
Sixty-six adult male Sprague Dawley rats obtained from Charles River were used in this study. All rats were housed in pairs prior to surgery and were housed individually after surgical procedures. All rats had ad libitum access to water throughout the study and ad libitum access to food until they acclimated (2 days post-arrival), and were fed 23g of food per day (Purina RMH3000), which is the recommended diet from the manufacturer (LabDiet, St. Louis MO). Experimental manipulations commenced after rats had been in the housing colony for at least five days. Rats were on a 12-hour light/dark cycle, with all experimentation occurring during the animals’ light cycle. All experiments were approved by the University of Delaware Institutional Animal Care and Use Committee, following guidelines established by the NIH.
2.1.2 Surgery
All surgeries were conducted in a Kopf stereotaxic surgical frame. We adopted previously described protocols to induce selective cholinergic lesions (Conner et al. 2003; Frick et al. 2004; Knox and Berntson 2006). Efferent projections of MS/vDBB cholinergic neurons were targeted by infusing the selective cholinergic toxin 192-IgG saporin (Advanced Targeting Systems, San Diego CA) directly into the vHipp, dHipp, and/or mPFC. Acetylcholine in the Hipp originates from the MS/vDBB (Woolf et al. 1983; Mesulam et al. 1983a; Mesulam et al. 1983b; Woolf et al. 1984), thus infusing 192-IgG saporin into the vHipp and dHipp selectively results in loss of MS/vDBB input to these respective areas of the Hipp. Even though the mPFC receives cholinergic input from the MS/vDBB and nucleus basalis (Woolf et al. 1983; Mesulam et al. 1983a; Mesulam et al. 1983b; Chandler et al. 2013; Zaborszky et al. 2015), cholinergic lesions in the nucleus basalis and horizontal limb of the diagonal bands of Broca have no effects on fear or extinction memory in the Pavlovian fear conditioning paradigm (Conner et al. 2003; Frick et al. 2004; Knox and Keller 2016). Thus, infusing 192-IgG saporin directly in the mPFC selectively targets a group of MS/vDBB cholinergic neurons that could be critical for fear or extinction memory.
Rats were administered xylazine (12mg/kg, subcutanteously) and general anesthesia was induced using 5% isoflurane in air. Rats were then placed in a stereotaxic apparatus (David Kopf, Tujunga CA) and maintained at a surgical plane using .5 – 2% isoflurane in air. Burr holes were drilled in the skull to allow for insertion of a 5μL Hamilton syringe with a 26-gauge needle into the brain. The coordinates used for drilling the holes were taken with respect to Bregma from the atlas of Paxinos and Watson (1998). Coordinates were as follows. dHipp: DV −3.2mm, ML +/− 2.1mm, and +/− 2.4mm, AP −3.4mm and −2.4mm; vHipp: DV −6.2mm, ML +/− 5.2mm and +/− 5.4mm, AP −5.2mm and −6mm; mPFC: DV −4.4mm, ML +/− 0.8mm, AP +3.00mm. In another group of rats cholinergic lesions were induced in the dHipp and mPFC together (COM-lesion). 192-IgG saporin was infused into all brain regions at a concentration of .2μg/μL dissolved in .2M phosphate buffered saline (PBS). The total volume of each injection was .5μL. Sham surgeries were accomplished using the same volume (.5μL) of PBS.
2.1.3 Behavioral Testing
All sessions were conducted in identical rodent observation chambers constructed of aluminum and Plexiglas (30 × 24 × 21 cm; MED Associates, St. Albans, VT), situated in sound-attenuating chambers and located in an isolated room. Fear conditioning and extinction training was conducted as previously described (Knox et al. 2012). Briefly, a 10s auditory CS (2kHz, 80 dB) co-terminated with a footshock US (1s, 1mA) five times in a distinct context (fear conditioning context). One day after fear conditioning, auditory extinction training started and consisted of 30 CS-only presentations in a distinct context (extinction context). Baseline levels of freezing prior to CS presentation in the extinction context was used as a measure of contextual fear memory discrimination (Knox and Keller 2016). Three hours after auditory extinction training, rats were exposed to the extinction context for one hour. Adopting this procedure lowers baseline freezing in an extinction test (Chang et al. 2009; Knox and Keller 2016). One day after extinction training all animals were tested for extinction in the extinction context by presenting 10 CSs. Distinct contexts were achieved by manipulating auditory, visual, tactile, and odor stimulation as previously described (Knox et al., 2012).
One day after extinction testing, rats were euthanized via rapid decapitation and brains were removed, frozen in isopentane that had been chilled on dry ice, and stored in a −80 °C freezer until further processing.
2.2.1 Acetylcholinesterase staining
To verify lesions, acetylcholinesterase (AChE) stains were performed to measure cholinergic fiber loss. Brains were sliced in a cryostat at a temperature of −13 °C at a thickness of 30μm. Slices were mounted onto glass slides and stored in at −80 °C until staining. Glass slides with mPFC, dHipp, and vHipp sections were treated for visualization of AChE in order to measure the cholinergic fiber loss in these brain regions. AChE histology was conducted as previously described (Tago et al. 1986), with some modifications. Slides were fixed for 2 hours in 4% paraformaldehyde in .2M PBS, rinsed with .1 M maleate buffer (pH 6.0), and incubated for 45 minutes in a solution consisting of 20 mg of acetylthiocholine iodide, 448 mg sodium citrate, 100 mg copper sulfate, and 65.6 mg potassium ferricyanide in 200 mL of .1 M maleate buffer. Sections were then rinsed in .1M Tris buffered saline (TBS) and incubated for 10 minutes in a solution consisting of 100 mg diaminobenzidine (DAB), 750 mg nickel ammonium sulfate, and 20μL of a 30% H2O2 solution in 250mL of TBS. Slides were then rinsed with TBS, dehydrated in ethanol, left for two to four hours in xylene, and coverslipped using DPX mountant (Sigma-Aldrich Inc).
