Abstract
Ovulation, the process of releasing a mature oocyte from the ovary, is crucial for animal reproduction. In order for the process of ovulation to occur, a follicle must be fully matured and signaled to rupture from the ovary. During follicle rupture in both mammals and Drosophila, somatic follicle cells are enzymatically degraded to allow the oocyte to be liberated from the follicle. Here, we describe a detailed protocol of our newly developed ex vivo follicle rupture assay in Drosophila, which represents a first assay allowing direct quantification of follicles’ capacity to respond to ovulation stimuli and rupture. This assay can be modified to stimulate rupture with other reagents (for example, ionomycin) or to query enzymatic activity (in situ zymography). In addition, this assay allows genetic or pharmacological screens to identify genes or small molecules regulating follicle rupture in Drosophila.
Keywords: Drosophila, Ovulation, Follicle rupture, Octopamine, in situ zymography , Follicle cells
Background
The study of ovulation in Drosophila has largely been limited due to technical challenges in direct visualization and quantification of ovulation events. In the last several decades, multiple indirect methods have been developed with limitations. The first method for the study of ovulation in Drosophila is to push the female’s abdomen and record if an egg was ejected from the ovipositor ( Aigaki et al., 1991 ; Lee et al., 2003 ). This method is a rough estimate of whether there’s an ovulated egg inside the uterus. The second common assay is to measure the egg-laying capacity of female flies after mating. The egg-laying process is complex, involving development and maturation of a follicle, ovulation, transportation of the ovulated egg through the oviduct, selection of an appropriate egg-laying substrate, and oviposition (Spradling, 1993; Bloch Qazi et al., 2003 ). If any of these processes are impaired, it will result in defective egg laying. Another indicator that ovulation is impaired is an egg-retention phenotype ( Monastirioti et al., 1996 ; Monastirioti, 2003; Cole et al., 2005 ). If a female has an excess of mature follicles within her ovaries, it indicates an anovulatory phenotype. However, this can be caused directly by an ovulation defect or indirectly by a defect downstream of ovulation in the egg-laying process. On the other hand, a lack of egg-retention phenotype does not necessarily mean a lack of ovulation defect. A fourth type of assay used to study ovulation is to examine if an egg is present in the reproductive tract ( Heifetz et al., 2000 ; Lee et al., 2009 ; Lim et al., 2014 ). Variations in this assay range from quantifying ovulation rate by the percentage of females with an egg in their lower oviduct/common oviduct/uterus post mating ( Lee et al., 2009 ; Lim et al., 2014 ), to examining the distribution of eggs in each of these separate regions over time ( Heifetz et al., 2000 ). However, each of these assays could be influenced by the speed of oogenesis, ovulation, and oviposition. To account for all the possible drawbacks of each individual assay, we recently combined the egg-retention assay, the egg-laying assay, and the egg location in the female reproductive tract to estimate the average time for ovulating an egg (ovulation time; Sun and Spradling, 2013; Deady et al., 2015; Deady and Sun, 2015; Knapp and Sun, 2017; Deady et al., 2017 ). However, this method is tedious and also relies on the indirect measurements of ovulation.
We recently characterized ovulation at a cellular level and discovered that Drosophila ovulation involves a follicle rupture process. During ovulation, posterior follicle cells activate matrix metalloproteinase 2 (Mmp2), which degrades posterior follicle cells allowing for the encased oocyte to rupture into the oviduct ( Deady et al., 2015 ). We also found that this process is initiated by direct octopamine (OA) and octopamine receptor in mushroom body (Oamb) signaling in follicle cells, and the entire process can be recapitulated in our ex vivo culture system (Deady and Sun, 2015). We named this assay ex vivo follicle rupture, in which mature follicles are isolated from the ovary and stimulated with OA to induce follicle rupture. Percent of follicles ruptured can be reported at the end of a short three-hour incubation, which is a direct quantification of follicle rupture. This assay allows for a relatively simple, high-throughput examination of follicle rupture in Drosophila, and is ideal for genetic and pharmacological screens. However, this experiment is done ex vivo, and results should be verified in vivo using some of the assays described above.
