Abstract
Objective
F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) has been increasingly used in myocardial viability imaging. In routine PET viability studies, oral glucose and intravenous insulin loading is commonly utilized. In an optimal study, glucose and insulin loading is expected to cause FDG uptake both in hibernating and normal myocardium. However, in routine studies it is not uncommon to see absent or reduced FDG uptake in normal myocardium. In this retrospective study we further analyzed our PET viability images to evaluate FDG uptake status in myocardium under the oral glucose and intravenous insulin loading protocol that we use in our hospital.
Methods
Patients who had both myocardial perfusion single photon emission computed tomography (SPECT) and FDG PET cardiac viability studies were selected for analysis. FDG uptake status in normal and abnormal myocardial segments on perfusion SPECT was evaluated. Based on SPECT and PET findings, patients were divided into two main groups and four subgroups. Group 1 included PET viable studies and Group 2 included PET-nonviable studies. Subgroups based on FDG uptake in normal myocardium were 1a and 2a (normal uptake) and 1b and 2b (absent or significantly reduced uptake).
Results
Seventy-one patients met the inclusion criteria. Forty-two patients were PET-viable and 29 were PET-nonviable. In 33 of 71 patients (46.4%) there was absent or significantly reduced FDG uptake in one or more normal myocardial segments, which was identified more in PET-viable than PET-nonviable patients (59.5% vs. 27.5%, p = 0.008). This finding was also more frequent in diabetic than nondiabetic patients (53% vs. 31.8%), but the difference was not significant (p = 0.160).
Conclusions
In nearly half of our patients, one or more normal myocardial segments showed absent or significantly reduced FDG uptake. This finding, particularly if it is diffuse, could be from suboptimal study, inadequacy of current glucose and insulin loading protocols, or various other patient-related causes affecting FDG uptake both in the normal and hibernating myocardium. In cases with significantly reduced FDG uptake in normal myocardium, PET images should be interpreted cautiously to prevent false-negative results for viability.
Keywords: Fluorodeoxyglucose, Glucose loading, Insulin loading, Myocardial viability, Positron emission tomography
Abbreviations
- FDG
F-18 fluorodeoxyglucose
- PET
Positron Emission Tomography
- SPECT
Single photon emission computed tomography
- Tl-201
Thallium-201
- GLUTs
Glucose transporters
- AMI
Acute myocardial infarction
- CAD
Coronary artery disease
- EHI
Euglycemic hyperinsulinemic
- FBG
Fasting blood glucose
- LVEF
Left ventricular ejection fraction
- NIDDM
Noninsulin-dependent diabetes mellitus
Introduction
Approximately 50% of the patients with chronic obstructive coronary artery disease (CAD) and chronic contractile dysfunction have hibernating (viable but ischemic and dysfunctional) myocardium [1], [2]. Hibernating myocardium is potentially salvageable, and functional improvement can occur with appropriate treatments and revascularization. There are various methods to detect hibernating myocardium, such as single photon emission computed tomography (SPECT) with Tl-201- and Tc-99m-labeled agents, positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose (F-18 FDG or FDG), rubidium-82, nitrogen-13 ammonia, and carbon-11 acetate, cardiac magnetic resonance imaging, stress echocardiography, and cardiac computed tomography (CT).
Currently, FDG PET myocardial viability imaging is considered the gold standard and has mostly replaced Tl-201 SPECT imaging in many centers. As glucose is the major energy source for an ischemic/hibernating myocardium, F-18 FDG, a radiolabeled glucose analog, is used in PET myocardial viability studies. FDG uptake in myocardial cells is mediated by insulin-sensitive glucose transporters. Within the cell, FDG is phosphorylated into FDG-6-phosphate by hexokinase enzyme. FDG-6-phosphate does not undergo subsequent metabolism (glycogen synthesis or aerobic glycolysis) but only minimal dephosphorylation [3]. In the region of the fixed perfusion defect, FDG uptake (perfusion–metabolism mismatch) usually indicates viability, whereas lack of FDG uptake usually indicates nonviability, although false-negative or false-positive results have been reported [3], [4], [5], [6], [7], [8], [9], [10]. Inflammatory reaction following recent myocardial infarction can cause false-positive FDG uptake, providing misleading information about the viability of the myocardium [8]. In the subacute phase following reperfusion of acute myocardial infarction (AMI), decreased FDG uptake relative to perfusion (reverse perfusion–metabolism mismatch) is seen [11], [12], [13], [14], [15]. In reperfused AMI patients, the presence of reverse blood flow–glucose metabolism mismatch is indicative of preserved oxygen consumption and free fatty acid (FFA) metabolism, despite suppression of glucose metabolism [15]. In addition to reperfused AMI, reverse mismatch was also reported in chronic multivessel CAD, postischemic myocardial dysfunction (stunning), and in the septum of patients with left bundle branch block [11], [16], [17].
To increase FDG uptake in the hibernating myocardium various protocols have been used such as fasting, oral glucose loading, low carbohydrate diet, intravenous (i.v.) or oral glucose and i.v. insulin loading, acimipox administration (to reduce myocardial fatty acid metabolism), and euglycemic hyperinsulinemic (EHI) clamping [3], [18], [19], [20], [21], [22]. Among these protocols, EHI clamping is considered to be the best way to improve FDG uptake in the hibernating myocardium. The goal is to provide euglycemia and hyperinsulinemia at the time of FDG injection, as insulin increases glucose uptake in ischemic tissues. In routine cardiac FDG PET viability studies glucose/insulin loading is commonly used [3]. In an optimal FDG PET viability study under glucose and insulin loading it is expected to see normal FDG uptake in normally perfused myocardium. However, in routine studies, it is not uncommon to see reduced or absent FDG uptake in the normal myocardium. This is believed to be either suboptimal protocol or various other patient-related reasons including diabetes and insulin resistance. Our goal in this retrospective study was to further analyze our PET viability images to evaluate FDG uptake status in normal myocardium under the oral glucose and i.v. insulin loading protocol that we use in our hospital.
