Abstract
Background
Cytokines are key immune mediators in physiological and disease processes, whose increased levels have been associated with the physiopathology of hematopoietic malignancies, such as myeloproliferative neoplasms.
Methods
This study examined the plasma cytokine profiles of patients with essential thrombocythemia, primary myelofibrosis, polycythemia vera and of healthy subjects, and analyzed correlations with JAK2 V617F status and clinical-hematological parameters.
Results
The proinflammatory cytokine levels were increased in myeloproliferative neoplasm patients, and the presence of the JAK2 V617F mutation was associated with high IP-10 levels in primary myelofibrosis patients.
Conclusions
Essential thrombocythemia, primary myelofibrosis, and polycythemia vera patients exhibited different patterns of cytokine production, as revealed by cytokine network correlations. Together, these findings suggest that augmented cytokine levels are associated with the physiopathology of myeloproliferative neoplasms.
Keywords: Ph-negative myeloproliferative neoplasms; Inflammation, Plasma cytokines; JAK2 V617F
Introduction
Myeloproliferative neoplasms (MPN) are hematological disorders characterized by increased proliferation and accumulation of mature myeloid cells in the bone marrow and peripheral blood. MPN are classified as Philadelphia (Ph) chromosome-positive or -negative. The present investigation focusses on three Ph-negative MPN: essential thrombocythemia (ET), primary myelofibrosis (PMF), and polycythemia vera (PV).1, 2 These MPN are clonal disorders that result from malignant transformations of hematopoietic stem cells, which raise the number of mature myeloid blood cells, especially megakaryocytes/platelets in ET, erythrocytes in PV, and both megakaryocytes/platelets and granulocytes in PMF. Nearly 10–15% of ET, PMF, and PV patients progress to acute myeloid leukemia.1, 2
Constitutive activation of the Janus kinase 2 (JAK2)/signal transducer and activator of transcription (STAT) signaling pathway due to acquired somatic mutations in the JAK2, calreticulin or MPL genes may drive the course of MPN. The JAK2 V617F mutation, which is detected in more than 90% of PV patients and in about half of ET and PMF patients, is located in the pseudokinase domain of the JAK2 protein and elicits a ‘pre-activation state’.3, 4 This mutation seems to confer the advantage of survival and proliferation to myeloid hematopoietic cells independently of stimulatory signals, leading to clonal expansion of myeloid progenitors and mature cells.4 In MPN, the hematopoietic cells display an exacerbated response to cytokines and growth factors such as erythropoietin, granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 3 (IL-3), and insulin-like growth factor-1 (IGF-1).5 Autocrine stimulation may occur due to either defective cytokine receptors or constitutive activation of the JAK2, STAT5, phosphoinositide 3-kinase (PI3K), signal-regulated kinases extracellular (ERK), and protein kinase B (PKB) signaling pathways.5
MPN are considered onco-inflammatory disorders. The concept of ‘onco-inflammation’ proposes a crosstalk between cancer cells and their inflammatory microenvironment suggesting that this plays a relevant role in the initiation and progression of cancers.6, 7 MPN are characterized by abnormal immune system activity, increased monocyte/macrophage compartment, expansion of myeloid-derived suppressor cells, abnormal frequency of regulatory T cells, and dysfunction of natural killer and T CD4+ cells.8 In line with this concept, chronic inflammation in MPN has been described as a potential facilitator for premature atherosclerosis development and clonal evolution to acute myeloid leukemia.9 In addition, fibrotic transformation, commonly found in PMF patients and in myelofibrosis secondary to ET and PV, seems to be associated with an exacerbated inflammation status: cytokines secreted by bone marrow cells, in particular megakaryocytes, probably activate endothelial cells and fibroblasts in the bone marrow microenvironment, contributing to the establishment of myelofibrosis.10
Elevated levels of cytokines have also been described in other hematological malignancies including chronic myeloid leukemia (CML), non-Hodgkin lymphoma, multiple myeloma and myelodysplastic syndromes.11, 12, 13, 14, 15, 16 Moreover, the high plasmatic levels of inflammatory cytokines are associated with the response of CML patients to tyrosine-kinase inhibitors and with the prognosis of the aforementioned hematological malignancies.11, 12, 13, 14, 15, 16
Considering the critical role that cytokines play in different hematological diseases and the contribution of inflammation to cancer development, the present investigation examined the differences between the cytokine profiles of ET, PMF and PV patients and analyzed their correlation with the patients’ clinical laboratory data.
Methods
Ethical approval
The Ethics Committee for Human Research of the Faculdade de Ciências Farmacêuticas de Ribeirão Preto (FCFRP-USP) and Hospital das Clínicas, Faculdade de Medicina de Ribeirão Preto (HC-FMRP-USP), Universidade de São Paulo, Ribeirão Preto, SP, Brazil, approved the study protocol (number 348). All the patients and healthy subjects signed informed written consent forms in compliance with the guidelines established by the Brazilian National Health Council (law 466/2012).
Blood samples
This study enrolled 11 ET patients, 16 PMF patients, and 20 PV patients without any prior treatment, consulted at HC-FMRP-USP and at Hospital Estadual de Transplantes Euryclides de Jesus Zerbini (São Paulo, SP, Brazil) and 34 healthy subjects (Control Group; CTRL) from the community of Ribeirão Preto.
Peripheral blood was collected from patients and healthy subjects using EDTA tubes (Vacutainer®; Becton, Dickinson and Company). The plasma samples were obtained after blood centrifugation at 400 × g for 10 min at 4 °C (Eppendorf 5810R Centrifuge), and stored at −80 °C until analysis. Patient's samples were collected at diagnosis, without any previous therapy. The MPN were classified using the World Health Organization (WHO) 2016 classification.17
Analysis of hematological parameters
The Red blood cell count (RBC), white blood cell count (WBC), platelet count, hemoglobin concentration (Hb), hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and red cell distribution width (RDW) were determined using the automatic ABX Micros 60 equipment (HORIBA ABX SAS).
