Abstract
Background
The large diversity of red blood cell antigens favors, especially in multi-transfused patients, the occurrence of autoimmunization and alloimmunization with the risk of hemolytic transfusion reactions. Thus, this study aimed to determine the rates of alloimmunization and autoimmunization in these individuals, as well as the types of alloantibodies and their systems, clinical and epidemiological aspects and the frequency of autoimmunity in alloimmunized and non-alloimmunized patients.
Methods
In a retrospective study, 153 multi-transfused patients from 2006 to 2014 were evaluated. Sixty-eight had onco-hematological diseases, 64 had hemoglobinopathies and 21 had chronic renal failure. Descriptive analyses were carried out with the proportions being compared using the chi-square test, with the significance level set at 5%.
Results
The Rh system was the most frequently involved (53.11%) and anti-E and anti-K (Kell system) were the most prevalent alloantibodies (21.87% each). Autoantibodies were found in ten patients (6.54%) with the percentages of autoimmunization in alloimmunized and non-alloimmunized individuals being 29.16% and 2.32%, respectively (p = 0.0001). There was a significant difference between autoimmunization and the number of transfusions (16.21% in 6–10 vs. 5.26% <6 vs. 2.56% >10; p = 0.0203) and diseases (19.04% in chronic renal failure vs. 6.25% in hemoglobinopathies vs. 2.94% in onco-hematological diseases; p = 0.0329).
Conclusion
The results show a strong correlation between alloimmunization and autoimmunization. Moreover, they reinforce the need for further studies on the clinical and epidemiological profile of multi-transfused patients in relation to alloimmunity and autoimmunity, especially the latter, for a better understanding of its etiopathogenesis and physiopathogenesis.
Keywords: Multi-transfused patients, Red blood cell antibodies, Alloimmunization, Autoimmunization
Introduction
Red blood cell (RBC) transfusion is an essential resource in the treatment of onco-hematological diseases, hemoglobinopathies and chronic renal failure (CRF). As a consequence of multiple transfusions, RBC alloimmunization is a common complication among these patients.1, 2 The antigenic difference between donor and recipient, the patient's immune status and the immunogenicity of the RBC antigen are some of the factors that influence the formation of alloantibodies.3
More than 300 RBC antigens have already been discovered and organized in 36 systems, in particular ABO, Rh, Kell, Duffy, Kidd and MNS.4 This high number of antigens increases the risk of RBC alloimmunization, making it difficult to obtain compatible RBCs, which can result in hemolytic transfusion reactions of variable severity including fatal in some cases.2
Patients with hemoglobinopathies present frequencies of RBC alloimmunization of between 4% and 50%.5, 6 In individuals with onco-hematological diseases the percentage varies from 9% to 13%7 and in patients with CRF it is from 6.1% to 13.1%.8
RBC autoimmunization may occur in association with alloimmunization. Autoantibodies have been identified after transfusions even without alloantibodies.9 However, there are cases, such as in autoimmune hemolytic anemia (AIHA), that RBC autoimmunization can occur without any history of transfusions. Among the possible causes of this are the depression/modification of the immune system by viruses and drugs, alteration in the T cell balance and cross-reactions of antibodies induced by infectious agents with RBC surface antigens.10 The presence of RBC autoantibodies may interfere in pre-transfusion blood compatibility tests between donor and recipient (cross-matching).11
Therefore, the aims of this study were to determine the percentages of RBC alloimmunization and autoimmunization in multi-transfused patients, as well as the types of alloantibodies and their systems, clinical and epidemiological aspects and frequency of RBC autoantibodies in alloimmunized and non-alloimmunized individuals.
Methods
This retrospective study evaluated 153 patients treated in the Hemocentro Regional de Uberaba (HRU)/Fundação Hemominas or Hospital de Clínicas da Universidade Federal do Triângulo Mineiro from 2006 to 2014, who had been transfused with at least three RBC bags on different occasions. Sixty-eight (44.44%) had onco-hematological diseases (acute leukemia, multiple myeloma, myelodysplastic syndrome), 64 (41.83%) had hemoglobinopathies (sickle cell anemia and thalassemia) and 21 (13.73%) had CRF.
Data were collected from patient files at the HRU focused on the production of alloantibodies (types and systems) and autoantibodies. The epidemiological and clinical profiles of alloimmunized and autoimmunized patients were characterized according to gender, age group, diagnosis and number of transfusions before the identification of antibodies.
