Abstract
Background
Previous studies have demonstrated that members of Trichoderma are able to generate appreciable amount of extracellular amylase and glucoamylase on soluble potato starch. In this study the α-amylase was purified and characterized from Trichoderma pseudokoningii grown on orange peel under solid state fermentation (SSF).
Results
Five α-amylases A1-A5 from Trichodrma pseudokoningii were separated on DEAE-Sepharose column. The homogeneity of α-amylase A4 was detected after chromatography on Sephacryl S-200. α-Amylase A4 had molecular weight of 30 kDa by Sephacryl S-200 and SDS-PAGE. The enzyme had a broad pH optimum ranged from 4.5 to 8.5. The optimum temperature of A4 was 50 °C with high retention of its activity from 30 to 80 °C. The thermal stability of A4 was detected up to 50 °C and the enzyme was highly stable till 80 °C after 1 h incubation. All substrate analogues tested had amylase activity toward A4 ranged from 12 to 100% of its initial activity. The Km and Vmax values of A4 were 4 mg starch/ml and 0.74 μmol reducing sugar, respectively. The most of metals tested caused moderate inhibitory effect, except of Ca2+ and Mg2+ enhanced the activity. Hg2+ and Cd+ 2 strongly inhibited the activity of A4. EDTA as metal chelator caused strong inhibitory effect.
Conclusions
The properties of the purified α-amylase A4 from T. pseudokoningii meet the prerequisites needed for several applications.
Keywords: α-Amylase, Trichoderma pseudokoningii, Purification, Characterization
Background
α-Amylases are produced by plants, animals and a wide variety of bacteria and fungi. Microbial sources of α-amylases are cost effective and appropriate for industrial demands [1–5]. Amylases have the major world market share of enzymes in several applications. Amylases are used in industrial processes like detergents, food, textiles and paper. They can also use for pharmaceutical and fine chemical [6, 7]. Interestingly, it was reported that α-amylase is composed in around 90% of all liquid detergents. Moreover, the usage of α-amylases in detergents used for automatic dishwashing is increased [8].
Several works has been performed to improve the efficient utilization of agro-industrial residues in order to produce enzymes from microorganism which have commercial importance [9, 10]. Previous studies have demonstrated that members of Trichoderma are able to generate appreciable amount of extracellular amylase and glucoamylase on soluble potato starch [11, 12]. The susceptibility of starch to amylase attack depends on the properties of the specific starch, such as e.g. degree of gelatinization, and the characteristics of the specific amylase [13]. Therefore, in this study the α-amylase was purified and characterized from Trichoderma pseudokoningii grown on orange peel under solid state fermentation.
Methods
Trichoderma pseudokoningii
Trichoderma pseudokoningii was provided from Plant Pathology Unit, National Research Centre, Cairo, Egypt.
Solid-state fermentation
Two g dried orange peel was soaked with 2 ml distilled water in 25 ml Erlenmeyer flask and autoclaved. T. pseudokoningii was inoculated in the flask and incubated at 28 °C for seven days.
Purification of α-amylase
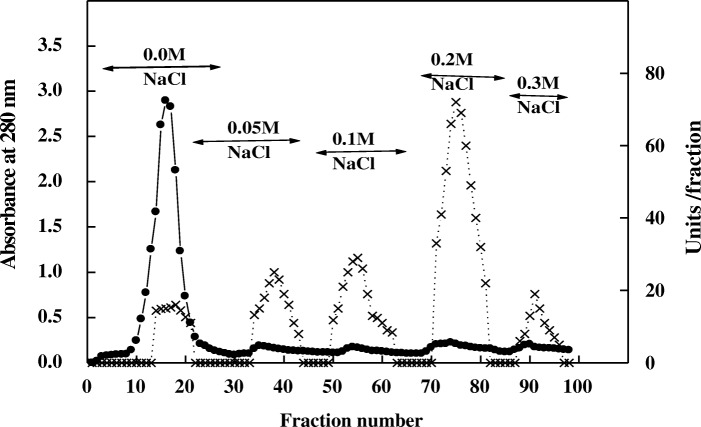
T. pseudokoningii α-amylase was extracted in distilled water and centrifuged at 12,000 rpm for 10 min. The supernatant (crude extract) was dialyzed against 20 mM Tris-HCl buffer, pH 7.2. The crude extract was loaded on a DEAE- Sepharose column (12 × 1.6 cm i.d.). Five protein peaks were separated with different concentrations of NaCl (0.0–0.3 M). These peaks had α-amylase activity (A1 – A5). A4 with highest activity was loaded on Sephacryl S-200 column (90 × 1.6 cm i.d.).
