The miR390/TAS3/ARF4 module is involved in the regulation of lateral root development under salt stress in poplar by auxin signaling.
Abstract
Salt-induced developmental plasticity in a plant root system strongly depends on auxin signaling. However, the molecular events underlying this process are poorly understood. MicroRNA390 (miR390), trans-actin small interfering RNAs (tasiRNAs), and AUXIN RESPONSE FACTORs (ARFs) form a regulatory module involved in controlling lateral root (LR) growth. Here, we found that miR390 expression was strongly induced by exposure to salt during LR formation in poplar (Populus spp.) plants. miR390 overexpression stimulated LR development and increased salt tolerance, whereas miR390 knockdown caused by a short tandem target mimic repressed LR growth and compromised salt resistance. ARF3.1, ARF3.2, and ARF4 expression was inhibited significantly by the presence of salt, and transcript abundance was decreased dramatically in the miR390-overexpressing line but increased in the miR390-knockdown line. Constitutive expression of ARF4m harboring mutated trans-acting small interfering ARF-binding sites removed the salt resistance of the miR390 overexpressors. miR390 positively regulated auxin signaling in LRs subjected to salt, but ARF4 inhibited auxin signaling. Salinity stabilized the poplar Aux/IAA repressor INDOLE-3-ACETIC ACID17.1, and overexpression of an auxin/salt-resistant form of this repressor suppressed LR growth in miR390-overexpressing and ARF4-RNA interfering lines in the presence of salt. Thus, the miR390/TAS3/ARFs module is a key regulator, via modulating the auxin pathway, of LR growth in poplar subjected to salt stress.
Due to climate change and agricultural practice, increase in soil salinity is a major threat to crop yields around the world (Munns and Tester, 2008). Salt generally damages plants through osmotic stress in the rhizosphere, interfering with water and nutrient uptake and causing cells to be subjected to ionic toxicity (Hasegawa et al., 2000; Zhang and Shi, 2013). Developmental plasticity in the root system is an important strategy allowing plants to cope with environmental stresses, including salinity (Galvan-Ampudia and Testerink, 2011). Salt-induced plasticity occurs in a number of root development processes, such as primary root elongation, lateral root (LR) growth, root hair formation, and root tropism (Sun et al., 2008; Wang et al., 2008, 2009; Galvan-Ampudia et al., 2013). Relatively little is known about the genetic and molecular events underlying salt-induced root plasticity, even though morphological modifications in roots are very important to the adaptabilities of higher plants to the presence of salt.
Salt responses in plant roots require sophisticated and coordinated spatial-temporal transcriptional programs and the regulation of hormones, including auxin, abscisic acid, and GA (Duan et al., 2013; Galvan-Ampudia et al., 2013; Geng et al., 2013; Liu et al., 2015). Auxin is a well-known phytohormone that triggers root genesis and salt-induced root developmental plasticity (Kazan, 2013). Meristematic activity and root pattern formation are determined by the distal auxin maximum in Arabidopsis (Arabidopsis thaliana) root tips (Sabatini et al., 1999; Blilou et al., 2005), orchestrated by polar auxin transport (Palme and Gälweiler, 1999; Friml et al., 2002). Decreases in meristem size when plants suffer from salt stress inhibit root cell proliferation and elongation (West et al., 2004), and salt-restricted auxin accumulation occurs in the root tips (Liu et al., 2015). Halotropism, which allows roots to bend away from areas with high salt concentrations, requires the active redistribution of auxin in the root tips, mediated by PIN-FORMED2 (PIN2; Galvan-Ampudia et al., 2013). The sensitivity of root tissues to salt can be modified by polar auxin transport modulation and changes in the protein turnover of Aux/IAAs (a family of transcriptional repressors involved in auxin signaling; Wang et al., 2009; Liu et al., 2015). Auxin perception starts with the direct binding of auxin to its receptors TIR1 (TRANSPORT INHIBITOR RESPONSE1)/AFB (AUXIN SIGNALING F-BOX; Dharmasiri et al., 2005; Kepinski and Leyser, 2005), which are posttranscriptionally targeted by microRNA393 (miR393; Si-Ammour et al., 2011). The miR393-dependent regulation of TIR1/AFBs is a critical checkpoint in auxin signaling, coordinating auxin signaling with root growth under saline conditions (Iglesias et al., 2014). The Salt Overly Sensitive (SOS) pathway is a well-understood module coupling salt signals with sodium homeostasis; it plays a critical role in salt-induced plastic LR development via the modulation of auxin distribution in the presence of salt (Zhao et al., 2011; Ji et al., 2013). Thus, auxin plays key roles in integrating a salt stimulus into root development programs. A comprehensive understanding of salt-induced root plasticity requires the identification of more novel components and pathways within the salt/auxin-mediated molecular framework (Galvan-Ampudia and Testerink, 2011).
