Abstract
Background
Major depressive disorder is a common psychological problem affecting up to 20% of adults in their lifetime. The majority of people treated for depression receive antidepressant medication through their primary care physician. This commonly results in low rates of recovery. Failure points in the process of care contributing to poor outcomes include patient non-adherence to medications, failure of physicians to optimize dose and absence of communication between patients and physicians.
Objective
This pilot study evaluated the feasibility of a systemic digital intervention (MedLink) designed to address failure points and improve treatment of depression in primary care among patients during the first eight weeks of initiating a new course of antidepressant therapy.
Methods
Participants were provided with the MedLink mobile app that provided dose reminders, information and surveys of symptoms and side effects. A cellularly enabled pillbox monitored antidepressant medication adherence. Reports were provided to physicians and participants to prompt changes in medication regimen. Study outcomes were assessed via self-report and interview measures at baseline, week 4 and week 8.
Results
Medication adherence detected by the MedLink system was 82%. Participants demonstrated significant decreases in depressive symptoms on the patient health questionnaire-9 (PHQ-9) (p = 0.0005) and the Quick Inventory of Depressive Symptomatology (p = 0.0008) over the eight-week trial. Usability was generally rated favorably.
Conclusions
The MedLink system demonstrated promise as an intervention to address failure points in the primary care treatment of major depressive disorder. Current findings support the further development of MedLink through a randomized controlled trial to evaluate the efficacy of improving processes of care, patient adherence and symptoms of depression.
Keywords: mental health, mHealth, medication adherence, depression, primary care
Introduction
Major depressive disorder (MDD) is a common psychiatric disorder affecting 7% of the general population in any given year and 20% of adults in their lifetime.1 Depression imposes a very high societal burden in terms of cost, morbidity, suffering and mortality2,3 and is a leading cause of disability worldwide.4 The majority of people treated for depression receive pharmacotherapy through primary care.5,6 Unfortunately, response and recovery rates among people treated with antidepressants in general medicine are very low.7–9
A large research literature points to three broad, potentially modifiable failure points in the process of care that contribute to poor outcomes once treatment for depression has been initiated in primary care.10,11 These failure points, illustrated in Figure 1, include patient factors, provider factors and communication. On the patient side, a key contributor to poor treatment outcome is that patient adherence to prescribed antidepressants is poor, with more than 70% discontinuing their medication in the first 3 months12 and more than 80% discontinuing prior to six months response.13 Recommendations advise that patients should remain on antidepressants at least six months after response to treatment.14
Figure 1.
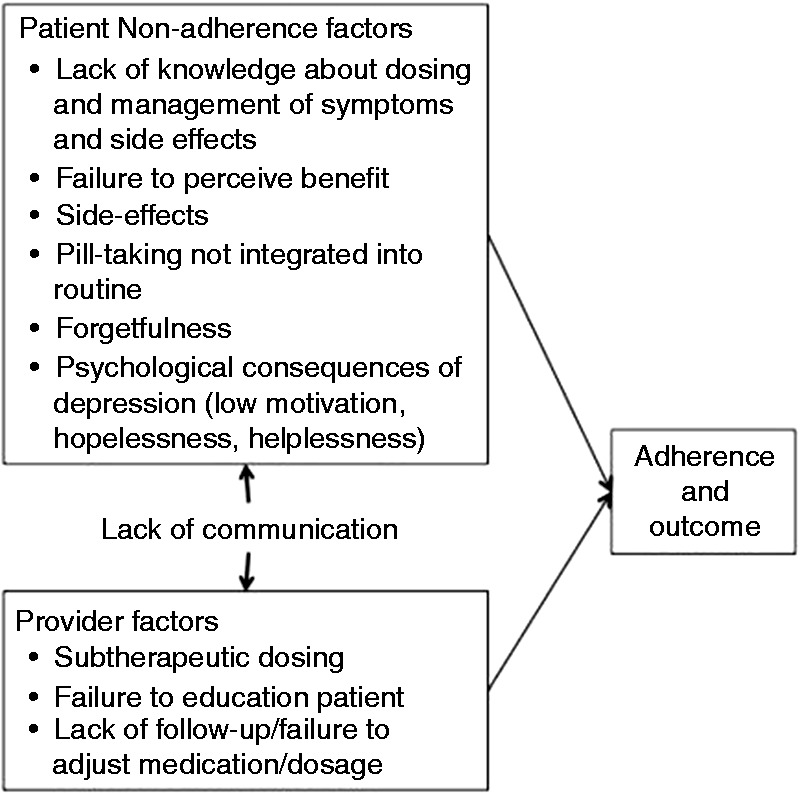
Modifiable failure points in pharmacotherapy for depression.