α-Amylase assay
α-Amylase activity was measured by determination the liberated reducing sugars as end products according to the method of Nelson [14].
Protein determination
Bradford [15] method was used to determine the protein.
M Wt
The M Wt of α-amylase was determined by Sephacryl S-200 and SDS-PAGE [16].
Characterization of α-amylase.
Effect of temperature
At temperature range of 30-70 °C, the activity of α-amylase was investigated.
pH optimum
Different buffers were used to examine α-amylase activity at several pH’s.
Substrate specificity
The enzyme was tested to determination a preference for different substrates such as starch, amylopectin, amylose, glycogen, β-cyclodextrin and α- cyclodextrine.
Metal cations and EDTA
Fe2+, Co2+, Ca2+, Cu2+, Ni2+, Zn2+, Hg2 + and EDTA were incubated with enzyme for 15 prior to substrate addition. One hundred percent of activity has been taken as an enzyme activity without metal ions.
Results and discussion
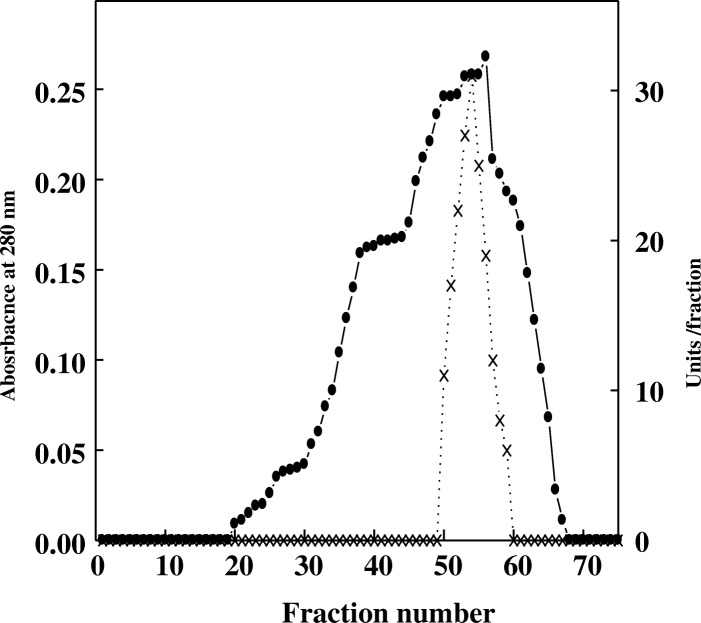
Extracellular α-amylase was produced by T. pseudokoningii grown on orange peel using solid state fermentation. It was purified by DEAE-Sepharose and Sephacryl S-200 columns. Five α-amylases A1-A5 from T. pseudokoningii were separated on DEAE-Sepharose column (Fig. 1). The homogeneity of α-amylase A4 was detected after chromatography on Sephacryl S-200 with specific activity of 550 units/mg protein and fold purification of 15.7 (Figs. 2, 3 and Table 1). α-Amylase A4 had M Wt of 30 kDa by Sephacryl S-200 and SDS-PAGE (Fig. 3). The similar M Wt was detected in T. matsutake (34 kDa) [17]. The higher M Wt of α-amylase was detected for T. harzianum (70 kDa) [18].
Fig. 1.
Chromatography of T. pseudokoningii α-amylase on DEAE-Sepharose column. (•___•) Absorbant at 280 nm, (x ___ x) units/fraction
Fig. 2.