Material and methods
In this retrospective study, patients who had both myocardial perfusion SPECT and FDG PET cardiac viability studies from 2011 to 2016 were selected for further analysis. This retrospective study was approved by Kuwait Ministry of Health.
Stress and rest myocardial perfusion SPECT images were obtained using a 2-day protocol following i.v. injection of 740 MBq (20 mCi) Tc-99m tetrofosmin. Images were obtained either on SPECT (Infinia; General Electric, Milwaukee, WI; Symbia S and C-cam; Siemens Medical Solutions, Illinois, USA) or SPECT/CT (Infinia Hawkeye 4 and Discovery 670–16 Slice; General Electric, Milwaukee, WI) cameras. Normal-sized or thin patients were imaged on SPECT whereas obese or large patients on SPECT/CT cameras for attenuation correction.
FDG PET cardiac viability study was performed after overnight fasting. The patient’s fasting blood glucose (FBG) level was measured. In nondiabetic patients, 50 g oral glucose was administered if FBG was ≤150 mg/dL, and 25 g glucose if FBG level was 151–250 mg/dL. In diabetic patients, 25 g glucose was administered if FBG was ≤150 mg/dL, and 12.5 g glucose for FBG of 151–250 mg/dL. No glucose was administered if FBG was >250 mg/dL. Blood glucose levels were monitored every 15–30 minutes. Regular insulin was administered i.v. in patients with FBG >250 mg/dL and in glucose-loaded patients. The i.v. insulin doses for blood glucose levels of 141–160 mg/dL, 161–180 mg/dL, 181–200 mg/dL, 201–220 mg/dL, 221–240 mg/dL, 241–260 mg/dL, 261–280 mg/dL, and 281–300 mg/dL were 1 U, 2 U, 3 U, 4 U, 5 U, 6 U, 7 U, and 8 U, respectively. The physician was notified if blood glucose (BG) was >300 mg/dL. When the BG level was <140 mg/dL, 185–370 MBq (5–10 mCi) of i.v. F-18 FDG was administered. The patients were asked to eat a light meal 15 minutes after FDG injection. BG was checked every 15 minutes after FDG injection to monitor hypoglycemia.
PET images were obtained 60–90 minutes after FDG injection using Phillips Gemini Time of Flight 64 PET/CT camera (Philips Medical Systems, Best, the Netherlands). Duration of PET acquisition was 15–20 minutes following a low-dose CT scan for attenuation correction. In some cases with high blood pool activity, delayed PET images were also obtained.
SPECT and PET images were evaluated visually by two readers. SPECT and PET images were evaluated using short axis, horizontal and vertical long axis images as well as bullseye polar maps, and the left ventricle was divided into nine segments (apical, anterior, anteroseptal, anterolateral, septal, inferoseptal, inferior, inferolateral, and lateral). The size (small, moderate, and large) and severity (mild, moderate, and significant) of reversible or fixed perfusion defects as well as left ventricular ejection fraction (LVEF) and left ventricle (LV) wall motion, thickening, and cavity size were noted on SPECT images.
On FDG PET/CT images, myocardial viability was assessed with visual analysis (mild, moderate, and significant). Viability was considered mild if it involved less than one-third of the area of perfusion defect, moderate if between one-third and two-thirds, and significant if more than two-thirds of the perfusion defect area. Visual evaluation and segmented bullseye polar maps were used to compare the size of perfusion defect and the viable area. In cases with mismatch of segments in polar maps on SPECT and PET, visual analysis was used to estimate the size of the perfusion defect and viability and the area of involvement.
Based on SPECT and PET findings, patients were divided into two main groups and four subgroups: 1a: FDG uptake in the region of fixed perfusion defect (PET-viable), and normal FDG uptake in normally perfused myocardium; 1b: FDG uptake in the region of fixed perfusion defect (PET-viable) and absent or significantly reduced FDG uptake in normally perfused myocardium (one or more segments); 2a: no FDG uptake in the region of fixed perfusion defect (PET-nonviable), and normal FDG uptake in normally perfused myocardium; and 2b: no FDG uptake in the region of fixed perfusion defect (PET-nonviable), and absent or significantly reduced FDG uptake in normally perfused myocardium (one or more segments).
Statistical analysis (χ2 test) was used to see if there is any difference in between PET-viable and PET-nonviable and in between diabetic and nondiabetic patients with regard to number of patients with normal and reduced/absent FDG uptake in normally perfused myocardium. Average BG, age, and LVEF values between groups was also compared.
Results
Our study included 71 patients (14 female and 57 male) with a mean age of 63.7 years, ranging from 23 years to 86 years. All the patients had one or more risk factors, such as diabetes mellitus, hypertension, hyperlipidemia, obesity, and angina. All the patients had fixed perfusion defects and 19 of them also had stress-induced ischemia.
In 33 patients (46.4% of total) there was reduced or absent FDG uptake in normally perfused myocardium. Absent or reduced FDG uptake in normal myocardium was in one segment in 13 patients, two segments in six patients, three segments in one patient, four segments in one patient, five segments in four patients, six segments in four patients, seven segments in two patients, and eight segments in two patients. In some patients with diffusely reduced FDG uptake in the left ventricle there was also high blood pool activity.
In 42 patients there was a varying degree of FDG uptake in the region of fixed perfusion defect (Group 1). Seventeen of them (40.4%) showed normal (Fig. 1) and 25 (59.5%) had absent or reduced FDG uptake in normally perfused myocardial segments (Fig. 2, Table 1).
