Primary cardiac lymphoma (PCL) is a rare entity [1] and defined as a lymphoma with the main bulk localized to the heart. The prognosis is poor since diagnosis is often delayed and median survival is less than 12 months. More than 90% of PCL is classified as diffuse large B-cell lymphoma (DLBCL).
Combined 18 F-fluorodeoxyglucose positron emission tomography (FDG-PET)/computed tomography (CT) scan has been extensively used in the diagnostic evaluation, staging, and treatment monitoring of different malignant tumors [2]. Immunochemotherapy is nowadays the most effective treatment for PCL [3]. Regarding cardiac lymphoma remission, PET study is suggested to be more accurate than magnetic resonance imaging (MRI) and echocardiography [4].
The aim of our study was to elucidate the usefulness of echocardiographic monitoring in the clinical management through the reports of three emblematic cases of PCL that were successfully treated, focusing on changes associated with disease regression and identification of potential side effects.
We observed 3 PCL cases among 458 newly diagnosed lymphomas from 2002 to 2014. PCL accounted for 0.6% out of all lymphomas. Diagnosis was performed by heart mass biopsy in two patients and by mediastinal lymph node biopsy in one patient.
At the time of diagnosis, Patients 1, 2, and 3 were aged 46 years (male), 74 years (male), and 70 years (female), respectively. Histology confirmed the diagnosis of DLBCL in all PCL cases. None had a previous history of cardiac disease or acquired human immunodeficiency virus. Clinical presentation included shortness of breath, chest pain, palpitations, and cough; two patients developed systemic manifestations such as fever. All patients underwent combined FDG-PET/CT scan for initial staging, which revealed increased glucose metabolism in correspondence of the cardiac lesions. At the time of diagnosis, serial echocardiography was performed (Fig. 1A) prior to and after each course of chemotherapy. The lymphoma was localized at the level of the right chambers in all patients: the soft tissue density was localized at the level of the free wall of the right ventricle (RV), and one patient also presented also a lateral right atrial wall involvement. All patients had right ventricular dysfunction, and the one with pericardial effusion had RV wall hypokinesia (Figure 1, Figure 2). Left ventricular ejection fraction and volumes were normal in all the patients. We used sulfur hexafluoride contrast agent (SonoVue, Bracco Imaging, Milan, Italy) to enhance the visualization of the RV cavity, demonstrate tumor infiltration, and evaluate tumor vascularity (Fig. 2, see supplementary material video). Prompt treatment was initiated in all the patients receiving pretreatment with steroids and vincristine in order to avoid acute complications, in particular cardiac perforation due to tumor cytolysis. Next, six cycles of R-COMP (Myocet not pegylated liposomal doxorubicin, rituximab, vincristine, and prednisone) were administered. No acute cardiac event occurred during chemotherapy. In all the patients, a significant reduction of the mass dimension (>50%) was appreciable at transthoracic echocardiography (TTE) after the first cycle of COMP.
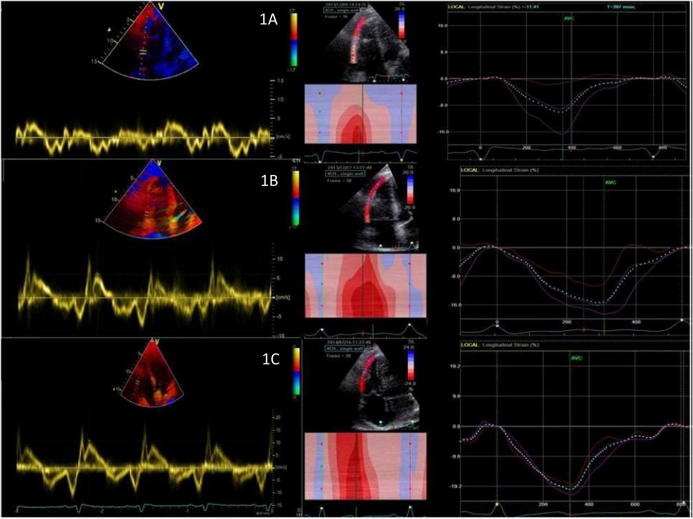
Figure 1.
