Highlights
-
•
Ultrasonography or CT guided biopsy is useful for diagnosis of liver tumors.
-
•
Some tumors were not detectable with any modality except only MRI.
-
•
3D simulation liver imaging can be created with CT and MRI images.
-
•
Excisional biopsy using preoperative 3D simulation could provide a certain biopsy.
-
•
Present biopsy method is effective for tumors that cannot be detected by CT or US.
Abbreviations: CT, computed tomography; EOB-MRI, gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging; FNH, focal nodular hyperplasia; Gd-MRI, gadolinium-enhanced magnetic resonance imaging; MDCT, multiple detector computed tomography; US, ultrasonography
Keywords: Laparoscopic hepatectomy, 3D simulation imaging, Liver biopsy, FNH-like lesion, Case report
Abstract
Introduction
The imaging diagnostics of liver tumor are difficult. There are no effective biopsy examinations for liver tumors that cannot be detected even by ultrasonography (US) and computed tomography (CT). We report a remarkably useful biopsy method for such tumors.
Presentation of case
A 67-year-old man with hepatitis C underwent gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging, and the images revealed multiple delayed enhanced masses that showed high signal intensity in the hepatobiliary phase. The possibility of malignancy could not be ruled out due to the trend towards increased size of the masses. Percutaneous liver biopsy was considered impossible because CT and US could not detect the masses. Laparoscopic liver biopsy with preoperative simulation using 3D imaging was performed. The 3D imaging provided accurate information of liver surface irregularities with cirrhosis change. The tumor location was confirmed, and adequate tumor excisional biopsy was performed. Histological assessment revealed the tumor to be a focal nodular hyperplasia-like nodule.
Discussion and conclusions
Laparoscopic liver biopsy has been widely used because of its safety and accuracy. It enables accurate resection of tumors that are undetectable with CT and US by employing preoperative 3D imaging while maintaining the less-invasiveness.
1. Introduction
The imaging diagnostics of liver tumors are difficult. Some cases are not clear whether they are benign or malignant, and they often require ultrasonography (US)-guided biopsy [[1], [2], [3], [4], [5]]. In cases in which tumors are not detectable by US, computed tomography (CT)-guided biopsy examination is used. However, there are presently no effective biopsy examinations for liver tumors that are undetectable even by using CT-guided biopsy.
We report a case of a focal nodular hyperplasia (FNH)-like nodule showing progression, which did not rule out the possibility of malignancy. The patient could not undergo biopsy of the tumor using US or CT because the tumors were not detectable with any modality except gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (EOB-MRI). We performed an excisional biopsy of the tumor using analysis of the liver surface based on the preoperative 3D simulation and visualization. The work has been reported in line with the SCARE criteria [6].
2. Presentation of case
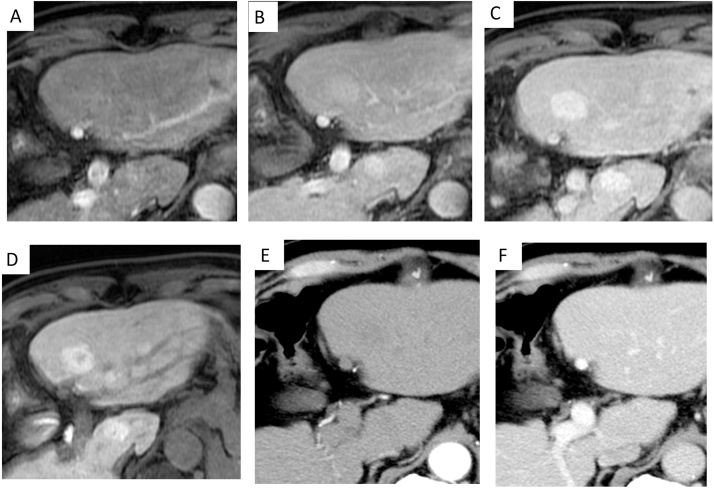
A 67-year-old man was diagnosed as having type C chronic hepatitis and had been treated at our hospital. After diagnosis, the patient was scheduled for regular follow-up examinations. From October 2009, contrast-enhanced CT and gadolinium-enhanced magnetic resonance imaging (Gd-MRI) were periodically checked; however, no tumor was detected. In April 2014, EOB-MRI was performed for the first time and showed delayed enhanced multiple tumors in the bilateral lobes of the liver. In the hepatobiliary phase, these nodules appeared hyperintense, and the largest nodule had a central scar (Fig. 1). Though we checked the contrast-enhanced US with a perflubutane microbubble contrast agent (Sonazoid; Daiichi-Sankyo, Tokyo, Japan), each vascular phase and the Kupffer phase did not clearly show the nodules that had been detected by EOB-MRI. He showed no hepatitis B virus, but hepatitis C virus infection (anti-hepatitis C virus antibody) was positive. The laboratory tests of liver function and tumor markers were all negative.
