Abstract
Clinical guidelines for the prevention of stroke in patients with nonvalvular atrial fibrillation (NVAF) are available from several international cardiology associations. Patients with NVAF in the Middle East and North Africa (MENA) region present unique challenges and opportunities related to differences in geography, practice patterns, and patient demographics that are as yet unaddressed in practice guidelines. This review aims to offer a practical perspective on the management of NVAF in patients in MENA and draws on evidence-based guidelines as well as real-world evidence and expert opinion. The literature was searched for relevant original research articles, systematic reviews, meta-analyses, and guideline recommendations addressing the prevention of stroke in patients with NVAF with a focus on issues relevant to the MENA region. Guideline recommendations, best practices, and expert opinion were discussed and agreed on by a working group consisting of cardiologists from across the MENA region. The incidence of stroke secondary to atrial fibrillation in patients across the MENA region is higher than rates reported globally, and this might be attributed to a higher incidence of vascular risk factors and underuse of anticoagulants in patients in the MENA. The available evidence supports the established role of non-vitamin K antagonist oral anticoagulants (NOACs) in the prevention of stroke in patients with NVAF. There is a consistent body of clinical trial and real-world evidence supporting their efficacy for stroke prevention in NVAF, with more favorable bleeding risk profiles relative to vitamin K antagonists, such that guidelines now recommend the use of NOACs in preference over vitamin K antagonists. There are important opportunities to improve the management of NVAF outcomes for patients with NVAF by applying evidence-based guidelines for stroke prevention. Growing experience with NOACs in the MENA region will help guide patient selection and elucidate optimal dosing strategies to maximize the clinical benefits of the NOACs.
Keywords: Non-valvular atrial fibrillation, NOACs, Stroke prevention, Middle East and North Africa
Abbreviations
- ACC/AHA
American College of Cardiology/American Heart Association
- ACS
Acute coronary syndrome
- AF
Atrial fibrillation
- ASA
Acetylsalicylic acid
- CAD
Coronary artery disease
- CHADS2
Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke
- CHA2DS2-VASc
Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65-74 years, Sex category
- CI
Confidence interval
- CrCl
Creatinine clearance
- EHRA
European Heart Rhythm Association
- ESC
European Society of Cardiology
- FIIa
Activated factor II
- FXa
Factor Xa
- GI
Gastrointestinal
- HAS-BLED
Hypertension, Abnormal renal function, Stroke, Bleeding, Labile INR, Elderly, Drug or alcohol use
- HR
Hazard ratio
- ICH
Intracranial hemorrhage
- INR
International normalized ratio
- LAA
Left atrial appendage
- MENA
Middle East and North Africa
- MI
Myocardial infarction
- NOAC
Non-vitamin K antagonist oral anticoagulant
- NVAF
Non-valvular atrial fibrillation
- OAC
Oral anticoagulation
- P-gp
P-glycoprotein
- RCT
Randomized controlled trial
- TIA
Transient ischemic attack
- TTR
Time in therapeutic range
- VKA
Vitamin K antagonist
Introduction
In the past decade, non-vitamin K oral anticoagulants (NOACs) have emerged as alternatives to vitamin K antagonist (VKA) therapies for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF). As opposed to VKAs, NOACs are direct and specific inhibitors of a single factor in the coagulation cascade. To date, three agents have been approved in most countries in the Middle East and North Africa (MENA) region: dabigatran is an activated factor II (FIIa or thrombin) inhibitor, whereas rivaroxaban and apixaban are antagonists of activated factor X (FXa). A third FXa antagonist, edoxaban, was not yet approved at the time these practical perspectives were developed.
Although warfarin, the most commonly used VKA, has been the therapeutic standard for stroke prevention in patients with NVAF for several decades, it presents many limitations to clinical use. NOACs have the potential to address clinical needs in prevention of stroke and systemic embolism for patients with NVAF that are still unmet by VKAs, and this is supported by their widespread uptake in recent years. Several papers have summarized the pharmacology and pharmacokinetics of NOACs and provided clinical guidelines and recommendations on their use [1], [2], [3], [4], [5]. The management of patients with NVAF in the MENA region presents some unique challenges and opportunities related to differences in geography, practice patterns, and patient demographics, relative to the Phase III studies that have largely been carried out in North America, Europe, and Asia.
Using a convenient question and answer format, this review aims to provide practical guidance to cardiologists in the MENA to implement NOACs in their routine management of stroke prevention in NVAF.
Material and methods
A group of expert cardiologists from the MENA region convened in March 2016 to discuss regional issues related to stroke prevention in patients with NVAF. Current evidence and perceived gaps in regional knowledge were identified and discussed. A literature review was subsequently conducted; PubMed, Google Scholar, and conference abstracts were searched for relevant original research articles, systematic reviews, meta-analyses, and guideline recommendations to address the clinically relevant topics that were identified by this expert group.
Results and discussion
What is NVAF?
The European Heart Rhythm Association (EHRA) defines NVAF as atrial fibrillation (AF) that is not accompanied by moderate-to-severe mitral stenosis (usually of rheumatologic origin) nor by mechanical prosthetic heart valves [6]. Although there has been recent debate in the literature regarding the heterogeneous definitions of NVAF used in clinical trials [7], it is beyond the scope of this review to discuss unapproved indications for NOACs (e.g., in patients with aortic stenosis or mitral regurgitation).
What is the incidence, prevalence, and burden of NVAF?
The estimated number of patients with AF in 2010 was 33.5 million worldwide, and the global AF-associated burden of disease increased by 18% between 1990 and 2010 [8]. In MENA countries, the prevalence of AF in 2010 was similar to the prevalence in developed countries globally [8]. However, epidemiological studies suggest a differential prevalence of some classic stroke risk factors. In the Saudi Atrial Fibrillation Survey registry, patients with AF had high rates of smoking (24%), hypertension (63%), diabetes (48%), and dyslipidemia (44%) [9]. In contrast, a meta-analysis of observational studies from Europe and North America reported the following prevalence rates: smoking, 12–43%; hypertension, 39–68%; diabetes mellitus, 5–18%; prior stroke/transient ischemic attack (TIA), 4–17%; and coronary artery disease (CAD), 5–32% [10].
Limited data on NVAF-associated thromboembolism specific to the MENA region suggest the 1-year rate of stroke or TIA in patients admitted to the hospital with AF is higher (4.2%) [11] than that in Western countries (0.5–2%) [12], [13], [14]. Adherence to antithrombotic guidelines in a European study was significantly correlated with better outcomes with respect to mortality, thromboembolism and bleeding [15]. Therefore, the relatively high risk of stroke in patients with AF in the MENA may be attributable, at least in part, to suboptimal management of anticoagulation therapy. These data highlight important opportunities to improve AF management specifically in the MENA.
How should NVAF patients be risk stratified for oral anticoagulation?
Accurate risk stratification of patients with NVAF may help optimize prescription patterns for antithrombotic therapy, address the underuse of this therapy in MENA countries, and ultimately, lessen the burden of stroke and its complications in patients with NVAF. In patients with NVAF, the newer CHA2DS2-VASc score is recommended for stroke risk assessment [13], [16], [17], whereas the HAS-BLED [Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile INR (international normalized ratio), Elderly, Drugs/alcohol concomitantly] score is recommended for bleeding risk assessment [1]. Bleeding risk estimation should not exclude patients from antithrombotic therapy [1]. Instead, it should be used to inform bleeding risks and suggest which patients would benefit from closer monitoring and/or from modifying risk factors [1].
It should be noted that many of the risk factors that make up the CHA2DS2-VASc and the HAS-BLED scores overlap such that patients at the highest risk of stroke are also at the highest risk of bleeding [18].
What are the key advantages and disadvantages of VKA therapy?
VKAs such as warfarin have been the hallmark of stroke prevention in NVAF for more than six decades. They have well-characterized advantages but also many disadvantages (Table 1), which may account for suboptimal use of VKAs across the world [19], [20], [21], [22]. This underscores the need and opportunity for antithrombotic treatment options that are easier to manage and with better risk–benefit profiles. Indeed, the most recent European guidelines recommend that patients on VKAs whose time in therapeutic range is not well controlled (<60–70%) despite good adherence and without contraindications, should be considered for NOAC treatment [1].
Table 1.
Advantages and limitations of vitamin K antagonist (VKA) therapy.
| Advantages | Limitationsa |
|---|---|
| Proven efficacy for stroke prevention | Risk of bleeding complications |
| Historical standard of care | Routine monitoring required |
| Long half-life, once-daily dosing | Dose adjustments frequently needed |
| Slow onset of action | |
| Narrow therapeutic window | |
| Dietary restrictions | |
| Numerous drug interactions | |
| Variability in patient response |
Note. From “New oral Xa and IIa inhibitors: updates and clinical trial results,” by S. Haas, 2008, J Thromb Thrombolysis, 25, p. 52–60. Copyright 2007, Springer Science+Business Media, LLC. Adapted with permission.
Some of the listed disadvantages may exist with other oral anticoagulants.
What do the guidelines recommend for oral anticoagulation in NVAF?