Measurement of cytokines and chemokines
A customized multiplex assay kit was used to determine the plasma levels of: granulocyte-macrophage colony stimulating factor (GM-CSF), interferon (IFN)-α2, IFN-γ, IFN γ-induced protein 10 (IP-10), monocyte chemoattractant protein (MCP)-1, MIP-1α, MIP-1β, regulated on activation normal T cells expressed and secreted (RANTES), tumor necrosis factor (TNF)-α, and the interleukins IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12p70, and IL-17A (16-plex, EMD Millipore Corporation, MA, USA). Fluorescence was recorded in a fluorescent bead-based plate reader (Luminex1 MAGPIX1 System; Luminex Corporation, TX, USA). Data were analyzed using the Milliplex Analyst software v3.5 (Millipore; VigeneTech Inc., Boston, MA, USA), and a three-parameter logistic curve was fit.
Data analyses
Conventional statistical analyses were performed using GraphPad Prism 5.0 software (Graph-Pad Software, San Diego, CA, USA). Considering the nonparametric nature of all data sets, the Mann–Whitney test was used to compare the experimental groups, and the Spearman correlation test was used to analyze the correlation between cytokine/chemokine levels and the hematological parameters and clinical data. Statistical differences were considered significant when p-values were <0.05.
The correlations of cytokine/chemokine levels were compiled using the open source software Cytoscape (version 2.8; http://www.cytoscape.org).16 The networks were constructed using a circular layout, where the edges display the score of the correlation index (r) as negative (r < 0), weak (r ≤ 0.35), moderate (0.36 ≥ r ≤ 0.67) or strong (r ≥ 0.68).18
Additionally, the cytokine profile of each individual was analyzed. First, all data from each cytokine/chemokine were used to calculate the overall median value, which was used as a cut-off point to classify each individual as a ‘high’ or ‘low’ producer of a given cytokine/chemokine. Next, the data were organized in black-and-white scale diagrams to calculate the frequency of high producers in each clinical group. Relevant data (≥50%), previously characterized by Luiza-Silva et al.19 were highlighted in both bold and underline format. Radar charts were built using Microsoft Excel (Microsoft Office 2013, Las Vegas, USA) to characterize the overall frequency of individuals expressing high levels of each cytokine/chemokine in each study group.
The analysis of overall cytokine profile was assessed to characterize the general cytokine pattern of each group as previously suggested by Vitelli-Avelar et al.20 but modified as follows: the percentages of cytokine/chemokine production were compiled using a four step platform, in this manner:
-
(i)
Conventional statistical analysis;
-
(ii)
Establishment of the overall median of cytokine/chemokine production for each group;
-
(iii)
Classification of each group as low or high cytokine-producer using the overall median values as the cut-off point;
-
(iv)
Creation of black-and-white scale diagrams for each group.
These approaches have been shown as relevant to detect, with high sensitivity, minor putative changes in the immunological profiles that are not detectable by conventional statistical approaches.19, 21, 22
Results
Clinical and demographic characteristics of patients and controls
The median ages were similar in the ET (54 years), CTRL (57.2 years), PV (66 years) and PMF (64 years) Groups. The gender distribution in the CTRL Group was 42% male and 58% female, in the ET Group it was 37% male and 63% female, in PMF it was 75% male and 25% female and in the PV Group it was 55% male and 45% female (Table 1). The JAK2 V617F mutation was detected in 36%, 68%, and 100% of ET, PMF, and PV patients, respectively (Table 1).
Table 1.
Hematological parameters and mutational status of patients and demographic characteristics of controls and patients.
| CTRL | ET | PMF | PV | |
|---|---|---|---|---|
| Age (years) – n (range) | 57.2 (31–83) | 54 (20–81) | 65 (44–80) | 63 (39–83) |
| Gender – n (%) | ||||
| Male | 14 (42) | 5 (55) | 13 (76) | 9 (39) |
| Female | 20 (58) | 4 (44) | 4 (23) | 14 (60) |
| JAK2 V617F carriers – n (%) | – | 5 (55) | 12 (70) | 17 (74) |
| Hemoglobin (g/dL) – n (range) | – | 13.01 (10.1–17.1) | 11.47 (5.88–17.4) | 14.95 (9.9–21.5) |
| Platelets (× 103/μL) – n (range) | – | 818 (399–1293) | 334 (38.5–1474) | 425 (161–772) |
| WBC (× 106/L) – n (range) | – | 9.02 (3.96–15.6) | 11.71 (1.46–31.4) | 10.21 (3.6–21.3) |
CTRL: control (healthy subjects); ET: essential thrombocythemia; PMF: primary myelofibrosis; PV: polycythemia vera; WBC: white blood cell count.
Essential thrombocythemia, primary myelofibrosis, and polycythemia vera present elevated levels of inflammatory cytokines/chemokines
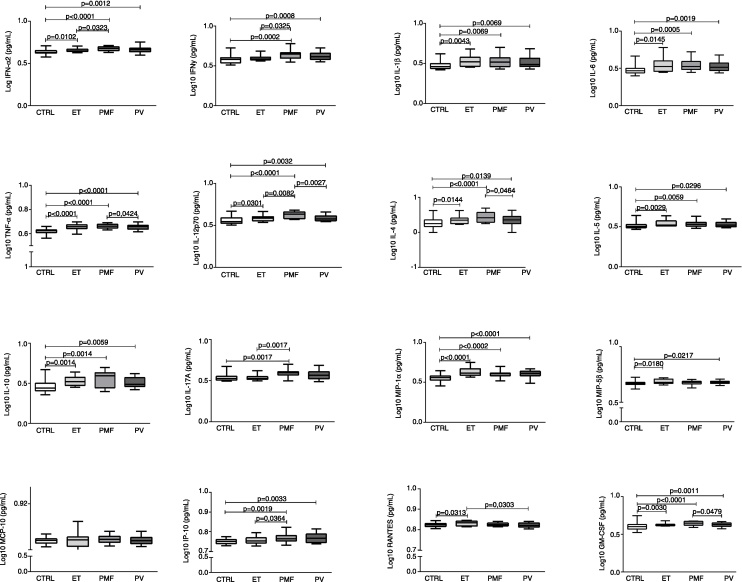
The immune profiles of ET, PMF, and PV patients were analyzed by determining the plasma levels of 16 cytokines and chemokines (Figure 1). Compared with the control group, the three MPN patient groups exhibited elevated levels of GM-CSF, IL-1β, IL-4, IL-5, IL-6, IL-10, IFN-α2, MIP-1α, IL-12p70 and TNF-α. PMF patients also exhibited an increased level of IL-17A compared to the CTRL Group, increased levels of IFN-γ, IL-12p70, IL-17A and IP-10 compared to ET patients and increased levels of IL-12p70, IL-4 and GM-CSF compared to PV patients. ET patients exhibited an increased level of RANTES compared to the CTRL and PV Groups as well as an increased level of MIP-1β compared to the CTRL Group. PV patients had a higher level of MIP-1β compared to the CTRL Group. The MCP-1 level was similar among the four study groups (Figure 1).