Fig. 1.
Finding of clinical imaging (the largest tumor in segment 3 of the liver) (A–C) EOB-MRI showed delayed enhanced multiple tumors in the bilateral lobes of the liver. In the hepatobiliary phase, these nodules appeared hyperintense, and (D) the largest nodule had a central scar. (E,F) Dynamic CT did not detect any tumors.
The patient was followed up as an outpatient. Dynamic CT in May 2014 and Gd-MRI in August 2014 were performed, however, other than with EOB-MRI the tumors remained undetectable. EOB-MRI was performed again in March 2015, which showed multiple tumors in the bilateral lobes of the liver had increased in size. The maximum tumor diameter increased from 26 mm to 29 mm. Therefore, the possibility of a malignant tumor could not be ruled out, and a liver biopsy was considered to make a definitive diagnosis.
2.1. Surgical technique
Tumor tissues are needed to make pathological diagnoses, however, a percutaneous liver biopsy was impossible because neither CT nor US could detect the masses. Therefore, we planned a laparoscopic partial liver excisional biopsy using analysis of the liver surface based on the preoperative 3D simulation and visualization. In order to create preoperative simulations, the volume analyzer SYNAPSE VINCENT by Fujifilm, in its Liver Analysis Application, was used. First, the liver surface was created with the MDCT (multiple detector CT) images employing the 3D region growing image segmentation method. The tumor location was determined by reflecting the information from the EOB-MRI into the 3D simulation (Fig. 2). The four noticeable surface irregularity points around the tumor were defined, and we determined that the tumor was completely resected by maintaining the extent of resection including these points at a depth of 2 centimeters.
Fig. 2.
(A,B) The preoperative 3D simulation and visualization was created preoperatively by the SYNAPSE VINCENT. There were multiple tumors in the bilateral lobes of the liver. (C) The salient peripheralis tumor in segment 6 that was detected with MRI imaging in the hepatobiliary phase appeared as the easiest to resect and was therefore selected for resection.
3. Results
The patient’s informed consent was obtained for the surgical procedure. The operative time was 155 min, and blood loss was insignificant. The location of each liver surface tumor was visually indeterminable neither could intraoperative laparoscopic US detect any of the tumors. However, 3D imaging provides accurate information of liver surface irregularities with cirrhotic change. We could, therefore, identify the tumor location and perform an adequate tumor excisional biopsy (Fig. 3). Macroscopic findings showed the tumor was 1.2 cm in diameter with a relatively clear margin against the surrounding liver tissue. A soft green nodule was resected with a sufficient surgical margin (Fig. 4). In the background liver, the hepatic parenchyma had a noticeable cirrhotic change. The pathological diagnosis of this nodule was an FNH-like nodule.
Fig. 3.
The location of tumors even on the surface were visually indeterminable intraoperatively. Tumor excisional biopsy using the 3D imaging provided accurate information of liver surface irregularities with cirrhotic change. (A) Simulation imaging of the target tumor. (B) The resection line was marked with an electrocautery. The asterisks denote the surface irregularities around the tumor. (C) The appearance after resection.
Fig. 4.
Macroscopic findings showed the tumor was 1.2 cm in diameter with a relatively clear margin against the surrounding liver tissue. (A,B) A soft green nodule was resected with a sufficient surgical margin. In the background liver, the hepatic parenchyma had a noticeable cirrhotic change. (C) The tumoral tissue is seen in the left side of the frame and the normal liver tissue is to the right. Hematoxylin and eosin stain, ×40. Compared with the background liver, the nodule showed higher cellular density. The pathological diagnosis of this nodule was an FNH-like nodule.
The clinical course after partial hepatectomy was good and regular check-ups were performed. Surprisingly, in June 2017, follow-up EOB-MRI showed that each tumor continued to grow.