miR390 is an evolutionarily conserved microRNA (miRNA) that specifically binds to and then cleaves transcripts of the noncoding trans-acting short interfering RNA3 (TAS3; Allen et al., 2005). A number of molecular components involved in small interfering RNA biogenesis, including RNA-DEPENDENT RNA POLYMERASE6, SUPPRESSOR OF GENE SILENCING3, and DICER-LIKE4, allow the cleaved TAS3 transcript fragments to produce trans-acting small interfering RNAs (Xie et al., 2005). The TAS3-derived trans-acting small interfering RNAs can mediate the cleavage of transcripts encoding AUXIN RESPONSE FACTOR2 (ARF2), ARF3, and ARF4, so they are also called trans-acting small interfering (tasi) ARFs (Marin et al., 2010). ARF proteins are key components of the auxin signaling cascade and directly control the transcription of primary auxin-responsive genes (Guilfoyle and Hagen, 2007). The miR390/TAS3/ARFs module is functionally diverse, regulating a number of plant development processes. The tasiARFs derived from miR390-cleaved TAS3 transcripts are key regulators of leaf patterning, leaf polarity, leaf expansion, and developmental phase transition (Adenot et al., 2006; Fahlgren et al., 2006; Garcia et al., 2006; Yifhar et al., 2012). The miR390/TAS3/ARFs pathway is part of the auxin-mediated molecular network that orchestrates LR formation in Arabidopsis (Marin et al., 2010; Yoon et al., 2010). This pathway also is required for the initiation of the shoot meristem in rice (Oryza sativa; Nagasaki et al., 2007). In legume species, the miR390/TAS3 pathway regulates lateral organ development and leaf margin formation (Zhou et al., 2013) and also plays an important role in root nodule symbiosis (i.e. facilitating plant-microbe interactions; De Luis et al., 2012; Li et al., 2014; Cabrera et al., 2016). The miR390/TAS3 pathway modulates LR growth but encourages nodule development in legumes (Hobecker et al., 2017). However, it is still unclear whether the miR390/TAS3/ARFs module is involved in the responses of plants to abiotic stresses.
Poplar (Populus spp.) are fast-growing woody plants of great economical and ecological importance (Dickmann, 2006). Different poplar species and ecotypes have very different salt tolerances, allowing the Populus genus to be used as an ideal model when studying physiological and genetic salt acclimation strategies (Chen and Polle, 2010). A number of transcription factors, including GRAS (GIBBERELLIN-INSENSITIVE, REPRESSOR OF GAI, and SCARECROW)/SCL (SCARECROW-LIKE7), ERF (ETHYLENE RESPONSE FACTOR76), HDG (HOMEODOMAIN GLABROUS11), and WRKY70 (the homologs of which have been found to regulate stress responses in other species), have been found to participate in the transcriptional regulation of salt tolerance in poplar (Ma et al., 2010; Yao et al., 2016; Yu et al., 2016; Zhao et al., 2017). Studies of transgenic poplar indicate that the SOS pathway is involved in salt tolerance, as it is in herbaceous Arabidopsis (Tang et al., 2010; Yang et al., 2015; Ke et al., 2017). Little is known about the underlying molecular processes involved in salt tolerance in Populus spp. miRNAs in Populus euphratica, a salt-tolerant poplar species, have been comprehensively profiled, and salt-induced expression of a series of miRNAs, including miR390, occurs in root tissues (Si et al., 2014). Salt-responsive modulation of the miR390/ARF unit has been found in a comprehensive small RNA profiling survey of cotton (Gossypium hirsutum; Yin et al., 2017). Here, we determine the salt-responsive expression of miR390 in poplar during LR formation and functionally characterize miR390/ARF4 under saline conditions using genetically transformed poplar plants. The results indicate that the miR390/TAS3/ARF module, an evolutionarily conserved pathway, is involved in salt-induced LR development regulation in poplar via the auxin pathway.