2.2.2 Choline Acetyltransferase immunostaining
Immunocytochemistry was used to visualize choline acetyltransferase (ChAT) cells in MS/vDBB regions. Slides were fixed for 2-3 hours in 4% paraformaldehyde solution, and then incubated in .1% Triton X-100 in TBS. Slides were next incubated in a 3% goat serum solution in TBS. Slides were washed in TBS and exposed to a primary rabbit ChAT polyclonal antibody (Millipore Inc., AB143) at a concentration of 1:500 (in PBS) overnight at 4°C. After this slides were washed in TBS and visualization of the ChAT primary antibody was accomplished using an ABC kit (Vector Lab, Burlingame CA, pk-6101) with a goat anti-rabbit IgG secondary antibody according to the manufacturer’s instructions. Sections were then dehydrated in ethanol, left for four hours in xylene, and coverslipped using DPX mountant.
2.3.1 Data Analysis
Freezing was scored using Any-maze software (Stoelting Inc., Kiel WI) as previously described (Knox et al. 2012) and averaged across CS presentation and a corresponding ITI (e.g. CS1 and ITI1) for each CS presentation. Freezing during fear conditioning was divided into baseline freezing and fear conditioning (FC) trials (FC trials 1-5). Baseline freezing was subjected to t-test (lesion vs. sham), while FC trials were analyzed using a surgery (lesion vs. sham) x FC trial (1-5) factor design. Freezing during extinction training was divided into baseline, an auditory fear memory retrieval (FMR) trial that consisted of averaged freezing during the first four CS presentations, and extinction trials that were comprised of CS presentations 5-30 averaged into blocks of two trials (Blocks 1-13). Freezing during baseline and the FMR trial was analyzed using a surgery x FMR trial (baseline vs. FMR) factor design. Freezing during the extinction training trials were analyzed using a surgery x block (Extinction training block 1-13) factor design. Freezing during extinction testing was divided into baseline and extinction testing trials that were comprised of CS-induced freezing averaged into blocks of two trials. Baseline freezing was analyzed using t-test. Freezing during extinction testing trials was analyzed using a surgery x extinction testing block (1-5) factor design.
AChE and ChAT sections were imaged with a 2.5× objective using a Leitz Dialux 20 microscope with attached 20MB Cannon Rebel T5i camera. AChE fiber density was scored with ImageJ software. AChE density was scored using a previously defined method (Knox and Keller 2016). Briefly, the optical density (OD) of AChE fiber staining was compared to OD values in white matter sections from the corpus callosum. All values were then normalized relative to OD values of sham rats. When there is an absence of AChE fibers in cortical or hippocampal regions these normalized scores never approach zero, because OD values in these regions are always higher than OD values in whiter matter. Nevertheless using OD values in cortical and hippocampal regions to characterize AChE loss provides an objective unbiased estimate of AChE loss (Knox and Keller 2016). These normalized OD scores were then subjected to t-test (lesion vs. sham) for each brain region analyzed.
ChAT images were imported into ImageJ. A 100 × 100 unit square was then placed into different places in either the MS or vDBB and ChAT positive cells within the square were manually counted. Placement of the square was non-overlapping and a minimum of eight MS and vDBB brain sections were analyzed using this method. The average cell/unit square was then analyzed using t-test (lesion vs. sham).
Main and simple effects were analyzed using analysis of variance (ANOVA) while main and simple comparisons were analyzed using t-test with a Bonferroni correction applied where needed. A p-value of less than .05 was used as the statistical criterion of significance for all statistical tests.
3.1 Results
3.1.1 vHipp cholinergic lesions disrupt auditory fear memory
The density of AChE fibers in the vHipp was lower in vHipp-lesion rats (n = 9) in comparison to vHipp-sham rats (n = 6) [vCA1 t(12) = −2.256, p = .044, vDG t(12) = −3.074, p = .01]. In this experiment, and in all other experiments, AChE loss was only observed in the targeted brain region (e.g. vHipp) and was not observed in other MS/vDBB efferent targets (e.g. mPFC) (i.e. no collateral loss of AChE fibers (see Supplemental material)). There was no detectable loss of ChAT positive cells in the MS/vDBB in vHipp-lesion rats (p > .05). These results are illustrated in Figures 2A-B.
Figure 2.