The key antithrombotic recommendations from the European Society of Cardiology (ESC) and the American College of Cardiology/American Heart Association are summarized in Table 2. Similar messages for oral anticoagulation (OAC) in NVAF emerge from the Saudi Arabia Ministry of Health guidelines [23]. All available NOACs are indicated for the prevention of stroke and systemic embolism in patients with NVAF who are candidates for OAC therapy.
Table 2.
Summary and comparison of the European Society of Cardiology (ESC) and American College of Cardiology/American Heart Association (ACC/AHA) recommendations for antithrombotic therapy in patients with atrial fibrillation (AF).
| ESC guidelines [1] | ACC/AHA guidelines [2] |
|---|---|
| Prevention of thromboembolism—general | |
| Antithrombotic therapy based on shared decision making, discussion of risks of stroke and bleeding, and patient’s preferences (Level 1C) | |
| The CHA2DS2-VASc score is recommended for stroke risk prediction in patients with AF (Level IA) | CHA2DS2-VASc score recommended to assess stroke risk (Level IB) |
| In general, patients without clinical stroke risk factors (CHA2DS2-VASc = 0) do not need antithrombotic therapy (Level IIIB) | With NVAF and CHA2DS2-VASc score of 0, it is reasonable to omit antithrombotic therapy (Level IIa B) |
| OAC therapy is recommended in all patients with a CHA2DS2-VASc score ≥2 (men) or ≥3 (women) (Level IA) | With prior stroke, TIA, or CHA2DS2-VASc score ≥2, OACs recommended. Options include:
|
| In patients with a CHA2DS2-VASc score of 1 (men) or 2 (women), OAC should be considered to prevent thromboembolism, considering individual characteristics and patient preferences (Level IIa B) | With NVAF and a CHA2DS2-VASc score of 1, no antithrombotic therapy or treatment with OACs or aspirin may be considered (Level IIb C) |
| When OAC is initiated in a patient with AF who is eligible for a NOAC (apixaban, dabigatran, edoxaban, or rivaroxaban), a NOAC is recommended in preference to a VKA (Level IA) | Selection of antithrombotic therapy based on risk of thromboembolism (Level 1B) |
| When patients are treated with a VKA, time in therapeutic range (TTR) should be kept as high as possible and closely monitored (Level IA) | With warfarin, determine INR at least weekly during initiation and monthly when stable (Level IA) |
| Antiplatelet monotherapy is not recommended for stroke prevention in AF patients, regardless of stroke risk [Level IIIA (harm)] | |
| Combinations of OACs and platelet inhibitors increase bleeding risk and should be avoided in AF patients without another indication for platelet inhibition [Level IIIB (harm)] | |
| Renal function | |
| The assessment of kidney function by serum creatinine or creatinine clearance is recommended in all AF patients to detect kidney disease and to support correct dosing of AF therapy (Level IA) All AF patients treated with OAC should be considered for at least yearly renal function evaluation to detect chronic kidney disease (Level IIa B) |
Evaluate renal function prior to initiation of direct thrombin or factor Xa inhibitors, and reevaluate when clinically indicated and at least annually (Level 1B) |
| Direct thrombin dabigatran and factor Xa inhibitor rivaroxaban are not recommended in patients with AF and end-stage chronic kidney disease (CKD) or on dialysis because of a lack of evidence from clinical trials regarding the balance of risks and benefits (Level IIIC) | |
| With moderate-to-severe CKD and CHA2DS2-VASc scores ≥ 2, reduced doses of direct thrombin or factor Xa inhibitors may be considered (Level IIb C) | |
| With CHA2DS2-VASc score ≥ 2 and end-stage CKD (CrCl < 15 mL/min) or on hemodialysis, it is reasonable to prescribe warfarin for OAC (Level IIa B) | |
| If VKA is not an option | |
| AF patients already on treatment with a VKA may be considered for NOAC treatment if TTR is not well controlled despite good adherence, or if patient preference without contraindications to NOAC (e.g., prosthetic valve) (Level IIb A) | Direct thrombin or factor Xa inhibitor recommended if unable to maintain therapeutic INR (Level 1C) |
| Mechanical heart valves and mitral stenosis | |
| VKA therapy (INR 2.0–3.0 or higher) is recommended for stroke prevention in AF patients with moderate-to-severe mitral stenosis or mechanical heart valves (Level 1B) | Warfarin recommended for mechanical heart valves and target INR intensity based on type and location of prosthesis (Level IB) |
| Direct thrombin inhibitor dabigatran should not be used with a mechanical heart valve [Level IIIB (harm)] | |
Classes of recommendation: I, evidence and/or general agreement that a given treatment or procedure is beneficial, useful, effective; II, conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given treatment or procedure; IIa, weight of evidence/opinion is in favor of usefulness/efficacy; IIb, usefulness/efficacy is less well established by evidence/opinion; III, evidence or general agreement that the given treatment or procedure is not useful/effective, and in some cases may be harmful.
Levels of evidence: A, data derived from multiple randomized clinical trials or meta-analyses; B, data derived from a single randomized clinical trial or large nonrandomized studies; C, consensus of opinion of the experts and/or small studies, retrospective studies, registries.
INR = international normalized ratio; NOACs = non-vitamin K antagonist oral anticoagulants; NVAF = nonvalvular atrial fibrillation; OAC = oral anticoagulation; TIA = transient ischemic attack; TTR = time in therapeutic range; VKA = vitamin K antagonist.
The guidelines differ with respect to recommendations for specific anticoagulation therapy. Whereas the ESC and Saudi guidelines recommend using one of the NOACs in preference to a VKA for most patients with NVAF (Level IA) [1], the American guidelines do not prefer NOACs over VKAs; “if patients are stable, their condition is easily controlled, and they are satisfied with warfarin, it is not necessary to change to a new agent” [2]. However, American guidelines state that “for patients with NVAF unable to maintain a therapeutic international normalized ratio (INR) level with warfarin, use of a direct thrombin or factor Xa inhibitor is recommended” (Level IC) [2].
The European guidelines do not recommend antiplatelet monotherapy for stroke prevention in AF patients, regardless of stroke risk [Level IIIA (harm)] [1], because the evidence suggests that single or dual antiplatelet therapy is inferior to VKA therapy with a higher risk of bleeding. In comparison, the American guidelines, which were published 2 years earlier in 2014, acknowledge the lower risk–benefit of antiplatelet therapy but do not include a formal recommendation [2]. The Saudi guidelines acknowledge that some patients at low risk of stroke (CHADS2 score = 0) will choose antithrombotic therapy particularly if they place a high value on stroke prevention and low value on bleeding risk [23]. In such patients, the Saudi guidelines recommend that acetylsalicylic acid (ASA; 75 to 325 mg once daily) be used rather than OAC (weak recommendation, moderate quality evidence) [23].
What are the absolute and relative contraindications to NOAC therapy?
Patients with severe impairment of renal function were excluded from all NOAC registration trials; therefore, these agents are not approved for patients with creatinine clearance (CrCl) <30 mL/min (dabigatran) and CrCl <15 mL/min (FXa inhibitors) [17]. The different CrCl threshold reflects the renal elimination of dabigatran, whereas FXa inhibitors are only partially eliminated via the kidneys. Anticoagulation in patients with advanced kidney disease remains a clinical challenge; the ongoing Compare Apixaban and Vitamin-K Antagonists in Patients With Atrial Fibrillation and End-Stage Kidney Disease and Renal Hemodialysis Patients Allocated Apixaban Versus Warfarin in Atrial Fibrillation trials are evaluating the safety and efficacy of apixaban in patients with end-stage kidney disease and in patients on hemodialysis, respectively. Patients with moderate-to-severe mitral stenosis or with a mechanical prosthetic valve were also excluded from registration trials; therefore, NOACs should be solely used in NVAF until further data supporting expanded indications are available. Clinically active severe bleeding [i.e., major, intracranial or gastrointestinal (GI)], spontaneous or pharmacological impairment of hemostasis, and pregnancy and lactation should be considered absolute contraindications to NOAC therapy.
Clinically relevant bleeding risk, liver disease associated with coagulopathy, and presence of organic lesions at risk of bleeding, are relative contraindications to NOACs [4].
What are the key differences between the NOACs?
As opposed to VKAs, NOACs are specific inhibitors of a single factor in the coagulation cascade (Table 3). Despite different targets, all NOACs have rapid onset of action (peak plasma levels in 2–4 hours) and relatively short half-lives (8–12 hours), and—compared to VKAs—carry minimal food restrictions and drug interactions (reviewed by Fontana et al. [4]).
Table 3.
