Abstract
Background
Women with diabetes are at increased risk of adverse maternal and fetal outcomes. Preconception care can improve pregnancy outcomes and is paramount to minimise complications, but, current provision is sub-optimal. Mobile technology, particularly smartphones and apps have the potential to improve preconception care provision but research is lacking in this area. The need to use modern technologies to improve preconception care knowledge and awareness led to the development of a preconception and diabetes information app in Stage A of this study.
Objective
The aim of this paper, Stage B of the study, is to explore the feasibility and acceptability of the Preconception and Diabetes Information app to improve preconception care knowledge and attitudes in women with diabetes, and explore the potential for wider implementation.
Methods
A mixed-methods study design adopting a quasi-experimental approach will assess women's knowledge and attitudes related to preconception care, and level of patient activation (knowledge and confidence for self-management of health) before and after the three-month intervention period. A log of activity will be used to determine engagement with the app and semi-structured interviews will explore women's experiences.
Conclusions
This is the first study to explore the acceptability and feasibility of a preconception and diabetes information app for women with diabetes. The app has potential to change the way preconception care is delivered, improve pregnancy outcomes and be widely implemented both in developed and developing countries. This is important given the considerable shortfalls in current preconception care services in the United Kingdom and around the world.
Keywords: Preconception care, education, diabetes mellitus, women, smartphones, mobile applications, mobile health, technology
Introduction
Diabetes mellitus (DM) has been deemed a global emergency.1,2 Worldwide, 415 million adults have DM and by 2040, this is predicted to rise to 642 million adults.2 There are two main types of DM that affect women before pregnancy: Type 1 (the pancreas does not produce any insulin) and Type 2 (the amount of insulin produced by the pancreas is insufficient or the body develops a resistance to insulin produced).1,2 DM is the most common pre-existing medical condition that can complicate pregnancy creating considerable risks both for mother and child.3–5 Poorly controlled DM together with unplanned pregnancy increases the risk of maternal and fetal complications. In addition to the increased risk of fetal and maternal death, common complications include miscarriage, birth defects, preterm labour and birth injury,3–6 the risks of which can be minimised through preconception care (PCC). This is targeted medical care and advice about pregnancy planning which can improve pregnancy outcomes by increasing knowledge and supporting women to adopt healthy behaviours, e.g. blood glucose monitoring and folic acid use before pregnancy.3,5,7–12 Clinical guidelines recommend that PCC is provided to all women with DM once they reach adolescence.3,6,13,14 However, evidence suggests that PCC is hindered by poor implementation of clinical guidelines,11,15,16 provider- and patient-level barriers,10,12,16 sub-optimal uptake10,17 and inadequate knowledge of PCC.8,12 These have negative implications for women with DM around the world, especially those in developing countries who are at greatest risk of adverse pregnancy outcomes.18
There is therefore a need to adopt more innovative approaches to delivering PCC. Evidence suggests that while PCC delivered by multimedia, i.e. compact discs with read-only memory (CD-ROMs) and digital video discs (DVDs)19–22 is feasible and effective in raising awareness of PCC in women with DM,12 these multimedia technologies are now outdated. Consequently they offer limited scope to the many women without access to computers or DVD players who increasingly rely on apps delivered by smartphones to access health information.23 The smartphone is the most popular mobile device, with over a billion users worldwide.23 It is technologically advanced, easily accessible, mobile and offers the opportunity to penetrate a larger population.23,24 In most developed countries, smartphones are ingrained into daily life while in developing economies, usage is proliferating rapidly and is expected to approach levels seen in developed countries.24 Most smartphone functionalities are aided by apps (software designed to run on smartphones), and in a recent qualitative study, women with DM suggested development of a PCC app to help convey PCC education, increase knowledge of diabetes and pregnancy and improve PCC uptake.8
The increasing availability of health information in easily accessible digital format is rapidly changing the health education and information paradigm.25 There is some evidence to suggest that apps can help improve women's knowledge of family planning and contraception26 and to reduce body mass index (BMI).27 They have also contributed to healthy behavioural changes in other areas – medication management, diet control, physical activity, lifestyle improvement, smoking cessation and diabetes management.28 The acceptance, coverage and effectiveness of apps in improving knowledge and enhancing behaviour change, coupled with the need for increased PCC awareness and uptake in women with DM, led to the development of a Preconception and Diabetes Information (PADI) app in Stage A of this study. The PADI app is designed to provide flexible and easy access to information about PCC, blood glucose monitoring and support for women with DM to optimise their health from preconception to early pregnancy. This paper presents Stage B of the study, the primary aim of which is to test the feasibility and acceptability of the PADI app and explore its potential for wider implementation.