There are a number of specific patient factors related to non-adherence. Patients frequently do not know how to take their medications due to lack of explanation from their prescribing physician or failure to remember information that was provided. Patients may also be unaware that they must take medications consistently for at least four weeks before they are likely to experience benefits.11,15 Many patients interpret the lack of improvement in the first weeks as evidence that the treatment is ineffective and discontinue.16,17 Side effects, which usually occur shortly after beginning treatment, have consistently been shown to be a cause of non-adherence,13,17,18 Many of the symptoms of depression, such as decreased motivation, hopelessness, forgetfulness and a sense that nothing will get better, paradoxically also contribute to non-adherence.15 Thus, patients beginning treatment for depression have little knowledge, initially experience side effects, expect but receive little benefit and are prone to forgetfulness, low motivation, hopelessness and helplessness.
Specific factors related to care provided by primary care physicians (PCPs) also contribute to non-adherence with antidepressants. Treatment guidelines require regular follow-up visits during the acute treatment phase (ranging from every 2–8 weeks depending on the guideline), aimed at optimizing treatment medication and dosage and managing side effects.19–21 However, PCPs rarely follow these guidelines, due to a variety of reasons, including time constraints, management of multiple chronic conditions and practice patterns that do not always fit with guidelines. The median number of patient visits to PCPs for treatment of MDD is 1, when the initial prescription is made.22 Thus, there is little communication between the patient and physician, leaving the PCP unaware of response or non-response to the antidepressant, side effects or to other patient factors that can lead to non-adherence. Even when patients continue to take their medications, dosing by PCPs is commonly inadequate to achieve response.23
Scheduling frequent follow-up visits for depression does not fall into the normal workflow for most physicians. Without these follow-up visits to promote communication, patient and physician issues that contribute to poor treatment outcome are exacerbated. Given the number of patient factors driving non-adherence, the failure of physicians to optimize the treatment regimen, and lack of communication between the two, it is not surprising that adherence rates are low and treatment outcomes are poor for antidepressant medications in general medicine.
There is strong evidence that a variety of care management programs can improve adherence and outcomes for treatment in primary care. However, these programs require staff reassignment or resources that are not available.24–26 Technology has the potential to perform many of the functions of these more intensive interventions. Most of these technologies have targeted either patients or providers. SMS reminders have been shown to improve adherence to many medications, although this has not been investigated for antidepressants.27 Devices that monitor pill taking can also improve adherence if they are integrated into the process of care, such as providing adherence information to the care team,28 but these have also not been tried specifically with antidepressants. Interventions aimed at improving physician care with antidepressants through decision support tools in the electronic medical record (EMR) have generally failed to improve quality of care and outcomes.29 Given that the failure points of treatment for depression in primary care are systemic, technological solutions that address only one aspect (e.g. forgetfulness) are unlikely to fully resolve the problem.
The aim of this study was to evaluate the feasibility of a systemic digital intervention, MedLink, which comprehensively and systematically addresses modifiable patient, physician and communication failure points during the early acute phase of treatment for depression in primary care.
Methods
Participants
Recruitment of participants occurred from April 2014 to December 2014, through Northwestern Medicine’s General Internal Medicine Clinic. The study was approved by the Northwestern University Institutional Review Board, and registered at www.clinicaltrials.gov (NCT01909973). A best practice alert was launched in the EMR when a PCP made a new prescription for an antidepressant, asking if patients would be willing to be contacted by study staff. When the physician indicated ‘yes’, an alert was sent to study staff, who then contacted the patient and described the study over a telephone call. Interested participants verbally consented to a research assistant over the telephone and signed a written consent online prior to the full screening assessment. Those who met all entry criteria were enrolled in the study.
Participants were included in the study if they were at least 18 years old, able to speak and read English, were willing to use the smartphone-based MedLink app and WisePill box for the duration of the study period, were beginning a new course of antidepressant medication treatment with their PCP and had not been on it for more than two weeks at screening. Participants were excluded if they had visual or hearing impairments that would prevent participation, met diagnostic criteria for a severe psychiatric disorder (e.g. bipolar or psychotic disorders) or other comorbid disorder for which participation would be inappropriate or reported severe suicidality including plan and intent. Participants who did not have an Android phone with operating system 4.2 or higher or who requested a study-provided smartphone were provided a study phone.
MedLink
MedLink is a behavioral intervention technology30 digital support system intended to enhance antidepressant treatment processes and outcomes in primary care, targeting the failure points described in Figure 1. MedLink builds on Wagner’s Chronic Care Model,31 which suggests that a care delivery system is most effective when: (1) the patient is informed and activated to perform the prescribed tasks; (2) decision support tools are available to the care team to ensure guideline-congruent care; (3) the care team is provided with up-to-date information on the patient’s status.