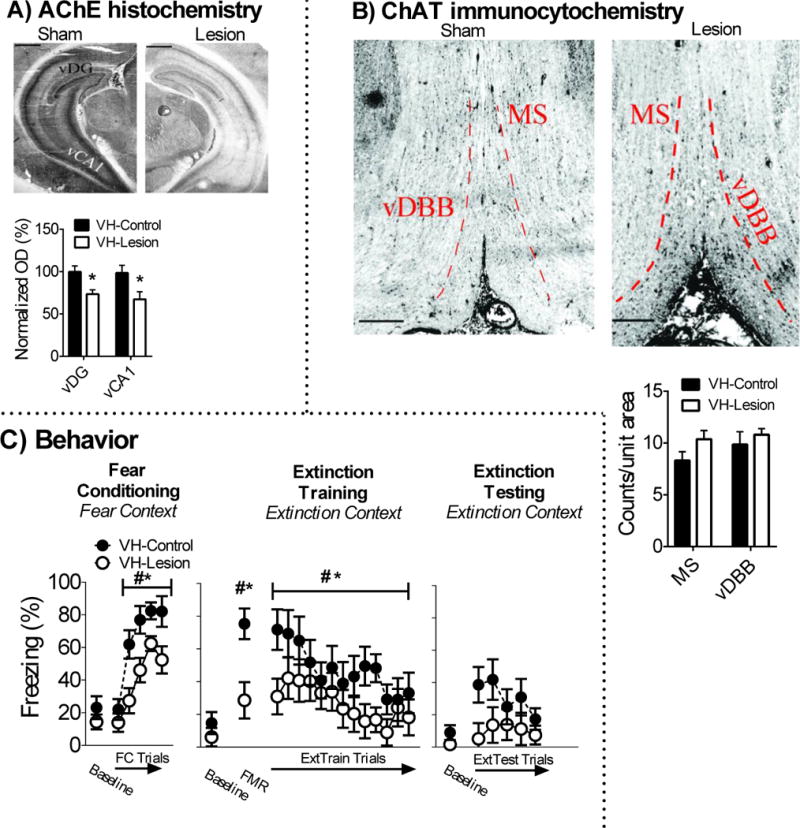
Cholinergic lesions in the vHipp disrupt auditory fear memory. A) Infusion of 192-IgG saporin into the vHipp attenauted AChE fiber expression in the vHipp, but B) had no effect on ChAT positive cell counts in the MS/vDBB. C) vHipp cholinergic lesions disrupted acquisition and retention of fear memory. vHipp cholinergic lesions had no effect on baseline freezing during extinction training in the extinction context (i.e. contextual fear memory discrimination) or acquistion and retention of extinction memory. # indicates main effect of trial while * indicates main effect of surgery. Scale bar on images represent 1mm.
Baseline freezing was equivalent between vHipp-lesion and vHipp-sham rats in the fear conditioning context (p > .05). All rats acquired conditioned fear in the fear conditioning context, which was revealed by a main effect of FC trial [F(4,52) = 32.874, p < .001]. vHipp cholinergic lesions disrupted acquisition of conditioned fear. This was revealed by a main effect of surgery for FC Trials [F(1,13) = 7.132, p = .019] and no significant difference for baseline freezing (p > .05). Baseline freezing was low in the extinction context and was not significantly different between vHipp-lesion and sham rats (p > .05), which suggests vHipp cholinergic lesions had no effect on contextual fear memory discrimination. All rats expressed conditioned fear, which was revealed by a main effect of FMR trial [F(1,14) = 54.058, p < .001]. vHipp cholinergic lesions disrupted expression of conditioned fear. This was revealed by a surgery x FMR trial interaction [F(1,14) = 11.371, p = .005], which was driven by attenuated freezing in vHipp lesion rats during the FMR trial, but not at baseline (p > .05). All rats acquired fear extinction during extinction training, which was revealed by a significant main effect of extinction training block [F(12,168) = 4.457, p = .001]. There was a significant surgery x extinction training block interaction on the cubic trend analysis [F(1,14) = 4.673, p = .048]. This was driven by lower levels of freezing in vHipp-lesion rats during the start of extinction training. However, there was no significant surgery or surgery x extinction testing block interaction during extinction testing (ps > .05). These results are illustrated in Figure 2C.
3.1.2 dHipp cholinergic lesions alter auditory fear memory expression without having any effects on fear or extinction memory
dHipp AChE fiber staining was lower in dHipp-lesion (n = 7) rats in comparison to dHipp-sham (n = 7) rats [CA1 t(11) = −5.438, p < .001, CA3 t(11) = −3.944, p = .002, DG t(11) = −5.126, p < .001]. There was no detectable loss of ChAT positive cells in the MS/vDBB of dHipp-lesion rats (ps > .05). These results are illustrated in Figures 3A-B.
Figure 3.
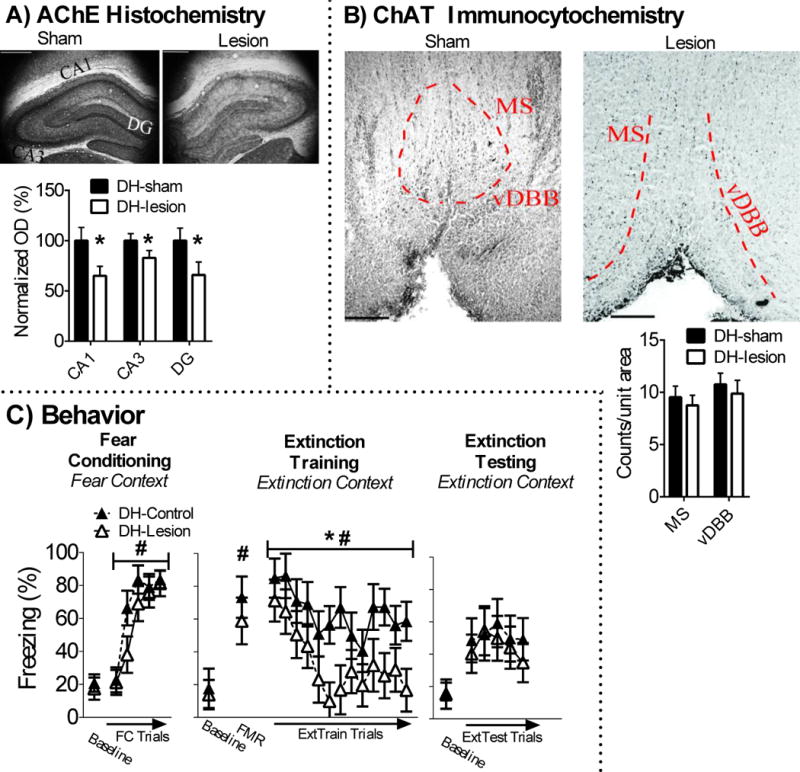
Cholinergic lesions in the dHipp have no effect on fear or extinction memory. A) Infusion of 192-IgG saporin into the dHipp attenauted AChE fiber expression in the dHipp, but B) had no effect on ChAT positive cell counts in the MS/vDBB. C) dHipp cholinergic lesions had no effect on conditioned freezing during fear conditioning, but decreased conditioned freezing during extinction training trials. However, dHipp cholinergic lesions had no effect on conditioned freezing during extinction testing, which suggests this treatment had no effect on extinction memory.