Clinically relevant pharmacological properties and pharmacokinetics of NOACs.
| Dabigatran | Rivaroxaban | Apixaban | |
|---|---|---|---|
| Direct target | Factor IIa (thrombin) | Factor Xa | Factor Xa |
| Pro-drug | Yes: dabigatran etexilate | No | No |
| Bioavailability (%) | 6–10 | 66% without food 80–100% with food |
50 |
| Time to peak plasma concentration (h) | 3 | 2–4 | 3 |
| Half-life (h) | 12–17 | 5–13 | 9–14 |
| Metabolism | P-gp | P-gp CYP3A4/3A5 CYP2J2 |
P-gp CYP3A4/3A5 |
| Renal elimination (%) | 80 | 33 | 27 |
Note. From “2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS,” by P. Kirchhof, S. Benussi, D. Kotecha, A. Ahlsson, D. Atar, B. Casadei, et al., 2016, Eur Heart J, 37, p. 2893–62. Copyright 2016, The European Society of Cardiology. From “Updated European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary,” by H. Heidbuchel, P. Verhamme, M. Alings, M. Antz, H.C. Diener, W. Hacke, et al, 2015, Europace, 17, p. 1467–507. Copyright 2016, The European Society of Cardiology. Adapted with permission.
NOACs = non-vitamin K antagonist oral anticoagulants; P-gp = P-glycoprotein.
Bioavailability and metabolism vary between the NOACs. Dabigatran is mostly (>80%) eliminated by the kidneys, whereas renal elimination of rivaroxaban and apixaban is considerably less at ∼50% [24], [25]. Assessment of renal function through CrCl is therefore necessary for all NOACs [1], [2], but especially for patients using dabigatran. The use of FXa inhibitors should be preferred to dabigatran in patients with impaired kidney function.
Absorption of all NOACs is dependent on intestinal P-glycoprotein (P-gp) [26]. Consequently, NOACs should be avoided in combination with any treatment that affects P-gp activity. Furthermore, FXa inhibitors, but not the FIIa inhibitor dabigatran, are metabolized by cytochrome P450 CYP3A4 and should therefore not be prescribed concurrently with strong modulators of the CYP3A4 pathway [6], [27]. Clinicians are encouraged to refer to specific product information for a complete listing of food–drug and drug–drug interactions for each NOAC.
What is the comparative efficacy and safety of NOACs versus VKAs?
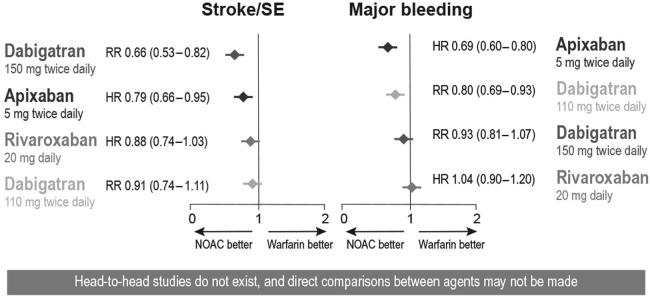
Several large-scale, Phase III clinical trials have evaluated the efficacy and safety of NOACs compared to warfarin (Table 4). Overall, the current data on NOACs support their noninferiority (dabigatran both doses, rivaroxaban, and apixaban) and superiority (dabigatran 150 mg and apixaban) in terms of preventing stroke and systemic embolism in patients with NVAF, and their superiority (dabigatran 110 mg and apixaban) in terms of major bleeding, compared to warfarin (Fig. 1) [25], [28]. Because of these net clinical benefits, European [1], Saudi [23], and Canadian [3] guidelines recommend using these new-generation antithrombotic agents over warfarin, whereas the American guidelines recommend using either VKAs or NOACs, with no preference over one class of drugs [2].
Table 4.
Rate of events [% patients/y, RR (95% CI), p value] for efficacy and safety endpoints in Phase III clinical trials comparing the effects of NOACs to warfarin.
| Dabigatran (RE-LY) [57] | Rivaroxaban (ROCKET-AF) [58] | Apixaban (ARISTOTLE) [59] | |||||
|---|---|---|---|---|---|---|---|
| Study design | Randomized, open-label | Randomized, double-blind | Randomized, double-blind | ||||
| Number of patients | 18,113 | 14,264 | 18,201 | ||||
| Groups | Dose-adjusted warfarin vs. blinded doses of dabigatran (150 mg b.i.d. or 110 mg b.i.d.) |
Dose-adjusted warfarin vs. rivaroxaban (20 mg q.d.) |
Dose-adjusted warfarin vs. apixaban (5 mg b.i.d.) |
||||
| W | D150 | D110 | W | R20 | W | A5 | |
| Incidence of stroke/systemic embolism | 1.72 | 1.12 [0.65 (0.52–0.81), p < 0.001 for noninferiority and superiority] | 1.54 [0.89 (0.73–1.09), p < 0.001 for noninferiority] | 2.4 | 2.1 [0.88 (0.75–1.03), p < 0.001 for noninferiority and p = 0.12 for superiority] | 1.6 | 1.27 [0.79 (0.66–0.95), p < 0.001 for noninferiority, p = 0.01 for superiority] |
| All-cause mortality | 4.13 | 3.64 [0.88 (0.77–1.00), p = 0.051] | 3.75 [0.91 (0.80–1.03), p = 0.13] | 2.21 | 1.87 [0.85 (0.70–1.02), p = 0.07] | 3.94 | 3.52 [0.89 (0.80–0.99), p = 0.047] |
| Major bleeding | 3.61 | 3.40 [0.94 (0.82–1.08), p = 0.41] | 2.92 [0.80 (0.70–0.93), p = 0.003] | 3.45 | 3.60 [1.04 (0.90–2.30), p = 0.58] | 3.09 | 2.13 [0.69 (0.60–0.80), p < 0.001] |
| Intracerebral hemorrhage | 0.77 | 0.32 [0.42 (0.29–0.61), p < 0.001] | 0.23 [0.29 (0.19–0.45), p < 0.001] | 0.74 | 0.49 [0.67 (0.47–0.93), p = 0.02] | 0.80 | 0.33 [0.42 (0.30–0.58), p < 0.001] |
| GI major bleeding | 1.09 | 1.60 [1.48 (1.19–1.86), p < 0.001] | 1.13 [1.04 (0.82–1.33), p = 0.74] | 1.24 | 2.00 [1.61 (1.30–1.99), p < 0.001] | 0.86 | 0.76 [0.89 (0.70–1.15), p = 0.37] |
Note. From “2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS,” by P. Kirchhof, S. Benussi, D. Kotecha, A. Ahlsson, D. Atar, B. Casadei, et al., 2016, Eur Heart J, 37, p. 2893–62. Copyright 2016, The European Society of Cardiology. Adapted with permission.
A5 = apixaban 5 mg b.i.d. = twice a day; CI = confidence interval; D150 = dabigatran 150 mg b.i.d.; D110 = dabigatran 110 mg b.i.d.; GI = gastrointestinal; NOACs = non-vitamin K antagonist oral anticoagulants; R20 = rivaroxaban 20 mg q.d. = once a day; RR = relative risk; W = warfarin.
Figure 1.
Net clinical benefit of non-vitamin K antagonist oral anticoagulants (NOACs) relative to vitamin K antagonists (VKAs). Note. From “New oral anticoagulant agents – general features and outcomes in subsets of patients,” by S. Schulman, 2014, Thromb Haemost, 111, p. 575–82. Copyright. Adapted with permission. HR = hazard ratio; RR = risk ratio; SE = systemic embolism.
There are no head-to-head studies directly comparing different NOACs. The heterogeneity of patients enrolled in various NOAC registration trials and other study design differences render it impossible to directly compare the efficacy and safety of these agents [29]. At this point, individual patient characteristics, comorbidities, tolerability, food–drug and drug–drug interactions, and cost constitute important decision-making factors when selecting an anticoagulation strategy.
What is the comparative efficacy of NOACs versus aspirin?
Only one clinical trial evaluated the efficacy and safety of a NOAC compared to aspirin. In the Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment study, patients who were unsuitable or not willing to take VKAs were randomized to either apixaban [5 mg, twice daily (b.i.d.)] or aspirin (81–324 mg/d) [30]. There were significantly lower rates of stroke/systemic embolism with apixaban, and the trial was terminated early, after a mean follow-up of 1.1 years. Rates of adverse bleeding events were comparable between the two arms [30]. Based on these data, apixaban shows net clinical benefit compared to aspirin in patients who are not suitable candidates for VKA.
What is the real-world evidence for NOACs for stroke prevention in NVAF?
Although randomized controlled trials (RCTs) remain the gold standard for the evaluation of new therapeutics, there is increasing focus on the important contributions of real-world evidence [31]. Real-world evidence can be described as insights on diseases, products, and patient populations derived from the analysis of data generated from nonexperimental sources (e.g., databases, electronic medical records, postmarketing registries, pragmatic trials). As such, real-world evidence complements data from RCTs by evaluating a new therapeutic approach under conditions more representative of the routine clinical setting. Guidelines typically do not account for real-world evidence in their recommendations; therefore, these practical perspectives are unique in considering the applicability of real-world evidence to clinical practice.