The results were stored in the Microsoft Excel program and organized into tables and graphs. The analysis was descriptive with proportions being compared by the chi-square test with the level of significance set at 5%.
The study was approved by the Research Ethics Committee of the Fundação Hemominas (#42047915.0.0000.5118).
Results
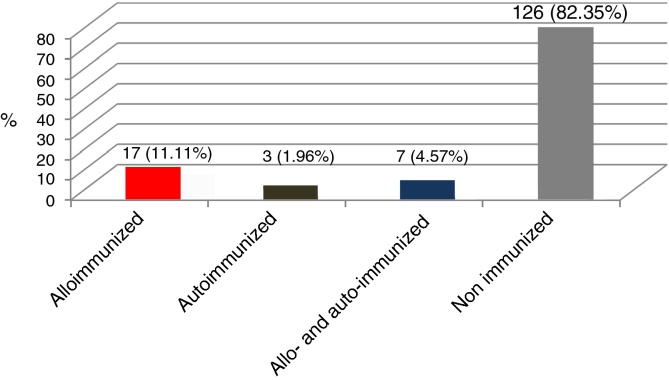
Of the 153 patients: 17 (11.11%) presented only alloimmunization, seven (4.57%) had RBC alloantibodies and autoantibodies and three (1.96%) had only autoantibodies (Figure 1).
Figure 1.
Percentages of alloimmunized, autoimmunized, alloimmunized and autoimmunized, and non-immunized individuals against red blood cell antigens in 153 multi-transfused patients.
Thirty-two alloantibodies were identified in 24 patients with six developing more than one. The most frequent were anti-E (Rh system) and anti-K (Kell system) (21.87% each), followed by anti-C (12.50%) and non-specific antibodies (9.37%). Rh was the system most involved (53.11%) (Table 1).
Table 1.
Systems and types of red blood cell alloantibodies.
| Rh | n | % |
|---|---|---|
| 17 | 53.11 | |
| Anti-E | 7 | 21.87 |
| Anti-C | 4 | 12.50 |
| Anti-c | 2 | 6.25 |
| Anti-D | 2 | 6.25 |
| Anti-Cw | 1 | 3.12 |
| Anti-e | 1 | 3.12 |
| Kell – Anti-K | 7 | 21.87 |
| Non-specific | 3 | 9.37 |
| Lewis | 2 | 6.25 |
| Anti-Lea | 1 | 3.12 |
| Anti-Leb | 1 | 3.12 |
| MNS | 2 | 6.25 |
| Anti-M | 1 | 3.12 |
| Anti-S | 1 | 3.12 |
| Lutheran – Anti-Lua | 1 | 3.12 |
| Total | 32* | 100 |
Total alloantibodies in 24 alloimmunized patients; six had more than one antibody.
Although not significant, the occurrence of alloimmunization was higher in men (17.56%), over 50-year olds (19.44%) and in those who received six to ten transfusions (24.32%). In relation to diseases, alloimmunization was more prevalent in CRF (23.80%), followed by individuals with hemoglobinopathies (17.18%) and onco-hematological diseases (11.76% – Table 2).
Table 2.
Clinical and epidemiological profiles of alloimmunized and non-alloimmunized multi-transfused patients.
| Alloimmunized (n = 24) | Non-alloimmunized (n = 129) | p-value | |
|---|---|---|---|
| Gender – n (%) | |||
| Female | 11 (13.92) | 68 (86.08) | 0.6915 |
| Male | 13 (17.56) | 61 (82.44) | |
| Age group – n (%) | |||
| <30 | 14 (15.91) | 74 (84.09) | 0.6025 |
| 30–50 | 3 (10.34) | 26 (89.66) | |
| >50 | 7 (19.44) | 29 (80.56) | |
| Diagnosis – n (%) | |||
| Hemoglobinopathies | 11 (17.18) | 53 (82.82) | 0.3777 |
| Onco-hematological diseases | 8 (11.76) | 60 (88.24) | |
| Chronic renal failure | 5 (23.80) | 16 (76.20) | |
| Transfusions – n (%) | |||
| <6 | 4 (10.52) | 34 (89.48) | 0.2231 |
| 6–10 | 9 (24.32) | 28 (75.68) | |
| >10 | 11 (14.10) | 67 (85.90) | |
RBC autoantibodies were found in ten (6.53%) of the 153 individuals; in alloimmunized patients the occurrence was 29.16%, while in the non-alloimmunized group only 2.32% (p = 0.0001 – Table 3).