Materials and Reagents
Utility boxes, 500 ml, Nalgene (Thermo Fisher ScientificTM, catalog number: 5700-0500)
Aluminum foil
Paper towel
PYREXTM spot plates (9-well) (Corning, PYREX®, catalog number: 7220-85)
Stainless steel needles, 0.25 mm, 36 mm (Ted Pella, catalog number: 13561)
Plastic transfer pipets, disposable, 5.8 ml (Fisher Scientific, Fisherbrand, catalog number: 13-711-9CM)
1.5 ml Eppendorf tubes
Grace’s Media, with L-Glutamine (Genesee Scientific, catalog number: 25-516G)
Dry yeast, active (Genesee Scientific, catalog number: 62-103)
Cornmeal (Genesee Scientific, catalog number: 62-101)
-
Molasses (Fisher Scientific, catalog number: NC9109740)
Manufacturer: LabScientific, catalog number: FLY-8008-16.
Agar (Genesee Scientific, catalog number: 66-103)
Tegosept (Genesee Scientific, catalog number: 20-258)
Ethanol
Propionic acid (Sigma-Aldrich, catalog number: P1386-1L)
Fetal bovine serum (Atlanta Biologicals, catalog number: S11150)
Penicillin-streptomycin (10,000 U/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122)
Octopamine hydrochloride (Sigma-Aldrich, catalog number: O0250-1G)
Fluorescein-conjugated DQTM Gelatin From Pig Skin (Thermo Fisher Scientific, InvitrogenTM, catalog number: D12054)
Wet yeast paste (see Recipes)
Fly food (see Recipes)
Culture media mixture (see Recipes)
Octopamine stock solution (see Recipes)
Octopamine working solution (see Recipes)
Fluorescein-conjugated DQTM Gelatin stock solution (see Recipes)
Gelatin working solution (see Recipes)
Equipment
Dumont #5 forceps (Fine Science Tools, catalog number: 11252-20)
Pipetman kit G series (P20, P200, P1000) (Gilson, catalog number: F167900)
Modular stereo microscope for fluorescent imaging (Leica Microsystems, model: Leica MZ10 F)
sCMOS camera (PCO. 4.2)
29 °C incubator with humidity control–set to ~70% RH (Percival Scientific, model: DR-36VL)
CO2 tank, CD 50 (Airgas, model: CGA-320)
Autoclave
Flypad (Genesee Scientific, catalog number: 59-114)
Nutator (Fisher Scientific, model: 260100F)
Software
ImageJ (NIH) ( Schneider et al., 2012 )
Micro-Manager (NIH) ( Schneider et al., 2012 ; Edelstein et al., 2014 )
Procedure
-
Fly rearing
-
Genetic requirements
A fluorescent reporter expressed in mature follicle cells (stage 14) is required for isolating mature follicles with an intact follicle-cell layer. In our experiments, we used either R47A04-Gal4 or R44E10-Gal4 from the Janelia Gal4 collection ( Pfeiffer et al., 2008 ) to drive the expression of a UAS-RFP reporter specifically in stage-14 follicle cells.
Collect virgin females with correct genotype and age them for 5-6D at 29 °C.
Supplement food with ~1 teaspoon of fresh yeast paste 2-3D before the experiment.
-
-
Experimental preparation
Warm Grace’s media to room temperature (~25 °C).
Prepare the culture media mixture (see Recipes).
Line utility boxes with aluminum foil to shield inside from the light.
Add a damp paper towel to the bottom of the box to maintain moisture during the three-hour incubation.
-
Dissection and isolation (see Figure 1)
Make sure to minimize the amount of time from the start of dissection to the last step in Dissection and Isolation. Limit the entire Dissection and Isolation within one hour.
Fill one well in the PYREX spot plate with ~1 ml Grace’s media (Figure 1, ‘1. Initial dissection’).
-
Use forceps to dissect ovary pairs out of eight females in Grace’s media. Make sure not to damage ovaries and keep them intact.
Tip: Gently remove ovaries from the abdomen by grabbing the oviduct/uterus region instead of directly grabbing the ovaries.
Fill another well with ~1 ml Grace’s media and transfer all ovary pairs into this new well (Figure 1, ‘2. Holding ovary pairs’).
-
See Video 1. ‘Dissection Example’
Move ovary pairs (~two at a time) to a new well with ~1 ml Grace’s media (Figure 1, ‘3. Isolating follicles’).