Baseline freezing was equivalent between dHipp-lesion and dHipp-sham rats in the fear conditioning context (p > .05). All rats acquired conditioned fear in the fear conditioning context, which was revealed by a main effect of FC trial [F(4,48) = 47.57, p < .001]. There was no surgery or surgery x FC-trial interactions (ps > .05), which suggested that dHipp cholinergic lesions had no effect on acquisition of conditioned fear. Baseline freezing in the extinction context was not significantly different between dHipp-lesion and sham rats (p > .05), which suggests dHipp cholinergic lesions had no effect on contextual fear memory discrimination. All rats expressed conditioned fear, which was revealed by a main effect of FMR trial [F(1,12) = 38.324, p < .001]. There were no main or interaction effects of surgery (ps > .05), which suggests dHipp cholinergic lesions had no effect on expression of auditory fear memory. All rats acquired fear extinction during extinction training, which was revealed by a significant main effect of extinction training block [F(12,144) = 5.583, p < .001]. There was a significant main effect of surgery [F(1,12) = 5.105, p = .043]. This was driven by lower levels of freezing in dHipp-lesion rats during extinction training. There was no significant main or interaction effects of surgery during extinction testing (ps > .05), which suggests dHipp cholinergic lesions disrupted expression of conditioned fear during extinction training and not extinction memory. These results are illustrated in Figure 3C.
3.1.3 mPFC cholinergic lesions have no effects on fear or extinction memory
AChE fiber staining was lower in the ACC [t(16) = 2.967, p = .009] and IL [t(16) = 2.583, p = .02], but not PL [t(16) = 1.767, p = .09] of mPFC-lesion (n = 9) rats in comparison to mPFC-sham (n = 9) rats. There was no detectable loss of ChAT positive cells in the MS/vDBB of mPFC-lesion rats (p > .05). These results are illustrated in Figures 4A-B.
Figure 4.
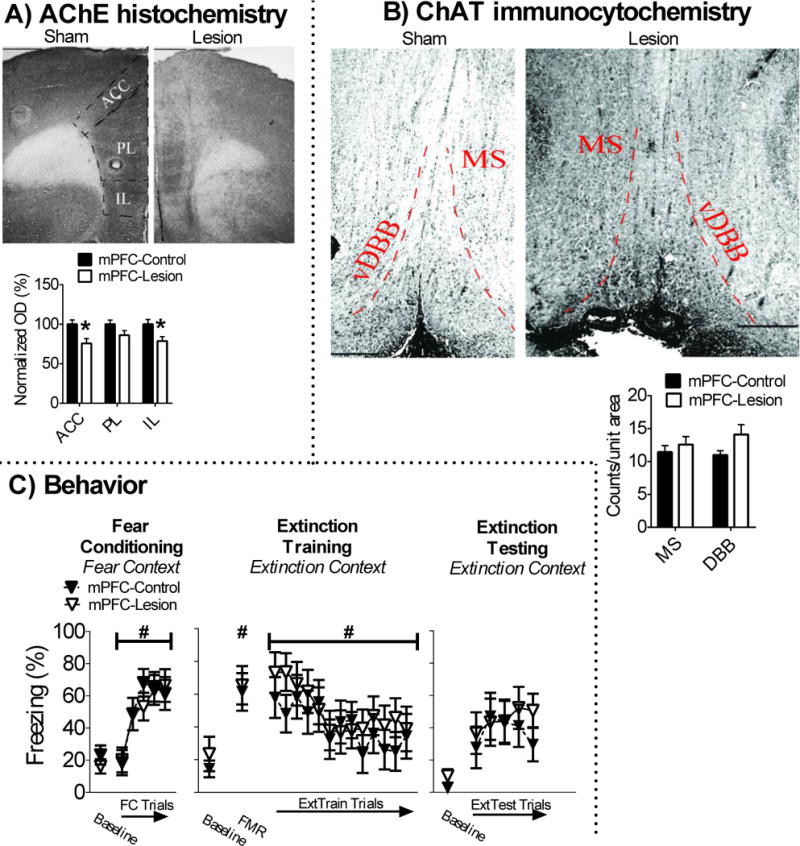
Cholinergic lesions in the mPFC have no effect on fear or extinction memory. A) Infusion of 192-IgG saporin into the mPFC attenauted AChE fiber expression in the ACC and IL, but not PL. B) This treatment also had no effect on ChAT positive cell counts in the MS/vDBB. C) mPFC cholinergic lesions had no effect on conditioned freezing during fear conditioning, extinction training, or extinction testing.