RESULTS
Salt-Induced Expression of miR390 during LR Development in Poplar
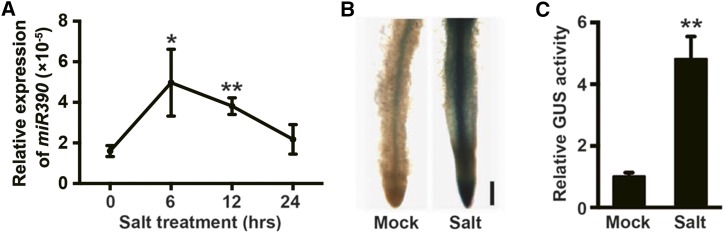
The miR390 family in genus Populus has four members that, despite some rather variable flanking sequences, have a 21-bp mature sequence identical to that in Arabidopsis miR390 (miRbase: http://www.mirbase.org; Supplemental Fig. S1A). As predicted by the RNAfold WebServer (Gruber et al., 2008), the precursors of the poplar miR390 members form stem-loop secondary structures and generate the same mature miRNA (Supplemental Fig. S1B). Reverse transcription-quantitative PCR (RT-qPCR) showed that mature miR390 is expressed throughout roots and leaves (Supplemental Fig. S2A). The GUS construct driven by the miR390a promoter was introduced into poplar plants, and histological staining indicated that miR390 was expressed weakly in the main roots but strongly in the LRs (Supplemental Fig. S2B). A genomic survey indicated a series of stress-associated cis-elements in the promoter region of each miR390 member (Supplemental Fig. S2C) and that miR390 is thus possibly involved in stress-response processes. Stem-loop RT-qPCR assays revealed that miR390 expression is induced by salt stress (Fig. 1A). Salt-induced miR390 expression patterns in different tissues were determined by histologically staining with the GUS reporter driven by the miR390 promoter (Fig. 1, B and C; Supplemental Fig. S2). No induced miR390 expression was detected in the leaves (Supplemental Fig. S2, D and E). Salt-responsive GUS activity driven by the miR390 promoter was not present in main root tips (Supplemental Fig. S2, F, G, and I), whereas miR390 was induced weakly by salt in the stele tissues of the main roots, although induction was not statistically significant (Supplemental Fig. S2, F, H, and I). In contrast, salt strongly stimulated PromiR390a activity in the LR tips (Fig. 1B). GUS fluorometric measurements showed that salt stress increased PromiR390a activity by 5-fold in LRs containing the PromiR390a:GUS construct (Fig. 1C). miR390 expression also was induced in LR primordia (Supplemental Fig. S2J). We conclude that salinity induces miR390 expression in poplar plants during LR formation.
Figure 1.