Methods
Study design
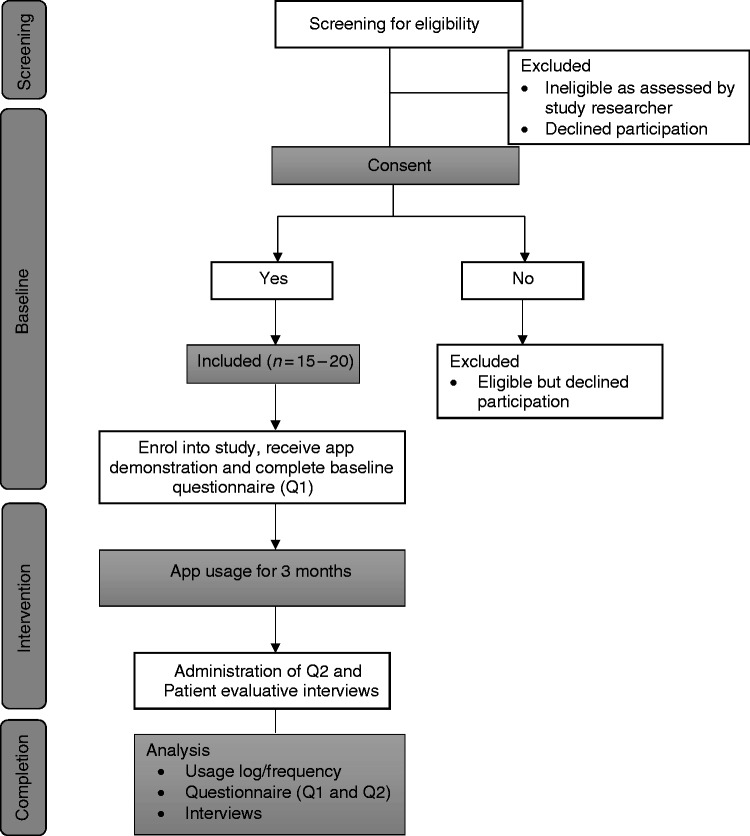
A mixed-methods29–31 study design incorporating several data-collection methods will be used to explore the feasibility of the PADI app for women with Type 1 DM (T1DM) and Type 2 DM (T2DM). A longitudinal quasi-experimental approach with two data collection points, at the beginning and end of three months of app usage (Figure 1), will be used to assess women's knowledge and attitudes to PCC. The quasi-experiment will not involve an external control group, outcomes will be measured before participants receive the intervention and again after the intervention, and any changes in measurement compared within the individuals over a period of time to determine the impact of the intervention.32,33 A log of activity will be used to determine engagement with the app, a simple visual scale will measure satisfaction and semi-structured interviews will explore women's experiences of app usage.
Figure 1.
Study flow diagram.
Conceptual framework
The Normalisation Process Theory (NPT)34 provides the theoretical basis for this study. The NPT provides a framework that is useful for assessing and enhancing the implementation potential of technological interventions. It addresses the factors for successful implementation and integration of health interventions into routine practice, a process known as normalisation. The four components of the NPT (Table 1) were considered before and during the development of the app, and incorporated into the design of this feasibility study.
Table 1.
Components of the Normalisation Process Theory.
| Component | Related Questions |
|---|---|
| 1. Coherence or sense-making (the meaning the intervention makes to participants) | Is the intervention easy to understand and what benefits will it bring and to whom? |
| 2. Cognitive participation or engagement (commitment made by participants) | Will the target user groups see it as a good idea and will they be prepared to invest time, energy and work into it? |
| 3. Collective action (effort invested in the intervention by participants) | How will the intervention affect the work of user groups, will it promote or impede their work, and what effect will it have on health services, e.g. consultation time? |
| 4. Reflexive monitoring (appraisal of the intervention by participants) | How will users perceive use of the intervention, can users contribute feedback about the intervention, and is there room for the intervention to be improved following user experience? |
Ethical review
Favourable ethical opinion was obtained from United Kingdom National Research Ethics Service (UK NRES) Committee East Midlands-Derby; REC reference 15/EM/0358; IRAS project ID: 178530.
Recruitment
Participants will be recruited from two National Health Service (NHS) hospitals in the South of England and via social media (i.e. Twitter). Eligible participants who attend the outpatient clinics at the hospitals will be approached by a member of the healthcare team when they come for their clinic appointments and asked if they are willing to speak to a researcher (CHN) about testing the PADI app. A Twitter advert will be sent out inviting eligible participants to contact the study researcher if they are interested in taking part in the study. If participants indicate interest, they will be asked to provide the researcher (CHN) with their contact details. Potentially eligible participants will receive an introduction to the study face to face, by mail or telephone. It will be made clear in all information provided to participants that they do not have to participate in the study and that they can withdraw from the study at any time without prejudicing their care. All potentially eligible participants will be screened for eligibility against the following inclusion criteria:
Woman of reproductive age (18–45 years) with T1DM or T2DM.
Diagnosis of diabetes by a healthcare professional for more than six months.
Currently are not pregnant, but are planning a future pregnancy (in the next five years) or want children sometime in the future.