Chromatography of T. pseudokoningii α-amylase A4 DEAE-Sepharose fraction on Sephacryl S-200 column. (•___•) Absorbant at 280 nm, (x ___ x) units/fraction
Fig. 3.
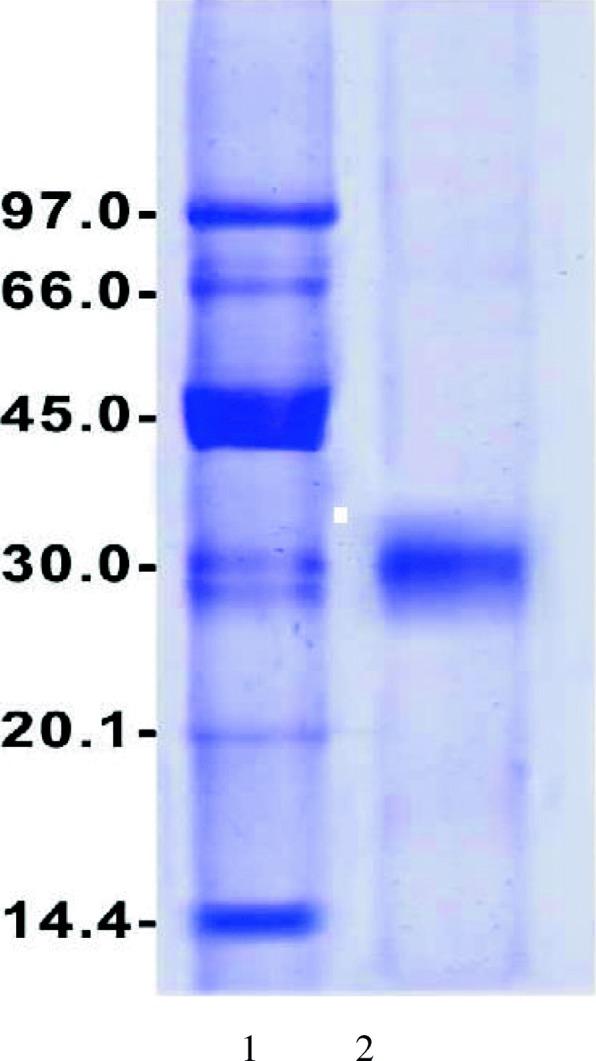
SDS-PAGE for T. pseudokoningii α-amylase A4. 1, molecular markers, 2, Sephacryl S-200 A4
Table 1.
Purification scheme for T. pseudokoningii α-amylase
| steps | T. units | T. Protein mg | S.A* | Fold purification | Recovery 100% |
|---|---|---|---|---|---|
| Crude extract | 605 | 17.3 | 34 | 1 | 100 |
| Chromatography DEAE-sepharose | |||||
| 0.0 M NaCl (A1) | 17.8 | 6.8 | 2.6 | 0.07 | 2.9 |
| 0.05 M NaCl(A2) | 28 | 0.39 | 71.8 | 2.05 | 4.6 |
| 0.1 M NaCl (A3) | 31 | 1 | 31 | 0.88 | 5.1 |
| 0.2 M NaCl (A4) | 422 | 1.04 | 405.7 | 11.6 | 69 |
| 0.3 M NaCl (A5) | 25 | 1.9 | 13 | 0.37 | 4.1 |
| Sephacryl S-200 A4 |
110 | 0.2 | 550 | 15.7 | 18 |
S.A*: Specific activity (total units/ mg protein)
The hydrogen ion concentration is one of the most fundamental factors affecting the enzymatic activity. Fig. 4 shows the maximal activity of A4, which had a broad pH optimum ranged from pH 4.5 to 8.5. α-Amylases from different fungi had sharp pH optima ranged from acidity to alkalinity [19–22].
Fig. 4.
