Abstract
Myocardial bridge is defined as the narrowing of any coronary artery segment in systole but a normal diameter in diastole. It is most frequently seen on left anterior descending (LAD) artery. Left circumflex artery (LCx) is very rare. A 62 year-old male patient presented with severe, squeezing chest pain. The electrocardiogram showed T wave inversion in V1–V4 and ST depression in DII, DIII, aVF. Coronary angiography showed complicated lesion on after S2 branches of LAD and myocardial bridge causing 100% systolic narrowing of fourth obtus marginal branch of LCx. Bare metal stent was placed to LAD lesions with no residual occlusion. The patient was discharged with beta-blocker therapy. He had no recurrent chest pain during six months of follow-up.
Keywords: Myocardial bridging, Coronary circulation
Introduction
Myocardial bridge is defined as the narrowing of any coronary artery segment in systole but a normal diameter in diastole. Myocardial bridging generally occurs in the left anterior descending artery (LAD); left circumflex artery (LCx) involvement is very rare [1]. Although it is a benign condition, myocardial bridging can manifest symptoms such as acute coronary syndrome, arrhythmias, and sudden cardiac death [2].
We report an incidentally determined nonsymptomatic myocardial bridge that caused 100% systolic narrowing of the LCx in a patient with acute coronary syndrome.
Case report
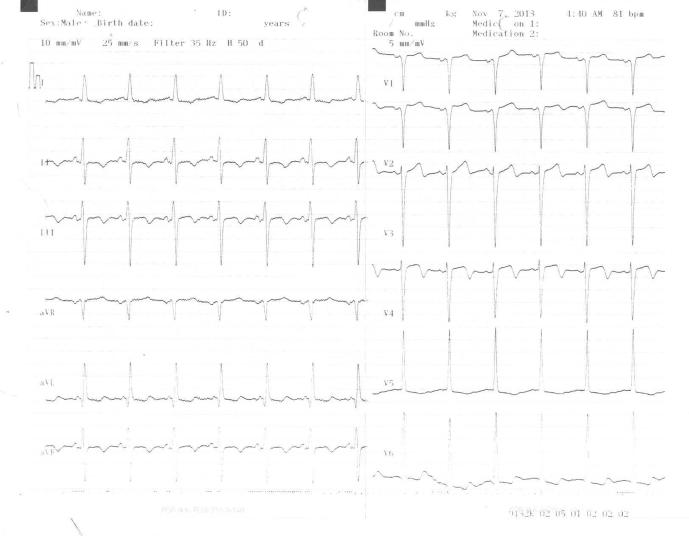
A 62-year-old male patient presented with severe, squeezing chest pain. Aortic 2/6 systolic murmur and 1/4 diastolic murmur were observed; the result of his physical examination was otherwise normal. Blood pressure and cardiac rate were in normal ranges. The electrocardiogram (ECG) showed T wave inversion and R wave progression loss in V1–V4, ST depression and T wave inversion in DII, DIII, aVF, and left ventricular hypertrophy, left axis (Fig. 1). Age, obesity, and smoking were present as risk factors for coronary heart disease. Cardiac enzymes levels were elevated. Creatine kinase (CK), myocardial band (MB) fraction of CK (CK-MB), and troponin T levels were high with values of 181 IU/L (normal range, 0–17 1U/L), 21 U/L (normal range, <24 U/L), and 0.365 ng/mL (normal range, 0–0.014 ng/mL), respectively. Other hematological and biochemical tests revealed the following: prothrombin time of 11.3 seconds (normal range, 9.4–12.5 seconds), activated partial thromboplastin time of 36 seconds (normal range, 25.4–38.4 seconds), total cholesterol 200 mg/dL (normal range, 110–200 mg/dL), triglyceride 91 mg/dL (normal range, 0–200 mg/dL), and low density lipoprotein-cholesterol 141 mg/dL (normal range, 0–130 mg/dL).
Figure 1.
Electrocardiogram (ECG) readings obtained at admission of the patient.
Transthoracic echocardiography revealed a left ventricular ejection fraction of 40%, and hypokinesia in the anterior wall of the left ventricle and apex. Mild aortic stenosis and aortic regurgitation were identified. The patient was admitted to the coronary intensive care unit with a diagnosis of acute coronary syndrome. Cardiac catheterization was performed. Coronary angiography revealed a complicated lesion after S2 branches of LAD, coronary artery ectasia (CAE) involving the left main artery, LCx, and myocardial bridge causing 100% systolic narrowing of LCx (Figure 2, Figure. 3). LCx flow was normal in systole (Fig. 4). The right common artery was normal (Fig. 5). A bare metal stent was placed in the LAD lesions with no residual occlusion. Flow defined as grade 3 according to thrombolysis in myocardial infarction scale was achieved in the areas of intervention (Fig. 6). Medications administered in the catheterization laboratory and coronary care unit included aspirin, clopidogrel, heparin, and nitroglycerin protocol, and aspirin, clopidogrel, atorvastatin, ramipiril, and metoprolol protocol, respectively.