Patient-facing tools aim to: (1) provide standardized education to support patient knowledge; (2) monitor medication adherence and provide reminders when non- adherence is detected; (3) monitor side effects and treatment response; (4) activate the patient to contact the prescribing physician when changes to medication regimen may be needed. The primary care team was supported and activated through the provision of: (1) suggested guideline-congruent actions; (2) timely information regarding the patient’s status.
MedLink elements
MedLink was designed to be very simple and efficient to use. Participants received weekly alerts to read a very brief lesson and answer a short set of questions. Otherwise, users received reminder notifications via android pop-ups only as reminders to take their medications when MedLink did not detect that they had taken their medication. Participants could access lessons and feedback at any time. We describe each of the MedLink elements below.
Lessons
The MedLink smartphone app provided brief unique didactic lessons each week consisting of information on antidepressants, dosage and how to take them, strategies for adherence, as well as general self-management skills such as goal setting, problem solving, behavioral activation and cognitive restructuring. Lessons (written by authors D.C.M. and J.D.) were revised and shortened based on lab usability evaluation32 to be readable in 1–3 minutes. The purpose of these lessons was to ensure knowledge about antidepressants and impart information that would be useful and maintain patient engagement over the intervention period.
Within app assessment
Each week, the MedLink smartphone app prompted participants to complete the patient health questionnaire-8 PHQ-833 for depression and the Patient Report Inventory of Side Effects (PRISE) and Frequency, Intensity and Burden of Side Effects Ratings (FIBSER)34 to evaluate side effects. These data were collected for the MedLink system, and not as part of study outcome measurement, which is described below. The PHQ-8 was used based on focus groups with PCPs, who did not want to receive information from the PHQ-9 suicide item as it only assesses frequency of suicidal ideation, and thus would be a liability without sufficient information to know if outreach is required. The FIBSER consists of three items measuring the overall frequency, severity and impact on functioning of all side effects. The PRISE consists of checklists of 3–5 side effects in each of six side effect domain areas. Individual side effects were rated only if the participant indicated problems in that domain area.
Additional app features
Graphical feedback was provided in the app from longitudinal PHQ-8 scores and medication adherence. Contact information for both the prescribing physician and study research assistant was viewable under contacts.
Wisepill pill box
Wisepill is an electronic pill dispenser that is cellularly enabled so that a signal is sent over the cellular network in real time, informing the MedLink system that the pill box has been opened. In the absence of a detectable medication adherence event within 10 minutes of their dose time, patients receive medication prompts (i.e. ‘Have you taken your medication today?’) to their smartphone. This prompt was persistent and thus remained on the screen until the participant responded or actively declined to answer. This allowed participants to indicate that they had taken their medication in instances of system failures. This system was intended to provide reminders only when the system did not detect a dose change, thereby avoiding notification fatigue.
Reports to physicians and patients
PCP report content and format was informed by two focus groups of 10 PCPs. Physicians were uniform in wanting weekly PHQ-8 scores, with an indicator of severity level (mild, moderate, severe) and a specific notification if there was an increase in score. As noted above, physicians were concerned that the PHQ-9 item assessing suicidal ideation is too non-specific to be actionable and therefore would frequently trigger needless outreach to the patient, significantly increasing burden on the clinic staff and the patient. Information on the severity of side effects (FIBSER) along with the specific side effect symptoms (PRISE) were also seen as important. Adherence rates as a percentage of days since the previous visit were requested. There were mixed feelings about specific treatment recommendations, with some stating it would be useful and some expressing concerns that clear recommendations could present a legal liability if their clinical judgment led them to a course of action that was different from the recommendation. We elected to retain the treatment recommendation because failure to optimize the medication regimen is a common physician failure point and EMR-based decision support is increasingly common.
There was no consensus on where such information could be provided in the EMR so that it would be noticed. It was decided that, for this early field trial, paper reports would be provided to physicians. Three sample mock-ups were produced, with input from a general physician that included all requested elements. Information on guideline congruent treatment was softened to recommendations. These were presented to the group of physicians in the second focus group meeting, who uniformly preferred one report format and their suggested minor modifications were incorporated. Reports were provided to physicians every 4 weeks. Reports containing the same information were provided to patients along with contact information for the physician, when an action was recommended.
All patient-facing elements of MedLink were subjected to in-lab usability testing with 23 patients taking medications (12 with MDD taking antidepressants and 11 without MDD, some taking an antidepressant and some taking a non-psychiatric medication). These findings, reported elsewhere,32 identified minor problems, which were corrected. Patients indicated that they were uncomfortable with specific daily information on adherence being provided to their physician, such as a calendar representing on which days they were adherent or not adherent. However, they felt that providing overall adherence rates to physicians was acceptable.