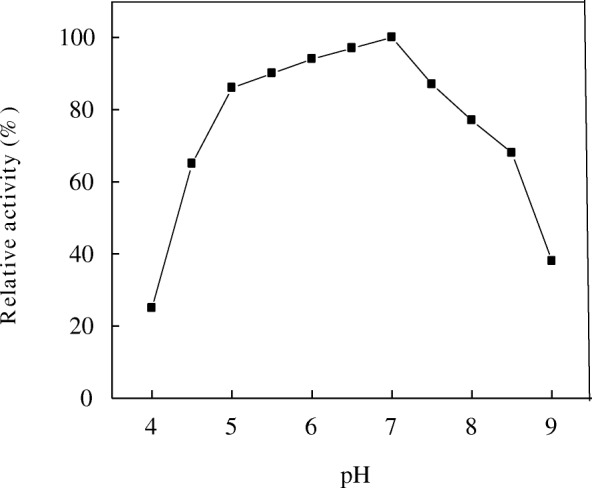
Optimum pH of T. pseudokoningii α-amylase A4
Maximal activity of A4 was recorded at 50 °C with high retention of its activity from 30 to 80 °C (Fig. 5). At 20 and 90 °C the enzyme lost the most of its activity. Optimum temperature of α- amylase isolated from several microorganisms was obtained at temperatures ranging from 45 °C to 65 °C [8, 21–23]. The thermal stability of A4 was detected up to 50 °C and the enzyme was highly stable till 80 °C after 1 h incubation (Fig. 6). Similarly, T. harzianum α-amylase had thermal stability up to 50 °C [11].
Fig. 5.
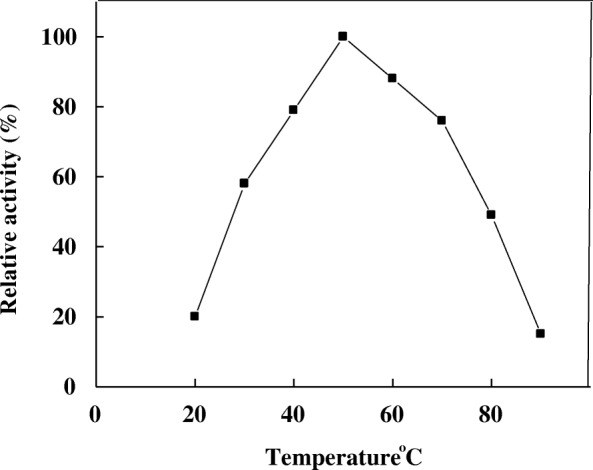
Optimum temperature of T. pseudokoningii α-amylase A4
Fig. 6.
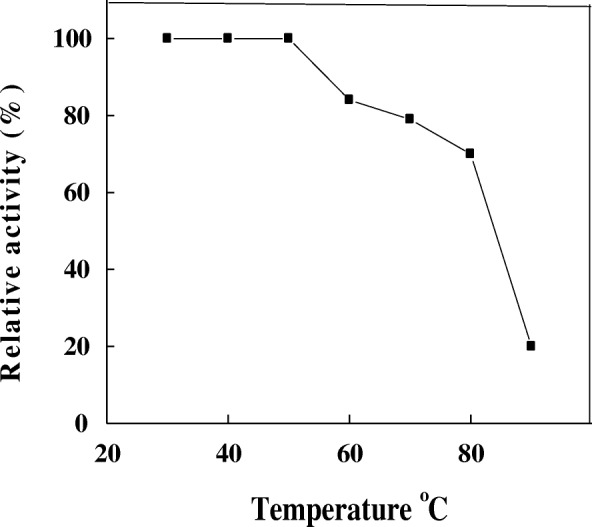
Thermal stability of T. pseudokoningii α-amylase A4
The substrate specificity was studied to evaluate the ability of A4 to catalyze the hydrolysis of some analogues structurally related to starch. Table 2 shows that A4 catalyzed the hydrolysis of substrates in the order of starch < glycogen < amylopectin < amylose < α- cyclodextrin < β-cyclodextrin. These results indicating that the substrates with high molecular weight had high affinity toward enzyme. de Azevedo et al. [11] showed that α-amylase of T. harzianum degraded the starch, glycogen and amylopectin but not cellobiose, α-cyclodextrin or β-cyclodextrin. The starch and amylopectin were the substrates preferentially hydrolyzed by A. tamarii α-amylase [24]. In a decreasing order, A. sporosulcatum α-amylase catalyzed the degradation of starch > glycogen > dextrin [25]. The Km and Vmax values of A4 were 4 mg starch/ml and 0.74 μmol reducing sugar, respectively (Fig. 7). Similarly, the Km values of α-amylases from T. harzianum (3.5 mg/ml) [11] and Geobacillus thermodenitrificans (3.05 mg /ml) were detected [19].