Figure 1.
Cytokine and chemokine plasma levels in patients with myeloproliferative neoplasms and healthy subjects. The plasma levels of GM-CSF, IFN-α, IFN-γ, IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-17A, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES, and TNF-α were measured in healthy subjects (CTRL; n = 34) and patients with essential thrombocythemia (ET; n = 11), primary myelofibrosis (PMF; n = 16), and polycythemia vera (PV; n = 20). Statistical differences are represented in each graph (p < 0.05; Mann–Whitney test).
JAK2 V617F mutation status is associated with an increased level of IP-10 in primary myelofibrosis
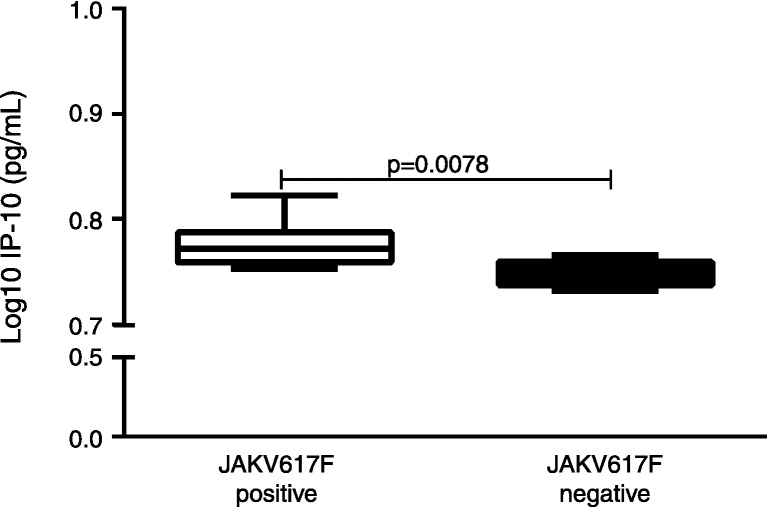
Considering the pivotal role of the JAK2 V617F mutation in the physiopathology of MPN,23 we evaluated the potential association of the JAK2 V617F mutation and the altered cytokine/chemokine levels. Compared with PMF patients negative for the JAK2 V617F mutation, JAK2 V617F-positive PMF patients exhibited an increased level of IP-10 (p = 0.0078; Figure 2). There was no association between the JAK2 V617F mutation status and the cytokine/chemokine levels in ET patients.
Figure 2.
The JAK2 V617F status influences cytokine production in primary myelofibrosis (PMF) patients. The IP-10 pro-inflammatory chemokine plasma level is increased in JAK2 V617F-positive PMF patients (n = 5) compared with JAK2 V617F-negative PMF patients (n = 11) (p < 0.05; Mann–Whitney test).
Alteration of the cytokine/chemokine biomarker network in myeloproliferative neoplasm patients
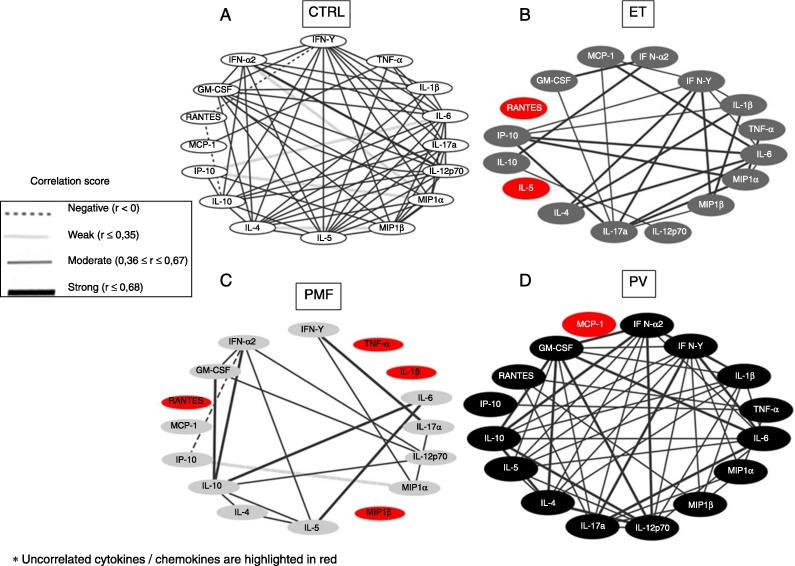
To analyze the strength of correlation between the levels of the studied cytokines/chemokines in the CTRL Group and in the three MPN patient groups, we designed a network using Spearman's correlation test (Figure 3). The network of the CTRL Group presented strong connections between IL-12p70, IFN-α2, MIP-1α, and MIP-1β and weak interactions between IL-4, IL-6, IFN-α2, IP-10, MIP-1α, and MIP-1β; and negative correlations between IL-10, IFN-γ and RANTES (Figure 3A).
Figure 3.
Systemic interactions between immunological biomarkers is abnormal in patients with myeloproliferative neoplasms. The network analysis shows significant correlations (p < 0.05) between cytokines and chemokines in healthy subjects (CTRL, Panel A) and in patients with primary myelofibrosis (PMF; Panel B), polycythemia vera (PV; Panel C), and essential thrombocythemia (ET; Panel D). The ‘r’ values obtained after the Spearman's correlation analysis were used to determine the correlation strength: negative (r < 0), weak (r ≤ 0.35), moderate (0.36 ≤ r ≤ 0.67), or strong (r ≥ 0.68).