NOACs have been commercially available for several years now, and the accumulating real-world evidence on their use broadly confirms the results of the landmark NOAC registration trials. Many studies and registries are ongoing and reporting data, such that the field is rapidly evolving. It is beyond the scope of this review to discuss all of the available data, but a few studies merit attention. For example, an ongoing American study of more than 134,000 Medicare patients newly treated with dabigatran or warfarin confirmed that dabigatran significantly lowers the risk of ischemic stroke [hazard ratio (HR) = 0.80; 95% confidence interval (CI), 0.67–0.96] and intracranial bleeding (HR = 0.34; 95% CI, 0.26–0.46) compared to warfarin [32]. However, in contrast to the RCTs, there was a significantly lower risk of all-cause mortality (HR = 0.86; 95% CI, 0.77–0.96) and a significantly higher risk of major GI bleeding (HR = 1.28; 95% CI, 1.14–1.44), whereas there was no difference in risk of acute myocardial infarction between the two drugs (HR = 0.92; 95% CI, 0.78–1.08) [32].
Although systematic reviews and meta-analyses of RCTs provide indirect evidence supporting relatively comparable efficacy profiles for the NOACs [33], real-world evidence may elucidate differentiating features between these agents. For instance, real-world evidence from a Danish registry of all NVAF patients (n = 61,678) newly treated with OACs from August 2011 to October 2015 suggests that the annual risk of death over 1 year of follow-up was significantly lower with apixaban and dabigatran compared with warfarin [HR = 0.65 (95% CI, 0.56–0.75) and 0.63 (95% CI, 0.48–0.82), respectively] but not with rivaroxaban [34]. All three NOACs were similarly effective as warfarin for prevention of ischemic stroke, whereas only rivaroxaban was associated with a lower risk of ischemic stroke or systemic embolism compared with warfarin (HR = 0.83; 95% CI, 0.69–0.99). In this same registry, the combined endpoint of any bleeding was significantly lower with apixaban and dabigatran versus warfarin [HR = 0.63 (95% CI, 0.53–0.76) and 0.61 (95% CI, 0.51–0.74), respectively] whereas rivaroxaban was not significantly different [34].
The results of several retrospective US registries have been recently reported at major congresses and are contributing evidence for differences between the NOACs with respect to bleeding risk and hospitalizations (Table 5). The growing body of real-world evidence provides reassurance to clinicians that the potential bleeding risks of NOACs as reported in their respective registration trials have been relatively consistent with routine clinical populations. Although real-world studies inherently have limitations and confounding factors, most [35], [36], [37], [38], [39], [40], [41], but not all [42], of the available data support the superiority of apixaban and dabigatran compared to rivaroxaban or VKAs in terms of bleeding risks.
Table 5.
Real-world evidence from US registries of NOACs: risk of bleeding.
| Lip et al. [38] | Lin et al. [39] | Tepper et al. [40] | Deitelzweig et al. [41] | |
|---|---|---|---|---|
| Data source | US Truven MarketScan commercial and Medicare supplemental databases | US Humedica deidentified EHR data | US Truven MarketScan Earlyview insurance claims database | US Premier Hospital database |
| Study population | Age ≥18 y Newly prescribed NOAC or warfarin with NVAF diagnosis |
Age ≥18 y ewly prescribed NOAC or warfarin with NVAF diagnosis |
Age ≥18 y Switched from warfarin or newly prescribed a NOAC during study period |
Age ≥18 y Hospital discharge code indicating primary or secondary diagnosis of AF Prescribed NOAC during hospitalization |
| Study drug (n) | A (n = 2402) R (n = 10,050) D (n = 4173) W (12,713) |
A (n = 2038) R (n = 6407) D (n = 2440) W (24,872) |
A (n = 8785) R (n = 30,529) D (n = 20,963) |
A (n = 4138) R (n = 37,754) D (n = 32,838) |
| Study period | Jan. 1, 2012–Dec. 31, 2013 (includes 1 y baseline) | Jan. 1, 2013–Jun. 30, 2014 | Jan. 1, 2013–Oct. 31, 2014 | Jan. 1, 2012–March 31, 2014 |
| Follow-up | Up to 1 y | Up to 180 d | Up to 6 mo | Up to 1 mo following hospitalization for NVAF |
| Endpoint(s): Adjusted HR (95% CI) |
Major bleeding W vs. A: 1.93 (1.12–3.33) R vs. A: 2.19 (1.26–3.79) D vs. A: 1.71 (0.94–3.1) |
Any bleeding W vs. A: 1.34 (1.13–1.58) R vs. A: 1.46 (1.23–1.75) D vs. A: 0.91 (0.73–1.13) |
Major bleeding D vs. A: HR 0.99 (0.88–1.10) R vs. A: 1.36 (1.23–1.52) CRNM D vs. A: 1.07 (0.98–1.15) R vs. A: 1.43 (1.34–1.54) Any bleeding D vs. A: 1.06 (0.99–1.13) R vs. A: 1.41 (1.32–1.50) |
Bleeding-related hospital readmission within 1 mo D vs. A: 1.2 (0.9–1.6) R vs. A: 1.4 (1.1–1.9) |
| Bleeding definition/diagnosis | Bleeding requiring hospitalization with a bleeding diagnosis code as the first listed ICD-9-CM code | At least one encounter with an ICD-9-CM code indicative of a major or CRNM bleed in any setting | Based on ICD-9-CM diagnostics codes, CPT and HCPCS procedure codes | ICD-9-CM codes |
Hazard ratios in bold are statistically significantly different for comparison.
A = apixaban; CI = confidence interval; CPT = Common Procedural Terminology; CRNM = clinically relevant nonmajor; D = dabigatran; EHR = electronic health record; HCPCS = Healthcare Common Procedure Coding System; HR = hazard ratio; ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification; NOAC = non-vitamin K antagonist oral anticoagulant; NVAF = nonvalvular atrial fibrillation; R = rivaroxaban; W = warfarin.
Real-world evidence for NOACs in the MENA region is sorely lacking. In the Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA-AF) Phase 2 study, which includes more than 15,000 NVAF patients, less than 600 (roughly 4%) were from the MENA region [43]. Given the differences in prevalence and risk factors for stroke and NVAF in the MENA region, there is a need to better understand the real-world efficacy and safety of NOACs in this population.
As the real-world evidence base continues to expand, it is likely that clinicians will be better able to tailor specific treatments to individual patients to maximize net clinical benefits and minimize potential harms.
How should NOACs be dosed in clinical practice? What dosing adjustments are required?
The label dosing recommendations of NOACs reflect the dosages evaluated in the Phase III registration trials for the individual agents (Table 6). Dosing of NOACs is relatively straightforward with advantages over VKAs, notably rapid onset of action after oral intake, fixed dosing regimens, and no need for anticoagulation monitoring [6]. Although there are few food interactions with the NOACs relative to VKAs, food intake increases the bioavailability and absorption of rivaroxaban by 39% [26]. It is therefore recommended that rivaroxaban be taken with food [44] whereas the other NOACs can be taken with or without food.
Table 6.
Recommended dosing and dose adjustments of NOACs for stroke prevention in patients with NVAF.
| Drug | Dabigatran | Rivaroxaban | Apixaban |
|---|---|---|---|
| Dose (mg) | 150 | 20 | 5 |
| Dosing frequency | Twice daily | Once daily | Twice daily |
| Approved for CrCl ≥a | 30 mL/min | 15 mL/min | 15 mL/min |
| Dosing recommendation | CrCl ≥50 mL/min: 150 mg b.i.d. | CrCl ≥50 mL/min: 20 mg q.d. | Serum creatinine ≥1.5 mg/dL: 5 mg b.i.d. |
| Dosing if renal impairment | When CrCl 30–49 mL/min, 150 mg b.i.d. is possible (SmPC) but 110 mg b.i.d. should be consideredb | When CrCl 15–49 mL/min, 15 mg q.d. | When CrCl 15–29 mL/min, 2.5 mg b.i.d. If two out of three: SCr ≥ 1.5 mg/dL, age ≥80 y, weight ≤60 kg,c 2.5 mg b.i.d. |
| Not recommended if CrCl <… | 30 mL/min | 15 mL/min | 15 mL/min |
| Absorption with food | No effect | 39% increase | No effect |
| Intake recommended with food? | No | Mandatory | No |
Note. From “Updated European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary,” by H. Heidbuchel, P. Verhamme, M. Alings, M. Antz, H.C. Diener, W. Hacke, et al, 2015, Europace, 17, p. 1467–507. Copyright 20XX, Name of the Copyright Holder. Adapted with permission.
b.i.d = twice a day; CrCl = creatinine clearance; NOACs = non-vitamin K antagonist oral anticoagulants; NVAF = nonvalvular atrial fibrillation; SmPC = summary of product characteristics.
Estimated according to the Cockroft–Gault formula: CrCl = [(140 − age) × weight/creatinine level] × k, where k = 1.23 (men) or 1.03 (women).
75 mg b.i.d. approved in the United States only.
If age >80 years and/or weight <60 kg.
What dosage adjustments are required with the NOACs based on renal impairment?
Renal impairment is an independent risk factor for both stroke/systemic embolism and bleeding events in patients with NVAF [6]. Therefore, the net clinical benefit of anticoagulation in NVAF patients with renal disease must be carefully assessed prior to initiating antithrombotic therapy. The Phase III RCTs for the NOACs included patients with mild-to-moderate renal impairment with protocol-mandated dose reductions, but patients with severe renal impairment or end-stage renal disease requiring dialysis (CrCl <30 mL/min and <15 mL/min, respectively) were excluded. Therefore, these agents are not approved for patients with CrCl <30 mL/min (dabigatran) and CrCl <15 mL/min (FXa inhibitors) [17].