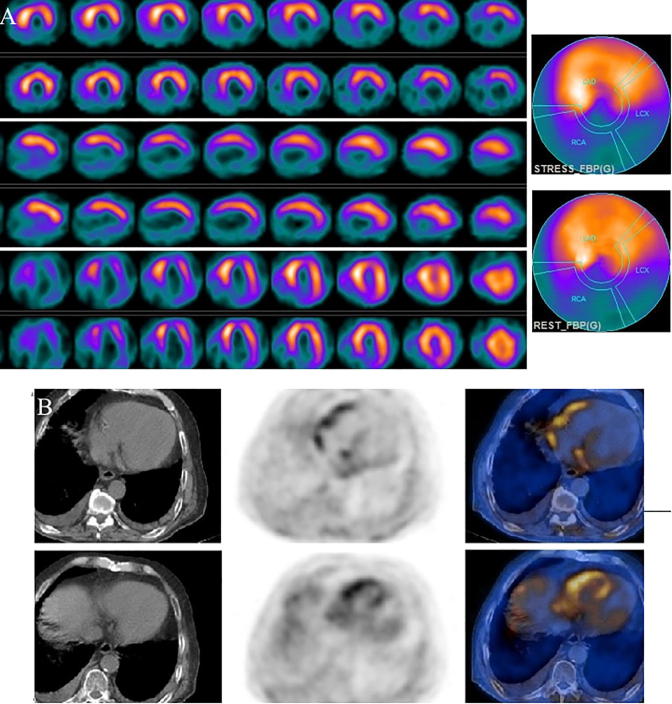
Figure 1.
(A) Stress and rest myocardial perfusion SPECT short, vertical, and horizontal long axis slices and polar maps demonstrate anterior and anteroseptal fixed perfusion defect and mild peri-infarct ischemia. (B) FDG PET slices and polar map shows significant viability in the anterior/anteroseptal region. FDG uptake is normal in other normally perfused walls. SPECT = single photon emission computed tomography; FDG = fluorodeoxyglucose; PET = positron emission tomography.
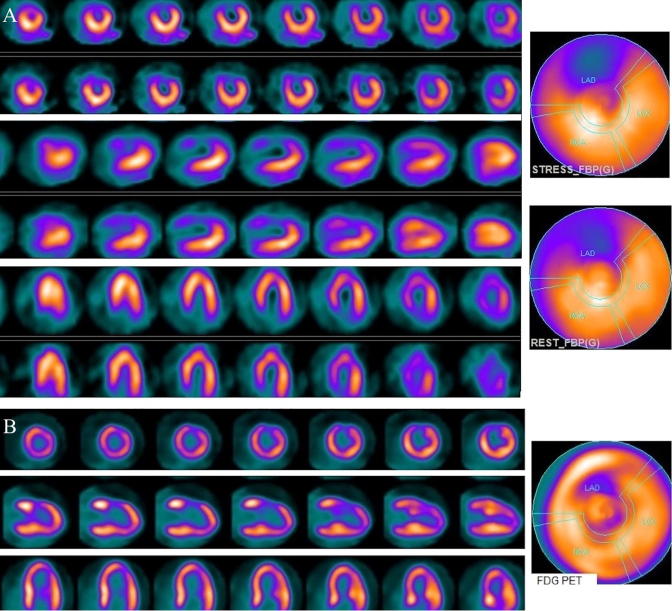
Figure 2.
(A) Stress and rest myocardial perfusion SPECT short, vertical, and horizontal long axis slices and polar maps demonstrate a large area of fixed perfusion defect in the LAD distribution (apex, anterior, and anteroseptal). Mild fixed decreased activity in the inferior wall is likely secondary to diaphragm attenuation. (B) FDG PET slices and polar map shows significant viability in the LAD distribution (PET-viable) but markedly decreased FDG uptake in the rest of myocardium. SPECT = single photon emission computed tomography; FDG = fluorodeoxyglucose; LAD = left anterior descending artery; PET = positron emission tomography.
Table 1.
Number of patients in each group and subgroups.
| Group 1 | Group 2 | |
|---|---|---|
| (PET-viable) | (PET-nonviable) | |
| FDG uptake in normal myocardium | ||
| Normal (a) | 17 | 21 |
| Reduced/absent (b) | 25 | 8 |
| Total | 42 | 29 |
FDG = fluorodeoxyglucose; PET = positron emission tomography.
In 29 patients there was no FDG uptake in the region of fixed perfusion defect (Group 2). In 21 of these patients (72.4%) there was normal (Fig. 3) and in eight (27.5%) there was reduced or absent FDG uptake (Fig. 4) in normally perfused myocardial segments (Table 1).
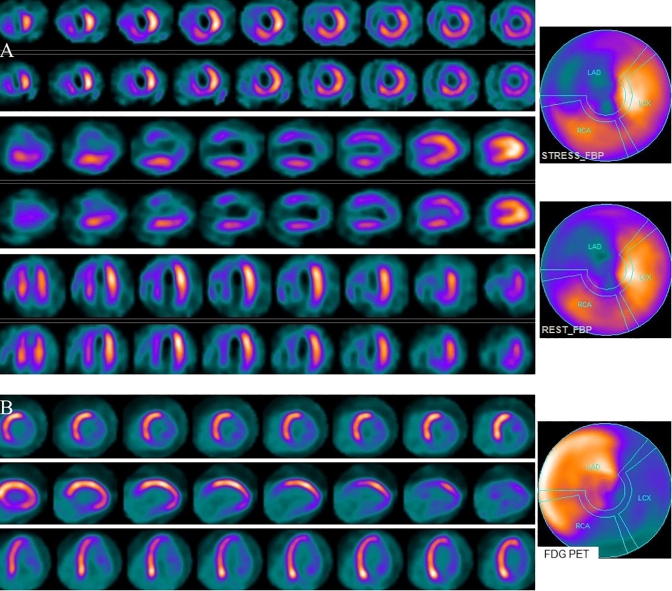
Figure 3.