The ET patient network displayed many strong connections involving GM-CSF, IL-4, IL-6, IL-10, IL-17A, IFN-α2, IL-1β, TNF-α, MIP-1α, MIP-1β, IP-10 and IFN-γ; (Figure 3B). In contrast to ET and CTRL, the PMF patient network showed very few connections: strong interactions involving IFN-γ, IL-6, IL-17A, GM-CSF, IL-6, IL-10, and IFN-α2. The PMF patients also presented a weak correlation between MIP-1α and IP-10 and a negative correlation between GM-CSF and IP-10 (Figure 3C). The PV patient network displayed strong interactions involving GM-CSF, IL-4, IL-6, IL-10, IL-12p70, IL-17A, IFN-γ and IFN-α2 (Figure 3D).
Myeloproliferative neoplasm patients presented an inflammatory status
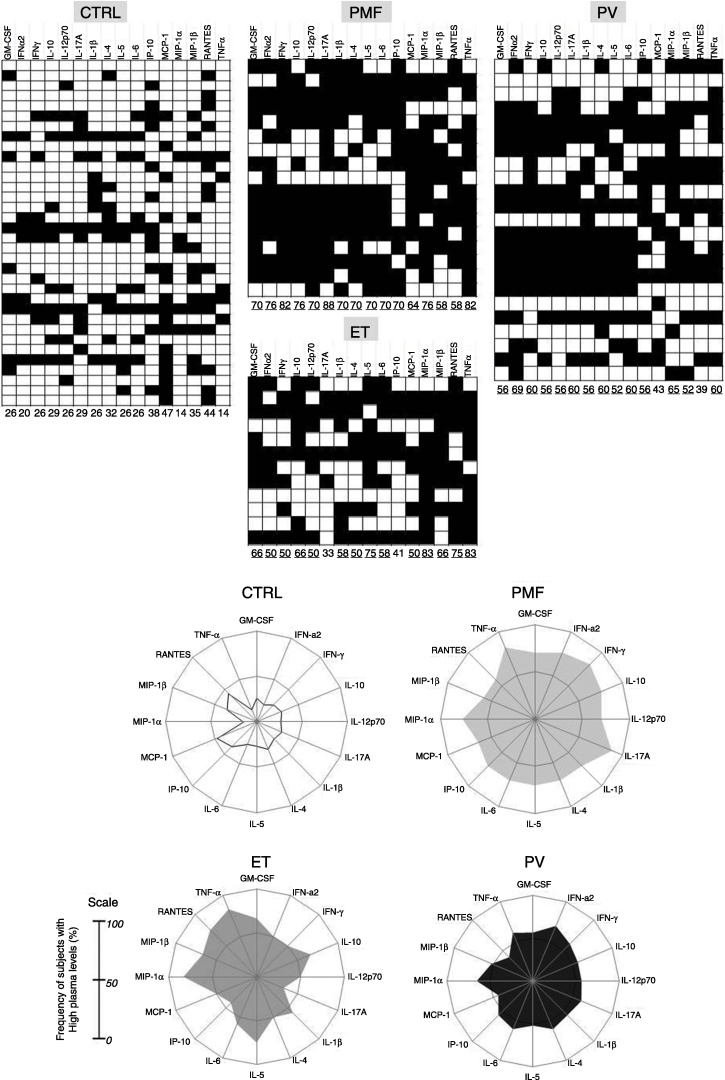
To better understand and characterize the relationship between the different MPN patient groups and their respective immune profiles, categorical analysis was performed and the data compiled in black-and-white scale diagrams (Figure 4A). For this purpose, the overall median for each cytokine/chemokine subset was calculated taking the whole range of values obtained from the CTRL, PV, ET and PMF Groups. The overall median percentage of each cytokine/chemokine was used as the cut-off point to group individuals into two categories, low and high producers. The results indicate that the CTRL Group was composed of low cytokine/chemokine producers, but the three MPN patient groups exhibited the opposite profile: they presented high frequencies of high producers of most cytokines/chemokines; this finding was more evident in PMF patients.
Figure 4.
Immune profile of healthy subjects (CTRL) and patients with primary myelofibrosis (PMF), polycythemia vera (PV), and essential thrombocythemia (ET). (A) Plasma levels of GM-CSF, IFN-α, IFN-γ, IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-17A, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES, and TNF-α were used for categorical classification of CTRL, PMF, PV and ET groups as high or low producers of a given cytokine/chemokine. Black and white blocks represent high and low producers of each cytokine/chemokine, respectively. Each column represents a cytokine/chemokine, and each block represents each individual's cytokine/chemokine production pattern. The numbers below each column represent the frequency of high producers of the cytokine/chemokine tested.
(B) Radar chart representation of the immune profile of healthy subjects (CTRL) and patients with essential thrombocythemia (ET), primary myelofibrosis (PMF), and polycythemia vera (PV). Radar charts summarize the percentage of high producers of each cytokine/chemokine in the study groups. The plasma levels of GM-CSF, IFN-α, IFN-γ, IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-17A, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES, and TNF-α were used for categorical classification of the CTRL, PMF, PV, and ET groups as high and low producers of a given cytokine/chemokine.
Subsequently, radar charts were plotted to compare the overall cytokine/chemokine profile of the high cytokine/chemokine producers in the four study groups (Figure 4B). We considered a cytokine/chemokine to be relevant in the disease process when the frequency of high producers was greater than 50%.19 In the CTRL Group, the frequencies of high cytokine/chemokine producers for all the cytokines/chemokines evaluated were less than 50%. The ET patients presented high production frequencies for 12 of the 16 cytokines/chemokines analyzed. The PMF and PV patient groups exhibited the highest frequencies among all the study groups, showing high cytokine/chemokine productions for 16 and 14, respectively of the 16 cytokines/chemokines tested.