Own a smartphone (iOS or Android).
Those who meet the inclusion criteria will be provided with a written participant information sheet (PIS) informing them about the study. They will be given the opportunity to ask questions and written consent to participate in the study will be obtained prior to study commencement. Only eligible participants who provide written informed consent will be enrolled in the study.
Sample
A purposive sample of patients with T1DM and T2DM will be recruited into the study, from two NHS hospitals in the South of England and via social media. For feasibility studies, a sample size of 12 is considered sufficient.35 Participants for the intervention phase will comprise (n = 15–20) women with DM, with attrition (or drop-outs) estimated at 20%.36 Figure 1 provides a flowchart of participants through the study.
Intervention
All eligible participants enrolled in the study will receive the intervention. It is anticipated that participants will utilise the PADI app for at least five minutes a day, 35 minutes a week and seven hours over three months to (1) access preconception information and (2) record their blood glucose level.
PADI app development
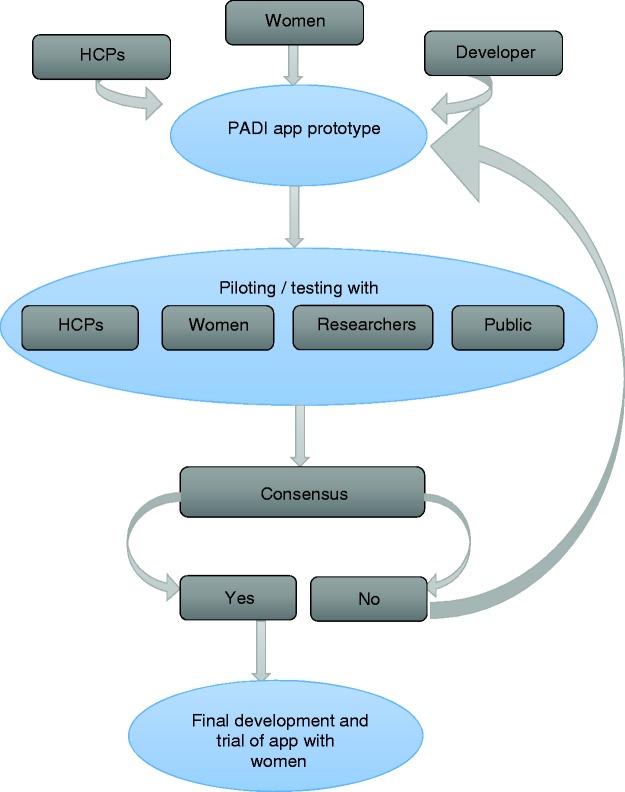
A co-design approach was used to develop the PADI app (Figure 2) to help ensure that it met the needs of women and that its content was appropriate and acceptable.37–40 The PADI development team included women with DM (app development working group), healthcare professionals (expert advisory group) and a digital agency (Netsells Ltd).
Figure 2.
Diagrammatic representation of PADI app development process.
PADI: Preconception and Diabetes Information; HCPs: healthcare providers.
Content development
The PADI app content was based on a series of focus groups and interviews with the expert advisory group and app development working group, the majority of whose members were supportive of using technology to support PCC. The app content was also informed by previous research in this area (Holmes et al.21) and the findings from a recent systematic review.10
Expert advisory group
The expert advisory group comprised doctors (general practitioner, obstetrician, endocrinologist) and nurses (research and specialist) who suggested including the following information: importance of planning pregnancy and using contraception until stable HbA1c is achieved, pregnancy-related risks for the woman and baby, and general health advice for the preconception period.
App development working group
The app development working group included women of childbearing age with T1DM and T2DM, age range 22–43 years, with and without prior pregnancies. They expressed desire for easily accessible and comprehensive PCC information that was provided using positive and supportive language.
Both groups were of the opinion that the information should extend beyond preconception to include diabetes and pregnancy, particularly what to expect during pregnancy and delivery. They suggested including a blood glucose diary, reminder and graph to help women track and visualise their blood glucose levels in the run up to pregnancy, and wanted a PCC app that was attractive, simple and user friendly.
PADI app design and prototype development
The app design was discussed with the app developer (Netsells), which then created wireframes, a visual guide representing the skeletal framework of the app. The wireframes were used to detail the app layout, prioritise components and determine how the app screens will link together. They took into account the functionality of the screen, content, layout, app behaviour and sequencing of the app's function. The output of the wireframe work was then used to create an initial prototype, a design model of the final user interface (UI), which gave the first detailed impression of how the UI or app screens would look and work. The app prototype was designed to provide a series of informational pages and a place to submit and view blood glucose readings by day, week and month. This initial design output, however, highlighted potential usability issues regarding the layout, colours and navigation. The prototype was then revised several times to ensure that the final UI was simple, user friendly and attractive.