Use one of the forceps to hold the anterior end of the ovary, near the germarium. Use the other forceps to gently separate ovarioles at the posterior end of the ovary near the calyx.
Next, use the other forcep to gently squeeze follicles out of the posterior end. This process is to liberate follicles from surrounding ovariole sheath. Be careful not to puncture follicles.
Identify mature follicles according to the fluorescent reporter and make a pile of intact mature follicles using the needle.
Transfer the intact mature follicles using the P20 pipette to a new well filled with ~1 ml culture media (Figure 1, ‘4. Pool of isolated, intact S14 follicles’). Coat the pipet tip with culture media first to prevent follicles from sticking to the pipet tip.
Repeat Steps C4-C7 until you have enough follicles or until around ~40 min have passed since initial dissection.
Remove any younger-stage follicles in the pool and double check that every follicle in the pool remains intact. Younger-stage follicles should not express the fluorescent reporter when viewed under fluorescent light but can be viewed under the bright-field light.
Use the needle to pile ~25-35 intact follicles together. Transfer piled follicles in culture medium to new wells with a P20 pipette. These follicles should not be dried–they should remain in the ~20 μl culture media in which they were transferred. Repeat this step until all of selected follicles are dispensed in new wells in ‘groups’ of 25-35, or until a total of ~50 min have passed since initial dissection (Figure 1, ‘~25-35 intact S14 follicles in ~20 μl CM’).
Prepare octopamine working solution (see Recipes)–1 ml per each ‘group’.
Add 1 ml of octopamine working solution to each well to stimulate ex vivo rupture OR 1 ml of culture media to each well as a negative control.
Put the PYREX spot plate into a foil-lined utility box (prepared in Procedure B), cover the box, and put the box into a 29 °C incubator for three hours.
-
Image acquisition
Remove the box from the incubator after three hours.
Use the needle to pile follicles toward the center of each well under a microscope. Do not overlap follicles with each other.
Use Micro-Manager to capture both bright-field and fluorescent images.
-
Modify for in situ zymography
Follow Steps C1-C10 to prepare isolated mature follicles.
Prepare gelatin working solution (see Recipes)–1 ml per each ‘group’.
Add 1 ml of gelatin working solution to each well to stimulate ex vivo rupture and detect gelatinase activity. A negative control should be gelatin working solution without octopamine.
After three hours incubation at 29 °C, wash out gelatin working solution from each well by pipetting out the solution and replacing with fresh culture media (be careful not to remove follicles from each well).
Use the needle to pile follicles toward the center of each well under the microscope. Do not overlap follicles with each other.
Identify follicles with posterior enzymatic activity (exhibiting green fluorescence at the posterior end) and segregate them to one side. See Video 2 for an example of identifying follicles with posterior enzymatic activity.
Use the micromanager to capture bright-field, green-fluorescent, and red-fluorescent images.
Figure 1. PYREX 9-well spot plate setup.
Video 1. Dissection example.
Video 2. Analysis of in situ zymography assay.
Open images acquired at the end of each experiment (both bright-field and fluorescent images) in ImageJ. Count the total number of follicles using the multi-point tool in the bright-field image. In this example, there are a total of 29 follicles. Then, count the total number of follicles with posterior green fluorescence in the green-fluorescent image. Before taking the image, most of the follicles identified with enzymatic activity were segregated toward the top of the image. In this example, there are a total of 12 follicles with posterior green fluorescence; therefore, 41.4% of follicles had enzymatic activity.
Data analysis
Data are plotted as ‘Ruptured follicles (%)’. Each data point is one ‘group’ or well: count the total number of follicles in the well and the total number of ruptured follicles (see Video 3, Analysis of ex vivo follicle rupture assay). A follicle is categorized as ‘ruptured’ if > 80% of the oocyte is exposed. Divide the ruptured follicles by the total number of follicles to calculate ‘Ruptured follicles (%)’ ( Deady et al., 2015 ).
Video 3. Analysis of ex vivo follicle rupture assay.
Open images acquired at the end of each experiment (both bright-field and fluorescent images) in ImageJ. Count the total number of follicles using the multi-point tool in the bright-field image. In this example, there are a total of 27 follicles. Then, count the total number of ruptured follicles in the fluorescent image. In this example, there are a total of 20 ruptured follicles; therefore, 74% of follicles ruptured in this example.