Table 2.
Substrate specificity of T. pseudokoningii α-amylase A4
| Substrate | Relative activity % |
|---|---|
| Potato starch | 100 |
| Glycogen | 85 |
| Amylopectin | 73 |
| Amylose | 55 |
| α-Cyclodextrin | 13 |
| β-Cyclodextrin | 12 |
Fig. 7.
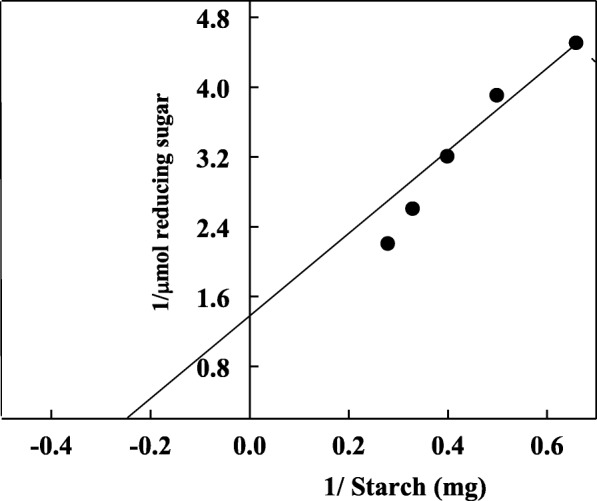
linewever burkplot of T. pseudokoningii α-amylase A4 using different concentration of starch
Many of amylases had Ca2+ cation in active site, therefore they are metal ion-dependent [26, 27]. The most of metals tested caused moderate inhibitory effect, except of Ca2+ and Mg2+ enhanced the activity of A4. Hg2+ and Cd+ 2 strongly inhibited the activity of A4 (Table 3). The similar results were detected in α-amylase T. harzianum concerning the effect of metal ions [11]. The A4 can be used as a biosensor for heavy metals Hg2+ and Cd2+ as its activity is greatly affected by the two and an enzymatic biosensor can be most sensitive and specific. EDTA as metal chelator caused strong inhibitory effect on activity of A4 suggesting that the active site of the enzyme contained metal ions.
Table 3.
Influence of metal and EDTA at 5 mM on T. pseudokoningii α-amylase A4
| Metal cations | Relative activity % |
|---|---|
| Control | 100 |
| Ca2+ | 152 |
| Mg2+ | 134 |
| Ni2+ | 100 |
| Pb2+ | 80 |
| Co2+ | 72 |
| Hg2+ | 38 |
| Cu2+ | 75 |
| Zn2+ | 65 |
| Cd2+ | 37 |
| EDTA | 28 |
Conclusions
In the present study, the purified α-amylase A4 from T. pseudokoningii, grown on agro-industrial residues under SSF, characterized by broad pH optima, thermal stability, all substrate analogues tested had amylolytic activity and moderate tolerance towards some metal ions. Similarly, α-amylases produced from Bacillus licheniformis, Bacillus stearothermophilus, and Bacillus amyloliquefaciens show broad pH optimum and thermal stability and promising potential in a number of industrial applications in processes such as food, fermentation, textiles and paper industries [28, 29].These findings make α-amylase A4 useful in the several applications.