PADI was developed both in Swift (programming language for iOS) and Java (android development language) in order to run both on Apple's iOS and Google's Android platforms. An application programming interface (API) was also developed to allow the storage and retrieval of individual records of information. The API routine connects PADI to Netsells' remote server and along with the creation of user accounts, allows authentication with the data in the database. The API was developed using one of the most popular programming languages for web application backend development, Hypertext Preprocessor (PHP) framework Laravel.41 The resulting prototype was tested by Netsells in-house to ensure optimal functionality before being released for further testing. The final prototype was presented to a selection of participants (healthcare professionals, women with DM, researchers and members of the public) to test the full app functionality for a period of 14 days and provide feedback. The piloting comprised two cycles of feedback and resulted in minor textual changes, modification of the blood glucose diary, and rectification of an error with the graphical display of blood glucose readings. This process was deemed complete when the modifications made were tested by participants and their needs satisfied.
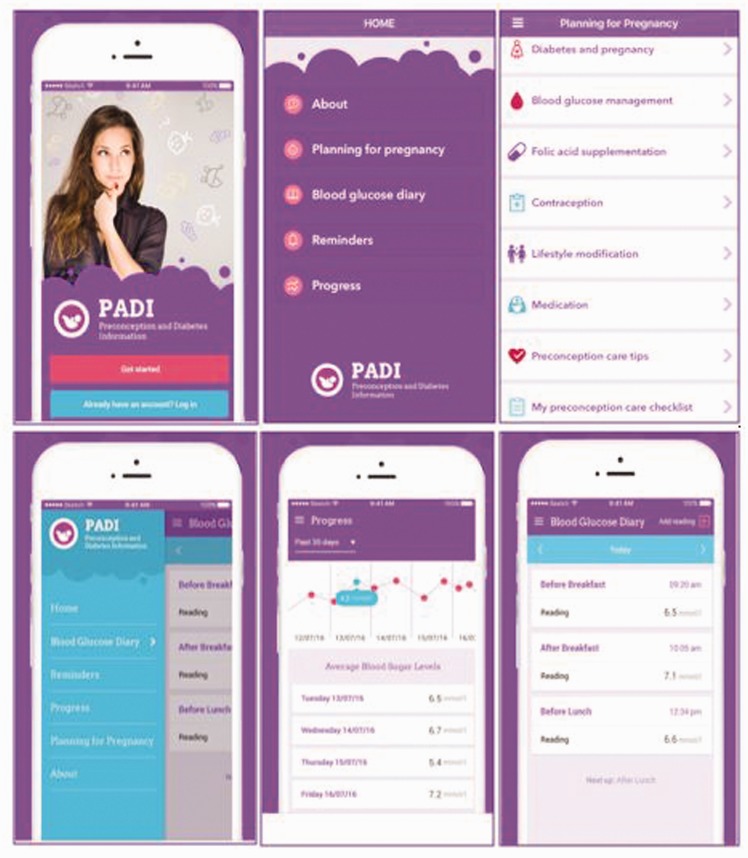
Features of the final PADI app
Screenshots of the final app (Figure 3) show the following features: Planning for pregnancy and blood glucose diary with graphical display and reminder. The core aspects of these features and their purpose within the app are shown in Table 2.
Figure 3.
Screenshots of the final Preconception and Diabetes Information (PADI) app.
Table 2.
Preconception and Diabetes Information (PADI) app feature description.
| Features | Aspects of the feature | Purpose |
|---|---|---|
| Planning for pregnancy drafted in line with National Institute for Clinical Excellence (NICE) preconception care (PCC) guidelines3 | This feature comprises 13 sections with information on the various aspects of preparing for pregnancy and what to expect during pregnancy and delivery. In addition to textual information, it contains videos and uniform resource locators (URLs) that users can click on to view more information on a specific topic. These URLs take users out of the app and to reliable external websites such as Diabetes UK,42 NICE guidelines,3 Family Planning Association (FPA)43 and womenwithdiabetes.net.21,44 PADI app icon will remain at the top of the page and users can use this to navigate back to their current session in the app. | Promote knowledge of PCC and pregnancy planning. |
| Blood glucose diary (represented both in text format and through data visualisation) | Blood glucose (BG) readings throughout the day are recorded. Frequency depends on number of times BG reading is taken and as advised by a healthcare professional. Users are able to select the current time of day from a drop-down menu, e.g. before breakfast. The time of reading is automatically set and users have the option of being reminded to take their next reading before saving the BG reading. The list of entries is then displayed starting with the oldest to the most recent. | To record and keep track of BG reading. |
| Reminders | These are set when a new reading is added. A notification is sent to the user's phone at their set reminder time. Users have the option to deactivate set reminders by toggling the switch. | Reminder to take BG reading and help keep a regular BG reading schedule. |
| Progress | The inputted BG information is further broken down into daily averages and represented in a graph. BG readings outside recommended PCC target range (4–9 mmol/l) trigger a pop-up feature and highlights the reading. Clicking on the pop-up redirects users to the BG management page within the app. Users can choose how they want their progress displayed, e.g. today, past seven days or 30 days. | To display the user's progress and help monitor trends. |
Outcome measures
As a feasibility study evaluating the process and/ acceptability of the intervention to users there is no primary outcome measure.45,46 The study will explore women's satisfaction and experiences of using the PADI app, and provide preliminary outcome estimates, i.e. knowledge of preconception care, patient activation measure (PAM) and attitudinal change to PCC. Evaluative semi-structured interviews and log of activity after the three months of app use will seek to explore perceived acceptability, utility and usability of the app.