What dosage adjustments are required with the NOACs during periods of fasting?
Patients observing the holy month of Ramadan may require adjustments to their medication regimens. There is no direct evidence from RCTs to provide guidance on dosing adjustments with fasting during Ramadan; however, clinical practice suggests that drugs taken once or twice daily, such as NOACs, do not need to be adjusted [45].
Clinicians should be aware that many patients alter their normal drug intake patterns to accommodate fasting and some may stop taking their medications altogether [46], placing NVAF patients at risk of stroke or systemic embolism. Clinicians should preemptively discuss routines surrounding drug taking behaviors during periods of fasting, and allow for flexibility in dosing by 2–3 hours (authors’ expert opinion). Patients taking rivaroxaban should be reminded of the importance of taking this NOAC with food even during periods of fasting.
What dosage adjustments are required based on body weight?
Dosage adjustments are recommended for patients with very high or low body weight. According to product labels, the recommended dose of apixaban is 2.5 mg taken orally twice daily in patients with NVAF and at least two of the following characteristics: age ≥80 years, body weight ≤60 kg, or serum creatinine ≥1.5 mg/dL (133 μmol/L). Close clinical surveillance is recommended with dabigatran in patients <50 kg. There are no recommended dosage adjustments based on body weight for rivaroxaban.
There is little clinical evidence to guide NOAC dosing in obese patients, because patients weighing >100 kg were largely underrepresented in the Phase III clinical studies (<20%). Some experts have suggested that VKAs should be preferred over NOACs in patients whose body weight exceeds 130 kg based on this lack of evidence, although this cut-off is arbitrary [4]. Further studies evaluating the efficacy and safety of NOACs in the obese population are needed. This is particularly true in the MENA region, where obesity is a common risk factor among patients with NVAF [8].
What dosage adjustments are required in the elderly?
NOACs are indicated for adults older than 18 years. There are age-associated changes in bleeding risk and in drug metabolism and clearance that should be considered when prescribing NOACs to elderly patients. According to product labels, dabigatran should be dosed at 110 mg b.i.d. in patients ≥80 years of age, and this reduced dose may be considered for patients aged 75–80 years if their thromboembolic risk is low and bleeding risk is high. For apixaban, no dosage adjustment is required based on age alone; however, patients meeting two of three criteria (i.e., body weight ≤60 kg, age ≥80 years, and serum creatinine is elevated ≥1.5 mg/dL) should be dose-reduced to 2.5 mg b.i.d. There are no dosage adjustments for rivaroxaban in the elderly.
How should dosing errors be managed?
Patients taking NOACs should be advised on how to manage missed or forgotten doses. The recommended management strategies depend on the half-life of the particular NOAC and when the missed dose is remembered during the dosing cycle. The EHRA suggests that forgotten doses may be taken up until halfway through the dosing interval (i.e., up to 12 hours for rivaroxaban and up to 6 hours for dabigatran or apixaban) [6]. If the forgotten dose is remembered beyond this time frame, the dose should be skipped and the patient should continue with their next scheduled dose. If there is a suspected overdose in the absence of bleeding, a “watchful waiting” approach is appropriate in most cases as the plasma half-life of the NOACs is relatively short.
Underdosing of NOACs in real-world practice
Concerns regarding increased risk of bleeding have contributed to underdosing of NOACs in some situations, placing patients at risk of subtherapeutic dosing and inadequate protection against stroke/systemic embolism. Indeed, a propensity-weighted cohort study by Neilsen et al. [47] showed a trend toward higher rates of ischemic stroke and systemic embolism when low-dose apixaban (2.5 mg b.i.d.) was used compared with VKA in AF patients who were naïve to OAC treatment. In this same study, bleeding rates were not significantly different between the two groups, underscoring the importance of correct dosing to achieve a net clinical benefit [47]. Yet, in a recent real-world study of 176 patients treated with NOACs for stroke prevention in NVAF (n = 134) or prevention of venous thromboembolism (n = 40), 34% received an inappropriate dose based on age and renal impairment [48]. Among these, 38% were prescribed a lower dose than recommended. Similarly, a study of 174 consecutive patients receiving NOACs in an outpatient clinic reported that 30.4% received low-dose NOACs despite a low risk of bleeding whereas only 6.8% received a higher dose than recommended [49]. These data suggest that prescribing physicians are hesitant to use the label dosages of NOACs in a substantial proportion of patients who meet indications for antithrombotic therapy without the need for dosage adjustment. Closer adherence to guideline recommendations and a careful evaluation of net clinical benefit are warranted to promote evidence-based use of NOACs in the clinical setting.
How should NVAF patients be switched between VKAs and NOACs?
Switching from VKAs to NOACs is relatively straightforward: patients can be switched immediately if the INR is ≤3 (rivaroxaban) or ≤2 (dabigatran or apixaban) [6]. If the INR is between 2 and 2.5, the NOAC can be started immediately or the next day. If the INR is ≥3, switching should be postponed until the INR falls to <2.5.
Switching from NOACs to VKAs is more complex owing to the slow onset of action of VKAs. It may take 5–10 days for a patient to reach a therapeutic INR when switched to a VKA; therefore, the EHRA recommends continuing the NOAC until the INR reaches 2–3 [6]. Close monitoring of INR is recommended during the transition and for up to 1 month or until three consecutive INR readings of 2–3 are recorded. FXa inhibitors affect INR values; therefore, measurements should be taken immediately prior to the next administration of NOAC.
There is limited evidence to guide switching between the NOACs. The EHRA recommends starting the first dose of apixaban, rivaroxaban, or dabigatran when the next dose of the preceding NOAC would have been due [6]. In situations where higher than therapeutic plasma concentrations are expected such as in patients with renal impairment or the frail elderly, a longer interval between stopping the current NOAC and starting the new one may be considered.
How should patients on NOACs be monitored?
Assessment of renal function is recommended prior to initiation and routinely during maintenance therapy [6]. Regular monitoring of renal function is particularly important in patients receiving dabigatran owing to its predominant renal clearance.
Other assessments that are recommended prior to initiation of any antithrombotic therapy include hemoglobin, hematocrit and coagulation parameters (i.e., prothrombin time, activated partial thromboplastin time, and fibrinogen), and liver function tests if liver dysfunction is confirmed or suspected [4].
Are reversal antidotes available for NOACs?
Only one NOAC antidote, idarucizumab, which specifically targets dabigatran [50], was commercially available (only in the United Arab Emirates) at the time these practical perspectives were developed [51]. Several other antidotes were in clinical development at the time these perspectives were developed, including andexanet alpha, which targets FXa inhibitors [52], and ciraparantag, a potentially “universal antidote” [53].
How should a bleeding event be prevented and managed in patients on NOACs?
The most common adverse event associated with all anticoagulants is bleeding [17]. This is a dynamic process and a patient’s status can change rapidly. In general, reversible risk factors for bleeding should be appropriately managed to reduce the risk of bleeding. Co-administration of drugs that further increase the risk of bleeding, such as antiplatelet therapies, should be avoided, if possible. The European guidelines recommend that patients at high risk of GI bleeding should be treated with a VKA or a NOAC other than dabigatran 150 mg b.i.d. or rivaroxaban 20 mg once-a-day (q.d.) (Level IIa B) owing to the higher risk of major GI bleeding with those agents [1].
Major guidelines recommend a graded approach comprising largely supportive strategies for bleeding events (Table 7) since the relatively short half-lives of NOACs make “time the most important antidote of the NOACs” [6].
Table 7.
Recommended approach to the management of bleeding.
| Type of bleed | Direct thrombin inhibitors (dabigatran) | FXa inhibitors (apixaban, rivaroxaban) |
|---|---|---|
| Nonlife-threatening | Estimate normalization of hemostasis (12–≥48 h depending on renal function) | Normalization of hemostasis 12–24 h |
| General supportive measures | General supportive measures | |
| Consider tranexamic acid, desmopression, and/or dialysis | Consider tranexamic acid and/or desmopressin | |
| Charcoal hemoperfusion not recommended | ||
| Life-threatening | All of the above | All of the above |
| Idarucizumab (where available) | PCC 25 U/kg | |
| Activated factor VII (rFVIIa; 90 μg/kg) | Activated PCC 50 IE/kg (max 200 IE/kg/d) if available | |
| Activated factor VII (rFVIIa; 90 μg/kg) |
Note. From “Updated European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary,” by H. Heidbuchel, P. Verhamme, M. Alings, M. Antz, H.C. Diener, W. Hacke, et al, 2015, Europace, 17, p. 1467–507. Copyright 20XX, Name of the Copyright Holder. Adapted with permission.
PCC = prothrombin complex concentrate.
What should I tell my surgical and dental colleagues about the perioperative management of NOACs?