Baseline freezing was equivalent between mPFC-lesion and mPFC-sham rats in the fear conditioning context (p > .05). All rats acquired conditioned fear in the fear conditioning context, which was revealed by a main effect of FC trial [F(4,64) = 24.497, p < .001]. There was no main or interaction effects of surgery (ps > .05), which suggests that mPFC cholinergic lesions had no effect on acquisition of conditioned fear. Baseline freezing in the extinction context was not significantly different between mPFC-lesion and sham rats (p > .05), which suggests mPFC cholinergic lesions had no effect on contextual fear memory discrimination. All rats expressed conditioned fear, which was revealed by a main effect of FMR trial [F(1,16) = 42.87, p < .001]. There were no main or interaction effects of surgery (ps > .05), which suggest mPFC cholinergic lesions had no effect on expression of auditory fear memory. All rats acquired fear extinction during extinction training, which was revealed by a significant main effect of extinction training block [F(12,192) = 5.167, p < .001]. There were no main or interaction effects of surgery (ps > .05). There were also no significant main or interaction effects of surgery during extinction testing (ps > .05). These findings suggest that mPFC cholinergic lesions had no effect on extinction memory. These results are illustrated in Figure 4C.
3.1.4 Combined mPFC and dHipp cholinergic lesions have no effects on fear or extinction memory
dHipp [CA1 t(17) = −2.168, p = .045, CA3 t(17) = −1.429, p = .171, DG t(17) = −1.295, p = .273] and mPFC [ACC t(16) = 2.741, p = .015, PL t(16) = 1.685, p = .111, IL t(16) = 1.904, p = .075], AChE fiber staining was lower in COM-lesion (n = 9) rats in comparison to COM-sham (n = 10) rats, although most AChE loss occurred in the CA1 of the dHipp and in the ACC of the mPFC. There was no detectable loss of ChAT positive cells in the MS/vDBB of COM-lesion rats relative to sham rats (p > .05). These results are illustrated in Figures 5A-B.
Figure 5.
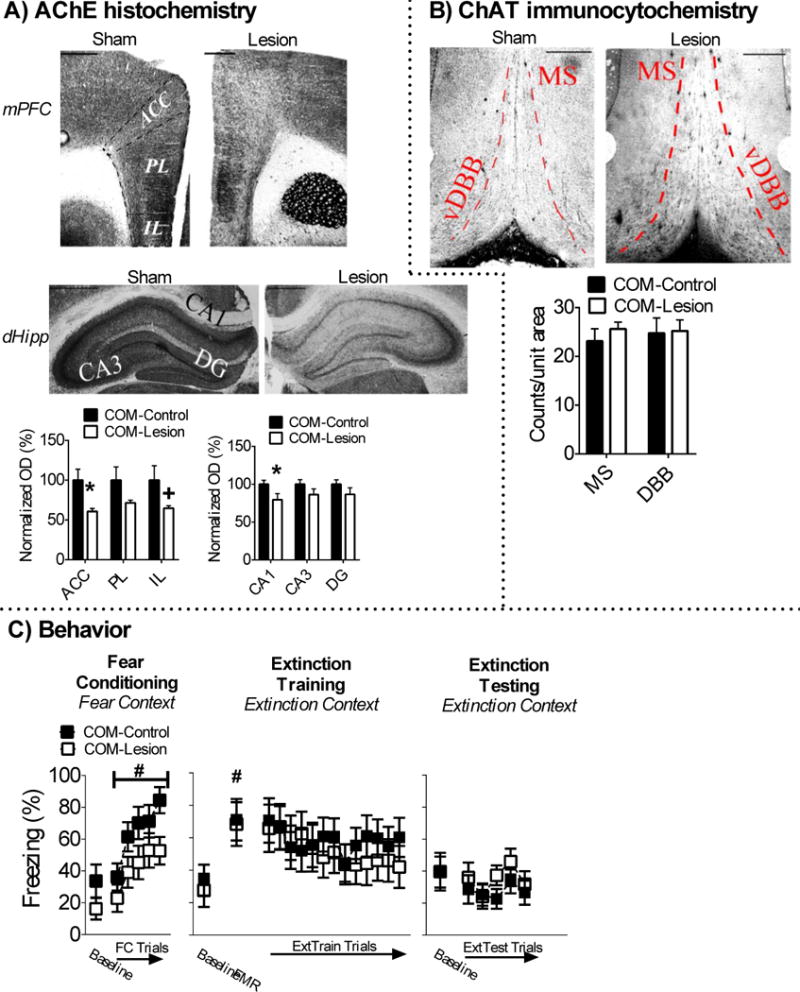
Cholinergic lesions in the dHipp and mPFC (COM-lesion) have no effect on fear or extinction memory. A) Infusion of 192-IgG saporin into the dHipp (CA1, but not CA3 and DG) and mPFC (ACC, approached statistical difference in IL, not PL) attenauted AChE fiber expression. B) These treatments had had no effect on ChAT positive cell counts in the MS/vDBB. C) COM-lesions had no effect on conditioned freezing during fear conditioning, extinction training, or extinction testing.
Baseline freezing in the fear conditioning context was equivalent between COM-lesion and COM-sham rats (p > .05). All rats acquired conditioned fear in the fear conditioning context, which was revealed by a main effect of FC trial [F(4,68) = 22.266, p < .001]. There was no main or interaction effects of surgery (ps > .05), which suggests that combined cholinergic lesions did not alter acquisition of auditory fear memory. Baseline freezing in the extinction context was not significantly different between COM-lesion and sham rats (p > .05), which suggests that the combined mPFC and dHipp cholinergic lesions had no effect on contextual fear memory discrimination. All rats expressed conditioned fear, which was revealed by a main effect of FMR trial [F(1,17) = 23.802, p < .001]. There were no main or interaction effects of surgery (ps > .05), which suggests that the combined cholinergic lesions had no effect on expression of auditory fear memory. There was no main effect of extinction training trial, because levels of conditioned freezing did not decrease across extinction training block (ps > .05). Nevertheless, further analysis revealed that all rats did acquire extinction memory (see Supplemental data). There were no main or interaction effects of surgery (ps > .05) during extinction training. There were also no significant main or interaction effects of surgery during extinction testing (ps > .05). These findings suggest that combined cholinergic lesions had no effect on extinction memory. These results are illustrated in Figure 5C.