Procedures
Following enrollment into the study, the MedLink app was installed on the participants’ phones for those who had a compatible Android device. A trained research assistant called participants and, when applicable, guided them through the installation of the app. Participants were also sent the WisePill device via express shipping. For participants using a study-provided smartphone, the shipment also included an Android device with a full data and call plan, along with the MedLink app pre-installed. All participants received an extensive walkthrough of the MedLink app and WisePill device and expectations of use were outlined. Participants were instructed to begin storing their antidepressants in the WisePill device and using the MedLink app for the duration of the eight-week field trial. Participants were informed that a research assistant may periodically contact them to update the app or troubleshoot problems and were encouraged to contact study staff if they experience any problems using the system (e.g. bugs, Wi-Fi connectivity).
Study assessments
Study assessments occurred at baseline, and weeks 4 and 8. To avoid reporting biases that might occur with in-app assessments that are intended for clinical communication, participants were informed that these data were for study purposes only and would not be provided to their physician. Self-report measures were administered via a REDCap survey, which included demographics and the PHQ-935 for depression. Interviewer-based depression severity was measured using the Quick Inventory of Depressive Symptomatology – Clinician version (QIDS-C).36 The QIDS-C was administered by telephone by research assistants who received training and monitoring by a PhD-level psychologist.
To assess usability, participants were administered questions drawn from the USE questionnaire37 at weeks 4 and 8 asking them to rate likability, ease of use, learnability and usefulness on a seven-point Likert scale (1 = strongly disagree, 7 = strongly agree) for the overall MedLink system, lessons and Wisepill box. Semi-structured user feedback interviews were conducted by a research assistant inquiring about participants’ experience, potential design flaws and suggested improvements.
Physicians were given questionnaires at the conclusion of the study asking about the usefulness of the MedLink reports. Anticipating that, in a busy practice, some of the physicians may not recall receiving only a few feedback sheets, questions about the potential utility of the feedback were asked.
Statistical analysis
Demographic and study characteristics were reported as frequencies and percentages for categorical variables and mean and SD for continuous variables. Usability of the MedLink app was described using event frequencies. Due to the small sample size, non-parametric analysis methods were used to analyze study outcomes. Wilcoxon’s signed rank test was used to compare adherence and usability ratings at week 4 and week 8. The Cochran–Mantel–Haenszel test was used to evaluate the change in PHQ-9 and QIDS total scores over the eight-week study period. All statistical analyses were performed using SAS, version 9.4 (Cary, NC, USA).
Results
Participants
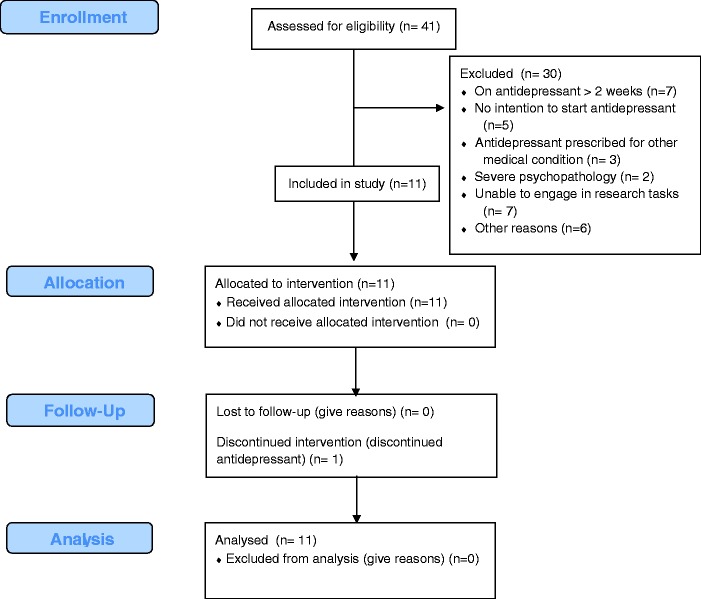
Of the 41 individuals screened for eligibility, 11 (29%) were enrolled. The flow of participants is shown in Figure 2. On average, participants were enrolled 16.5 ± 3.1 days after receiving their prescription. All participants completed all assessments in the eight-week trial. There were no adverse events. Baseline characteristics are presented in Table 1. Four participants used personal Android smartphone and seven participants used study-provided Android smartphones for the duration of the study.
Figure 2.
CONSORT flow diagram.
Table 1.