Salt-induced expression of miR390 in poplar LRs. A, Time-course assay of mature miR390 expression under salt stress. Four-week-old wild-type seedlings grown on Woody Plant Medium (WPM) were subjected to 200 mm NaCl for 0, 6, 12, and 24 h, and total roots were collected for RNA extraction. The miR390 expression level was determined by stem-loop RT-qPCR. The U6 snRNA was used as a reference to normalize the expression data of miR390. Asterisks indicate significant differences with respect to the value for 0 h (one-way ANOVA followed by Dunnett’s test for pairwise comparisons: *, P < 0.05 and **, P < 0.01; n = 3). B, Histological staining of the LR tip of transgenic poplar harboring the GUS reporter gene driven by the promoter of miR390a under salt treatment. Four-week-old wild-type seedlings were subjected to 200 mm NaCl for 6 h for GUS staining. Bar = 500 μm. C, Quantitative measurement of salt-induced GUS activity driven by the miR390a promoter in the reporter line. Salt treatment was performed as indicated in B. The values under mock treatment were normalized to 1. Error bars represent sd. Asterisks indicate significant differences with respect to the control (Student’s t test: **, P < 0.01; n = 4).
miR390 Promotes LR and Shoot Growth in Poplar
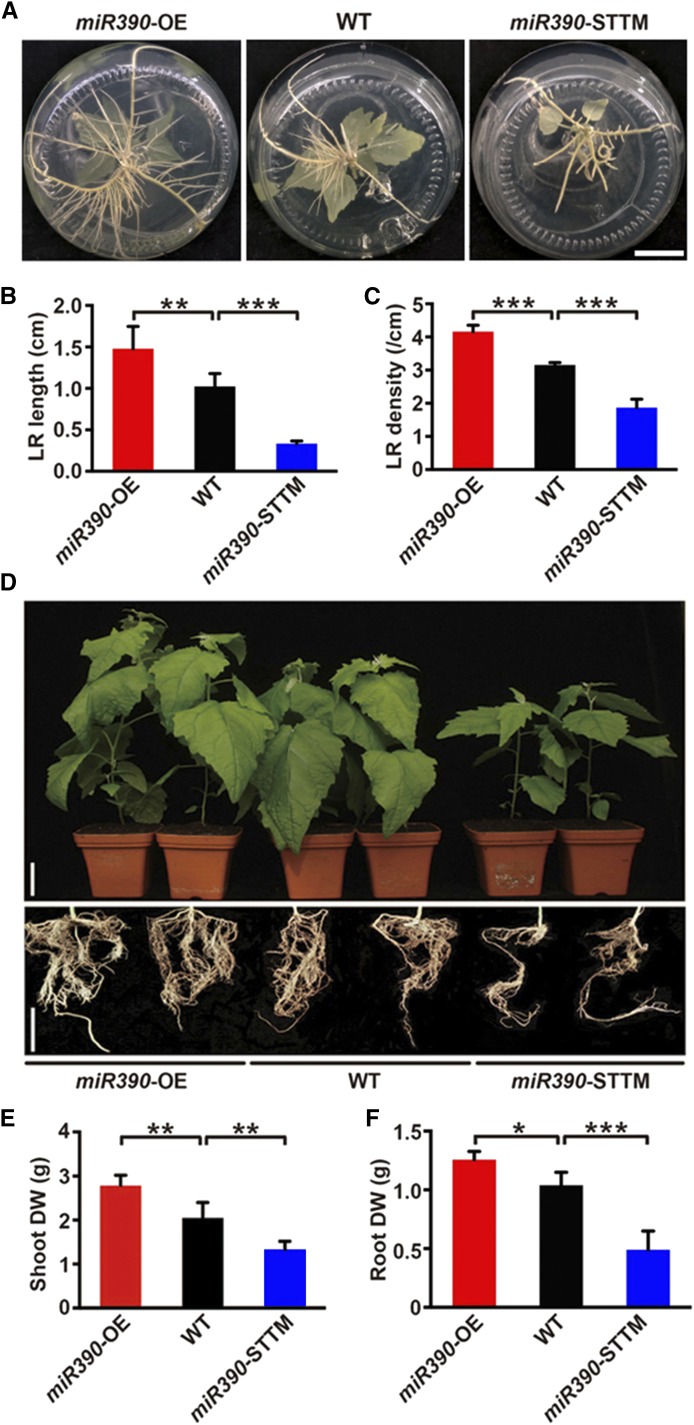
To determine whether miR390 regulates root growth in poplar, overexpressing (OE) and short tandem target mimic (STTM)-based knockdown transgenic miR390 poplar were generated to allow functional identification (Supplemental Fig. S3, A–C). LR development was significantly different in wild-type and transgenic 4-week-old seedlings cultivated in medium (Fig. 2A). LR length and density were significantly higher in the miR390-OE seedlings than in the wild-type seedlings but lower in the miR390-STTM lines (P < 0.01 and P < 0.001; Fig. 2, B and C). The lengths and numbers of main roots in 4-week-old miR390 transgenic plants cultivated on medium were not affected (Supplemental Fig. S3, D and E). For 10-week-old plants cultivated in soil, the miR390-OE transgenic plants were larger than the wild-type plants (Fig. 2D). In contrast, miR390 knockdown repressed shoot and root growth in the miR390-STTM lines (Fig. 2D). Shoot and root biomasses were 36.3% and 16.1% higher, respectively, in the miR390-OE lines than in the wild-type plants and 36.1% and 52.9% lower, respectively, in the miR390-STTM lines than in the wild-type plants (Fig. 2, E and F). We conclude that miR390 positively regulates root (especially LR) growth and shoot growth in poplar plants.