The rapid onset of action and short half-life of NOACs simplifies periprocedural management strategies compared with VKAs. Nevertheless, cardiologists are often consulted by their noncardiology colleagues regarding the need for interruption of NOACs or bridging with heparin when patients require invasive interventions. NOACs may be safely continued without interruption for procedures that carry only a minor risk of bleeding (e.g., tooth extraction, cataract surgery, pacemaker insertion, and AF ablation) [6], [17]. In these cases, the intervention should ideally be performed at trough concentration of the NOAC (i.e., 12 hours after last intake of dabigatran or apixaban, 24 hours after last intake of rivaroxaban). Recommendations on antithrombotic dosing in patients undergoing specific electrophysiological and device implantation procedures have been published by the EHRA, and have been endorsed by the ESC, Heart Rhythm Society and Asia Pacific Heart Rhythm Society [54].
Other same-day procedures carry a higher risk of bleeding (e.g., some biopsies), and short interruption of NOACs may be appropriate based on a careful assessment of a patient’s risk of bleeding and thromboembolism, the risk of bleeding associated with the procedure, the patient’s renal function, and pharmacokinetics of each NOAC [17], [54]. Evidence from the Phase III clinical trials of NOACs suggests the risk of thromboembolic events with short-term interruption of NOACs is low (≤0.5%) [17]. Preoperative heparin bridging is not recommended because it increases bleeding risk [1], [54]. Clinicians are encouraged to consult their individual institutional guidelines; general recommendations on when to stop NOACs prior to planned interventions are provided in Table 8.
Table 8.
Recommended timing of last NOAC intake prior to elective surgical procedures.
| CrCl (mL/min) |
Dabigatran |
Apixaban, rivaroxaban |
||
|---|---|---|---|---|
| No important bleeding risk and/or adequate local hemostasis possible: perform at trough level (i.e., ≥12 h or 24 h after last intake) | ||||
| Risk levela | Low risk | High risk | Low risk | High risk |
| ≥80 | ≥24 h | ≥48 h | ≥24 h | ≥48 h |
| 50–80 | ≥36 h | ≥72 h | ≥24 h | ≥48 h |
| 30–50b | ≥48 h | ≥96 h | ≥24 h | ≥48 h |
| 15–30b | Not indicated | ≥36 h | ≥48 h | |
| <15 | No official indication for use | |||
Note. From “North American thrombosis forum, AF action initiative consensus,” by. C.T. Ruff, J.E. Ansell, R.C. Becker, E.J. Benjamin, D.J. Deicicchi, M. Estes, et al, 2016, Am J Med, 129, p. S1–29. Copyright 20XX, Name of the Copyright Holder. From “Updated European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary,” by H. Heidbuchel, P. Verhamme, M. Alings, M. Antz, H.C. Diener, W. Hacke, et al, 2016, Eur Heart J, pii: ehw058 [Epub ahead of print]. Copyright 20XX, Name of the Copyright Holder. Adapted with permission.
b.i.d = twice a day; NOACs = non-vitamin K antagonist oral anticoagulants.
Many of these patients may be on lower dose NOAC, i.e., dabigatran 110 mg b.i.d., apixaban 2.5 mg b.i.d., or rivaroxaban 15 mg q.d.
Low risk, surgery with low risk of bleeding aiming for mild-to-moderate residual anticoagulant effect at surgery (<12–25%); high risk, surgery with high risk of bleeding aiming for no or minimal residual anticoagulant effect (<3–6%) at surgery.
Recommendations regarding when to restart NOAC dosing also varies by level of patient-related and procedural risk, and level of hemostasis achieved (Table 9). In general, bridging with heparin is recommended only in exceptional cases, for example, after the procedure in patients who cannot tolerate oral medications [17] or in cases requiring immobilization [6].
Table 9.
Suggested management approach to resumption of NOACs after intervention.
| NOAC | Low-risk surgery | High-risk surgerya |
|---|---|---|
| Dabigatran | 150 mg b.i.d. starting 24 h postoperatively | 150 mg b.i.d. starting 48–72 h postoperativelyb |
| Rivaroxaban | 20 mg q.d. starting 24 h postoperatively | 20 mg q.d. starting 48–72 h postoperatively |
| Apixaban | 5 mg b.i.d. starting 24 h postoperatively | 5 mg b.i.d. starting 48–72 h postoperativelyb |
Note. From “North American thrombosis forum, AF action initiative consensus,” by. C.T. Ruff, J.E. Ansell, R.C. Becker, E.J. Benjamin, D.J. Deicicchi, M. Estes, et al, 2016, Am J Med, 129, p. S1–29. Copyright 20XX, Name of the Copyright Holder. Adapted with permission.
b.i.d = twice a day; NOACs = non-vitamin K antagonist oral anticoagulants; q.d = once a day.
Low risk, surgery with low risk of bleeding aiming for mild-to-moderate residual anticoagulant effect at surgery (<12–25%); high risk, surgery with high risk of bleeding aiming for no or minimal residual anticoagulant effect (<3–6%) at surgery.
For patients at high risk for thromboembolism, consider administering a reduced dose of NOAC (110 mg dabigatran; 2.5 mg apixaban) on evening after surgery and on following day (i.e., 1st postoperative day) after surgery.
Nonelective, urgent surgical procedures should be deferred, where possible, until 12–24 hours after the last dose of NOAC was taken [6]. Coagulation tests may be performed to assess the residual level of anticoagulant effect prior to the procedure.
Can antithrombotic therapy be safely combined with antiplatelet therapy?
Patients fulfilling the indications for both antithrombotic therapy for stroke prevention as well as antiplatelet therapy for CAD require a careful evaluation of net clinical benefit because both classes of medications are associated with an increased risk of bleeding [17].
There is currently a dearth of data on the efficacy and safety of dual or triple therapy in patients with AF and CAD to guide clinical practice. Several ongoing trials are evaluating the combination of antiplatelet therapies with NOACs in patients with AF after an acute coronary syndrome or stent placement, and the results will help inform the optimal clinical management of these challenging patients.
The American guidelines recommend that after coronary revascularization in patients with CHA2DS2-VASc scores of ≥2, the concurrent use of clopidogrel with OAC but without ASA may be reasonable (Level IIb B) [2]. In contrast, the ESC guidelines recommend triple therapy (OAC plus clopidogrel plus ASA) for 1 month after stenting in patients with stable CAD at risk of stroke (Level IIa B) and for 1–6 months after an acute coronary syndrome with stent implantation (Level IIa C) [1]. Both the ESC guidelines and EHRA guidelines advise a planned schedule for step-down therapy to dual therapy (OAC plus single antiplatelet drug) after 1 to 6 months, and then to OAC monotherapy after 1 year in patients who are stable, as a prespecified strategy to minimize the risk of bleeding [1], [6].
When should an NVAF patient be referred for left atrial appendage closure?
Left atrial appendage (LAA) closure or partitioning may be indicated for patients with AF and CAD who are at high risk of stroke/systemic embolism and anticoagulant-related bleeding [17]. Surgical closure of the LAA at the time of other surgical procedures, for example, coronary artery bypass grafting, has demonstrated favorable results. The ESC guidelines recommend that LAA occlusion or exclusion be considered for stroke prevention in patients with AF and contraindications to long-term OAC (Level IIb B) [1]. They further recommend that OAC therapy be continued in at-risk patients after surgical occlusion or exclusion of the LAA (Level IB) [1]. The American guidelines recommend consideration of surgical excision of the LAA in patients undergoing cardiac surgery (Level IIb C) [2]. There is some evidence for percutaneous technologies (e.g., the Watchman device) [55], [56], which suggest noninferiority to VKA treatment for stroke prevention in AF patients at moderate risk for stroke, and possibly lower risk of bleeding [1].
How should patients presenting with acute stroke while on NOACs be managed?
The management of patients presenting with acute stroke or TIA while on NOAC therapy is largely based on clinical experience and expert opinion because prospective data are missing. Guidelines recommend the prompt correction of coagulation status in patients receiving NOACs who suffer an intracranial hemorrhage (ICH) [6]. Thrombolytic therapy is not recommended in patients who present with acute ischemic stroke if a NOAC was administered within the past 48 hours, in accordance with the half-lives of these agents. Instead, mechanical recanalization of occluded vessels is recommended. The European guidelines recommend that aspirin be considered in AF patients who suffer a stroke until the initiation or resumption of OAC therapy [1]. Combination therapy of OAC and antiplatelet therapy is not recommended after a stroke or TIA [Level IIIB (harm)] [1]. In patients with a previous stroke, the ESC recommends NOACs in preference to VKA or aspirin for secondary stroke prevention (Level 1B) [1].
When should a NOAC be restarted after an intracerebral hemorrhage?
OAC therapy may be restarted 4–8 weeks after an ICH provided the cause of bleeding or relevant risk factor has been treated or controlled [1]. The EHRA recommends that NOACs may be restarted as early as 10–14 days after an ICH if the patient’s risk of recurrent ICH is deemed to be low and risk of recurrent thromboembolism is high [6]. The same factors that increase risk of embolic stroke also increase risk of ICH, namely, advanced age, hypertension, and previous stroke. Anticoagulants including NOACs are contraindicated in patients with a history of spontaneous ICH unless the cause has been identified and reversed. Closure of the LAA may be an appropriate nonpharmacological intervention for patients at risk of recurrent ICH [6].