Patient characteristics (N = 11).
| Variable | N (%)/Mean (SD) |
|---|---|
| Age – Mean ± SD | 45.1 (16.7) |
| Gender | |
| Male | 3 (27%) |
| Female | 8 (73%) |
| Race | |
| African American | 5 (45%) |
| Asian | 1 (9%) |
| White | 4 (36%) |
| Ethnicity | |
| Hispanic or Latino | 1 (9%) |
| Not Hispanic or Latino | 9 (82%) |
| Declined to report | 1 (9%) |
| Marital | |
| Single | 7 (64%) |
| Living with significant other | 1 (9%) |
| Married/Domestic partner | 3 (27%) |
| Education | |
| Some high school | 1 (9%) |
| Completed high school | 1 (9%) |
| 4-year college (BA, BS) | 5 (45%) |
| Master's degree | 4 (36%) |
| Employment status | |
| Employed | 7 (64%) |
| Unemployed | 1 (9%) |
| Retired | 2 (18%) |
| Student | 1 (9%) |
| Insurance (more than 1 could be selected) | |
| Private | 9 (82%) |
| Medicare | 2 (18%) |
| Medicaid | 1 (9%) |
| No insurance | 0 (0%) |
| Device | |
| Personal device | 4 (36.4%) |
| Study provided device | 7 (63.6%) |
Medication adherence
Medication adherence was calculated based on expected dose days. This excluded days for the patient who informed her physician she was discontinuing. Mean medication adherence detected by the MedLink system or participant self-report was 82.0% over the eight-week trial. Medication adherence did not decline significantly over the eight weeks, starting at 88.5% during the first four weeks of the trial and dropping slightly to 73.0% in the last four weeks (p = 0.16).
At the end of the trial, 10 (91%) of participants continued to take their medications. The one participant who discontinued reported that she did so due to side effects. She also informed a research assistant that discontinuation was under the advice of her doctor. However, her doctor reported that, while she was informed of the discontinuation, she had advised the patient to continue taking the antidepressant medication.
Physician encounters
The EMR revealed a range of 0–5 for all follow-up encounters per participant with 10 having at least one encounter. This included a range of 0–2 for office visits, 0–2 for phone contacts and 0–2 for patient portal contacts. Five participants had a change in medication or dosage as a result of these encounters. Of these five, four participants experienced an increase in dosage of the same medication and one participant had a change in medication.
Depression outcomes
All participants completed all outcome assessments and showed substantial improvement in depression (see Table 2). There was a significant decrease in both depression outcomes, including the PHQ-9 (p = 0.0005) and the QIDS (p = 0.0008).
Table 2.
Depression outcomes.
| Baseline Mean (SD) | Week 4 Mean (SD) | Week 8 Mean (SD) | p | |
|---|---|---|---|---|
| PHQ-9 | 12.0 (4.0) | 5.3 (4.6) | 3.5 (6.5) | .0005 |
| QIDS | 11.6 (3.7) | 7.6 (5.6) | 4.6 (4.0) | .0008 |
Use and usability
MedLink was launched an average of 23.8 times (SD = 10.5) per user over the eight weeks. The user feedback questionnaire outcomes, evaluating the overall usability of the MedLink system, are displayed in Table 3. The responses were generally favorable, ranging from a mean of 5.6 (SD = 0.09) for likability to 6.6 (SD = 0.05) for learnability on a seven-point Likert scale. These ratings did not change significantly over time (ps > 0.75).
Table 3.
Usability ratings across the trial (1 = strongly disagree; 7 = strongly agree).
| Statement | N | Mean ± SD |
|
|---|---|---|---|
| Week 4 | Week 8 | ||
| Overall | |||
| I like it. | 11 | 5.7 (1.2) | 5.6 (0.9) |
| It was easy to use. | 11 | 6.4 (0.8) | 6.3 (0.8) |
| I learned how to use it quickly. | 10 | 6.2 (1.2) | 6.6 (0.5) |
| It was useful/helpful. | 11 | 5.8 (1.5) | 5.8 (1.1) |
| Lessons | |||
| I like it. | 11 | 5.5 (1.3) | 5.9 (0.9) |
| It was easy to use. | 11 | 6.7 (0.5) | 6.3 (0.8) |
| I learned how to use it quickly. | 11 | 6.7 (0.5) | 6.5 (0.7) |
| It was useful/helpful. | 11 | 5.7 (1.5) | 5.9 (0.9) |
| Wisepill box | |||
| I like it. | 11 | 5.7 (1.3) | 5.5 (1.2) |
| It was easy to use. | 11 | 5.9 (1.8) | 6.5 (0.5) |
| I learned how to use it quickly. | 11 | 5.9 (1.8) | 6.7 (0.5) |
| It was useful/helpful. | 11 | 6.2 (1.3) | 5.7 (1.5) |
| MedLink guidance helps me better communicate my needs to my physician. | 10 | 4.8 (1.8) | 5.7 (1.1) |
Lessons were viewed an average of 12.5 (SD = 10.5) per user. Lessons were rated favorably, ranging from 5.5 (SD = 1.3) for likability to 6.7 (SD = 0.05) for ease of use and learnability, with non-significant changes over time (p > 0.18). Comments generally reflected the usefulness of the information; although one patient who was in concurrent psychotherapy commented that there was nothing new in the information.