(A) Stress and rest myocardial perfusion SPECT short, vertical, and horizontal long axis slices and polar maps demonstrate a large area of fixed perfusion defect in the apex, extending to mid anterior, inferior, and septal walls, and fixed decreased activity in the anterior base. (B) FDG PET slices and polar map shows no significant FDG uptake in the region of the fixed perfusion defect (PET-nonviable). FDG uptake in the anterior base is higher than the uptake on perfusion images. In the rest of the left ventricle there is normal FDG uptake. SPECT = single photon emission computed tomography; FDG = fluorodeoxyglucose; PET = positron emission tomography.
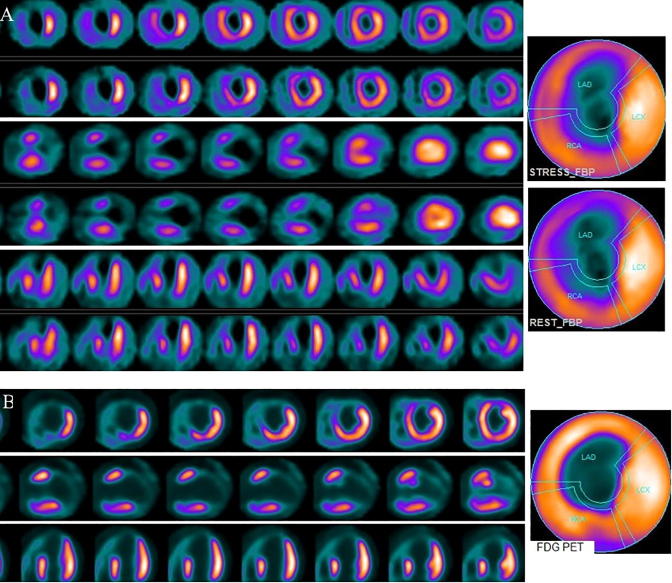
Figure 4.
(A) Stress and rest myocardial perfusion SPECT short, vertical, and horizontal long axis slices and polar maps demonstrate large area of fixed perfusion defect in the inferior, inferolateral, and inferoseptal segments from apex to base. (B) Selected FDG PET/CT transaxial slices show diffusely decreased activity in the left ventricle. FDG uptake is seen in both atria and right ventricle. SPECT = single photon emission computed tomography; FDG = fluorodeoxyglucose; PET/CT = positron emission tomography/computed tomography.
Overall, absent, or reduced FDG uptake in normal myocardium was seen more in Group 1 (PET-viable) patients than Group 2 (PET-nonviable) (59.5% vs. 27.5%) and this was statistically significant (p = 0.008). Normal FDG uptake in normal myocardium was more common in Group 2 than in Group 1 patients (72.4% vs. 40.5%), which was statistically significant (p = 0.008). Absent or reduced FDG uptake in normal myocardium was also seen more in diabetic patients than in nondiabetics (53% vs. 31.8%), but it was not statistically significant (p = 0.160).
Forty-nine of our patients had diabetes. Patients’ diabetic status was determined based on referring physicians’ notes or patients’ history. Thirty patients showed FDG uptake in the region of fixed perfusion defect and 19 showed no FDG uptake (Table 2). Overall, 26 (53%) diabetic patients showed absent or reduced FDG uptake in the normal myocardium, which was in 19 of PET-viable diabetic patients (63.3%) and in seven of PET-nonviable diabetic patients (36.8%).
Table 2.
Results in diabetic and nondiabetic patients (number).
| Group 1 |
Group 2 |
Total | |||
|---|---|---|---|---|---|
| 1a | 1b | 2a | 2b | ||
| Diabetic | 1 | 19 | 12 | 7 | 49 |
| Nondiabetic | 6 | 6 | 9 | 1 | 22 |
In 22 nondiabetic patients, 12 had viability on PET and 10 had no viability (Table 2). In six of PET-viable patients (50%), and one of PET-nonviable patients (10%) there was reduced or absent FDG uptake in normal myocardium. Overall, 31.8% of nondiabetic patients showed absent/reduced FDG uptake in the normal myocardium.
In patients with stress-induced ischemia, it was peri-infarct ischemia (PIS) in eight patients, in other walls in nine, and both PIS and ischemia in other walls in two patients. In 42 patients with viability on PET, only six had PIS. In 29 patients with nonviable tissue on PET, two had PIS. In 11 patients with stress-induced ischemia in other walls, eight showed good FDG uptake and three showed no uptake in the ischemic region.
The average BG level at the time of injection, age, and LVEF for each group are shown in Table 3. There was no significant difference between groups with regard to average BG, age, and LVEF values (p = 0.056, 0.799, and 0.732, respectively).
Table 3.
Average BG at time of FDG injection, age, and LVEF in main groups and subgroups.
| Group 1 | 1a | 1b | Group 2 | 2a | 2b | p* | |
|---|---|---|---|---|---|---|---|
| Average BG, mmol/L | 6.83 | 6.91 | 6.77 | 6.68 | 6.59 | 6.95 | 0.056 |
| Average age, y | 63.19 | 62.35 | 63.76 | 64.48 | 63.19 | 67.87 | 0.799 |
| Average LVEF, % | 39.47 | 40.29 | 38.92 | 37.41 | 40.5 | 35.87 | 0.732 |
BG = blood glucose; FDG = fluorodeoxyglucose; LVEF = left ventricular ejection fraction.
p values between Groups 1 and 2.