For in situ zymography assay, data are plotted as ‘follicles with posterior green fluorescence (%)’. Count the total number of follicles with posterior green fluorescence and divide by the total number of follicles in the well ( Deady et al., 2015 ). (See Video 2, Analysis of in situ zymography assay).
Notes
Ruptured follicles (%) from wild-type flies will vary depending upon the fluorescent reporter used. For example, if a reporter only labels the most mature follicles (stage-14C; such as with the R47A04-Gal4 driver; Deady et al., 2017 ), one would expect to see ~80% of follicles ruptured at the end of the three-hour culture. In contrast, if all stage-14 follicles are labeled and isolated (such as with the R44E10-Gal4 driver; Deady et al., 2017 ), you would expect to observe ~50% of follicles ruptured. The reduced rupture rate is likely due to the isolation of slightly younger stage-14 follicles that are not competent to OA-induced follicle rupture ( Deady et al., 2017 ).
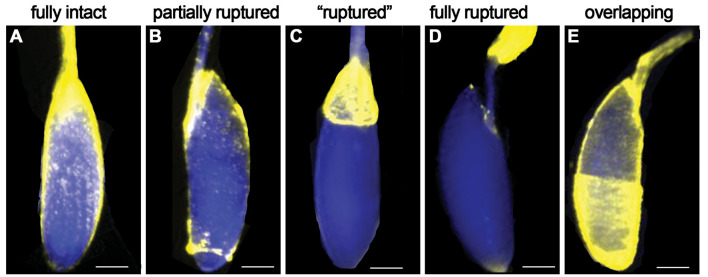
The ruptured follicles (%) in negative control groups (without octopamine) should be less than 10%. Greater than 10% ruptured follicles in negative controls will indicate that some of the isolated follicles have already initiated the rupture process (such as the breakdown of posterior follicle cells) before adding octopamine working solution. When setting up ex vivo follicle rupture, it is imperative that all follicles are completely intact. For an example of intact, partially ruptured, and ruptured follicles, see Figure 2.
Finish ‘Dissection and Isolation (Procedure C)’ within one hour.
Minimize the amount of endogenous octopamine exposure that follicles receive during isolation. Don’t isolate follicles in the same media that the flies were dissected in; rather, use fresh Grace’s media.
When transferring ~25-35 selected follicles to dry well, make sure they have enough media that they don’t dry out. Usually, the follicles are in ~15-30 μl culture media before adding the octopamine working solution.
Thoroughly mix the octopamine working solution or other drugs before beginning the culture.
During dissections, some follicle-cell layers can envelop the anterior egg chamber in the ovariole (see Figure 2E). Don’t select these egg chambers; they will not rupture.
Figure 2. Examples of follicle cell layer coverage of oocyte.
Scale bar is estimated 100 μm.
Recipes
-
Wet yeast paste
Mix active dry yeast in distilled water. Consistency of the wet yeast paste should be semi-solid, all yeast granules should be dissolved yet yeast should not be watery
-
Fly food (3 L)
Yeast (g): 61
Cornmeal (g): 163
Molasses (ml): 203
Agar (g): 22
Water (ml): 3,000
5% Tegosept (50 g Tegosept in 1,000 ml ethanol) (ml): 36
Propionic acid (ml): 13
Measure out all ingredients (except Tegosept and Propionic acid), mix thoroughly, and autoclave at 250 °F for 30 min. Once cooled to 70 °C, Tegosept and Propionic acid are added and mixed thoroughly before dispensing to the fly vials
-
Culture media mixture
Add 1 ml of fetal bovine serum and 100 μl of penicillin-streptomycin (10,000 U/ml) to 9 ml of Grace’s media
-
Octopamine stock solution (10 mM)
Dissolve octopamine hydrochloride powder in distilled water to 10 mM. Freeze aliquots for up to six months
-
Octopamine working solution (20 μM)
For each well: 1 ml of culture media mixture + 2 μl of octopamine stock solution. Rock on the nutator for 1~3 min before use
-
Fluorescein-conjugated DQTM gelatin stock solution (1 mg/ml)
To make aqueous solution, dissolve 1 mg DQTM gelatin powder in 1 ml distilled water and store the solution in fridge covered with foil
-
Gelatin working solution (25 μg/ml)
For each well: 1 ml of culture media mixture + 25 μl of DG gelatin stock solution + 20 μl of octopamine stock solution. Rock on the nutator for 1~3 min before use
Acknowledgments
We thank current and past members of the Sun lab for suggestions and technical assistance. We are very grateful for Dr. Laurinda Jaffe for initial suggestion of ex vivo culture. JS is supported by the University of Connecticut Start-up fund, NIH/National Institute of Child Health and Human Development Grant R01-HD086175, and Bill and Melinda Gates Foundation. This protocol was implemented in previously published studies (Deady and Sun, 2015; Knapp and Sun, 2017; Deady et al., 2017 ) and used to identify genes required for ovulation in Drosophila.