Inflammatory cytokine levels and hematological parameters are correlated in myeloproliferative neoplasms
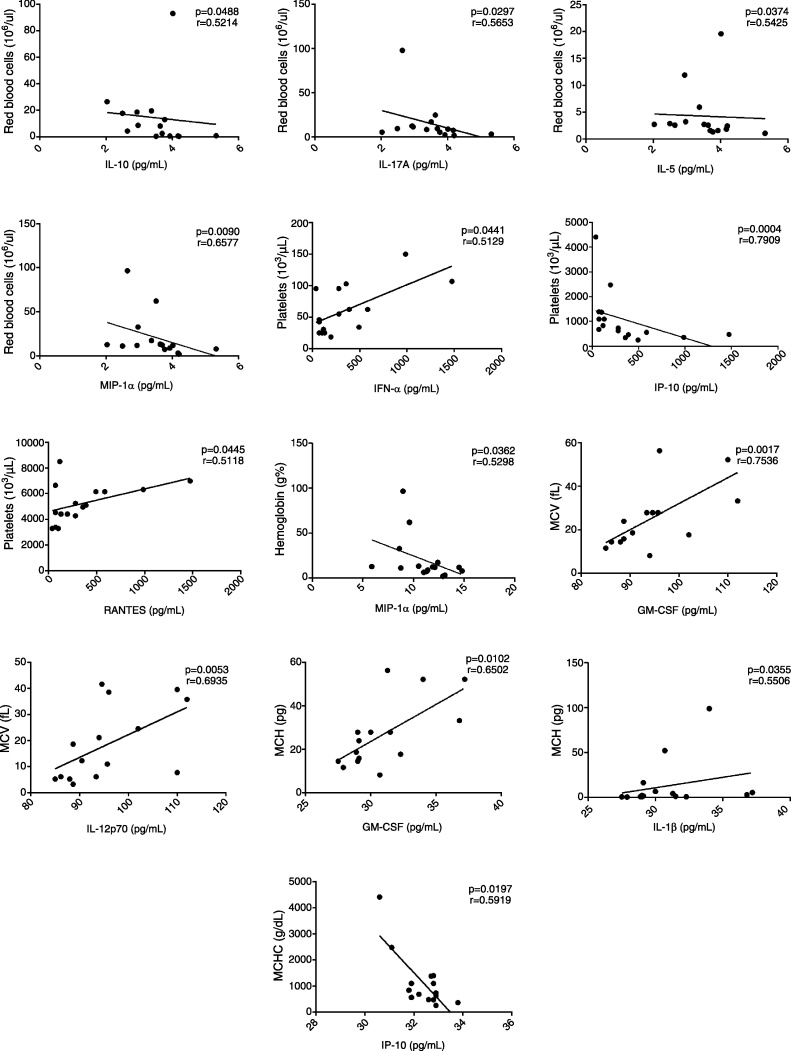
This study tested if the cytokine/chemokine levels correlate to hematological parameters in MPN patients. In PMF patients (Figure 5), GM-CSF and IL-12p70 levels correlated positively with MCV; GM-CSF and IL-1β levels correlated positively with MCH; and RANTES, IP-10 and IFN-α levels correlated positively with the platelet count. IP-10 levels correlated positively with MCHC, IP-10, IL-17A, IL-5 and MIP-1α levels were negatively correlated with RBC, while MIP-1α positively correlated with Hb.
Figure 5.
Correlation between cytokine/chemokine plasma levels and hematological parameters in primary myelofibrosis patients (n = 16). The figure depicts only the significant correlations (p < 0.05; non-parametric Spearman's correlation).
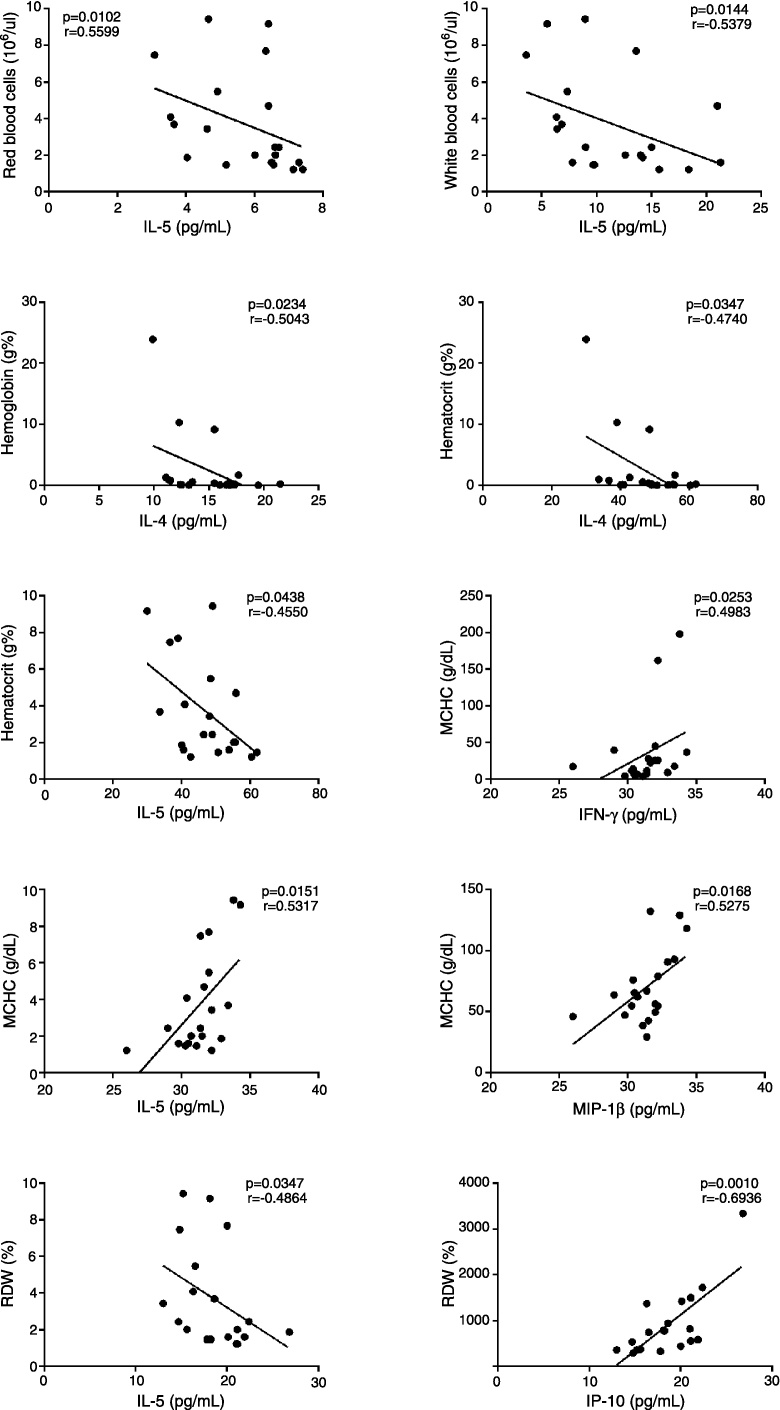
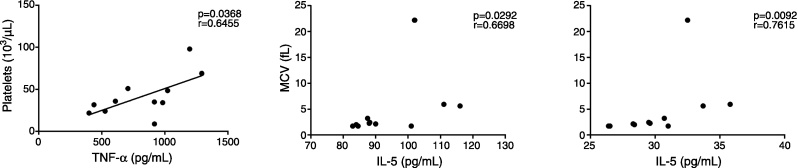
In PV patients (Figure 6), RDW correlated positively to IP-10 levels and negatively with IL-5 levels. IL-5 levels also correlated negatively to RBC, WBC, Hb and MCHC. IL-4 levels correlated negatively to Hb and hematocrit and MCHC positively correlated to IFN-γ and MIP-1β. In ET patients (Figure 7), IL-5 levels correlated positively to MCV and MCH and TNF-α correlated positively to platelet counts.