(A) Tissue Doppler of tricuspid annulus and right ventricular strain imaging of Patient 1 at the time of diagnosis showing very low myocardial velocities. (B) Tissue Doppler of tricuspid annulus and right ventricular strain imaging of Patient 1 at the end of the first chemotherapy cycle showing improvement of myocardial velocities. (C) Tissue Doppler of tricuspid annulus and right ventricular strain imaging of Patient 1 at the end of chemotherapy treatment showing normalization of myocardial velocities.
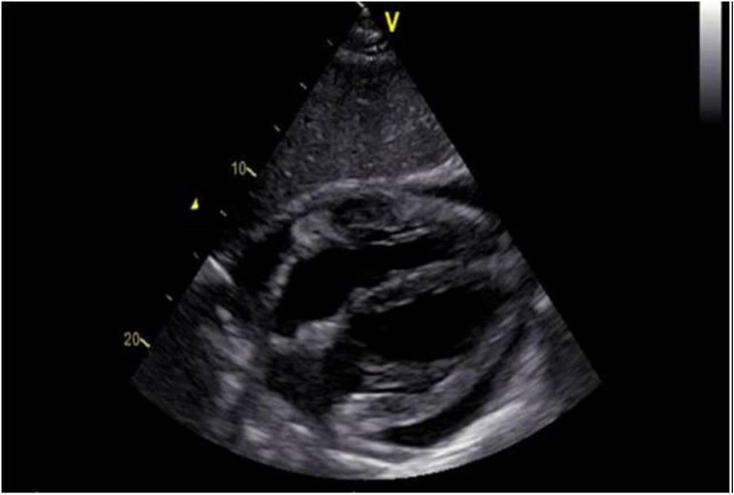
Figure 2.
Transthoracic echocardiographic subcostal view showing Patient 1 lymphoma prevalent and localized into the right ventricular free wall with moderate pericardial effusion at the time of diagnosis.
We also performed tissue Doppler-derived strain and 2D-strain imaging of the RV with evidence of progressive improvement of RV myocardial function (Fig. 1). When no evidence of cardiac involvement was demonstrated by TTE, a second FDG-PET/CT scan confirmed the complete regression of the tumor involvement of the heart. After restaging, all three patients were considered to have had a complete remission (CR). After a median follow-up of 25 months, two patients are alive and in continuous CR, whereas one patient experienced an early extra-cardiac relapse, underwent a salvage therapy, and received an autologous transplantation, thus achieving a second CR. Two patients developed late cardiac toxicity during post-remission surveillance: one patient started low dose angiotensin-converting enzyme-inhibitor due to mild biventricular dysfunction, while one patient started beta-blocker to treat non-sustained ventricular tachycardia associated with initial left ventricular dilation without ventricular dysfunction. In accordance with current literature [5], the incidence of PCL among newly diagnosed lymphomas is rare with a preferred right-sided localization. The novel findings in our experience are the demonstration of an immediate response with evidence of mass regression since the first pretreatment with steroids, prolonging median survival without acute cardiac complications. Symptoms related to cardiac lymphoma, such as dyspnea, arrhythmias, and congestive heart failure, are unspecific or subclinical, sometimes leading to a diagnostic delay. TTE is frequently the first-line imaging method used for diagnosis capable to show myocardial infiltration of the PCL. Even if previous studies have shown a relatively low diagnostic sensitivity and specificity [6], serial TTE can provide valuable information regarding the size, shape, location, and mobility of the tumor pre- and post-treatment, as observed in these cases. They can assess and monitor the presence of R-COMP therapy cardiotoxic effects, such as left ventricular systolic dysfunction, pericardial effusion/tamponade due to cardiac rupture, and embolization originating from tissue necrosis with potential life-threatening consequences [7]. All these information can be easily provided by TTE without exposure to ionizing radiation and at low costs. As drawbacks, echocardiography cannot provide tissue characterization and is less powerful than cardiac MRI in defining relationships with surrounding structures, particularly pericardial infiltration. During restaging phase, cardiac MRI may document contrast enhancement (possibly related to residual inflammation or scar) even if the tumor is no longer metabolically active, leading to false-positive findings. For this reason FDG-PET/CT imaging is the preferred modality for assessing treatment response [8].