Table 3.
Frequencies of red blood cell autoantibodies in alloimmunized and non-alloimmunized patients.
| Total (n = 153) | Alloimmunized (n = 24) | Non-alloimmunized (n = 129) | p-value | |
|---|---|---|---|---|
| Autoantibodies n – (%) | 10 (6.53) | 7 (29.16) | 3 (2.32) | 0.0001 |
RBC autoantibodies were more common in women (10.12%), over 50-year olds (11.11%) and in those who received six to ten transfusions (16.21%; p = 0.0203). In relation to diseases, autoantibodies were more common in CRF (19.04%), followed by individuals with hemoglobinopathies (6.25%) and onco-hematological diseases (2.94%; p = 0.0329 – Table 4).
Table 4.
Clinical and epidemiological profiles of autoimmunized and non-autoimmunized multi-transfused patients.
| Autoimmunized (n = 10) | Non-autoimmunized (n = 143) | p-value | |
|---|---|---|---|
| Gender – n (%) | |||
| Female | 8 (10.12) | 71 (89.88) | 0.1262 |
| Male | 2 (2.70) | 72 (97.30) | |
| Age group – n (%) | |||
| <30 | 4 (4.54) | 84 (95.48) | 0.4044 |
| 30–50 | 2 (6.89) | 27 (93.11) | |
| >50 | 4 (11.11) | 32 (88.89) | |
| Diagnosis – n (%) | |||
| Hemoglobinopathies | 4 (6.25) | 60 (93.75) | 0.0329 |
| Onco-hematological diseases | 2 (2.94) | 66 (97.06) | |
| Chronic renal failure | 4 (19.04) | 17 (80.96) | |
| Transfusions – n (%) | |||
| <6 | 2 (5.26) | 36 (94.74) | 0.0203 |
| 6–10 | 6 (16.21) | 31 (83.79) | |
| >10 | 2 (2.56) | 76 (97.44) | |
Discussion
Of the 153 multi-transfused patients, 24 (15.69%) presented RBC alloantibodies; a higher frequency compared to a study by Alves et al.12 who found, in the same service as this study, a rate of 10.49% in 143 patients in acute conditions, however the need was heterogeneous regarding the diagnosis and they mostly had simultaneous transfusions.
The most common antibodies were against the Rh (53.11%) and Kell (21.87%) systems similar to the literature which reports antibodies against the Rh system in 9.52–53.40% of cases and against the Kell system in 18.20–33.33%.6, 8, 13, 14 This may be explained by the fact that these systems are the most immunogenic.15
The rate of alloimmunization was similar between genders. We must remember, however that women with chronic diseases generally have a lower parity index and, consequently, fetal-maternal alloimmunization than in the general population, which might explain this result. Thompson et al.6 reported similar results (17.5% in women and 15.8% in men) when they evaluated 697 multi-transfused patients with thalassemia. Martins et al.16 reported that 72.83% of all alloimmunized individuals are women; however, individuals in acute conditions were also evaluated.
Regarding the diagnosis, this study found that the alloimmunization rate was lower in onco-hematological diseases albeit without significance; this result is in agreement with the generally lower sensitization in these individuals due to the immunosuppression caused by the disease and its chemotherapy and radiotherapy treatments.7 Similar data are found in the literature, with the percentage of alloimmunization varying from 9 to 13%7; in hemoglobinopathies, the frequencies range from 18% to 47%.5
Considering the number of transfusions, although not significant, there was a higher rate of alloimmunization in those who received six to ten transfusions (24.32%), which corroborates the findings of Martins et al.16 that most alloantibodies are produced early (by the 10th transfusion). Even so, other studies have shown that sensitization was higher in patients who received more than ten transfusions12, 17, 18, 19; it is believed that there is an individual predisposition, possibly of an inherited nature, which is present in the first exposures to foreign antigens.20 In addition, Higgins and Sloan21 demonstrated that alloimmunization is poorly correlated with the number of transfusions, with strong evidence of a subgroup of patients who present an increased risk of developing alloantibodies.