Data collection
Background information
Information will be collected on participants' demographics and baseline characteristics. A section containing questions aimed at capturing these background data will be included in the patient questionnaires (Q1) administered on enrolment.
Patient questionnaires
These will be administered at two time points, on enrolment (at baseline) and following three months of PADI app usage, and will be informed by previously validated tools involving PAM,47,48 reproductive health and diabetes instrument (RHAB)49 and knowledge of PCC.21 These evaluative questionnaires will be used to compare PAM, knowledge and attitudes to PCC before (pre-) and after (post)-intervention. At the end of the study, participants will be required to repeat the initial questionnaire (but without background information) (Q2).
Software log of activity
This will provide information on the frequency of use of the app for accessing informational pages and for self-monitoring activities, i.e. logging of blood glucose. This will be used to demonstrate uptake/utilisation of PADI's features. PADI has inbuilt analytics that support collection of these data, which will be stored on a secure server accessible only to the app development company who will then ensure secure transfer to the researcher (CHN) to allow data analysis.
Patient interviews
Semi-structured face-to-face or telephone interviews will be undertaken at the end of the study, 40–60 minutes with the first six to eight participants30 from the intervention who consent to being interviewed. Interviews will explore their experience of using PADI and from this, common themes will be identified regarding acceptability, impediments to regular usage and suggestions for improvement.40 The interview schedule (Appendix) was informed by previously published protocols for exploring participant experience of using an app.50
Data management and analysis
Microsoft Excel© will be used for the organisation, collation and recording of the quantitative data from each participant. Processing of the questionnaire will be consistent and a clear record of the returned questionnaires will be kept. Each participant will be assigned a number and the same number will be used throughout the study to allow the data on demographic information, knowledge and attitudes to PCC and PAM to be correlated. A coding manual will be developed for each questionnaire and used during the data entry process. The responses will be assigned numerical codes and recorded in the codebook. The data will then be entered into Excel spreadsheets and later imported into SPSS® version 22 for analysis. Descriptive statistics will provide preliminary estimates of key parameters, as recommended for feasibility studies.45,46
Content data from the open questions will be coded by assigning data to categories and then labelling the categories. Qualitative data (interviews) will be analysed using inductive thematic analysis.51 Interviews will be recorded, transcribed verbatim, and anonymised. QSR Nvivo 1052 will be used as a data handling management system for the data collected from the interviews. Nvivo will be used mainly for the advantages it offers in allowing large volumes of data to be handled with speed, preparing conceptual maps of coded data, facilitating exploration of relationships between codes, and aiding interpretation and theorising.33,51,52
Discussion
This paper presents a mixed-methods protocol that is designed to examine the acceptability and feasibility of the PADI app intervention. PADI incorporates both PCC information and self-monitoring of blood glucose (SMBG) functions. PCC education has been widely recommended as an effective strategy to promote PCC knowledge and encourage behaviour change.3,6,13,14,18,53 SMBG is a vital part of managing glycaemic control and optimised glycaemic control is a core component of PCC. Mobile phones have been shown to improve glycaemic control in patients with DM54–56 and the effectiveness of technological innovations in improving PCC knowledge and attitude have been demonstrated.19–22 PADI is designed to improve awareness of PCC and to support women with T1DM and T2DM adopt behaviours that support a healthy pregnancy and baby such as regular blood glucose monitoring, folic acid intake, lifestyle modification and use of contraception until optimum blood glucose levels are achieved.
Health interventions utilising mobile technologies offer many advantages including the potential for wide-scale implementation and dissemination.40 They are also able to penetrate disadvantaged or resource-poor settings including developing countries, thereby expanding the reach of health information to underserved communities in line with the United Nations sustainable development goals.57,58 Successful implementation of a technological innovation, however, requires careful consideration of several factors. For example, an intervention that is perceived as providing limited benefits, difficult to understand or fit into normal everyday practice would not appeal to healthcare professionals or policy makers.34,40 In order to maximise benefit, it is important for technological interventions to be accepted by the target population and normalised into practice.34,40 While a healthcare professional may recommend a smartphone app, it is ultimately up to those concerned to determine if the app is beneficial and worth an investment of their time.59 Therefore, to increase PADI's potential for wide-scale adoption and implementation, women with DM and their healthcare providers were involved in its design and development, and the NPT framework34 was used to optimise the app development and inform the study design.