4. Discussion
3D imaging is applied in various fields because of its distinct features. With it, it is now easier to understand the depth and the relationship with the surrounding structures compared to that using a conventional 2D image [7]. This also holds true in the field of surgery, and 3D simulation and visualization is often used in not only in liver operations but also for organs such as the pancreas and the esophagus [[8], [9]]. Previous reports focused on the 3D simulation of the vascular anatomy of each organ. However, the 3D simulation of the liver surface architecture was used in the present report. And, to our knowledge, there has not been any other report on this application using 3D simulation.
In a laparotomy approach, a liver excisional biopsy using 3D simulation imaging may be avoided as an alternate method of a percutaneous liver biopsy because of its high invasiveness. However, with a laparoscopic approach, there can be a broad clinical application providing minimally invasiveness, as in the present case. Of course, there are differences of abdominal situations between, for example, the resting state for a CT scan and laparoscopic surgery through the pneumoperitoneum. Furthermore, the intraoperative handling of organs could change the detailed liver figure. However, with regard to the cirrhotic hard liver, the surface architecture is insulated from the influence of such manipulation. The roughness of its surface might also have lead to the positive results in the present case. The background liver, which generated tumors like hepatocellular carcinoma, was generally a hard cirrhotic liver; therefore, this method may prove to be beneficial.
This excision biopsy method has some limitations. First, although it is effective for tumors on or near the surface, it is not applicable for all cases such as deep or central tumors. However, for central tumors, this will be resolved if the following method is used. Use image analysis to determine the positional relationship with the relatively deep noticeable vessels that surround the tumors, and puncture the target tumor or resect the appropriate size for a biopsy. Second, to calculate the resection line that involves the entire tumor is possible with image simulation of the liver surface, however, the cutting and confirmation of the image of the tumor is not done simultaneously. Therefore, the possibility of leaving the tumor stump should be considered. For this reason, at present, this method should only be used in certain cases such as when there is a risk of cutting into the tumor or when a positive margin is permissible. In the future, however, advances of imaging technology may make this method viable for not only tumor biopsies, but also radical excisions for malignant tumors. In this report, we used the preoperative 3D simulation, not real-time navigation. Real-time navigation may enable progression of the simulation and increase surface profile accuracy. Therefore, this surgical method will see improvements in accuracy and safety and will be appropriate as a radical excision method for superficial malignant tumors undetected by CT or US.
Recently, for the tumors that cannot be detected by either CT or US, MRI guided biopsy is done, and its efficiency is recognized in the breast and prostate regions [[10], [11]]. Regarding liver biopsies, however, the open magnet MRI device is necessary, but its deployment is expensive. The quality of the image is also limited [12]; and, the biopsy specimens often require diameters of 3 cm or larger.
A liver needle biopsy can be helpful in liver tumor diagnoses; however, there is the possibility that the biopsy specimens may mistakenly be taken from the surrounding normal tissue and not from a target lesion. Moreover, in pathologically benign liver tumors, it is not uncommon to observe dysplasia resembling normal liver tissues; therefore, it is difficult to determine whether or not the biopsy specimens were accurately taken from a target lesion. Furthermore, needle biopsy for tumors that are potentially malignant present the risk of dissemination. Therefore, partial liver excisional biopsy, as that done in the present report, is safer and more definitive than the commonly used needle biopsy.
Although the excised specimen was compatible with the FNH-like nodule in histopathologically and ruled out malignant disease, the tumors continued to increase in size even after the operation. FNH-like nodules are usually benign tumors that are not premalignant. There are, however, some reports in which the tumors underwent malignant transformation [13]. Therefore, regarding the progressive FNH-like nodules for which a risk of malignant transformation cannot be ruled out, as in the present case, a thorough follow-up is warranted.
5. Conclusion
We reported a case of multiple progressing FNH-like nodules in a man with hepatitis C. In this case, percutaneous liver biopsy was considered impossible because CT and US did not detect the masses. However, laparoscopic partial liver excisional biopsy using analysis of the liver surface based on the preoperative 3D simulation and visualization could provide accurate information of tumor location and an adequate excisional biopsy.
Conflicts of interest
All the authors have nothing to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This is a case report involving one patient and thus does not require IRB approval.
Consent
Written informed consent for the operative methods was obtained from the patient, and informed consent was also obtained from the patient for publication of this case report and any accompanying images.
Author contribution
SE performed the clinical work and drafted the manuscript. OI, HY, HU, SM, and SA participated in the clinical work. HO and MS performed pathological diagnosis. SE, OI and YK conceived of the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Registration of research studies
In concordance with the Helsinki declaration this case report does not require registration in the database, in this case there is no experimentation with humans.