Figure 6.
Correlation between cytokine/chemokine plasma levels and hematological data in polycythemia vera patients (n = 20). The figure depicts only the significant correlations (p < 0.05; non-parametric Spearman's correlation).
Figure 7.
Correlation between cytokine/chemokine plasma levels and hematological data in essential thrombocythemia patients (n = 11). The figure depicts only the significant correlations (p < 0.05; non-parametric Spearman's correlation).
Discussion
The hallmarks of immune deregulation and onco-inflammation have been reported mostly in solid cancer patients, but also identified in some MPN patients.24 Increased levels of cytokines in PV and ET patients have indicated that they play a critical role in the physiopathology of MPN and so the measurement of their plasma levels might be useful for the clinical and therapeutic stratification of patients.25 This study assessed the different patterns of cytokine/chemokine expression in patients with three types of MPN (ET, PMF, and PV), their correlations with hematological parameters, associations with mutation status and interactions within networks.
Elevated plasma levels of several cytokines and chemokines were detected in ET, PMF and PV patients compared to healthy subjects. This finding indicates that an inflammatory process may be involved in the physiopathology of MPN, as cytokines and chemokines act in autocrine, paracrine and endocrine manners, and may even affect the hematopoietic niche. The levels of two regulatory cytokines, IL-4 and IL-10, were increased in all patient groups, which suggests an attempt to counterbalance the excessive pro-inflammatory response. In addition, the levels of cytokine/chemokine expression differed between the three MPN groups. For instance, PMF patients exhibited higher plasma levels of IFN-γ, IL-12p70 and IL-17A than ET patients and higher plasma levels of TNF-α, IL-4, IL12-p70 and GM-CSF than PV patients. ET patients exhibited higher plasma levels of RANTES than CTRLs and PV, as well as higher levels of MIP-1β than PV patients. IL-12 and IL-17 are pro-inflammatory cytokines that participate in both innate and adaptive immune responses and whose altered levels have been correlated to the physiopathology of various diseases, including cancer.26, 27, 28 RANTES, an important chemokine associated with cancer progression and metastasis, acts as a growth factor, stimulates angiogenesis and participates in immune evasion mechanisms.29, 30 In this context, the increased levels of IL-12p70, IL-17A and RANTES in PMF patients suggest that they bear a more intense inflammatory process than ET or PV patients do.
The increased production of IP-10 in JAK2 V617F-positive PMF patients indicates a pro-inflammatory profile, as this cytokine, which is inducible by pro-inflammatory stimuli such as IFN-γ and TNF-α, presents pro-inflammatory properties.31 High levels of IP-10 seem to contribute to the pathogenesis of many organ-specific autoimmune diseases (such as autoimmune thyroiditis, type 1 diabetes, Graves’ disease and ophthalmopathy) and systemic autoimmune diseases (such as rheumatoid arthritis, systemic sclerosis, psoriatic arthritis, systemic lupus erythematosus and Sjögren syndrome), besides being an important chemokine involved in the alloimmune response against kidney allografts.32, 33
The MPN driven JAK2 V617F mutation stimulates the JAK-STAT3/5 pathway to boost inflammatory cytokine production through autocrine and paracrine mechanisms.34 Positivity for the JAK2 V671F mutation is associated with leukocyte and platelet activation, and represents a thrombogenic factor in MPN.35, 36 Little is known about the cytokine deregulation associated with thromboembolic events in MPN and their potential predictive values.10 Inflammation seems to be the major contributor to the prothrombotic state in MPN, as well as to bone marrow fibrosis, osteosclerosis, and angiogenesis.30 MPN patients also exhibit a massive upregulation of IFN-related genes, particularly the IFN-inducible gene IFI27, and severe deregulation of other inflammation and immune genes, as demonstrated by the whole-blood transcriptional profiling of several immune cells (granulocytes, monocytes, B cells, T cells, and platelets).37
Ho et al., using human cytokine array membranes combined to individual assets of enzyme-linked immunosorbent assay, enhanced chemiluminescence and high-throughput of microspot, evaluated plasmatic levels of 79 cytokines in controls and naïve PMF patients and described higher tissue inhibitor levels related to tissue inhibitor of matrix metalloproteinase-1 (TIMP-1), macrophage inflammatory protein-1β (MIP-1β), and insulin-like growth factor binding factor-2 (IGFBP-2).38 Differently from the current work they did not investigate these 79 cytokine plasmatic levels in PV and ET patients.39
Vaidya et al. showed by multivariable analysis and multiplex bead-based assaying that the elevated plasmatic levels of 13 cytokines (IL-1β, IL-4, EGF, IL-5, IL-7, IL-10, IL-17, TNF-α, IFN-α, GM-CSF, MIP-1β, MIP-1β and MCP-1) was associated with low overall survival in a group of 65 PV and 127 PMF patients. Moreover, similar to the current study, they showed that PMF and PV patients present different patterns of cytokine production.39
In the same context, Tefferi et al., using a multiplex biometric sandwich immunoassay and multivariable analysis reported increased levels of IL-1β, IL-1RA, IL-2R, IL-6, IL-8, IL-10, IL-12, IL-13, IL-15, TNF-α, G-CSF, IFN-α, MIP-1α, MIP-1β, HGF, IFN-γ–IP-10, monokines induced by IFN-γ (MIG), MCP-1 and vascular endothelial growth factor (VEGF) and decreased levels of IFN-γ in PMF patients compared to controls. The cytokine levels were associated with shorter survival in PMF patients.40
These authors studied a higher number of patients than the present study; however, they did not associate the patients’ mutation status and hematological parameters with the cytokine profile and did not compare the cytokine levels between the different MPN patient groups. The present study evaluated, 16 plasmatic cytokines/chemokines in ET, PMF, and PV patients at diagnosis using a multiplex bead-based assay to identify a disease signature/network and to associate it to the mutation status and hematological parameters. This study found that the studied MPN patients presented dysfunctional cytokine/chemokine production patterns characterized by high frequencies of ‘high’ cytokine/chemokine producers compared to healthy subjects. Furthermore, the correlation between the cytokine/chemokine levels and hematological parameters indicates that immune dysregulation may affect the production of hematological cells and favor their exacerbated proliferation.