4.1 Discussion
Our results suggest that MS/vDBB cholinergic projections to the vHipp are critical for auditory fear memory. The MS/vDBB is the sole source of acetylcholine in the vHipp (see Introduction) and thus cholinergic lesions applied in the vHipp removes MS/vDBB cholinergic input to the vHipp. Rats with vHipp cholinergic lesions showed weaker acquisition and expression of conditioned freezing, though they still exhibited acquisition and expression of conditioned fear (see Results). These findings suggest that vHipp cholinergic lesions did not disrupt freezing behavior, but instead disrupted acquisition and expression of auditory fear memory. This assertion is consistent with previous findings that suggest the vHipp is critical for fear memory (Fanselow and Dong 2010; Orsini et al. 2011; Sierra-Mercado et al. 2011; Orsini and Maren 2012). However, it is unknown if MS/vDBB cholinergic input to the vHipp facilitates fear memory formation or is critical for fear memory/retrieval expression. The vHipp has been implicated in auditory fear memory where temporarily disrupting neural activity in the vHipp during fear conditioning decreases retrieval of auditory fear memory when animals are drug free (Bast et al. 2001; Maren and Holt 2004; Hunsaker and Kesner 2008). However, there is no consensus about mechanisms via which the vHipp facilitates auditory fear memory formation. Enhanced neural synchrony is observed during fear memory formation and retrieval (Seidenbecher et al. 2003; Lesting et al. 2011). The vHipp has extensive outputs to many nodes within the fear circuit (Fanselow and Dong 2010; Orsini et al. 2011; Sotres-Bayon et al. 2012; Herry and Johansen 2014) and MS/DBB cholinergic neurons are critical for generating rhythmic activity (Smythe et al. 1992; Tsanov 2015; Dannenberg et al. 2016; Gu et al. 2017). MS/vDBB input to the vHipp could facilitate auditory fear memory formation by facilitating synchronous activity within the fear circuit. Other studies have suggested that the vHipp is critical for expression/retrieval of auditory fear memory (Zhang et al. 2001; Herry et al. 2008; Orsini et al. 2011). MS/vDBB neurons that project to the vHipp could modulate auditory fear memory by modulating neural activity in the vHipp critical for expression of auditory fear memory. Further research is needed to determine how MS/vDBB cholinergic input to the vHipp facilitates auditory fear memory.
Cholinergic lesions in none of the efferent targets of MS/vDBB cholinergic neurons had any effect on contextual fear memory discrimination or acquisition of fear extinction. This is surprising given the roles of the mPFC, dHipp, and vHipp in contextual memory, fear memory, and extinction memory (Maren 2001; Pare et al. 2004; Bouton et al. 2006; Orsini and Maren 2012; Maren et al. 2013). Also, other studies have observed that cholinergic manipulation in the dHipp and mPFC disrupts fear and extinction memory (for review see Knox 2016). Acetylcholine in the dHipp is enhanced during contextual fear conditioning (Nail-Boucherie et al. 2000; Kart et al. 2004) and pharmacological manipulation of nicotinic and muscarinic receptors in the dHipp modulates contextual fear memory (Izquierdo and Medina 1997; Wallenstein and Vago 2001; Rogers and Kesner 2004; Davis and Gould 2007; Kenney et al. 2012). Muscarinic receptor antagonism in the IL also disrupts extinction memory (Santini et al. 2012).
It is unclear why different methods of manipulating cholinergic input in the dHipp and mPFC produces contrasting results. One possibility could be that basal forebrain cholinergic input to the dHipp and mPFC modulates contextual fear memory and extinction memory, respectively, but this input is not necessary for either phenomena. Thus, temporarily manipulating cholinergic receptors in these neural substrates have effects, but permanent removal of cholinergic input to these brain regions does not, because a compensatory neural process facilitates fear and extinction memory with disrupted MS/vDBB input to the dHipp and/or mPFC. Another possibility is that more loss of MS/vDBB cholinergic input to efferent targets is needed to see effects on contextual fear memory discrimination and extinction memory. This is particularly so because in all experiments we targeted a relatively small percentage of MS/vDBB cholinergic neurons since none of the cholinergic lesions in efferent targets of MS/vDBB resulted in ChAT-positive cell loss in the MS/vDBB (see Results). In this study, infusing the cholinergic toxin into the mPFC did not result in AChE loss in the PL (see Results). This raises the possibility that MS/vDBB cholinergic input to the PL is critical for contextual fear memory discrimination and/or acquisition of fear extinction. This explanation is unlikely, because cholinergic lesions in the MS/vDBB do not result in AChE loss in the PL (Knox and Keller 2016). MS/vDBB cholinergic neurons innervate the medial habenula (though this is not the primary cholinergic efferent to the medial habenula (Woolf and Butcher 1986)). A recent study has shown that cholinergic neurons in the medial habenula are critical for inhibition of fear memory (Zhang et al. 2016). However, we have observed that infusing 192-IgG saporin into the medial dorsal thalamus (including medial habenula) has no effect on fear or extinction memory (Staib and Knox 2016). Another possibility is that cholinergic interneurons (which are present throughout the basal forebrain (Woolf et al. 1983; Mesulam et al. 1983a; Mesulam et al. 1983b; Woolf et al. 1984; Zaborszky et al. 2015)) in the MS/vDBB are critical for contextual fear memory discrimination and acquisition of fear extinction, but cholinergic projection neurons are not. Lastly, removal of MS/vDBB cholinergic input to the vHipp disrupted acquisition of fear memory and this may have generated a floor effect (i.e. too low levels of freezing), which made it difficult to examine if MS/vDBB cholinergic input to the vHipp also contributes to extinction memory. Of course, further research is needed to determine how MS/vDBB cholinergic neurons contribute to contextual fear memory discrimination and acquisition of fear extinction.