Figure 2.
miR390 positively regulates LR growth and biomass accumulation in poplar. A, LR phenotypes of seedlings of wild-type (WT), miR390-OE, and miR390-STTM lines. Cutting-propagated seedlings were cultivated in vitro for 4 weeks. Bar = 2 cm. B and C, Quantification of average LR length (B) and LR density (C) of the seedlings of wild-type, miR390-OE, and miR390-STTM lines cultivated in vitro for 4 weeks. D, Shoot and root phenotypes of wild-type, miR390-OE, and miR390-STTM plants cultivated in soil for 10 weeks. Bars = 5 cm. E and F, Quantitative measurements of shoot (E) and root (F) biomass (dry weight [DW]) of wild-type, miR390-OE, and miR390-STTM plants cultivated in soil for 10 weeks. For B, C, E, and F, error bars represent sd, and asterisks indicate statistically significant differences in one-way ANOVA followed by Dunnett’s test for pairwise comparisons between wild-type and transgenic lines (*, P < 0.05; **, P < 0.01; and ***, P < 0.001; n = 5).
miR390 Alleviates the Inhibition of LR Growth and Biomass Accumulation Caused by Salt Stress
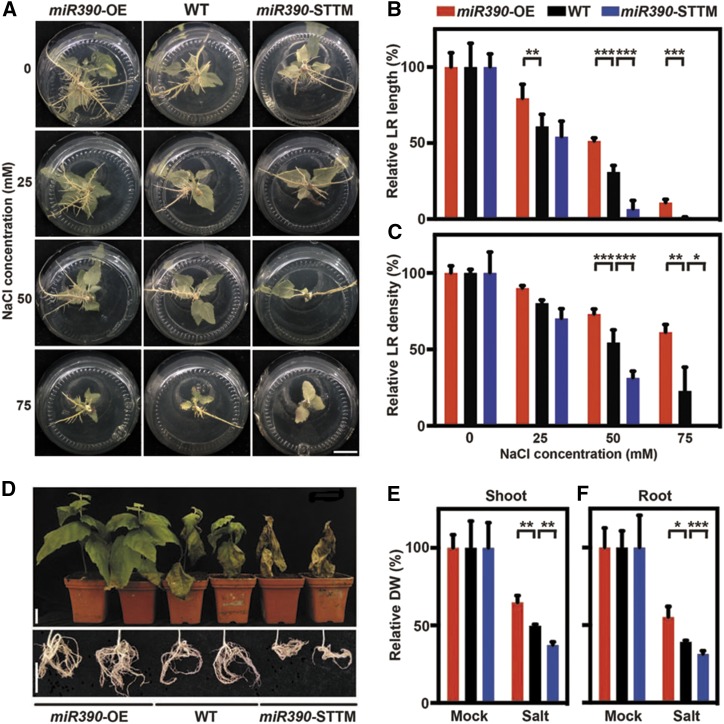
It was shown previously that miR390 regulates LR growth in Arabidopsis (Marin et al., 2010; Yoon et al., 2010). Our results indicate that salt induces miR390 expression during LR formation in poplar plants (Fig. 1, B and C; Supplemental Fig. S2D). Therefore, we wondered whether miR390 is involved in regulating LR development during salinity responses and the development of salt tolerance. Cutting-propagated wild-type, miR390-OE, and miR390-STTM seedlings were exposed to NaCl at concentrations of 0, 25, 50, and 75 mm for 4 weeks. LR growth was retarded in every seedling, but the wild-type and transgenic lines had different sensitivities to salt at high salt concentrations (Fig. 3A). LR length and density were inhibited slightly by 25 mm NaCl in all lines (Fig. 3, B and C; Supplemental Fig. S3F and G). The LR length and density were markedly better maintained in miR390-OE seedlings than in wild-type seedlings exposed to 50 and 75 mm NaCl, but 50 mm NaCl caused almost complete suppression of LR formation in the miR390-STTM seedlings (Fig. 3, A–C). The function of miR390 in response to salt stress was investigated further