Taken altogether, the data reported herein demonstrated that (i) the plasma cytokine/chemokine profile of ET, PMF, and PV patients are altered, which is most evident in PMF patients; and (ii) the JAK2 V617F status influences the cytokine/chemokine profile in PV patients. Our findings emphasize the overproduction of cytokines and chemokines in MPN patients, culminating in a pro-inflammatory state.
The increased levels of pro-inflammatory cytokines are associated with worse prognosis and shorter survival in MPN patients. Therapies targeting the pro-inflammatory cytokine profile seem to improve the quality of life and life expectancy of MPN patients. In this context, the JAK2 inhibitor ruxolutinib acts by decreasing the production of pro-inflammatory cytokines (inhibiting the cytokine gene transcription), thereby reducing the constitutional symptoms and splenomegaly (35%) in PMF patients.41, 42 However, the JAK2 inhibitor potential against cytokine production in this therapy is not curative and does not impair MPN progression to leukemia. Thus, we must continue to study the molecular mechanisms involved in MPN pathogenesis to search for new efficient therapies for these disorders.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
The authors wish to thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (grant n. 2014/04234-9, 2016/03265-3 and 2015/21237-4), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for their financial support.
References
- 1.James C., Ugo V., Le Couédic J.P., Staerk J., Delhommeau F., Lacout C. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A. Myeloproliferative neoplasms: a decade of discoveries and treatment advances. Am J Hematol. 2016;91(1):50–58. doi: 10.1002/ajh.24221. [DOI] [PubMed] [Google Scholar]
- 3.Levine R.L., Gilliland D.G. Myeloproliferative disorders. Blood. 2008;112(6):2190–2198. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speletas M., Katodritou E., Daiou C., Mandala E., Papadakis E., Kioumi A. Correlations of JAK2-V617F mutation with clinical and laboratory findings in patients with myeloproliferative disorders. Leuk Res. 2007;31(8):1053–1062. doi: 10.1016/j.leukres.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Vainchenker W., Constantinescu S.N. A unique activating mutation in JAK2 (V617F) is at the origin of polycythemia vera and allows a new classification of myeloproliferative diseases. Hematology Am Soc Hematol Educ Program. 2005:195–200. doi: 10.1182/asheducation-2005.1.195. [DOI] [PubMed] [Google Scholar]
- 6.Landskron G., De la Fuente M., Thuwajit P., Thuwajit C., Hermoso M.A. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rachidi S.M., Qin T., Sun S., Zheng W.J., Li Z. Molecular profiling of multiple human cancers defines an inflammatory cancer-associated molecular pattern and uncovers KPNA2 as a uniform poor prognostic cancer marker. PLoS ONE. 2013;8(3):e57911. doi: 10.1371/journal.pone.0057911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barosi G. An immune dysregulation in MPN. Curr Hematol Malig Rep. 2014;9(4):331–339. doi: 10.1007/s11899-014-0227-0. [DOI] [PubMed] [Google Scholar]
- 9.Hasselbalch H.C. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood. 2012;119(14):3219–3225. doi: 10.1182/blood-2011-11-394775. [DOI] [PubMed] [Google Scholar]
- 10.Mondet J., Hussein K., Mossuz P. Circulating cytokine levels as markers of inflammation in Philadelphia negative myeloproliferative neoplasms: diagnostic and prognostic interest. Mediat Inflamm. 2015;2015:670580. doi: 10.1155/2015/670580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stasi R., Zinzani L., Galieni P., Lauta V.M., Damasio E., Dispensa E. Clinical implications of cytokine and soluble receptor measurements in patients with newly-diagnosed aggressive non-Hodgkin's lymphoma. Eur J Haematol. 1995;54(1):9–17. doi: 10.1111/j.1600-0609.1995.tb01619.x. [DOI] [PubMed] [Google Scholar]
- 12.Niitsu N., Iijima K., Chizuka A. A high serum-soluble interleukin-2 receptor level is associated with a poor outcome of aggressive non-Hodgkin's lymphoma. Eur J Haematol. 2001;66(1):24–30. doi: 10.1034/j.1600-0609.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 13.Li Z.C., Sun M.D., Zheng Y.Q., Fu H.J. The low expression of IL-37 involved in multiple myeloma-associated angiogenesis. Med Sci Monit. 2016;22:4164–4168. doi: 10.12659/MSM.897451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsirakis G., Roussou P., Pappa C.A., Kolovou A., Vasilokonstantaki C., Miminas I. Increased serum levels of MIP-1alpha correlate with bone disease and angiogenic cytokines in patients with multiple myeloma. Med Oncol. 2014;31(1):778. doi: 10.1007/s12032-013-0778-2. [DOI] [PubMed] [Google Scholar]
- 15.Serio B., Risitano A., Giudice V., Montuori N., Selleri C. Immunological derangement in hypocellular myelodysplastic syndromes. Transl Med UniSa. 2014;8:31–42. [PMC free article] [PubMed] [Google Scholar]
- 16.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 18.Taylor R. Interpretation of the correlation coefficient: a basic review. J Diagn Med Sonogr. 1990:35–39. [Google Scholar]
- 19.Luiza-Silva M., Campi-Azevedo A.C., Batista M.A., Martins M.A., Avelar R.S., da Silveira Lemos D. Cytokine signatures of innate and adaptive immunity in 17DD yellow fever vaccinated children and its association with the level of neutralizing antibody. J Infect Dis. 2011;204(6):873–883. doi: 10.1093/infdis/jir439. [DOI] [PubMed] [Google Scholar]