5.1 Conclusion
Our results demonstrate that MS/vDBB cholinergic input to the vHipp is critical for auditory fear memory. A previous study has observed that MS/vDBB cholinergic lesions disrupt contextual fear memory discrimination and acquisition of fear extinction (Knox and Keller 2016). Together, these studies suggest that MS/vDBB cholinergic neurons are critical for emotional memory. More research is needed to elucidate the mechanisms via which MS/vDBB cholinergic neurons mediate emotional memory.
Finally we observed that infusing the cholinergic toxin, which targets the p75 receptor, into the mPFC did not result in loss of AChE fibers in the PL (see Results). This suggests that there are cholinergic neurons that innervate the PL that do not express the p75 receptor. Given the importance of the PL in fear memory expression (Corcoran and Quirk 2007; Sierra-Mercado et al. 2011), research examining the role of these cholinergic neurons in fear memory needs to be explored.
Supplementary Material
HIGHLIGHTS.
vHipp cholinergic lesions disrupt auditory fear memory
MS/vDBB cholinergic neurons are sole source of acetylcholine in vHipp
MS/vDBB cholinergic input to vHipp is critical for auditory fear memory
Acknowledgments
The work was supported by NIH grant 1P20GM103653 and start-up funding provided by the University of Delaware. We would also like to thank all of the undergraduate students who helped with scoring data in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AChE – Acetylcholinesterase
ANOVA – Analysis of variance
ChAT – Choline acetyltransferase
CS – Conditioned stimulus
DAB – diaminobenzedine
dHipp – Dorsal hippocampus
FC – Fear conditioning
FMR – Fear memory retrieval
Hipp – Hippocampus
mPFC – Medial prefrontal cortex
MS/vDBB – Medial septum and diagonal band of Broca
OD – Optical density
PBS – Phosphate buffered saline
PTSD – Post traumatic stress disorder
TBS – Tris buffered saline
US – Unconditioned stimulus
vHipp – Ventral hippocampus
vmPFC – Ventromedial prefrontal cortex
References
- Bast T, Zhang WN, Feldon J. The ventral hippocampus and fear conditioning in rats. Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABA(A) agonist muscimol. Exp Brain Res. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bowers ME, Ressler KJ. An Overview of Translationally Informed Treatments for Posttraumatic Stress Disorder: Animal Models of Pavlovian Fear Conditioning to Human Clinical Trials. Biological Psychiatry. 2015;78:E15–E27. doi: 10.1016/j.biopsych.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DJ, Lamperski CS, Waterhouse BD. Identification and distribution of projections from monoaminergic and cholinergic nuclei to functionally differentiated subregions of prefrontal cortex. Brain Res. 2013;1522:38–58. doi: 10.1016/j.brainres.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Knapska E, Orsini CA, Rabinak CA, Zimmerman JM, Maren S. Fear extinction in rodents. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.ns0823s47. Chapter 8: Unit8 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH. Lesions of the Basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron. 2003;38:819–829. doi: 10.1016/s0896-6273(03)00288-5. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg H, Hinman JR, Hasselmo ME. Potential roles of cholinergic modulation in the neural coding of location and movement speed. J Physiol Paris. 2016;110:52–64. doi: 10.1016/j.jphysparis.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2007;190:343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Monte FH, Quinones-Laracuente K, Quirk GJ. A temporal shift in the circuits mediating retrieval of fear memory. Nature. 2015;519:460–463. doi: 10.1038/nature14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes W, Skinner B. Some quantitative properties of anxiety. J Exp Psychol. 1941;29:390. [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Wassum KM. The Origins and Organization of Vertebrate Pavlovian Conditioning. Cold Spring Harb Perspect Biol. 2015;8:a021717. doi: 10.1101/cshperspect.a021717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Kim JJ, Baxter MG. Effects of complete immunotoxin lesions of the cholinergic basal forebrain on fear conditioning and spatial learning. Hippocampus. 2004;14:244–254. doi: 10.1002/hipo.10169. [DOI] [PubMed] [Google Scholar]
- Gu Z, Alexander GM, Dudek SM, Yakel JL. Hippocampus and Entorhinal Cortex Recruit Cholinergic and NMDA Receptors Separately to Generate Hippocampal Theta Oscillations. Cell Rep. 2017;21:3585–3595. doi: 10.1016/j.celrep.2017.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Griffin AL. Spatial working memory deficits accompany reductions in hippocampal-prefrontal synchrony following inactivation of the ventral midline thalamic reuniens and rhomboid nuclei. Society for Neuroscience; Washington DC: 2014. [Google Scholar]
- Hallock HL, Wang A, Shaw CL, Griffin AL. Transient inactivation of the thalamic nucleus reuniens and rhomboid nucleus produces deficits of a working-memory dependent tactile-visual conditional discrimination task. Behav Neurosci. 2013;127:860–866. doi: 10.1037/a0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci. 2014;17:1644–1654. doi: 10.1038/nn.3869. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol Learn Mem. 2008;89:61–69. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Kart E, Jocham G, Muller CP, Schlomer C, Brandao ML, Huston JP, de Souza Silva MA. Neurokinin-1 receptor antagonism by SR140333: enhanced in vivo ACh in the hippocampus and promnestic post-trial effects. Peptides. 2004;25:1959–1969. doi: 10.1016/j.peptides.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Raybuck JD, Gould TJ. Nicotinic receptors in the dorsal and ventral hippocampus differentially modulate contextual fear conditioning. Hippocampus. 2012;22:1681–1690. doi: 10.1002/hipo.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D. The role of basal forebrain cholinergic neurons in fear and extinction memory. Neurobiol Learn Mem. 2016;133:39–52. doi: 10.1016/j.nlm.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Berntson GG. Effect of nucleus basalis magnocellularis cholinergic lesions on fear-like and anxiety-like behavior. Behavioral Neuroscience. 2006;120:307–312. doi: 10.1037/0735-7044.120.2.307. [DOI] [PubMed] [Google Scholar]