Guarantor
Shigenori Ei, MD, PhD.
Osamu Itano, MD, PhD.
Acknowledgements
We thank Robert E. Brandt, Founder, CEO, and CME of MedEd Japan, for editing the manuscript.
Contributor Information
Shigenori Ei, Email: s.ei@med.kitasato-u.ac.jp.
Osamu Itano, Email: laplivertiger@gmail.com.
References
- 1.Wanless I.R., Mawdsley C., Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology. 1985;5:1194–1200. doi: 10.1002/hep.1840050622. [DOI] [PubMed] [Google Scholar]
- 2.Tajiri K., Tsuneyama K., Kawai K., Atarashi Y., Minemura M., Sawada S., et al. A case of progressing focal nodular hyperplasia and its molecular expression pattern. Clin. J. Gastroenterol. 2014;7:271–277. doi: 10.1007/s12328-014-0483-5. [DOI] [PubMed] [Google Scholar]
- 3.Nakashima O., Kurogi M., Yamaguchi R., Miyaaki H., Fujimoto M., Yano H., et al. Unique hypervascular nodules in alcoholic liver cirrhosis: identical to focal nodular hyperplasia-like nodules. J. Hepatol. 2004;41:992–998. doi: 10.1016/j.jhep.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Libbrecht L., Cassiman D., Verslype C., Maleux G., Van Hees D., Pirenne J., et al. Clinicopathological features of focal nodular hyperplasia-like nodules in 130 cirrhotic explant livers. Am. J. Gastroenterol. 2006;101:2341–2346. doi: 10.1111/j.1572-0241.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- 5.Sugimoto K., Kondo F., Furuichi Y., Oshiro H., Nagao T., Saito K., et al. Focal nodular hyperplasia-like lesion of the liver with focal adenoma features associated with idiopathic portal hypertension. Hepatol. Res. 2014;44:E309–15. doi: 10.1111/hepr.12273. [DOI] [PubMed] [Google Scholar]
- 6.Agha R.A., Fowler A.J., Saeta A., Barai I., Rajmohan S., Orgill D.P. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Satoshi Hata Y.S., Mitsuhara Masatoshi, Ikeda Ken-ichi, Nakashima Hideharu, Nakashima S.M.a.Y. Prospects of three-dimensional microstructural analysis techniques and their applications to structural materials. Sanyo Tech. Rep. 2012;19:9. [Google Scholar]
- 8.Hallet J., Gayet B., Tsung A., Wakabayashi G., Pessaux P. Systematic review of the use of pre-operative simulation and navigation for hepatectomy: current status and future perspectives. J. Hepatobiliary Pancreat Sci. 2015;22:353–362. doi: 10.1002/jhbp.220. [DOI] [PubMed] [Google Scholar]
- 9.Abe Y., Itano O., Kitago M., Shinoda M., Yagi H., Hibi T., et al. Computer assisted surgery, preoperative planning and navigation for pancreatic cancer. J. Hepatobiliary Pancreat Sci. 2014;21:251–255. doi: 10.1002/jhbp.84. [DOI] [PubMed] [Google Scholar]
- 10.McGrath A.L., Price E.R., Eby P.R., Rahbar H. MRI-guided breast interventions. J. Magn. Reson. Imaging. 2017;46:631–645. doi: 10.1002/jmri.25738. [DOI] [PubMed] [Google Scholar]
- 11.Velez E., Fedorov A., Tuncali K., Olubiyi O., Allard C.B., Kibel A.S., et al. Pathologic correlation of transperineal in-bore 3-Tesla magnetic resonance imaging-guided prostate biopsy samples with radical prostatectomy specimen. Abdom. Radiol. (N. Y.) 2017 doi: 10.1007/s00261-017-1102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanco Sequeiros R., Klemola R., Ojala R., Jyrkinen L., Lappi-Blanco E., Soini Y., et al. MRI-guided trephine biopsy and fine-needle aspiration in the diagnosis of bone lesions in low-field (0. 23 T) MRI system using optical instrument tracking. Eur. Radiol. 2002;12:830–835. doi: 10.1007/s003300101104. [DOI] [PubMed] [Google Scholar]
- 13.Ishak K.G., Goodman Z.D., Stocker J.T. In: Atlas of Tumor Pathology, 3rd Ser. Craig J.R., Peters R.L., Edmondson H.A., editors. Armed Forces Institute of Pathology; 2001. Tumor of the liver and intrahepatic bile ducts. [Google Scholar]