- 20.Vitelli-Avelar D.M., Sathler-Avelar R., Teixeira-Carvalho A., Pinto Dias J.C., Gontijo E.D., Faria A.M. Strategy to assess the overall cytokine profile of circulating leukocytes and its association with distinct clinical forms of human Chagas disease. Scand J Immunol. 2008;68(5):516–525. doi: 10.1111/j.1365-3083.2008.02167.x. [DOI] [PubMed] [Google Scholar]
- 21.Coelho-dos-Reis J.G., Passos L., Duarte M.C., Araújo M.G., Campi-Azevedo A.C., Teixeira-Carvalho A. Immunological profile of HTLV-1-infected patients associated with infectious or autoimmune dermatological disorders. PLoS Negl Trop Dis. 2013;7(7):e2328. doi: 10.1371/journal.pntd.0002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guimarães A.G., da Costa A.G., Martins-Filho O.A., Pimentel J.P., Zauli D.A., Peruhype-Magalhães V. CD11c+CD123Low dendritic cell subset and the triad TNF-α/IL-17A/IFN-γ integrate mucosal and peripheral cellular responses in HIV patients with high-grade anal intraepithelial neoplasia: a systems biology approach. J Acquir Immune Defic Syndr. 2015;68(2):112–122. doi: 10.1097/QAI.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 23.Them N.C., Kralovics R. Genetic basis of MPN: beyond JAK2-V617F. Curr Hematol Malig Rep. 2013;8(4):299–306. doi: 10.1007/s11899-013-0184-z. [DOI] [PubMed] [Google Scholar]
- 24.Hasselbalch H. Idiopathic myelofibrosis: a clinical study of 80 patients. Am J Hematol. 1990;34(4):291–300. doi: 10.1002/ajh.2830340411. [DOI] [PubMed] [Google Scholar]
- 25.Pourcelot E., Trocme C., Mondet J., Bailly S., Toussaint B., Mossuz P. Cytokine profiles in polycythemia vera and essential thrombocythemia patients: clinical implications. Exp Hematol. 2014;42(5):360–368. doi: 10.1016/j.exphem.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi M., Fitz L., Ryan M., Hewick R.M., Clark S.C., Chan S. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gately M.K., Renzetti L.M., Magram J., Stern A.S., Adorini L., Gubler U. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 28.Qian X., Chen H., Wu X., Hu L., Huang Q., Jin Y. Interleukin-17 acts as double-edged sword in anti-tumor immunity and tumorigenesis. Cytokine. 2017;89:34–44. doi: 10.1016/j.cyto.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Hanahan D., Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 30.Kershaw M.H., Westwood J.A., Darcy P.K. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13(8):525–541. doi: 10.1038/nrc3565. [DOI] [PubMed] [Google Scholar]
- 31.Gotsch F., Romero R., Friel L., Kusanovic J.P., Espinoza J., Erez O. CXCL10/IP-10: a missing link between inflammation and anti-angiogenesis in preeclampsia? J Matern Fetal Neonatal Med. 2007;20(11):777–792. doi: 10.1080/14767050701483298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antonelli A., Ferrari S.M., Giuggioli D., Ferrannini E., Ferri C., Fallahi P. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev. 2014;13(3):272–280. doi: 10.1016/j.autrev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Raza A., Firasat S., Khaliq S., Aziz T., Mubarak M., Naqvi S.A. The association of urinary interferon-gamma inducible protein-10 (IP10/CXCL10) levels with kidney allograft rejection. Inflamm Res. 2017;66(5):425–432. doi: 10.1007/s00011-017-1025-7. [DOI] [PubMed] [Google Scholar]
- 34.Koschmieder S., Mughal T.I., Hasselbalch H.C., Barosi G., Valent P., Kiladjian J.J. Myeloproliferative neoplasms and inflammation: whether to target the malignant clone or the inflammatory process or both. Leukemia. 2016;30(5):1018–1024. doi: 10.1038/leu.2016.12. [DOI] [PubMed] [Google Scholar]
- 35.Vannucchi A.M., Antonioli E., Guglielmelli P., Pardanani A., Tefferi A. Clinical correlates of JAK2 V617F presence or allele burden in myeloproliferative neoplasms: a critical reappraisal. Leukemia. 2008;22(7):1299–1307. doi: 10.1038/leu.2008.113. [DOI] [PubMed] [Google Scholar]
- 36.Vannucchi A.M., Guglielmelli P. JAK2 mutation-related disease and thrombosis. Semin Thromb Hemost. 2013;39(5):496–506. doi: 10.1055/s-0033-1343890. [DOI] [PubMed] [Google Scholar]
- 37.Hasselbalch H.C., Bjørn M.E. MPNs as inflammatory diseases: the evidence, consequences, and perspectives. Mediators Inflamm. 2015;2015:102476. doi: 10.1155/2015/102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho C.L., Lasho T.L., Butterfield J.H., Tefferi A. Global cytokine analysis in myeloproliferative disorders. Leuk Res. 2007;31(10):1389–1392. doi: 10.1016/j.leukres.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 39.Vaidya R., Gangat N., Jimma T., Finke C.M., Lasho T.L., Pardanani A. Plasma cytokines in polycythemia vera: phenotypic correlates, prognostic relevance, and comparison with myelofibrosis. Am J Hematol. 2012;87(11):1003–1005. doi: 10.1002/ajh.23295. [DOI] [PubMed] [Google Scholar]
- 40.Tefferi A., Vaidya R., Caramazza D., Finke C., Lasho T., Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29(10):1356–1363. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
- 41.Verstovsek S., Mesa R.A., Gotlib J., Levy R.S., Gupta V., DiPersio J.F. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrison C., Kiladjian J.J., Al-Ali H.K., Gisslinger H., Waltzman R., Stalbovskaya V. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]