The percentage of RBC autoantibodies, in relation to diseases, was significantly higher in CRF however, the low number of these patients should be considered. The rates in individuals with onco-hematological diseases and hemoglobinopathies are in agreement with the literature; Sanz et al.14 showed a lower rate in the first diagnosis, while Thompson et al.6 found a higher frequency in the second. We must reiterate that these findings are probably associated with immunosuppression and/or treatments inherent to onco-hematological diseases.7
Regarding the number of transfusions and RBC autoimmunization, this was significantly higher in those who received six to ten transfusions. We believe that, as in alloimmunization, these individuals are more likely to develop autoantibodies. However, Thompson et al.,6 on evaluating 697 patients with thalassemia using a different methodology, did not find any significant increase in RBC autoantibodies in terms of the duration of hemotherapy until the fifth decade.
This study found that the frequency of RBC autoantibodies was significantly higher in alloimmunized compared to non-alloimmunized patients, which is in agreement with Ahrens et al.22 These findings reinforce the evidence that individuals who have already developed alloantibodies produce more RBC autoantibodies.11 Ahrens et al. suggest the hypothesis that this sensitization results from an overactive immune response, stimulated by epitopes of the antigen causing the response (intramolecular) associated to other antigens (intermolecular). Such molecules, in large quantities, would contribute to the pathogenesis of chronic autoimmune diseases mediated by antibodies and T cells.22
As highlighted, the presence of RBC autoantibodies may interfere with pre-transfusion blood compatibility tests between donor and recipient (cross-matching),11 requiring an exhaustive search of compatible RBCs and delaying the start of the transfusion.
Conclusion
In summary, this study reports high levels of alloimmunization and autoimmunization in multi-transfused patients. Further studies on the clinical and epidemiological profiles of these individuals are necessary for a better understanding of the etiopathogenesis and physiopathogenesis of RBC alloimmunization and autoimmunization, especially the latter, due to the scarcity of reports in the literature.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We would like to thank all the collaborators of the research, from the patients to the employees of the Hemocentro Regional de Uberaba (HRU)/Fundação Hemominas/Universidade Federal do Triângulo Mineiro (UFTM). To Amanda de Oliveira Santos, student of Biomedicina at UFTM, for supporting and assisting the study. To the Fundação Hemominas and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for the scientific initiation scholarship.
References
- 1.McNerney M.E., Baron B.W., Volchenboum S.L., Papari M., Keith M., Williams K. Development of warm auto- and allo-antibodies in a 3-year old boy with sickle cell haemoglobinopathy following his first transfusion of a single unit of red blood cells. Blood Transfus. 2010;8(2):126–128. doi: 10.2450/2009.0105-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L.Y., Liang D.C., Liu H.C., Chang F.C., Wang C.L., Chan Y.S. Alloimmunization among patients with transfusion-dependent thalassemia in Taiwan. Transfus Med. 2006;16(3):200–203. doi: 10.1111/j.1365-3148.2006.00656.x. [DOI] [PubMed] [Google Scholar]
- 3.Bilwani F., Kakepoto G.N., Adil S.N., Usman M., Hassan F., Khurshid M. Frequency of irregular red cell alloantibodies in patients with thalassemia major: a bicenter study. J Pak Med Assoc (JPMA) 2005;55(12):563–565. [PubMed] [Google Scholar]
- 4.Castilho L., Pellegrino Junior J., Reid M.E. Editora Atheneu; São Paulo: 2015. Fundamentos de Imunohematologia; pp. 1–3. 56. [Google Scholar]
- 5.Matteocci A., Pierelli L. Red blood cell alloimmunization in sickle cell disease and in thalassaemia: current status, future perspectives and potential role of molecular typing. Vox Sang. 2014;106(3):197–208. doi: 10.1111/vox.12086. [DOI] [PubMed] [Google Scholar]