Figure 2.
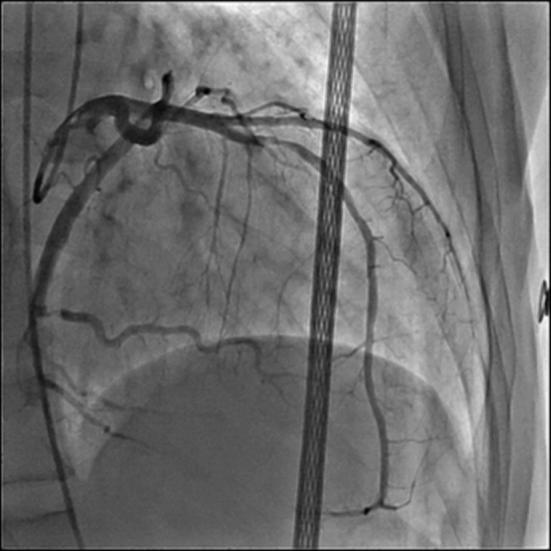
Coronary angiography of left anterior descending artery.
Figure. 3.
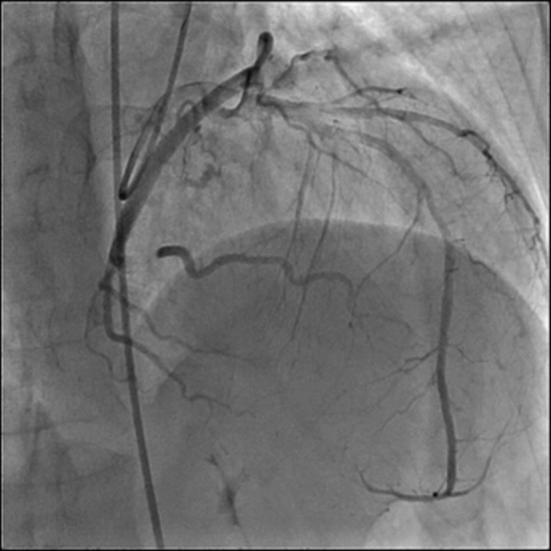
Myocardial bridge causing 100% systolic narrowing of left circumflex (LCx) artery.
Figure. 4.
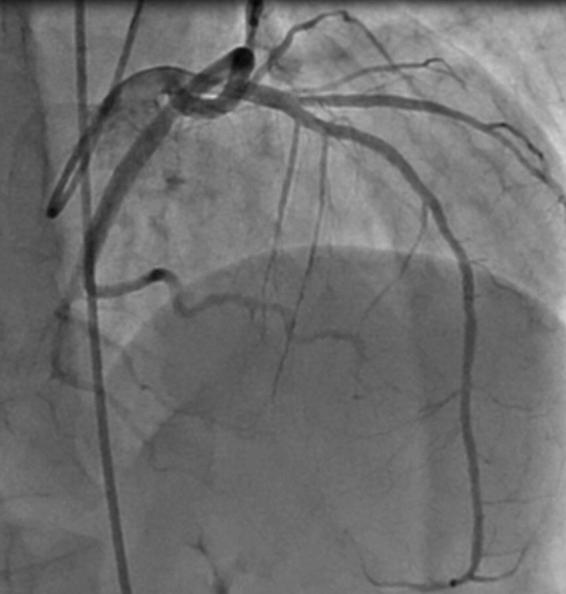
Coronary angiography of left circumflex (LCx) artery in diastole.
Figure. 5.
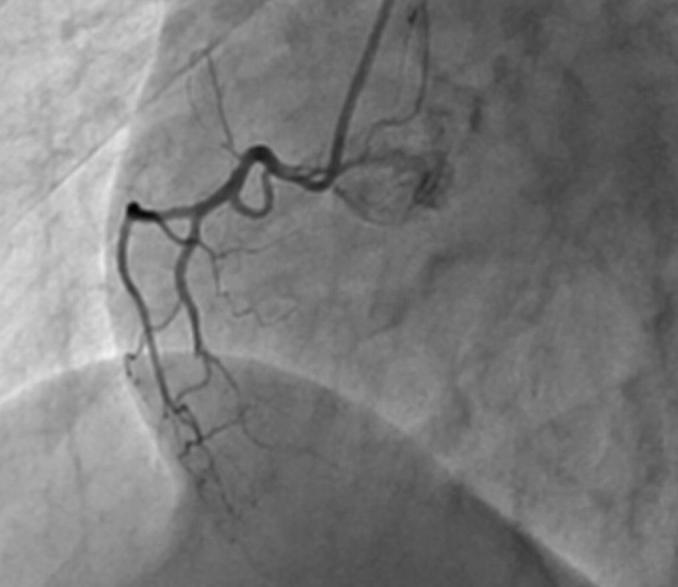
Coronary angiography of right coronary artery.