This is the first study to test the feasibility of a preconception app specifically developed for women with DM. Findings of the study will enhance understanding of the role of apps in PCC of women with DM and provide insight into their use for promoting PCC awareness and behaviour change. It is anticipated that PADI will provide an innovative way of providing more women with DM with information on PCC. This is important given the barriers associated with traditional PCC practice8,12,15,17 and the limited technology that is available to support PCC services.10 Given these limitations, the PADI app has been designed both for Apple iOS and Google Android, the two leading mobile operating systems.60 If this study is successful, it will provide the platform for a larger study to evaluate a potentially feasible and easy-to-implement intervention that can be integrated into healthcare and provide evidence of its effect on patient outcomes. However, for this study participants will be required to download the app onto their own phones and need to own a smartphone to be eligible to participate. Repeated examination of preliminary outcomes within three months may introduce some information bias into the study. Also, being a feasibility study, the sample size for the quantitative phase is quite small, limiting the generalisability of the study findings to other contexts and populations.
Conclusion
The prevalence of DM in women of reproductive age is growing, and improved PCC awareness is urgently required to reduce poor maternal and perinatal outcomes experienced by women with DM, particularly in low and middle income countries. Mobile technology, mainly smartphones and apps, have the potential to improve PCC and obstetric outcomes for women with DM around the world, but, research is lacking in this area. This is the first study to explore the acceptability and feasibility of a preconception app for women with DM, and the findings will inform the development of a larger study. PADI could be easily integrated into healthcare, scaled up and adapted into a sustainable PCC intervention programme which could be widely implemented, particularly in developing countries where women have the highest risk of adverse pregnancy outcomes. This is important given the considerable shortfalls in current PCC provision around the world and in the United Kingdom.
Acknowledgements
The authors would like to thank all the participants who contributed their time, experience and ideas towards the development of the PADI app.
Appendix
Semi-structured interview guide
- Experience of using the smartphone App
- How did you get on with using the App over the last 3 months?
- How did you find navigating the pages and finding information?
- Did you have any problems using the app?
- If you had problems, what were they?
- Was the app easy to use?
- Use of App for preconception care (PCC)
- How did you find using an App for PCC?
- Did you use the app regularly e.g. 5 minutes/day?
- • If no, why not.
- How did you find the information on preconception care (PCC)?
- Did you think the links to websites and videos were helpful?
- Did you use any other PCC services whilst in the study (e.g. GP, other website)?
- Is there any other information that you would have liked included in the App?
- Acceptability of features provided
- What do you think of the blood glucose diary?
- How did you find inputting blood glucose data?
- Did you use the reminders to record your blood glucose reading?
- • If no, why not.
- • If yes, were the reminders helpful?
- Did you use the progress feature to monitor trends?
- • If no, why not.
- • If yes, did it help you in your diabetes management?
- What features did you find useful?
- Why did you find these features useful?
- Satisfaction with the app
- What do you think about the overall content and functionality of the App?
- What did you like about having the PCC information delivered via an app?
- What did you dislike about having the PCC information delivered via an app?
- Feedback
- What improvements can be made to the App?
- Do you think any features could be removed or added to the app?
- Is there anything you would do differently following your participation in the study?
Contributorship
CHN researched the literature, developed the protocol, gained ethical approval and wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
The ethics committee of NHS Health Research Committee approved this study (REC number: 15/EM/0358).
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The primary author (CHN) was supported by a grant from Funds for Women Graduates (FfWG) until September 2016.
Guarantor
CHN.
Peer review
This manuscript was reviewed by Sheyu Li, Sichuan University West China Hospital; Margaret Allman-Farinelli, University of Sydney; Braulio Cezar Bonoto, Federal University of Minas Gerais; and one other individual who has chosen to remain anonymous.
References
- 1.Robinson A, Nwolise C, Shawe J. Contraception for women with diabetes: Challenges and solutions. Open Access J Contracept 2016; 7: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. Diabetes atlas, 7th ed http://www.diabetesatlas.org/ (2015, accessed 21 June 2016). [Google Scholar]
- 3.NICE Clinical Guideline NG3. Diabetes in pregnancy: Management from preconception to the postnatal period, http://nice.org.uk/guidance/ng3 (2015, accessed 29 September 2016).
- 4.McCance DR. Pregnancy and diabetes. Best Pract Res Clin Endocrinol Metab 2011; 25: 945–958. [DOI] [PubMed] [Google Scholar]
- 5.Knight M, Kenyon S, Brocklehurst P. Saving lives, improving mothers' care: Lessons learned to inform future maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–2012, Oxford: National Perinatal Epidemiology Unit, 2014. [Google Scholar]
- 6.NICE Clinical Guideline 63. Diabetes in pregnancy: Management of diabetes and its complications from preconception to the postnatal period, http://www.nice.org.uk/nicemedia/pdf/CG063Guidance.pdf (2008, accessed 29 September 2016).