The Wisepill box was also rated positively, ranging from a mean of 5.5 (SD = 1.2) for likability to 6.7 (SD = 0.05) for learnability, with no significant changes over time (p > 0.25). While we have no reason to believe that any Wisepill box openings were not detected, they were frequently delayed due to connectivity problems. The median time for the information from the opening of the WisePill device to reach the MedLink server was 12 minutes, ranging from under one minute to more than five days. Of the 493 pillbox openings, 203 (41.2%) were received in under 10 minutes, 120 (24.3%) were received between 10 and 30 minutes, 16 (3.2%) were received between 30 and 60 minutes and 154 (31.2%) were received more than 60 minutes after opening. When a WisePill opening was not detected within 10 minutes, MedLink launched a dialog box asking if the participant has taken their medication yet. When the first dialog box (‘Have you take your medication yet today?’) is answered ‘No’, they had the opportunity to tell MedLink if they were planning to take their medication later today. The first dialog box was launched over 300 times and was responded to 174 times, to which participants responded ‘Yes’ a total of 149 times (85.6%). Of the 25 ‘No’ responses, 21 times (84%) the participants said they were planning to take their medication that day.
Several of the participants commented on receiving reminders when they had taken their medications, saying that it resulted in confusion about how the prompts worked. There were also complaints about the reminder method. Many patients did not see the reminders in the tray and several did not like pop-up reminders, with one participant commenting that the prompts interrupted her use of the phone.
Finally, the participants rated the statement ‘MedLink guidance helps me better communicate my needs to my physician’ favorably with a rating of 5.7 (SD = 1.1) (n = 10).
Physician usability
Of the 10 physicians who had patients involved in the study, seven (70%) completed the feedback questionnaire. As anticipated, only four of the physicians remembered receiving the physician feedback sheets. Of those, three found them useful and one did not. Six physicians (86%) stated that the information on side effects and adherence would be useful and one stated it would not be useful. Six answered the question on the decision support (recommending changes to dose or medication), of whom five (80%) stated this would be useful and one said it would not.
There was no consensus on how this information could be supplied reliably to the physician, in a manner that fit into their workflow. Four indicated through the EMR inbox, three indicated as an EMR best practice alert, one stated as a note or report in the EMR and one stated on paper (note that more than one response was possible). One physician commented that it would be best if a care manager existed in the clinic who could serve as the point of contact.
Regarding the physician burden of the system, only one physician indicated it would add no extra burden, while three indicated it would add ‘a little’ burden and two indicated the added burden would be a more than ‘a little’.
Discussion
Participants generally liked MedLink, thought it was easy to use and found it useful. The use data indicated that patients used the system, for the most part consistently, over the eight weeks they were beginning their antidepressant treatment, when they are establishing their medication-taking routine and the risk of non-adherence and discontinuation is highest.12,13 While this field trial was not intended to test efficacy, adherence rates were good, with only one patient discontinuing her medications and 82% of doses taken across all participants. This is in stark contrast to the 70% discontinuation rates seen in other studies.12 Depression severity was significantly and substantially reduced at the end of the 8-week trial. These findings support the feasibility and potential utility of the MedLink system.
MedLink provides a novel approach to improving processes in pharmacotherapy delivered in primary care. The system targets actionable failure points in care, including patient factors (lack of knowledge and perceived benefit, side effects, forgetfulness and pill taking routine and psychological consequences of depression, such as low motivation), provider factors (sub-therapeutic dosing and failure to optimize regimen) and lack of communication between patient and provider.
Lessons, which were brief and released weekly, addressed patient factors such as lack of knowledge, lack of perceived benefit, hopelessness and low motivation and were accessed frequently and generally liked (with the exception of one participant who was in psychotherapy and found the information redundant). Because the educational content was valued, we believe the weekly release of lessons also served to keep people engaged with the MedLink system.
MedLink also appeared to affect processes of care. While treatment guidelines recommend follow-up visits every 2–8 weeks during the initiation of antidepressant therapy,19–21 PCPs typically do not follow up after the first prescription is made.22 Ten of the 11 participants in this trial saw their physician for at least one follow-up, which resulted in changes to dosage or medication for six of the participants. Several of the contacts occurred through the patient portal, indicating that patients were contacting their care providers, based on reports and recommendations provided to them.
Physicians appeared to find the system useful and helpful, but several concerns related to workflow and burden were uncovered. Physicians believed that MedLink would increase their work burden. While there is not sufficient data to indicate specifically where this burden would occur, we speculate that this would result in part from added patient contact. Given the prevalence of depression and the volume of patients that PCPs are expected to see, even this relatively small individual burden could end up being a substantial burden across an entire caseload. Hence, the physician’s suggestion that these follow-up tasks be shifted to a care manager may make such a tool more acceptable.