by irrigating 4-week-old wild-type and transgenic plants cultivated in soil with 200 mm NaCl every 2 d (Fig. 3D). This treatment caused all miR390-STTM plants to die within 6 weeks, but the miR390-OE seedlings grew well (Fig. 3D). While the wild-type plants survived, the older leaves started to wilt by this stage, indicating the effects of severe salt toxicity (Fig. 3D). Biomass accumulation in wild-type and transgenic plants exposed to salt was significantly different (P < 0.05, P < 0.01, and P < 0.001; Fig. 3, D–F; Supplemental Fig. S3H and I). Noticeably, shoot and root biomass was reduced by salt treatment to a greater extent in wild-type and STTM lines than in OE lines (Fig. 3, E and F). These results indicate that constitutive miR390 expression can increase salt tolerance in poplar plants.
Figure 3.
miR390 enhances the salt tolerance of LR growth and biomass accumulation in poplar. A, LR phenotypes of seedlings of wild-type (WT), miR390-OE, and miR390-STTM lines under salt treatment. Cutting-propagated seedlings were cultivated in vitro and subjected to 0, 25, 50, and 75 mm NaCl for 4 weeks. Bar = 2 cm. B and C, Relative quantification of total LR length (B) and LR density (C) in seedlings of wild-type, miR390-OE, and miR390-STTM lines under salt treatment. Cutting-propagated seedlings were cultivated in vitro and subjected to 0, 25, 50, and 75 mm NaCl for 4 weeks. The values for each genotype without NaCl treatment were normalized to 100%. Nonnormalized values are given in Supplemental Figure S3, F and G. D, Shoot and root phenotypes of 10-week-old wild-type, miR390-OE, and miR390-STTM plants under salt treatment. Poplar plants were cultivated in soil for 4 weeks and watered subsequently with 200 mm NaCl for 6 weeks. Bars = 5 cm. E and F, Relative quantification of shoot (E) and root (F) biomass (dry weight [DW]) of wild-type, miR390-OE, and miR390-STTM plants under salt treatment. Poplar plants were cultivated and salinity treated as described in D. The values for each genotype under mock treatment were normalized to 100%. Nonnormalized values are given in Supplemental Figure S3, H and I. For B, C, E, and F, error bars represent sd, and asterisks indicate statistically significant differences in two-way ANOVA followed by Dunnett’s test for pairwise comparisons between wild-type and transgenic lines (*, P < 0.05; **, P < 0.01; and ***, P < 0.001; n = 5).