- 7.Wahabi HA, Alzeidan RA, Bawazeer GA. Preconception care for diabetic women for improving maternal and fetal outcomes: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2010; 10: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Higgins S, McGuire BE, Mustafa E, et al. Barriers and facilitators to attending pre-pregnancy care services: The ATLANTIC-DIP experience. Diabet Med 2014; 31: 366–374. [DOI] [PubMed] [Google Scholar]
- 9.Mustafa E, Khalil S, Kirwan B, et al. A regional pre-pregnancy care (PPC) programme for women with Type 1 and Type 2 diabetes. Ir Med J 2012; 100: 11–13. [PubMed] [Google Scholar]
- 10.Nwolise CH, Carey N, Shawe J. Preconception care education for women with diabetes: A systematic review of conventional and digital health interventions. J Med Internet Res 2016; 18: e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes C, Spence D, Alderdice F, et al. Pre-conception care for women with diabetes: A public health issue. Br J Midwifery 2016; 24: 422–427. [Google Scholar]
- 12.Spence M, Alderdice FA, Harper R, et al. An exploration of knowledge and attitudes related to pre-pregnancy care in women with diabetes. Diabet Med 2010; 27: 1385–1391. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Preconception care of women with diabetes. Diabetes Care 2004; 27(Suppl 1): S76–S78. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2009; 32(Suppl 1): S13–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy HR, Roland JM, Skinner TC, et al. Effectiveness of a regional prepregnancy care program in women with Type 1 and Type 2 diabetes: Benefits beyond glycemic control. Diabetes Care 2010; 33: 2514–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yehuda I. Implementation of preconception care for women with diabetes. Diabetes Spectr 2016; 29: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Confidential enquiry into maternal and child health. Diabetes in pregnancy: Are we providing the best care? Findings of a national enquiry: England, Wales and Northern Ireland, London: CEMACH, 2007. [Google Scholar]
- 18.World Health Organization. Meeting to develop a global consensus on preconception care to reduce maternal and childhood mortality and morbidity, http://apps.who.int/iris/bitstream/10665/78067/1/9789241505000_eng.pdf (2013, accessed 31 December 2016).
- 19.Charron-Prochownik D, Ferons-Hannan M, Sereika S, et al. Randomized efficacy trial of early preconception counseling for diabetic teens (READY-Girls). Diabetes Care 2008; 31: 1327–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischl AF, Herman WH, Sereika SM, et al. Impact of a preconception counseling program for teens with Type 1 diabetes (READY-Girls) on patient-provider interaction, resource utilization, and cost. Diabetes Care 2010; 33: 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes VA, Spence M, McCance DR, et al. Evaluation of a DVD for women with diabetes: Impact on knowledge and attitudes to preconception care. Diabet Med 2012; 29: 950–956. [DOI] [PubMed] [Google Scholar]
- 22.Charron-Prochownik D, Fischl AF, Sereika SM, et al. Long-term effects of the booster-enhanced READY-Girls preconception counseling program on intentions and behaviors for family planning in teens with diabetes. Diabetes Care 2013; 36: 3870–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tripp N, Hainey K, Liu A, et al. An emerging model of maternity care: Smartphone, midwife, doctor? Women Birth 2014; 27: 64–67. [DOI] [PubMed] [Google Scholar]
- 24.Pew Research Center. Smartphone ownership and internet usage continues to climb in emerging economies, http://www.pewglobal.org/files/2016/02/pew_research_center_global_technology_report_final_february_22__2016.pdf (2016, accessed 31 December 2016).
- 25.Willcox CJ, Campbell KJ, McCarthy EA, et al. Testing the feasibility of a mobile technology intervention promoting healthy gestational weight gain in pregnant women (txt4two) – study protocol for a randomised controlled trial. Trials 2015; 16: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilliam ML, Martins SL, Bartlett E, et al. Development and testing of an iOS waiting room “app” for contraceptive counseling in a Title X family planning clinic. Am J Obstet Gynecol 2014; 211: 481.e1–481.e8. [DOI] [PubMed] [Google Scholar]
- 27.Carter MC, Burley VJ, Nykjaer C, et al. Adherence to a smartphone application for weight loss compared to website and paper diary: Pilot randomized controlled trial. J Med Internet Res 2013; 15: e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J, Freeman B, Li M. Can mobile phone apps influence people's health behavior change? An evidence review. J Med Internet Res 2016; 18: e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creswell JW, Plano Clark VL. Designing and conducting mixed methods research, 2nd ed Thousand Oaks, CA: Sage, 2011. [Google Scholar]
- 30.Creswell JW. Research design: Qualitative, quantitative and mixed methods approaches, International Student Edition, 4th ed Thousand Oaks, CA: Sage, 2014. [Google Scholar]
- 31.Bryman A. Integrating quantitative and qualitative research: How is it done? Qual Res 2006; 6: 97–113. [Google Scholar]
- 32.Bowling A. Research methods in health. Investigating health and health services, New York: Open University Press, 2009. [Google Scholar]
- 33.Babbie E. The Practice of social research, 13th international ed Canada: Wadsworth Centage Learning, 2013. [Google Scholar]
- 34.Murray M, Treweek S, Pope C, et al. Normalisation Process Theory: A framework for developing, evaluating and implementing complex interventions. BMC Med 2010; 8: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 2005; 4: 287–291. [Google Scholar]
- 36.National Institutes of Health (NIH). Quality assessment of controlled intervention studies, https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/rct (2014, accessed 12 May 2017).