Consistent with our findings from initial focus groups, physicians involved in the trial did not identify any reliable method of receiving this information within the EMR (Epic) used in this practice. These findings are also consistent with observations that, while it is technologically feasible to provide just-in-time decision support to care providers, such systems are frequently ineffective in practice due to the variability in provider workflow, the increasing volume of decision support alerts and the acceptability of such systems to providers.38 Thus, activating the patient to contact the provider when medication or dose changes may be indicated will remain a critical component in the continuing development of the MedLink system.
To provide just-in-time reminders, we used a cellularly enabled pill bottle to allow us to prevent reminders from being launched when we detected that a dose had been taken. This was intended to prevent notification fatigue, where repeated, automated messages become noise for the recipient. While many electronic pill bottles provide alerts in the bottle (as does Wisepill), this requires that the patient be near the bottle, which we believe is a likely failure point. The Wisepill device was selected because it can transmit pill bottle openings allowing MedLink to prevent the launch of reminders when a dose was taken, thereby minimizing notification fatigue on the part of the patient. However, this design did not function optimally when the pill bottle was in a low connectivity location, as our servers did not receive pill bottle opening data in a timely manner (although they were received). Thus, this system sometimes launched reminders when the person had already taken their medication, resulting in confusion for some people. One possible solution is to use multiple communication methods, such as transmitting the information via Bluetooth to the phone when the two devices are in range, thereby increasing the likelihood that the system detects pill bottle openings and decreasing the likelihood of unnecessary reminders.
Several participants did not like the use of tray notifications and pop-ups as a means of providing reminders, although this was acceptable and useful to others. Giving patients more control over how they receive the reminders would likely improve this. For example, patients could choose to receive reminders via tray notifications, pop-up messages, widget notifications, emails, automated phone calls or text messages.
Finally, these findings should be considered with a few caveats. As this was a single arm trial, no claims can be made about MedLink’s efficacy. It is entirely possible that patients recruited into this trial were those who would have likely been highly adherent regardless of exposure to MedLink. Furthermore, this study was conducted in a single academic practice. Thus, the findings may not be more broadly representative. Finally, physician reports suggest that, even if MedLink is successful in improving care processes, workflow issues and potential increase in physician burden, however small, would have to be resolved for this to be acceptable to providers.
In spite of these problems, the system functioned well. MedLink was easy for patients to use and they found it helpful. Physicians had follow-up contact with a majority of their patients and patients found it assisted them in their communication with their physicians. This study supports the general principles of disease-specific medication management systems that systematically address the patient, provider and communication failure points that impact the success of pharmacotherapy. These findings support the further development and evaluation of MedLink in a randomized controlled trial, to evaluate the efficacy and effectiveness of improving processes of care, patient adherence and improvement in depression, as well as its extension to cover other common problems in pharmacotherapy in primary care, such as hypertension.
Contributorship
D.C.M. conceptualized and oversaw all parts of the research and writing; M.E.C., E.K. and B.R. oversaw aspects of the research and wrote first draft of manuscript; H.L.P. conducted statistical analyses; C.B., M.B., and J.D. conceptualized the intervention and reviewed the manuscript; S.K. oversaw all aspects of research and human subjects, managed intervention and edited the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
Institutional Review Board for Northwestern University #STU00063382; Trial Registration: Clinicaltrials.gov NCT02583230.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article:This research was supported by a grant from the National Institute of Mental Health (R34 MH095907) to David C. Mohr, PhD.
Guarantor
D.C.M.
Peer review
This manuscript was reviewed by two individuals who have chosen to remain anonymous.