When should a NOAC be restarted after an ischemic stroke?
The optimal time to reinstitute administration of NOACs after an ischemic stroke is variable and largely dependent on the infarct size [6]. The European guidelines recommend that anticoagulation be interrupted for 3–12 days after a moderate-to-severe ischemic stroke based on a multidisciplinary assessment of the net risks of acute stroke and bleeding [1]. The EHRA recommends restarting NOAC dosing as soon as possible after a TIA without the need for bridging or aspirin; 3 days after a small, nondisabling embolic stroke; 6 days after a moderate stroke; and 2–3 weeks after a large infarct [6].
Conclusions
NOACs now have an established role in the prevention of stroke in patients with NVAF. There is a consistent body of clinical trial evidence supporting their comparable efficacy for stroke prevention in NVAF relative to VKAs, with dabigatran 150 mg and apixaban demonstrating superiority versus warfarin. NOACs offer important advantages including lower risk of major bleeding (dabigatran 110 mg and apixaban showed superiority vs. warfarin) and convenient dosing and administration. Real-world evidence is accumulating and providing reassurance that the NOACs are safe in the routine clinical setting across a diverse range of patients, with some differences emerging between individual agents. The optimal use of NOACs in patients with comorbid conditions such as CAD remains to be determined, and several ongoing clinical studies are poised to provide guidance to clinicians on the selection of specific NOACs for individual patients and clinical situations. Growing experience with these agents in the MENA region will help guide patient selection and elucidate optimal dosing strategies to maximize the clinical benefits of the NOACs.
Conflict of interest
These practical perspectives were prepared by Prof. Ahmad Hersi with input, review and approval of the final manuscript from a panel of expert cardiologists from North Africa and the Middle East. This panel met together under the auspices of a clinical consultancy meeting sponsored by Pfizer MENA. The meeting sponsor was not involved in drafting the manuscript. Professor A.S. Hersi, Dr M. Magdy and Professor S. Shaheen have no conflicts of interest to disclose. Dr A.K. Hamad has served as an advisor to Boehringer Ingelheim, Pfizer and Bayer, and has received honoraria for presentations from Boehringer Ingelheim and Bayer, all of which are unrelated to the submitted work. Dr H. Sabbour has been a member of a speaker bureau for Boehringer Ingelheim, Bayer and Bristol Myers Squibb, all of which are unrelated to the submitted work.
Acknowledgments
We wish to acknowledge MedPlan Communications for their editorial assistance in the preparation of this manuscript, which was funded by an unrestricted grant from Pfizer MENA.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37 doi: 10.1093/eurheartj/ehw210. 2893–62. [DOI] [PubMed] [Google Scholar]
- 2.January C.T., Wann L.S., Alpert J.S., Calkins H., Cigarroa J.E., Cleveland J.C., Jr 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation. Circulation. 2014;130:e199–267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macle L., Cairns J., Leblanc K., Tsang T., Skanes A., Cox J.L. 2016 focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2016;32:1170–1185. doi: 10.1016/j.cjca.2016.07.591. [DOI] [PubMed] [Google Scholar]
- 4.Fontana P., Robert-Ebadi H., Bounameaux H., Boehlen F., Righini M. Direct anticoagulants: a guide for daily practice. Swiss Med Wkly. 2016;146:w14286. doi: 10.4414/smw.2016.14286. [DOI] [PubMed] [Google Scholar]
- 5.Frost C., Wang J., Nepal S., Schuster A., Barrett Y.C., Mosqueda-Garcia R. Apixaban, an oral, direct factor Xa inhibitor: single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013;75:476–487. doi: 10.1111/j.1365-2125.2012.04369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidbuchel H., Verhamme P., Alings M., Antz M., Diener H.C., Hacke W. Updated European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Europace. 2015;17:1467–1507. doi: 10.1093/europace/euv309. [DOI] [PubMed] [Google Scholar]
- 7.Boriani G., Cimaglia P., Fantecchi E., Mantovani V., Ziacchi M., Valzania C. Non-valvular atrial fibrillation: potential clinical implications of the heterogeneous definitions used in trials on new oral anticoagulants. J Cardiovasc Med (Hagerstown) 2015;16:491–496. doi: 10.2459/JCM.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 8.Chugh S.S., Havmoeller R., Narayanan K., Singh D., Rienstra M., Benjamin E.J. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hersi A., Abdul-Moneim M., Almous’ad A., Al-Samadi F., AlFaqih A., Sweidan R. Saudi Atrial Fibrillation Survey: national, observational, cross-sectional survey evaluating atrial fibrillation management and the cardiovascular risk profile of patients with atrial fibrillation. Angiology. 2015;66:244–248. doi: 10.1177/0003319714529180. [DOI] [PubMed] [Google Scholar]
- 10.Hersi A.S., Alsheikh-Ali A.A., Zubaid M., Al Suwaidi J. Prospective observational studies of the management and outcomes in patients with atrial fibrillation: a systematic review. J Saudi Heart Assoc. 2012;24:243–252. doi: 10.1016/j.jsha.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridha M., Al-Sayed Amin A., Saleh S.A., Cherian B., Al-Kandari F., Redha F. The prevalence of atrial fibrillation among acute medical admissions in Kuwait. Med Princ Pract. 2005;14:136–139. doi: 10.1159/000084628. [DOI] [PubMed] [Google Scholar]
- 12.Nieuwlaat R., Capucci A., Camm A.J., Olsson S.B., Andresen D., Davis D.W. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26:2422–2434. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 13.Camm A.J., Breithardt G., Crijns H., Dorian P., Kowey P., Le Heuzey J.Y. Real-life observations of clinical outcomes with rhythm- and rate-control therapies for atrial fibrillation RECORDAF (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation) J Am Coll Cardiol. 2011;58:493–501. doi: 10.1016/j.jacc.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Kerr C.R., Boone J., Connolly S.J., Dorian P., Green M., Klein G. The Canadian Registry of Atrial Fibrillation: a noninterventional follow-up of patients after the first diagnosis of atrial fibrillation. Am J Cardiol. 1998;82:82N–85N. doi: 10.1016/s0002-9149(98)00589-x. [DOI] [PubMed] [Google Scholar]
- 15.Lip G.Y., Laroche C., Popescu M.I., Rasmussen L.H., Vitali-Serdoz L., Dan G.A. Improved outcomes with European Society of Cardiology guideline-adherent antithrombotic treatment in high-risk patients with atrial fibrillation. A report from the EORP-AF General Pilot Registry. Europace. 2015;17:1777–1786. doi: 10.1093/europace/euv269. [DOI] [PubMed] [Google Scholar]
- 16.Lane D.A., Lip G.Y.H. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126:860–865. doi: 10.1161/CIRCULATIONAHA.111.060061. [DOI] [PubMed] [Google Scholar]
- 17.Ruff C.T., Ansell J.E., Becker R.C., Benjamin E.J., Deicicchi D.J., Estes M. North American thrombosis forum, AF action initiative consensus. Am J Med. 2016;129:S1–29. doi: 10.1016/j.amjmed.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Lip G.Y., Andreotti F., Fauchier L., Huber K., Hylek E., Knight E. Bleeding risk assessment and management in atrial fibrillation patients: a position document from the European Heart Rhythm Association, endorsed by the European Society of Cardiology Working Group on Thrombosis. Europace. 2011;13:723–746. doi: 10.1093/europace/eur126. [DOI] [PubMed] [Google Scholar]
- 19.Glazer N.L., Dublin S., Smith N.L., French B., Jackson L.A., Hrachovec J.B. Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med. 2007;167:246–252. doi: 10.1001/archinte.167.3.246. [DOI] [PubMed] [Google Scholar]
- 20.Ogilvie I.M., Newton N., Welner S.A., Cowell W., Lip G.Y. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Zubaid M., Rashed W.A., Alsheikh-Ali A.A., Al-Zakwani I., Al Mahmeed W., Shehab A. Management and 1-year outcomes of patients with atrial fibrillation in the Middle East: Gulf survey of atrial fibrillation events. Angiology. 2015;66:464–471. doi: 10.1177/0003319714536980. [DOI] [PubMed] [Google Scholar]
- 22.Haas S. New oral Xa and IIa inhibitors: updates and clinical trial results. J Thromb Thrombolysis. 2008;25:52–60. doi: 10.1007/s11239-007-0108-7. [DOI] [PubMed] [Google Scholar]
- 23.Hersi A, Almusaad A, Suliman I, Bokhari F, Alghamdi B, Alfagih A, et al. Ministry of Health of Saudi Arabia and McMaster University clinical practice guidelines on the antithrombotic treatment of patients with non-valvular atrial fibrillation. Available at http://www.moh.gov.sa/depts/Proofs/Documents/5Atrial%20Fibrillation%20-%20Antithrombotic%20Treatment%20of%20Patients%20with%20Non-valvular%20Atrial%20Fibrillation.pdf. Issue date: April 2014.