Blood pool activity was high in comparison to myocardial wall uptake in two patients. Delayed imaging did not help in these patients.
All the patients demonstrated wall motion abnormality (hypokinesis, akinesis, or dyskinesis) in the region of the fixed perfusion defect. There was also global hypokinesis in 33 patients. Global hypokinesis was present in 14 PET-viable (1a: 7, 1b: 7) and 16 PET-nonviable (2a: 10 and 2b: 6) patients.
LV cavity was dilated at rest and stress in 11 patients.
Lack of attenuation correction in some of our SPECT images did not create a major problem as these patients were of normal size or were thin.
Discussion
A normal (nonischemic) myocardium utilizes FFA in the fasting state and glucose in the postprandial state. In ischemic myocardium, glucose is the main energy source. In fasting, there is reduced FDG uptake in normal myocardium due to low glucose and insulin levels and high FFA levels. Fasting decreases glucose transport into normal myocytes and causes a reduction in glucose metabolism. When the insulin level is low during fasting, there is an increase in lipolysis in peripheral tissue and increased plasma FFA levels. A study in pigs demonstrated that, in fasting condition, hibernating myocardium accumulated FDG at twice the rate of normal myocardium [23]. In routine FDG PET, whole-body oncological studies after 4–6 hours fasting, we often see variable FDG uptake in normal myocardium such as heterogeneous uptake, diffusely normal uptake, or diffusely reduced uptake. After glucose loading, increase in plasma glucose stimulates the release of endogenous insulin, which decreases the plasma FFA levels, increases glucose transporters, and facilitates the transport and utilization of FDG by the normal and hibernating myocardium [24]. Under fasting conditions, myocardial accumulation of FDG was heterogeneous with uptake in the septum and anterior wall averaging 80% of that in the lateral and posterior walls, but after glucose loading the regional distribution of myocardial FDG accumulation became more homogeneous [25]. In the same study, regional myocardial perfusion with O-15 water, oxidative metabolism and accumulation of C-11-acetate were homogeneous under both conditions. Fallavollita reported that in EHI clamping in pigs, FDG uptake in normal and hibernating myocardium increased significantly compared with fasting conditions [23]. In the fasting state, glucose uptake slightly increased in the dysfunctional regions compared with normal myocardium but during insulin clamping, a striking increase in glucose uptake by insulin was obtained in both the dysfunctional and the normal regions [26].
FDG uptake in normal myocardium may be affected by many factors, including but not limited to: the viability protocol used; duration of fasting; patient’s regular diet (fat-, carbohydrate- or protein-dominant diet vs. normal diet); patient’s glucose levels at fasting and at the time of FDG injection; amount of glucose loaded; amount of injected insulin; blood insulin level at the time of FDG injection; insulin resistance due to diabetes or other reasons; blood FFA levels at the time of FDG injection; utilization of substrates other than glucose; or suboptimal patient preparation.
In our study, in a significant number of patients there was absent or reduced FDG uptake in one or more normal myocardial segments (46.4%), which was higher than in previously reported studies using various protocols [27], [28]. Absent or reduced FDG uptake in normal myocardium was seen more in PET-viable than in PET-nonviable patients in our study. More FDG avidity of hibernating than normal myocardium could be one of the reasons for this.
The prevalence of insulin resistance was reported to be high in patients with diabetes (type 2), obesity, ischemic cardiomyopathy, and in other diseases [10], [29], [30], [31]. Most of our patients were diabetic. Diabetic patients have impaired myocardial glucose metabolism and FFA is the main substrates for energy production. Varying results have been reported on myocardial FDG uptake status in diabetics. Ohtake et al. [32] reported a slight decrease in myocardial glucose uptake rates during insulin clamp in patients with noninsulin-dependent diabetes mellitus (NIDDM), which might be from insulin resistance (glucose transporter-4 abnormality) [32]. In another study, FDG uptake in normal regions of myocardium after glucose load was not significantly different in patients with normal glucose tolerance, impaired glucose tolerance, and mild diabetes but it was lower in patients with severe diabetes [33]. A PET viability study after oral glucose load revealed an incidence of 28% of studies with inadequate myocardial FDG uptake in patients with diabetes mellitus (64% type 1 and 36% type 2) and CAD as compared with only 3% in patients with CAD but without known diabetes mellitus [34]. The same group later reported that hyperinsulinemic euglycemic clamp in young patients with insulin-dependent diabetes mellitus is associated with myocardial glucose uptake similar to that observed in the normal heart [35]. Under stable normoglycemic hyperinsulinemic conditions, myocardial glucose uptake was not reduced in patients with NIDDM and CAD in spite of peripheral insulin resistance [36]. Abbreviated hyperinsulinemic–euglycemic clamp produced similar myocardial glucose uptake values in normal resting myocardium in nondiabetic and diabetic patients [37]. In our study, absent or reduced FDG uptake in normal myocardium was identified more in diabetic than nondiabetic patients (53% vs. 31.8%), although the difference was not significant. Presence of diabetes could be one of the reasons for absent or reduced FDG uptake in normal myocardium in some of our patients but about one-third of our nondiabetic patients also showed absent or reduced FDG uptake in normal myocardium. However, some of our nondiabetic patients may have had unreported or unknown diabetes.
Suboptimal or inadequate glucose and insulin loading can affect FDG uptake in both normal and hibernating myocardium. There are various glucose/insulin loading protocols for viability studies and the protocol we used appears to be similar to the one described in the current Society of Nuclear Medicine guidelines [20]. As compared to oral glucose and i.v. insulin loading, EHI clamping maximizes myocardial glucose uptake and reduces the heterogeneity of FDG accumulation in viable myocardium [38]. EHI clamping improves the predictive accuracy of FDG PET for the assessment of viability [37]. Although EHI clamping is considered as a laborious and time consuming technique, some centers routinely use it [39].