Targets of tasiARFs Are Involved in LR Development
It is well known that miR390 directs noncoding TAS3 transcript cleavage, generating trans-acting small interfeing RNAs, which posttranscriptionally target ARF2, ARF3, and ARF4, and therefore are called tasiARFs (Allen et al., 2005; Axtell et al., 2006). This action mode is evolutionarily conserved in land plants including Populus spp. (Xia et al., 2017). The poplar genome has four TAS3 loci, and the TAS3 transcript has two miR390 target sites (Supplemental Fig. S4A), consistent with the two-hits model (Xia et al., 2017). TAS3.1 and TAS3.2 can generate tasiARFs at two neighboring sites, and the other poplar TAS3 members each contain a single centrally located tasiARF production site (Supplemental Fig. S4A). The poplar tasiARFs derived from TAS3 share high similarity in sequence (Supplemental Fig. S4B). As predicted by Xia et al. (2017), ARF3 (ARF3.1, ARF3.2, and ARF3.3) and ARF4 in poplar contain dual tasiARF-binding sites, whereas the ARF2 genes (ARF2.1 and ARF2.2) each contains a single tasiARF target site (Supplemental Fig. S5). ARF3.3 (Potri.011G059100.1) had truncated open reading frame sequences, so it was omitted from subsequent analyses.
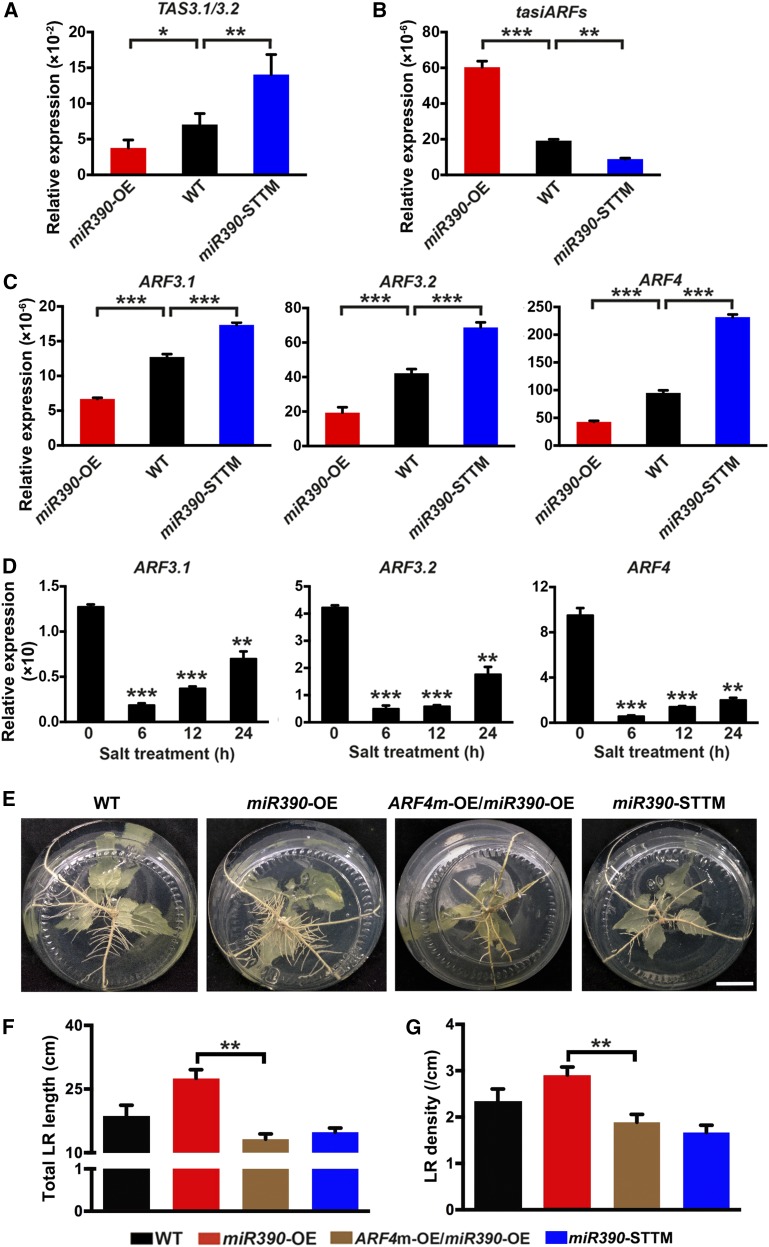
The miR390-mediated molecular context in poplar was studied by testing for expression of the downstream components in transgenic miR390-OE plants. The TAS3.1/3.2 transcript abundance was decreased significantly by miR390 overexpression but increased by miR390 knockdown (P < 0.05 and P < 0.01; Fig. 4A). Therefore, the tasiARFs derived from the TAS3 transcripts preferentially accumulated in the miR390-OE lines but not in the miR390-STTM lines (Fig. 4B), indicating that tasiARF production in poplar is positively regulated by miR390. The positive regulation of tasiARF1, tasiARF2, and tasiARF3 by miR390 also was detected using specific oligonucleotide primers to quantify the expression of these tasiARF members (Supplemental Fig. S6A). RT-qPCR showed that ARF3.1, ARF3.2, and ARF4 transcription was suppressed in the miR390-OE lines but enhanced in the miR390-STTM lines (Fig. 4C). In contrast, ARF2 member transcription was not affected by miR390 (Supplemental Fig. S6B). ARF3.1, ARF3.2, and ARF4 expression was suppressed to a significant degree when plants were exposed to salt for 6 h (Fig. 4D), unlike the salt-induced accumulation of miR390 (Fig. 1).