Figure. 6.
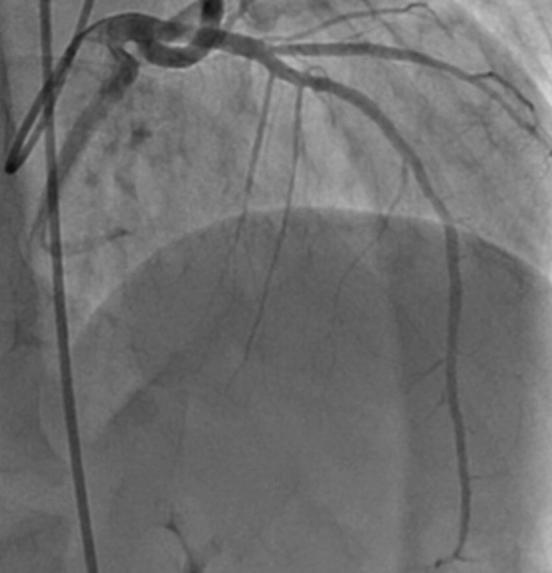
Coronary angiography after percutaneous coronary intervention.
A follow-up ECG showed resolution of the ECG changes. Chest pain did not recur in the patient following therapy in the coronary intensive care unit. One month later, the patient remained asymptomatic and achieved his target heart rate during a treadmill test. The patient had no further symptoms and no ischemic ECG changes. In the 6-month follow-up, the patient was asymptomatic.
Discussion
Myocardial bridging is manifested as a segment of an epicardial coronary artery traveling intramurally through the myocardium. Researchers have identified three types of myocardial bridges: intramural, mural, or tunneled. Myocardial bridge is commonly observed in the anterior interventricular branch of the left coronary artery with its middle segment. Bridging of the circumflex or the right coronary artery or one of their branches is not common and has been very rarely reported [3]. The first case of myocardial bridging of LCx was reported by Arjomand et al [4]. The other case was reported by Kumar at al [5]. In this case, they reported myocardial bridge involving the left main, left circumflex, and LAD coronary arteries. In the current case, the location of the myocardial bridge was in the middle portion of the circumflex artery and caused 100% narrowing. This specific location is very rare.
Myocardial bridge is generally clinically asymptomatic but has been associated with atherosclerosis, myocardial infarction, and sudden cardiac death. Myocardial bridge causes phasic systolic compression, increase in intracoronary velocity, increased sympathetic drive during stressor exercise, endothelial dysfunction, coronary artery spasm, and systolic kinking of the artery [4]. These pathophysiologic causes are related to the above-mentioned clinical conditions. Despite the 100% systolic narrowing of the LCx, the patient interestingly had no symptoms. If patients do not present with any symptoms, treatment may not be required. Similarly, we did not administer any medical therapy because of the myocardial bridge. By contrast, if patients are symptomatic, beta blockers, calcium channel blockers, coronary stents, minimally invasive coronary artery bypass grafting, and surgical myotomy may be suggested [3].
The present case showed that myocardial bridging may asymptomatic even with 100% systolic narrowing of the LCx. Myocardial bridge generally involves the LAD; however, it should be noted that myocardial bridge may be seen in other branches of coronary arteries as well.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Bourassa M.G., Butnaru A., Lesperance J., Tardif J.C. Symptomatic myocardial bridges: overview of ischemic mechanisms and current diagnostic and treatment strategies. J Am Coll Cardiol. 2003;41:351–359. doi: 10.1016/s0735-1097(02)02768-7. [DOI] [PubMed] [Google Scholar]
- 2.Bauters C., Chmait A., Tricot O., Lamblin N., Van Belle E., Lablanche J.M. Images in cardiovascular medicine. Coronary thrombosis and myocardial bridging. Circulation. 2002;105:130. doi: 10.1161/hc0102.100421. [DOI] [PubMed] [Google Scholar]
- 3.Loukas M., Von Kriegenbergh K., Gilkes M., Tubbs R.S., Walker C., Malaiyandi D. Myocardial bridges: a review. Clin Anat. 2011;24:675–683. doi: 10.1002/ca.21150. [DOI] [PubMed] [Google Scholar]
- 4.Arjomand H., AlSalman J., Azain J., Amin D. Myocardial bridging of left circumflex coronary artery associated with acute myocardial infarction. J Invas Cardiol. 2000;12:431–434. [PubMed] [Google Scholar]
- 5.Kumar B., Wardhan H., Nath R.K., Sharma A. A rare case of myocardial bridge involving left main, left circumflex, and left anterior descending coronary arteries. J Am Coll Cardiol. 2012;59:965. doi: 10.1016/j.jacc.2011.07.058. [DOI] [PubMed] [Google Scholar]