The authors declare no conflict of interest.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Aigaki T., Fleischmann I., Chen P. S. and Kubli E.(1991). Ectopic expression of sex peptide alters reproductive behavior of female D. melanogaster . Neuron 7(4): 557-563. [DOI] [PubMed] [Google Scholar]
- 2. Bloch Qazi M. C., Heifetz Y., Wolfner M. F.(2003). The developments between gametogenesis and fertilization: ovulation and female sperm storage in drosophila melanogaster. Dev Biol 256 195-211. [DOI] [PubMed] [Google Scholar]
- 3. Cole S. H., Carney G. E., McClung C. A., Willard S. S., Taylor B. J. and Hirsh J.(2005). Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility . J Biol Chem 280(15): 14948-14955. [DOI] [PubMed] [Google Scholar]
- 4. Deady L. D., Shen W., Mosure S. A., Spradling A. C. and Sun J.(2015). Matrix metalloproteinase 2 is required for ovulation and corpus luteum formation in Drosophila . PLoS Genet 11(2): e1004989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deady L. D. and Sun J.(2015). A follicle rupture assay reveals an essential role for follicular adrenergic signaling in Drosophila ovulation . PLoS Genet 11(10): e1005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deady L. D., Li W. and Sun J.(2017). The zinc-finger transcription factor Hindsight regulates ovulation competency of Drosophila follicles . Elife 6: e29887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edelstein A. D., Tsuchida M. A., Amodaj N., Pinkard H., Vale R. D. and Stuurman N.(2014). Advanced methods of microscope control using muManager software. J Biol Methods 1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heifetz Y., Lung O., Frongillo E. A. Jr.and Wolfner M. F.(2000). The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary . Curr Biol 10(2): 99-102. [DOI] [PubMed] [Google Scholar]
- 9. Knapp E. and Sun J.(2017). Steroid signaling in mature follicles is important for Drosophila ovulation . Proc Natl Acad Sci U S A 114(4): 699-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee H. G., Rohila S. and Han K. A.(2009). The octopamine receptor OAMB mediates ovulation via Ca2+/calmodulin-dependent protein kinase II in the Drosophila oviduct epithelium . PLoS One 4(3): e4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee H. G., Seong C. S., Kim Y. C., Davis R. L. and Han K. A.(2003). Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster . Dev Biol 264(1): 179-190. [DOI] [PubMed] [Google Scholar]
- 12. Lim J, Sabandal P. R., Fernandez A., Sabandal J. M., Lee H. G., Evans P. and Han K. A.(2014). The octopamine receptor Octβ2R regulates ovulation in Drosophila melanogaster . PLoS One 9: e104441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Monastirioti M.(2003). Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster . Dev Biol 264(1): 38-49. [DOI] [PubMed] [Google Scholar]
- 14. Monastirioti M., Linn C. E. Jr.and White K.(1996). Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine . J Neurosci 16(12): 3900-3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pfeiffer B. D., Jenett A., Hammonds A. S., Ngo T.-T. B., Misra S., Murphy C., Scully A., Carlson J. W., Wan K. H. and Laverty T. R. et al. (2008). Tools for neuroanatomy and neurogenetics in Drosophila . PNAS 105: 9715-9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schneider C. A., Rasband W. S. and Eliceiri K. W.(2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7): 671-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spradling A. C.(1993). Developmental genetics of oogenesis. Cold Spring Harbor Laboratory Press. [Google Scholar]
- 18. Sun J. and Spradling A. C.(2013). Ovulation in Drosophila is controlled by secretory cells of the female reproductive tract . Elife 2: e00415. [DOI] [PMC free article] [PubMed] [Google Scholar]