- 24.Kreutz R. A clinical and pharmacologic assessment of once-daily versus twice-daily dosing for rivaroxaban. J Thromb Thrombolysis. 2014;38:137–149. doi: 10.1007/s11239-013-1029-2. [DOI] [PubMed] [Google Scholar]
- 25.Schulman S. New oral anticoagulant agents – general features and outcomes in subsets of patients. Thromb Haemost. 2014;111:575–582. doi: 10.1160/TH13-09-0803. [DOI] [PubMed] [Google Scholar]
- 26.Stampfuss J., Kubitza D., Becka M., Mueck W. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther. 2013;51:549–561. doi: 10.5414/CP201812. [DOI] [PubMed] [Google Scholar]
- 27.Hellwig T., Gulseth M. Pharmacokinetic and pharmacodynamics drug interactions with new oral anticoagulants: what do they mean for patients with atrial fibrillation? Ann Pharmacother. 2013;47:1478–1487. doi: 10.1177/1060028013504741. [DOI] [PubMed] [Google Scholar]
- 28.Ruff C.T., Giugliano R.P., Braunwald E., Hoffman E.B., Deenadayalu N., Ezekowitz M.D. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Quesada C.J. Giugliano. Comparison of the phase III clinical trial designs of novel oral anticoagulants versus warfarin for the treatment of nonvalvular atrial fibrillation: implications for clinical practice. Am J Cardiovasc Drugs. 2014;14:111–127. doi: 10.1007/s40256-013-0062-z. [DOI] [PubMed] [Google Scholar]
- 30.Connolly S.J., Eikelboom J., Joyner C., Diener H.C., Hart R., Golitsyn S. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 31.Nallamothu B.K., Hayward R.A., Bates E.R. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008;118:1294–1303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- 32.Graham D.J., Reichman M.E., Wenecke M., Zhang R., Southworth M.R., Levenson M. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. doi: 10.1161/CIRCULATIONAHA.114.012061. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell S.A., Simon T.A., Raza S., Jakouloff D., Orme M.E., Lockhart I. The efficacy and safety of oral anticoagulants in warfarin-suitable patients with nonvalvular atrial fibrillation: systematic review and meta-analysis. Clin Appl Thromb Hemost. 2013;19:619–631. doi: 10.1177/1076029613486539. [DOI] [PubMed] [Google Scholar]
- 34.Larsen T.B., Skjøth F., Nielsen P.B., Kjaeldgaard J.N., Lip G.Y.H. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. doi: 10.1136/bmj.i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishak K.J., Phatak H., Rael M., Lanitis T., Hoog M., Kamble S. A simulated head to head comparison of stroke and major bleeding with apixaban versus rivaroxaban in high-risk NVAF patients. Eur Heart J. 2015;36:743–744. presented at ESC 2015. [Google Scholar]
- 36.Inoue H., Uchiyama S., Atarashi H., Okumura K., Koretsune Y., Yasaka M. Post-marketing surveillance on the long-term use of dabigatran in Japanese patients with nonvalvular atrial fibrillation: preliminary report of the J-dabigatran surveillance. J Arrhythm. 2016;32:145–150. doi: 10.1016/j.joa.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banergee A., Lane D.A., Torp-Pedersen C., Lip G.Y. Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus no treatment in a “real world” atrial fibrillation population: a modelling analysis based on a nationwide cohort study. Thromb Haemost. 2012;107:584–589. doi: 10.1160/TH11-11-0784. [DOI] [PubMed] [Google Scholar]
- 38.Lip G.Y., Keshishian A., Kamble S., Pan X., Mardekian J., Horblyuk R. Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin. A propensity score matched analysis. Thromb Haemost. 2016;116:975–986. doi: 10.1160/TH16-05-0403. [DOI] [PubMed] [Google Scholar]
- 39.Lin I, Masseria C, Mardekian J, et al. Real-world bleeding risk among non-valvular atrial fibrillation (NVAF) patients prescribed apixaban, dabigatran, rivaroxaban and warfarin: analysis of electronic health records. Poster presented at the 2015 European Society of Cardiology Congress 365, 29 August–2 September, London, UK (Poster P6215).
- 40.Tepper P., Mardekian J., Masseria C., Horblyuk R., Kamble S., Hamilton M. Real-world comparison of inpatient bleeding risk, bleeding-related hospitalization rates and costs among non-valvular atrial fibrillation patients on apixaban, dabigatran, rivaroxaban: cohorts comprising new initiators and/or switchers from warfarin. J Am Coll Cardiol. 2016;67:662. [Google Scholar]
- 41.Deitelzweig S., Bruno A., Trocio J., Tate N., Gupta K., Lin J. An early evaluation of bleeding-related hospital readmissions among hospitalized patients with nonvalvular atrial fibrillation treated with direct oral anticoagulants. Curr Med Res Opin. 2016;32:573–582. doi: 10.1185/03007995.2015.1131676. [DOI] [PubMed] [Google Scholar]
- 42.Bouillon K., Bertrand M., Maura G., Blotière P.O., Ricordeau P., Zureik M. Risk of bleeding and arterial thromboembolism in patient with non-valvular atrial fibrillation either maintained on a vitamin K antagonist or switched to a non-vitamin K-antagonist oral anticoagulant: a retrospective matched-cohort study. Lancet Haematol. 2015;2:e150–9. doi: 10.1016/S2352-3026(15)00027-7. [DOI] [PubMed] [Google Scholar]
- 43.Huisman M.V., Rothman K.J., Paquette M., Teutsch C., Diener H.-C., Dubner S.J. The changing landscape for stroke prevention in AF: findings from the GLORIA-AF Registry Phase 2. J Am Coll Cardiol. 2017;69:777–785. doi: 10.1016/j.jacc.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 44.Heidbuchel H., Verhamme P., Alings P., Antz M., Hacke W., Oldgren J. EHRA Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. 2013;34:2094–2106. doi: 10.1093/eurheartj/eht134. [DOI] [PubMed] [Google Scholar]
- 45.Alomi Y.A. General Administration of Pharmaceutical Care; Saudi Arabia Ministry of Health: 2015. Update 2015: Drug Therapy during Holy Month of Ramadan. [Google Scholar]
- 46.Aslam M., Assad A. Drug regimens and fasting during Ramadan: a survey in Kuwait. Public Health. 1986;100:49–53. doi: 10.1016/s0033-3506(86)80086-5. [DOI] [PubMed] [Google Scholar]
- 47.Neilsen P.B., Skjøth F., Søgaard M., Kjaeldgaard J.N., Lip G.Y.H., Larsen T.B. Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. Br Med J. 2017;356 doi: 10.1136/bmj.j510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pattullo C.S., Barras M., Tai B., McKean M., Donovan P. New oral anticoagulants (NOACs) – appropriateness of prescribing in real-world setting. Intern Med J. 2016;46:812–818. doi: 10.1111/imj.13118. [DOI] [PubMed] [Google Scholar]
- 49.Belen E., Canbolat I.P., Bayyigit A., Helvaci A., Pusuroglu H., Kilickesmez K. A new gap in the novel anticoagulants’ era: undertreatment. Blood Coagul Fibrinolysis. 2015;26:793–797. doi: 10.1097/MBC.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 50.Pollack C.V., Jr, Reilly P.A., Eikelboom J., Glund S., Verhamme P., Bernstein R.A. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373:511–520. doi: 10.1056/NEJMoa1502000. [DOI] [PubMed] [Google Scholar]
- 51.Tummala R., Kavtaradze A., Gupta A., Ghosh R.K. Specific antidotes against direct oral anticoagulants: a comprehensive review of clinical trials data. Int J Cardiol. 2016;214:292–298. doi: 10.1016/j.ijcard.2016.03.056. [DOI] [PubMed] [Google Scholar]
- 52.Siegal D.M., Curnutte J.T., Connolly S.J., Lu G., Conley P.B., Wiens B.L. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373:2413–2424. doi: 10.1056/NEJMoa1510991. [DOI] [PubMed] [Google Scholar]
- 53.Ansell J.E., Laulicht B.E., Bakhru S.H., Hoffman M., Steiner S.S., Costin J.C. Ciraparantag safely and completely reverses the anticoagulant effects of low molecular weight heparin. Thromb Res. 2016;146:113–118. doi: 10.1016/j.thromres.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 54.Heidbuchel H., Verhamme P., Alings M., Antz M., Diener H.C., Hacke W. Updated European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. 2016 doi: 10.1093/europace/euv309. pii: ehw058 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Reddy V.Y., Doshi S.K., Sievert H., Buchbinder M., Neuzil P., Huber K. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) trial. Circulation. 2013;127:720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 56.Belgaid D.R., Khan Z., Zaidi M., Hobbs A. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. Int J Cardiol. 2016;219:177–179. doi: 10.1016/j.ijcard.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 57.Connolly S.J., Ezekowitz M.D., Yusuf S., Eikelboom J., Oldgren J., Parekh A. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 58.Patel M.R., Mahaffey K.W., Garg J., Pan G., Singer D.E., Hacke W. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 59.Granger C.B., Alexander J.H., McMurray J.J., Lopes R.D., Hylek E.M., Hanna M. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]