Acknowledgments
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant No. (438–130-696). The author, therefore, acknowledge with thanks DSR for technical and financial support.
Availability of data and materials
All data generated or analyzed during this study are included in this published article or available from the corresponding author on reasonable request.
Abbreviations
- A
α-Amylase
- DEAE
Diethylaminoethyl
- EDTA
Ethylenediaminetetraacetic acid
- SDS-PAGE
Sodium dodicylsulphate-poly acrylamide gel electrophoresis
Authors’ contributions
WA designed the experiments, analyzed the data and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rahardjo YSP, Weber FJ, Haemers S, Sebastiaan J, Rinzema A. Aerial mycelia of Aspergillus oryzaeaccelerate α-amylaseproduction in a model solid-state fermentation system. Enzym Microb Technol. 2005;36:900–902. doi: 10.1016/j.enzmictec.2005.01.010. [DOI] [Google Scholar]
- 2.Gangadharan D, Sivaramakrishnan S, Madhavan KN, Pandey A. Solid culturing of Bacillus amyloliquefaciensfor alpha amylase production. Food Technol Biotechnol. 2006;44:269–274. [Google Scholar]
- 3.Mollania N, Khajeh K, Hosseinkhani S, Dabirmanesh B. Purification and characterization of thermostable phytate resistant α-amylase from Geobacillus sp. LH8. Int J Biol Macromol. 2010;46:27–36. doi: 10.1016/j.ijbiomac.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Sundarram A, Krishna Murthy TP. 훼-amylase production and applications: a review. J Appl Environ Microbiol. 2014;2:166–175. [Google Scholar]
- 5.Gopinath SCB, Anbu P, Arshad MK, Lakshmipriya T, Voon CH, Hashim U, Chinni SV. Biotechnological processes in microbial amylase production. Biomed Res Int. 2017;1272193:9. doi: 10.1155/2017/1272193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B. Microbial α-amylases: a biotechnological perspective. Process Biochem. 2003;38:1599–1616. doi: 10.1016/S0032-9592(03)00053-0. [DOI] [Google Scholar]
- 7.Gouda M, Elbahloul Y. Statistical optimization and partial characterization of amylase production. World J Agri Sci. 2008;4:359–368. [Google Scholar]
- 8.Ahmadia A, Ghobadia S, Khajehb K, Nomanpourc B, Dalfard B. Purification of α-amylase from Bacillus sp. GHA1 and its partial characterization. J Iran Chem Soc. 2010;7:432–440. doi: 10.1007/BF03246029. [DOI] [Google Scholar]
- 9.Ramachandran S, Patel AK, Nampoothiri KM, Chandran S, Szakacs G, Soccol CR, Pandey A. α-amylase from a fungal culture grown on oil cakes and its properties. Braz Arch Biol Technol. 2004;47:309–317. doi: 10.1590/S1516-89132004000200019. [DOI] [Google Scholar]
- 10.Saranraj P, Stella D. Fungal amylase—a review. Int J Microbiol Res. 2013;4:203–211. [Google Scholar]
- 11.de Azevedo AMC, De Marco JL, Felix CR. Characterization of an amylase produced by a Trichoderma harzianium isolate with antagonistic activity against Crinipellis perniciosa “the causal agent of witches” broom of cocoa. FEMS Microbiol Lett. 2000;188:171–175. doi: 10.1111/j.1574-6968.2000.tb09189.x. [DOI] [PubMed] [Google Scholar]
- 12.De Marco JL, Valadares-Inglis MC, Felix CR. Production of hydrolytic enzymes by Trichoderma isolates with antagonistic activity against Crinipellisperniciosa, the casual agent of witches’ broom of cocoa. Braz J Microbiol. 2003;34:33–38. doi: 10.1590/S1517-83822003000100008. [DOI] [Google Scholar]