Paternostro et al. [30] measured heart and skeletal muscle blood flow and glucose uptake during euglycemic hyperinsulinemia in patients with angiographically proven CAD and chronic regional wall motion abnormalities, and in healthy controls. There was markedly reduced FDG uptake in myocardium and skeletal muscles in patients as compared to controls, which suggests that patients with a history of myocardial infarction and a low ejection fraction are insulin resistant. Mean LVEF was around 40% in each group in our patients and there was no significant difference in LVEF between the groups with normal and reduced FDG uptake in normally perfused walls.
Most of our patients were obese. FFA metabolism increases in obese patients. Kim et al. [40] have indicated that visceral adiposity is strongly associated with alteration of myocardial glucose uptake evaluated by FDG-PET, and its association further relates to type 2 diabetes mellitus [40].
High-fat feeding and fasting increases circulating plasma FFA concentrations [41]. Some studies have reported no significant correlations between FDG uptake in the myocardium and fasting period [42], [43]. However, in a recent study, overnight fasting and restricted diet (low carbohydrate and fat-rich diet) for 2 days before FDG study suppressed myocardial FDG uptake more than only overnight fasting, and overnight fasting suppressed myocardial uptake more than 4–6 hours fasting [44]. All our patients fasted overnight and we did not know blood FFA levels at the time of FDG injection. Our population in Kuwait consumes high-fat and high-carbohydrate food.
In our study, normal perfusion status of myocardium was defined based on SPECT findings only, which was one of the limitations of our study. As it is well known, normal myocardial perfusion on rest and stress SPECT does not always indicate the lack of CAD. Various reasons such as low level coronary artery stenosis, inadequate exercise or inadequate/suboptimal pharmacologic stress, or balanced ischemia can cause normal SPECT, although there is coronary artery disease. Some of our patients with normal myocardial perfusion on SPECT may have had stress-induced ischemia. In a small number of our patients with stress-induced ischemia there was absent or reduced FDG uptake in the affected segments. Another limitation of our study was the lack of follow-up or correlation with other methods. However, our goal with this study was simply to test our PET viability protocol with oral glucose and i.v. insulin loading.
Conclusions
Cardiac use of FDG PET imaging has been increasing for diagnosing myocardial viability and it is important to obtain images in optimal condition for accurate results. In nearly half of our patients we observed reduced or absent FDG uptake in normally perfused myocardial segments by SPECT. In cases with lack of FDG uptake in the region of fixed perfusion defect as well as in normal myocardium, PET findings may not always indicate nonviability, as the study could be suboptimal or various other patient-related causes may affect FDG uptake both in normal and hibernating myocardium.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Schinkel A.F., Bax J.J., Poldermans D., Elhendy A., Ferrari R., Rahimtoola S.H. Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol. 2007;32:375–410. doi: 10.1016/j.cpcardiol.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Bonow R.O., Dilsizian V. Thallium 201 for assessment of myocardial viability. Semin Nucl Med. 1991;21:230–241. doi: 10.1016/s0001-2998(05)80043-1. [DOI] [PubMed] [Google Scholar]
- 3.Knuuti M.J., Nuutila P., Ruotsalainen U., Saraste M., Härkönen R., Ahonen A. Euglycemic hyperinsulinemic clamp and oral glucose load in stimulating myocardial glucose utilization during positron emission tomography. J Nucl Med. 1992;33:1255–1262. [PubMed] [Google Scholar]
- 4.Marshall R.C., Tillisch J.H., Phelps M.E., Huang S.C., Carson R., Henze E. Identification and differentiation of resting myocardial ischemia and infarction in man with positron computed tomography, 18 F-labeled fluorodeoxyglucose and N-13 ammonia. Circulation. 1983;67:766–778. doi: 10.1161/01.cir.67.4.766. [DOI] [PubMed] [Google Scholar]
- 5.Tillisch J., Brunken R., Marshall R., Schwaiger M., Mandelkern M., Phelps M. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med. 1986;314:884–888. doi: 10.1056/NEJM198604033141405. [DOI] [PubMed] [Google Scholar]
- 6.Tamaki N., Yonekura Y., Yamashita K., Saji H., Magata Y., Senda M. Positron emission tomography using fluorine-18 deoxyglucose in evaluation of coronary artery bypass grafting. Am J Cardiol. 1989;64:860–865. doi: 10.1016/0002-9149(89)90832-1. [DOI] [PubMed] [Google Scholar]
- 7.Marwick T.H., MacIntyre W.J., Lafont A., Nemec J.J., Salcedo E.E. Metabolic responses of hibernating and infarcted myocardium to revascularization. A follow-up study of regional perfusion, function, and metabolism. Circulation. 1992;85:1347–1353. doi: 10.1161/01.cir.85.4.1347. [DOI] [PubMed] [Google Scholar]