- Knox D, Keller SM. Cholinergic neuronal lesions in the medial septum and vertical limb of the diagonal bands of Broca induce contextual fear memory generalization and impair acquisition of fear extinction. Hippocampus. 2016;26:718–726. doi: 10.1002/hipo.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Nault T, Henderson C, Liberzon I. Glucocorticoid Receptors And Extinction Retention Deficits In The Single Prolonged Stress Model. Neuroscience. 2012;223:163–173. doi: 10.1016/j.neuroscience.2012.07.047. [DOI] [PubMed] [Google Scholar]
- Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, Pape HC. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS One. 2011;6:e21714. doi: 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Holmes A. Stress and Fear Extinction. Neuropsychopharmacology. 2015;41:58–79. doi: 10.1038/npp.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav Neurosci. 2004;118:97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983a;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983b;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Morici JF, Bekinschtein P, Weisstaub NV. Medial prefrontal cortex role in recognition memory in rodents. Behav Brain Res. 2015;292:241–251. doi: 10.1016/j.bbr.2015.06.030. [DOI] [PubMed] [Google Scholar]
- Nail-Boucherie K, Dourmap N, Jaffard R, Costentin J. Contextual fear conditioning is associated with an increase of acetylcholine release in the hippocampus of rat. Brain Res Cogn Brain Res. 2000;9:193–197. doi: 10.1016/s0926-6410(99)00058-0. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Pavlov I. Conditioned refelexes. Oxford University Press; London: 1927. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Pare D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A. Erasing fear memories with extinction training. J Neurosci. 2010;30:14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Experimental extinction. In: Mowrer RR, Klein SB, editors. Handbook of Contemporary Learning Theories. Lawrence Erlbaum Associates; Mahwah NJ: 2001. [Google Scholar]
- Rogers JL, Kesner RP. Cholinergic modulation of the hippocampus during encoding and retrieval of tone/shock-induced fear conditioning. Learn Mem. 2004;11:102–107. doi: 10.1101/lm.64604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Santini E, Sepulveda-Orengo M, Porter JT. Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction. Neuropsychopharmacology. 2012;37:2047–2056. doi: 10.1038/npp.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe JW, Colom LV, Bland BH. The extrinsic modulation of hippocampal theta depends on the coactivation of cholinergic and GABA-ergic medial septal inputs. Neurosci Biobehav Rev. 1992;16:289–308. doi: 10.1016/s0149-7634(05)80203-9. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76:804–812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staib JM, Knox D. Society for Neuroscience. Society for Neuroscience; San Diego CA: 2016. The role of cholinergic input from the medial septum in cued and contextual fear extinction memory. [Google Scholar]
- Tago H, Kimura H, Maeda T. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J Histochem Cytochem. 1986;34:1431–1438. doi: 10.1177/34.11.2430009. [DOI] [PubMed] [Google Scholar]
- Tsanov M. Septo-hippocampal signal processing: breaking the code. Prog Brain Res. 2015;219:103–120. doi: 10.1016/bs.pbr.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Wallenstein GV, Vago DR. Intrahippocampal scopolamine impairs both acquisition and consolidation of contextual fear conditioning. Neurobiol Learn Mem. 2001;75:245–252. doi: 10.1006/nlme.2001.4005. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Eckenstein F, Butcher LL. Cholinergic projections from the basal forebrain to the frontal cortex: a combined fluorescent tracer and immunohistochemical analysis in the rat. Neurosci Lett. 1983;40:93–98. doi: 10.1016/0304-3940(83)90285-9. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Eckenstein F, Butcher LL. Cholinergic systems in the rat brain: I. projections to the limbic telencephalon. Brain Research Bulletin. 1984;13:751–784. doi: 10.1016/0361-9230(84)90236-3. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, Nadasdy Z. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cereb Cortex. 2015;25:118–137. doi: 10.1093/cercor/bht210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Bissiere S, Hast TA, Bennett RZ, Abdipranoto A, Vissel B, Fanselow MS. Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proc Natl Acad Sci U S A. 2013;110:9938–9943. doi: 10.1073/pnas.1301691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tan L, Ren Y, Liang J, Lin R, Feng Q, Zhou J, Hu F, Ren J, Wei C, et al. Presynaptic Excitation via GABAB Receptors in Habenula Cholinergic Neurons Regulates Fear Memory Expression. Cell. 2016;166:716–728. doi: 10.1016/j.cell.2016.06.026. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behav Brain Res. 2001;126:159–174. doi: 10.1016/s0166-4328(01)00256-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