- 6.Thompson A.A., Cunningham M.J., Singer S.T., Neufeld E.J., Vichinsky E., Yamashita R. Red cell alloimmunization in a diverse population of transfused patients with thalassaemia. Br J Haematol. 2011;153(1):121–128. doi: 10.1111/j.1365-2141.2011.08576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schonewille H., de Vries R.R., Brand A. Alloimmune response after additional red blood cell antigen challenge in immunized hemato oncology patients. Transfusion. 2009;49(3):453–457. doi: 10.1111/j.1537-2995.2008.01980.x. [DOI] [PubMed] [Google Scholar]
- 8.Babiker H.A., Elsayed T.Y. Frequency of alloantibodies among chronic renal failure patients in Red Sea State. Indian J Hematol Blood Transfus. 2014;30(3):187–190. doi: 10.1007/s12288-013-0234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahrens N., Pruss A., Kähne A., Kiesewetter H., Salama A. Coexistence of auto antibodies and alloantibodies to red blood cells due to blood transfusion. Transfusion. 2007;47(5):813–816. doi: 10.1111/j.1537-2995.2007.01194.x. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira M.C., Oliveira B.M., Murao M., Vieira Z.M., Gresta L.T., Viana M.B. Clinical course of autoimmune hemolytic anemia: an observational study. J Pediatr (Rio J) 2006;82(1):58–62. doi: 10.2223/JPED.1438. [DOI] [PubMed] [Google Scholar]
- 11.Guirat-Dhouib N., Mezri M., Hmida H., Mellouli F., Kaabi H., Ouderni M. High frequency of autoimmunization among transfusion-dependent Tunisian thalassaemia patients. Transfus Apher Sci. 2011;45(2):199–202. doi: 10.1016/j.transci.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Alves V.M., Martins P.R., Soares S., Araújo G., Schmidt L.C., Costa S.S. Alloimmunization screening after transfusion of red blood cells in a prospective study. Rev Bras Hematol Hemoter. 2012;34(3):206–211. doi: 10.5581/1516-8484.20120051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obaid J.M., Abo El-Nazar S.Y., Ghanem A.M., El-Hadidi A.S., Mersal B.H. Red blood cells alloimmunization and autoimmunization among transfusion-dependent beta-thalassemia patients in Alexandria province, Egypt. Transfus Apher Sci. 2015;53(1):52–57. doi: 10.1016/j.transci.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Sanz C., Nomdedeu M., Belkaid M., Martinez I., Nomdedeu B., Pereira A. Red blood cell alloimmunization in transfused patients with myelodysplastic syndrome or chronic myelomonocytic leukemia. Transfusion. 2013;53(4):710–715. doi: 10.1111/j.1537-2995.2012.03819.x. [DOI] [PubMed] [Google Scholar]
- 15.Bordin J.O., Moreira Junior G. STD: imunohematologia eritrocitária. Belo Horizonte IEA Editora/SBHH; 1996. Sistemas Kell e Kx; pp. 137–143. [Google Scholar]
- 16.Martins P.R., Alves V.M., Pereira G.A., Moraes-Souza H. Frequência de anticorpos irregulares em politransfundidos no Hemocentro Regional de Uberaba-MG, de 1997 a 2005. Rev Bras Hematol Hemoter. 2008;30(4):272–276. [Google Scholar]
- 17.Pinto P.C., Braga J.A., Santos A.M. Risk factors for alloimmunization in patients with sickle cell anemia. Rev Assoc Med Bras (1992) 2011;57(6):668–673. doi: 10.1590/s0104-42302011000600014. [DOI] [PubMed] [Google Scholar]
- 18.Natukunda B., Schonewille H., van de Watering L., Brand A. Prevalence and specificities of red blood cell alloantibodies in transfused Ugandans with different diseases. Vox Sang. 2010;98(2):167–171. doi: 10.1111/j.1423-0410.2009.01241.x. [DOI] [PubMed] [Google Scholar]
- 19.Sakhalkar V.S., Roberts K., Hawthorne L.M., McCaskill D.M., Veillon D.M., Caldito G.C. Allosensitization in patients receiving multiple blood transfusions. Ann N Y Acad Sci. 2005;1054:495–499. doi: 10.1196/annals.1345.072. [DOI] [PubMed] [Google Scholar]
- 20.Novaretti M.C. Investigação Laboratorial em Pacientes com Anticorpos Eritrocitários. In: Bordin J.O., Langhi Júnior D.M., Covas D.T., editors. Hemoterapia: Fundamentos e Prática. Editora Atheneu; São Paulo: 2007. pp. 186–189. [Google Scholar]
- 21.Higgins J.M., Sloan S.R. Stochastic modeling of human RBC alloimmunization: evidence for a distinct population of immunological responders. Blood. 2008;112(6):2546–2553. doi: 10.1182/blood-2008-03-146415. [DOI] [PubMed] [Google Scholar]
- 22.Ahrens N., Pruss A., Mayer B., Genth R., Kiesewetter H., Salama A. Association between alloantibody specificity and autoantibodies to red blood cells. Transfusion. 2008;48(1):20–24. doi: 10.1111/j.1537-2995.2007.01505.x. [DOI] [PubMed] [Google Scholar]