- 8.Manabe O., Oyama-Manabe N., Naya M., Aikawa T., Sakakibara M., Tsutsui H. Pitfalls of (18)F-FDG PET for evaluating myocardial viability. J Nucl Cardiol. 2016;24:1110–1113. doi: 10.1007/s12350-016-0572-6. [DOI] [PubMed] [Google Scholar]
- 9.Manabe O., Ohira H., Yoshinaga K., Sato T., Klaipetch A., Oyama-Manabe N. Elevated (18)F-fluorodeoxyglucose uptake in the interventricular septum is associated with atrioventricular block in patients with suspected cardiac involvement sarcoidosis. Eur J Nucl Med Mol Imaging. 2013;40:1558–1566. doi: 10.1007/s00259-013-2460-5. [DOI] [PubMed] [Google Scholar]
- 10.Patil S., Lele V. Poor fluorodeoxyglucose uptake in myocardial viability study in nondiabetic Friedreich's ataxia patient. Indian J Nucl Med. 2014;29:262–263. doi: 10.4103/0972-3919.142638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamagishi H., Akioka K., Hirata K., Sakanoue Y., Takeuchi K., Yoshikawa J. A reverse flow-metabolism mismatch pattern on PET is related to multivessel disease in patients with acute myocardial infarction. J Nucl Med. 1999;40:1492–1498. [PubMed] [Google Scholar]
- 12.Anselm D.D., Anselm A.H., Renaud J., Atkins H.L., de Kemp R., Burwash I.G. Altered myocardial glucose utilization and the reverse mismatch pattern on rubidium-82 perfusion/F-18-FDG PET during the sub-acute phase following reperfusion of acute anterior myocardial infarction. J Nucl Cardiol. 2011;18:657–667. doi: 10.1007/s12350-011-9389-5. [DOI] [PubMed] [Google Scholar]
- 13.Piérard L.A., De Landsheere C.M., Berthe C., Rigo P., Kulbertus H.E. Identification of viable myocardium by echocardiography during dobutamine infusion in patients with myocardial infarction after thrombolytic therapy: comparison with positron emission tomography. J Am Coll Cardiol. 1990;15:1021–1031. doi: 10.1016/0735-1097(90)90236-i. [DOI] [PubMed] [Google Scholar]
- 14.Gropler R.J., Siegel B.A., Sampathkumaran K., Pérez J.E., Sobel B.E., Bergmann S.R. Dependence of recovery of contractile function on maintenance of oxidative metabolism after myocardial infarction. J Am Coll Cardiol. 1992;19:989–997. doi: 10.1016/0735-1097(92)90283-s. [DOI] [PubMed] [Google Scholar]
- 15.Fukuoka Y., Nakano A., Uzui H., Amaya N., Ishida K., Arakawa K. Reverse blood flow-glucose metabolism mismatch indicates preserved oxygen metabolism in patients with revascularised myocardial infarction. Eur J Nucl Med Mol Imaging. 2013;40:1155–1162. doi: 10.1007/s00259-013-2423-x. [DOI] [PubMed] [Google Scholar]
- 16.Perrone-Filardi P., Bacharach S.L., Dilsizian V., Marin-Neto J.A., Maurea S., Arrighi J.A. Clinical significance of reduced regional myocardial glucose uptake in regions with normal blood flow in patients with chronic coronary artery disease. J Am Coll Cardiol. 1994;23:608–616. doi: 10.1016/0735-1097(94)90744-7. [DOI] [PubMed] [Google Scholar]
- 17.Zanco P., Desideri A., Mobilia G., Cargnel S., Milan E., Celegon L. Effects of left bundle branch block on myocardial FDG PET in patients without significant coronary artery stenoses. J Nucl Med. 2000;41:973–977. [PubMed] [Google Scholar]
- 18.Soares J., Jr, Rodrigues Filho F., Izaki M., Giorgi M.C., Catapirra R.M. Low-carbohydrate diet versus euglycemic hyperinsulinemic clamp for the assessment of myocardial viability with 18 F-fluorodeoxyglucose-PET: a pilot study. Int J Cardiovasc Imaging. 2014;30:415–423. doi: 10.1007/s10554-013-0324-5. [DOI] [PubMed] [Google Scholar]
- 19.Schinkel A.F., Bax J.J., Valkema R., Elhendy A., van Domburg R.T., Vourvouri E.C. Effect of diabetes mellitus on myocardial 18 F-FDG SPECT using acipimox for the assessment of myocardial viability. J Nucl Med. 2003;44:877–883. [PubMed] [Google Scholar]
- 20.Dilsizian V., Bacharach S.L., Beanlands R.S., Bergmann S.R., Delbeke D., Dorbala S. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23:1187–1226. doi: 10.1007/s12350-016-0522-3. [DOI] [PubMed] [Google Scholar]
- 21.Vitale G.D., deKemp R.A., Ruddy T.D., Williams K., Beanlands R.S. Myocardial glucose utilization and optimization of (18)F-FDG PET imaging in patients with non-insulin-dependent diabetes mellitus, coronary artery disease, and left ventricular dysfunction. J Nucl Med. 2001;42:1730–1736. [PubMed] [Google Scholar]
- 22.Fragasso G., Chierchia S.L., Lucignani G., Landoni C., Conversano A., Gilardi M.C. Time dependence of residual tissue viability after myocardial infarction assessed by [18 F] fluorodeoxyglucose and positron emission tomography. Am J Cardiol. 1993;72:131G–139G. doi: 10.1016/0002-9149(93)90119-w. [DOI] [PubMed] [Google Scholar]
- 23.Fallavollita J.A. Spatial heterogeneity in fasting and insulin-stimulated (18)F-2-deoxyglucose uptake in pigs with hibernating myocardium. Circulation. 2000;102:908–914. doi: 10.1161/01.cir.102.8.908. [DOI] [PubMed] [Google Scholar]
- 24.Abel E. Glucose transport in the heart. Front Biosci. 2004;9:201–215. doi: 10.2741/1216. [DOI] [PubMed] [Google Scholar]
- 25.Gropler R.J., Siegel B.A., Lee K.J., Moerlein S.M., Perry D.J., Bergmann S.R. Nonuniformity in myocardial accumulation of fluorine-18-fluorodeoxyglucose in normal fasted humans. J Nucl Med. 1990;31:1749–1756. [PubMed] [Google Scholar]