Tissue Doppler-derived strain and 2D strain imaging provide valuable prognostic information in subclinical disease and are useful for the assessment of myocardial damage in ischemic heart disease; it was also found that 2D strain imaging is highly sensitive for the early detection of doxorubicin-induced cardiac injury [9].
Based on these small series, we demonstrated functional improvement of infiltrated myocardium detected by strain imaging in association with the regression too of myocardial infiltration. Therefore, deformation imaging could be an early indicator of therapy responsiveness. Our data suggest that PCL outcome has improved in the modern era, thanks to better diagnostic tools, which allow earlier diagnosis and more effective treatment. Serial accurate morphological and functional echocardiographic evaluation can provide valuable information in order to assess the extent of cardiac involvement, identifying potential complications, and monitoring the response to treatment.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
Right ventricular cavity enhanced with sulfur hexafluoride contrast agent and evaluation of tumor perfusion, which appears heterogeneous. Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jsha.2017.08.001.
Appendix A. Supplementary data
References
- 1.Jonavicius K., Salcius K., Meskauskas R., Valeviciene N., Tarutis V., Sirvydis V. Primary cardiac lymphoma: two cases and a review of literature. J Cardiothorac Surg. 2015;10:138. doi: 10.1186/s13019-015-0348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrich A., Cho S.I., Billett H. Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns. Cancer. 2011;117:581–589. doi: 10.1002/cncr.25444. [DOI] [PubMed] [Google Scholar]
- 3.Cresti A., Chiavarelli M., Glauber M., Tanganelli P., Scalese M., Cesareo F. Incidence rate of primary cardiac tumors: a 14-year population study. J Cardiovasc Med. 2016;17:37–43. doi: 10.2459/JCM.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 4.Mato A.R., Morgans A.K., Roullet M., Bagg A., Glatstein E., Litt H.I. Primary cardiac lymphoma: utility of multimodality imaging in diagnosis and management. Cancer Biol Ther. 2007;6:1867–1870. doi: 10.4161/cbt.6.12.5166. [DOI] [PubMed] [Google Scholar]
- 5.Ceresoli G.L., Ferreri A.J., Bucci E., Ripa C., Ponzoni M., Villa E. Primary cardiac lymphoma in immunocompetent patients. Cancer. 1997;80:1497–1506. doi: 10.1002/(sici)1097-0142(19971015)80:8<1497::aid-cncr18>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Miguel C.E., Bestetti R.B. Primary cardiac lymphoma. Int J Cardiol. 2011;149:358–363. doi: 10.1016/j.ijcard.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Kosugi M., Ono T., Yamaguchi H., Sato N., Dan K., Tanaka K. Successful treatment of primary cardiac lymphoma and pulmonary tumor embolism with chemotherapy. Int J Cardiol. 2006;111:172–173. doi: 10.1016/j.ijcard.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 8.Lee J.C., Platts D.G., Huang Y.T., Slaughter R.E. Positron emission tomography combined with computed tomography as an integral component in evaluation of primary cardiac lymphoma. Clin Cardiol. 2010;33:E106–E108. doi: 10.1002/clc.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Migrino R.Q., Aggarwal D., Konorev E., Brahmbhatt T., Bright M., Kalyanaraman B. Early detection of doxorubicin cardiomyopathy using two-dimensional strain echocardiography. Ultrasound Med Biol. 2008;34:208–214. doi: 10.1016/j.ultrasmedbio.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.