- 37.Sanders EBN. Design research in 2006, http://www.maketools.com/articles-papers/DesignResearchin2006_Sanders_06.pdf. (2006, accessed 31 December 2016).
- 38.Sanders EBN, Stappers PJ. Probes, toolkits and prototypes: Three approaches to making in codesigning. CoDesign 2014; 10: 5–14. [Google Scholar]
- 39.Liem A. Human-centred design workshops in collaborative strategic design projects: An educational and professional comparison. Des Tech Educ Int J 2013; 18: 72–86. [Google Scholar]
- 40.Mummah SA, Robinson TN, King AC, et al. IDEAS (integrate, design, assess, and share): A framework and toolkit of strategies for the development of more effective digital interventions to change health behavior. J Med Internet Res 2016; 18: e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bean M. Laravel 5 essentials, 1st ed Birmingham: Packt Publishing Ltd, 2015. [Google Scholar]
- 42.Diabetes UK. Pregnancy & diabetes, https://www.diabetes.org.uk/ (2017, accessed 22 May 2017).
- 43.Family Planning Association (FPA): The sexual health charity. Help, advice and information for you, www.fpa.org.uk. (2017, accessed 22 May 2017).
- 44.Queens University Belfast, http://www.womenwithdiabetes.net/ (2017, accessed 22 May 2017).
- 45.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: Recommendations for good practice. J Eval Clin Pract 2004; 10: 307–312. [DOI] [PubMed] [Google Scholar]
- 46.Arain M, Campbell MJ, Cooper CL, et al. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol 2010; 10: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hibbard J, Mahoney E, Stockard J, et al. Development and testing of a short form of the patient activation measure. Health Serv Res 2005; 40(6 Pt 1): 1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hibbard J and Gilburt H. The King's Fund. Supporting people to manage their health: An introduction to patient activation, http://www.kingsfund.org.uk/sites/files/kf/field/field_publication_file/supporting-people-manage-health-patient-activation-may14.pdf. (2014, assessed 31 December 2016).
- 49.Charron-Prochownik D, Wang SL, Sereika SM, et al. A theory-based reproductive health and diabetes instrument. Am J Health Behav 2006; 30: 208–220. [DOI] [PubMed] [Google Scholar]
- 50.Hebden L, Cook A, van der Ploeg H, et al. Development of smartphone applications for nutrition and physical activity behavior change. JMIR Res Protoc 2012; 1: e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braun V, Clarke V. Successful qualitative research: A practical guide for beginners, London: Sage, 2013. [Google Scholar]
- 52.Bazely P, Jackson K. Qualitative data analysis with Nvivo, 2nd ed London: Sage, 2013. [Google Scholar]
- 53.Johnson K, Posner SF, Biermann J. Recommendations to improve preconception health and health care – United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep 2006; 55: 1–23. [PubMed] [Google Scholar]
- 54.Pal K, Eastwood SV, Michie S, et al. Computer-based interventions to improve self-management in adults with type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2014; 37: 1759–1766. [DOI] [PubMed] [Google Scholar]
- 55.Kirwan M, Vandelanotte C, Fenning A, et al. Diabetes self-management smartphone application for adults with type 1 diabetes: Randomized controlled trial. J Med Internet Res 2013; 15: e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: A meta-analysis. Diabet Med 2011; 28: 455–463. [DOI] [PubMed] [Google Scholar]
- 57.Whittaker R, Merry S, Dorey E, et al. A development and evaluation process for mHealth interventions: Examples from New Zealand. J Health Commun 2012; 17(Suppl 1): 11–21. [DOI] [PubMed] [Google Scholar]
- 58.United Nations. Transforming our world: The 2030 agenda for sustainable development, http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E (2015, accessed 31 December 2016).
- 59.Groshek MR, Oldenburg J, Sarasohn-Kahn J, et al. mHealth app essentials: Patient engagement, considerations, and implementation, http://www.himss.org/mhealth-app-essentials-patient-engagement-considerations-and-implementation (2015, accessed 31 December 2016).
- 60.Gartner. Fierce battle between Apple and Samsung to hold the No. 1 global smartphone ranking, www.gartner.com/newsroom/id/3609817 (2017, accessed 12 May 2017).