- 26.Mäki M., Luotolahti M., Nuutila P., Iida H., Voipio-Pulkki L.M., Ruotsalainen U. Glucose uptake in the chronically dysfunctional but viable myocardium. Circulation. 1996;93:1658–1666. doi: 10.1161/01.cir.93.9.1658. [DOI] [PubMed] [Google Scholar]
- 27.Martin W.H., Jones R.C., Delbeke D., Sandler M.P. A simplified intravenous glucose loading protocol for fluorine-18 fluorodeoxyglucose cardiac single-photon emission tomography. Eur J Nucl Med. 1997;24:1291–1297. doi: 10.1007/s002590050154. [DOI] [PubMed] [Google Scholar]
- 28.Fronczewska-Wieniawska K., Chojnowski M., Mączewska J., Bąk M., Królicki L. Simplified protocol of cardiac 18 F-fluorodeoxyglucose positron emission tomography viability study in normoglycemic patients with known coronary artery disease. Clin Imaging. 2015;39:592–596. doi: 10.1016/j.clinimag.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Lee J.M., Okumura M.J., Davis M.M., Herman W.H., Gurney J.G. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29:2427–2432. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 30.Paternostro G., Camici P.G., Lammerstma A.A., Marinho N., Baliga R.R., Kooner J.S. Cardiac and skeletal muscle insulin resistance in patients with coronary heart disease. A study with positron emission tomography. J Clin Invest. 1996;98:2094–2099. doi: 10.1172/JCI119015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abel E.D., O'Shea K.M., Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the heart. Arterioscler Thromb Vasc Biol. 2012;32:2068–2076. doi: 10.1161/ATVBAHA.111.241984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohtake T., Yokoyama I., Watanabe T., Momose T., Serezawa T., Nishikawa J. Myocardial glucose metabolism in noninsulindependent diabetes mellitus patients evaluated by FDG-PET. J Nucl Med. 1995;36:456–463. [PubMed] [Google Scholar]
- 33.Hasegawa S., Kusuoka H., Uehara T., Yamaguchi H., Hori M., Nishimura T. Glucose tolerance and myocardial F-18 fluorodeoxyglucose uptake in normal regions in coronary heart disease patients. Ann Nucl Med. 1998;12:363–368. doi: 10.1007/BF03164926. [DOI] [PubMed] [Google Scholar]
- 34.vom Dahl J, Hicks RJ, Lee KS. Positron emission tomography myocardial viability studies in patients with diabetes mellitus. J Am Coll Cardiol 1991;17:121 A.
- 35.vom Dahl J, Herman WH, Hicks RJ, Ortiz-Alonso FJ, Lee KS, Allman KC, et al. Myocardial glucose uptake in patients with insulin-dependent diabetes mellitus assessed quantitatively by dynamic positron emission tomography. Circulation 1993;88:395–404. [DOI] [PubMed]
- 36.Mäki M., Nuutila P., Laine H., Voipio-Pulkki L.M., Haaparanta M., Solin O. Myocardial glucose uptake in patients with NIDDM and stable coronary artery disease. Diabetes. 1997;46:1491–1496. doi: 10.2337/diab.46.9.1491. [DOI] [PubMed] [Google Scholar]
- 37.Fallavollita J.A., Luisi A.J., Jr, Yun E., deKemp R.A., Canty J.M., Jr. An abbreviated hyperinsulinemic-euglycemic clamp results in similar myocardial glucose utilization in both diabetic and non-diabetic patients with ischemic cardiomyopathy. J Nucl Cardiol. 2010;17:637–645. doi: 10.1007/s12350-010-9228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bacharach S.L., Bax J.J., Case J., Delbeke D., Kurdziel K.A., Martin W.H. PET myocardial glucose metabolism and perfusion imaging: part 1 – guidelines for data acquisition and patient preparation. J Nucl Cardiol. 2003;10:543–556. doi: 10.1016/s1071-3581(03)00648-2. [DOI] [PubMed] [Google Scholar]
- 39.Hansen A.K., Gejl M., Bouchelouche K., Tolbod L.P., Gormsen L.C. Reverse mismatch pattern in cardiac 18 F-FDG viability PET/CT is not associated with poor outcome of revascularization: a retrospective outcome study of 91 patients with heart failure. Clin Nucl Med. 2016;41:e428–e435. doi: 10.1097/RLU.0000000000001312. [DOI] [PubMed] [Google Scholar]
- 40.Kim G., Jo K., Kim K.J., Lee Y.H., Han E., Yoon H.J. Visceral adiposity is associated with altered myocardial glucose uptake measured by (18)FDG-PET in 346 subjects with normal glucose tolerance, prediabetes, and type 2 diabetes. Cardiovasc Diabetol. 2015;14:148. doi: 10.1186/s12933-015-0310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopaschuk G.D., Ussher J.R., Folmes C.D., Jaswal J.S., Stanley W.C. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 42.Steinmetz A.P., Cronin B., Wierzbicki A.S., Lumb P.J., Maisey M.N. Relationship of myocardial 18-FDG uptake in oncologic PET imaging to plasma lipid and glucose metabolism [abstract] Eur J Nucl Med. 2000;27:902. [Google Scholar]
- 43.de Groot M., Meeuwis A.P., Kok P.J., Corstens F.H., Oyen W.J. Influence of blood glucose level, age and fasting period on non-pathological FDG uptake in heart and gut. Eur J Nucl Med Mol Imaging. 2005;32:98–101. doi: 10.1007/s00259-004-1670-2. [DOI] [PubMed] [Google Scholar]
- 44.Kumar P., Patel C.D., Singla S., Malhotra A. Effect of duration of fasting and diet on the myocardial uptake of F-18-2-fluoro-2-deoxyglucose (F-18 FDG) at rest. Indian J Nucl Med. 2014;29:140–145. doi: 10.4103/0972-3919.136559. [DOI] [PMC free article] [PubMed] [Google Scholar]