REFERENCES
- 1.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. JAMA 1989; 262: 914–919. [PubMed] [Google Scholar]
- 3.Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 2015; 76: 155–162. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med 2013; 10: e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 629–640. [DOI] [PubMed] [Google Scholar]
- 6.Regier DA, Narrow WE, Rae DS, et al. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry 1993; 50: 85–94. [DOI] [PubMed] [Google Scholar]
- 7.Schulberg HC, Block MR, Madonia MJ, et al. The ‘usual care' of major depression in primary care practice. Arch Fam Med 1997; 6: 334–339. [DOI] [PubMed] [Google Scholar]
- 8.Katon W, Von Korff M, Lin E, et al. Collaborative management to achieve treatment guidelines. Impact on depression in primary care. JAMA 1995; 273: 1026–1031. [PubMed] [Google Scholar]
- 9.Simon GE. Evidence review: efficacy and effectiveness of antidepressant treatment in primary care. Gen Hosp Psychiatry 2002; 24: 213–224. [DOI] [PubMed] [Google Scholar]
- 10.Pompili M, Venturini P, Palermo M, et al. Mood disorders medications: predictors of nonadherence – review of the current literature. Expert Rev Neurother 2013; 13: 809–825. [DOI] [PubMed] [Google Scholar]
- 11.Pompili M, Serafini G, Del Casale A, et al. Improving adherence in mood disorders: the struggle against relapse, recurrence and suicide risk. Expert Rev Neurother 2009; 9: 985–1004. [DOI] [PubMed] [Google Scholar]
- 12.Olfson M, Marcus SC, Tedeschi M, et al. Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry 2006; 163: 101–108. [DOI] [PubMed] [Google Scholar]
- 13.Hunot VM, Horne R, Leese MN, et al. A cohort study of adherence to antidepressants in primary care: The influence of antidepressant concerns and treatment preferences. Prim Care Companion J Clin Psychiatry 2007; 9: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulberg HC, Katon W, Simon GE, et al. Treating major depression in primary care practice: an update of the Agency for Health Care Policy and Research Practice Guidelines. Arch Gen Psychiatry 1998; 55: 1121–1127. [DOI] [PubMed] [Google Scholar]
- 15.Masand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther 2003; 25: 2289–2304. [DOI] [PubMed] [Google Scholar]
- 16.Bull SA, Hu XH, Hunkeler EM, et al. Discontinuation of use and switching of antidepressants: influence of patient-physician communication. JAMA 2002; 288: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 17.Linden M, Gothe H, Dittmann RW, et al. Early termination of antidepressant drug treatment. J Clin Psychopharmacol 2000; 20: 523–530. [DOI] [PubMed] [Google Scholar]
- 18.Fava M. Prospective studies of adverse events related to antidepressant discontinuation. J Clin Psychiatry 2006; 67(Suppl. 4): 14–21. [PubMed] [Google Scholar]
- 19.The MacArthur Foundation. The Macarthur initiative on depression and primary care at Darmouth and Duke: Depression management toolkit. Hanover, NH: Dartmouth, 2004.
- 20. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. American Psychiatric Association, 2010.
- 21.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006; 163: 28–40. [DOI] [PubMed] [Google Scholar]
- 22.Chen SY, Hansen RA, Farley JF, et al. Follow-up visits by provider specialty for patients with major depressive disorder initiating antidepressant treatment. Psychiatr Serv 2010; 61: 81–85. [DOI] [PubMed] [Google Scholar]
- 23.Simon GE, Lin EH, Katon W, et al. Outcomes of “inadequate” antidepressant treatment. J Gen Intern Med 1995; 10: 663–670. [DOI] [PubMed] [Google Scholar]
- 24.Williams JW, Jr, Gerrity M, Holsinger T, et al. Systematic review of multifaceted interventions to improve depression care. Gen Hosp Psychiatry 2007; 29: 91–116. [DOI] [PubMed] [Google Scholar]
- 25.van Servellen G, Heise BA, Ellis R. Factors associated with antidepressant medication adherence and adherence-enhancement programmes: A systematic literature review. Ment Health Fam Med 2011; 8: 255–271. [PMC free article] [PubMed] [Google Scholar]
- 26.Chong WW, Aslani P, Chen TF. Effectiveness of interventions to improve antidepressant medication adherence: A systematic review. Int J Clin Pract 2011; 65: 954–975. [DOI] [PubMed] [Google Scholar]
- 27.Vervloet M, van Dijk L, Santen-Reestman J, et al. SMS reminders improve adherence to oral medication in type 2 diabetes patients who are real time electronically monitored. Int J Med Inform 2012; 81: 594–604. [DOI] [PubMed] [Google Scholar]
- 28.Checchi KD, Huybrechts KF, Avorn J, et al. Electronic medication packaging devices and medication adherence: a systematic review. JAMA 2014; 312: 1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rollman BL, Hanusa BH, Lowe HJ, et al. A randomized trial using computerized decision support to improve treatment of major depression in primary care. J Gen Intern Med 2002; 17: 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohr DC, Burns MN, Schueller SM, et al. Behavioral intervention technologies: Evidence review and recommendations for future research in mental health. Gen Hosp Psychiatry 2013; 35: 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract 1998; 1: 2–4. [PubMed] [Google Scholar]
- 32. Montague E, Stiles-Shields C and Mohr DC. Usability evaluation of MedLink to improve pharmacotherapy in primary care. In: 58th international meeting of the human factors and ergonomics society, Chicago, IL, 2014.
- 33.Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009; 114: 163–173. [DOI] [PubMed] [Google Scholar]
- 34.Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009; 70(Suppl. 6): 26–31. [DOI] [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003; 54: 573–583. [DOI] [PubMed] [Google Scholar]
- 37.Lund AM. Measuring usability with the USE Questionnaire. STC Usability Newsletter 2001; 8.
- 38.Moxey A, Robertson J, Newby D, et al. Computerized clinical decision support for prescribing: provision does not guarantee uptake. J Am Med Inform Assoc 2010; 17: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]