- 13.Mukerjea R, Slocum G, Mukerjea R, Robyt JF. Significant differences in the activities of α-amylases in the absence and presence of polyethylene glycol assayed on eight starches solubilized by two methods. Carbohydr Res. 2006;341:2049–2054. doi: 10.1016/j.carres.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli UK. Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Kusuda M, Nagai M, Hur T-C, Ueda M, Terashita T. Purification and some properties of α-amylase from an ectomycorrhizal fungus, Tricholoma matsutake. Mycoscience. 2003;44:311–317. doi: 10.1007/S10267-003-0116-1. [DOI] [Google Scholar]
- 18.Mohamed SA, Azhar EI, Ba-Akdah MM, Tashkandy NR, Kumosani TA. Production, purification and characterization of α-amylase from Trichoderma harzianum grown on mandarin peel. Afr J Microbiol Res. 2011;5:930–940. doi: 10.5897/AJMR11.360. [DOI] [Google Scholar]
- 19.Ezeji TC, Bahi H. Purification, characterization, and synergistic action of phytate-resistant α-amylase and α-glucosidase from Geobacillus thermodenitrificans HRO10. J Biotechnol. 2006;125:27–38. doi: 10.1016/j.jbiotec.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Nouadri T, Meraihi Z, Shahrazed D, Leila B. Purification and characterization of the amylase isolated from Penicillium camemberti PL21. Afr J Biotechnol. 2010;4:155–162. [Google Scholar]
- 21.Femi-Ola TO. Olowe BM. Characterization of α-amylase from Bacillus subtilis BS5 isolated from Amitermes evuncifer Silvestri. Res J Microbiol. 2011;6:1–7. doi: 10.3923/jm.2011.1.24. [DOI] [Google Scholar]
- 22.Sindhu R, Suprabha GN, Shashidhar S. Purification and characterization of α-amylase from Penicillium janthinellum (NCIM 4960) and its application in detergent industry. Res Article Biotechnol Bioinf Bioeng. 2011;1:25–32. [Google Scholar]
- 23.Shafiei M, Abed-Ali Ziaee M, Amoozegar A. Purification and biochemical characterization of a novel SDS and surfactant stable, raw starch digesting, and halophilic α-amylase from a moderately halophilic bacterium, Nesterenkonia sp. strain F. Process Biochem. 2010;45:694–699. doi: 10.1016/j.procbio.2010.01.003. [DOI] [Google Scholar]
- 24.Moreira FB, Lenartovicz V, Peralta RM. A thermostable maltose-tolerant α- amylase from Aspergillus tamari. J Basic Microbiol. 2004;44:29–35. doi: 10.1002/jobm.200310302. [DOI] [PubMed] [Google Scholar]
- 25.Valaparla VK. Purification and properties of a thermostable amylase by Acremonium sporosulcatum. Int J Biotechnol Biochem. 2010;6:25–34. doi: 10.3844/ajbbsp.2010.25.31. [DOI] [Google Scholar]
- 26.Pandey A, Nigam P, Soccol CR, Soccol VT, Singh D, Mohan R. Advances in microbial amylases. Biotechnol Appl Biochem. 2000;31:135–152. doi: 10.1042/BA19990073. [DOI] [PubMed] [Google Scholar]
- 27.Normurodova T, Nurmatov Sh K, Alimova BK, Pulatova OM, Akhmedova ZR, Makhsumkhanov AA. Isolation and characteristics of highly active α-amylase from Bacillus subtilis-150. Chem Natu Comp. 2007;43:454–457. doi: 10.1007/s10600-007-0159-1. [DOI] [Google Scholar]
- 28.Konsoula Z, Liakopoulou-Kyriakides M. Co-production of alpha-amylase and beta-galactosidase by Bacillus subtilis in complex organic substrates. Bioresour Technol. 2007;98:150–157. doi: 10.1016/j.biortech.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Coronado MJ, Vargas C, Hofemeister J, Ventosa A, Nieto JJ. Production and biochemical characterization of an α-amylase from the moderate halophile Halomonas meridiana. FEMS Microbiol Lett. 2000;183:67–71. doi: 10.1111/j.1574-6968.2000.tb08935.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or available from the corresponding author on reasonable request.