Figure 4.
The tasiARF targets are involved in LR development. A to C, The expression levels of TAS3.1/3.2 (A), tasiARFs (B), and ARF3.1/3.2/4 (C) were quantitatively detected in wild-type (WT), miR390-OE, and miR390-STTM lines. LRs of 4-week-old seedlings cultivated in vitro were collected for RNA extraction. The expression levels of tasiARFs were determined by stem-loop RT-qPCR. U6 snRNA was used as a reference for normalizing the expression data of miR390. The expression levels of TAS3, ARF3.1, ARF3.2, and ARF4 were determined by RT-qPCR, and 18S rRNA was used as a reference for normalization. Error bars represent sd. Asterisks indicate statistically significant differences in one-way ANOVA followed by Dunnett’s test for pairwise comparisons between wild-type and transgenic lines (*, P < 0.05; **, P < 0.01; and ***, P < 0.001; n = 3). D, Time-course assays of ARF3.1, ARF3.2, and ARF4 expression levels to salt treatment. Four-week-old wild-type seedlings grown on WPM were subjected to 200 mm NaCl for 0, 6, 12, and 24 h, and LRs were collected for RNA extraction. The expression levels of ARFs were determined by RT-qPCR. 18S rRNA was used as a reference for normalization. Error bars represent sd. Asterisks indicate significant differences with respect to the values for 0 h (one-way ANOVA followed by Dunnett’s test for pairwise comparisons: **, P < 0.01 and ***, P < 0.001; n = 3). E, LR phenotypes of ARF4m transgenic seedlings in the miR390-OE background. Cutting-propagated seedlings of wild-type, miR390-OE, ARF4m-OE/miR390-OE, and miR390-STTM lines were cultivated on WPM for 4 weeks. Bar = 2 cm. F and G, Quantitative measurements of total LR length (F) and LR density (G) of ARF4m transgenic seedlings in the miR390-OE background. Seedlings of wild-type and transgenic lines were cultivated as described in E. Error bars represent sd. Asterisks indicate statistically significant differences in one-way ANOVA followed by Dunnett’s test for pairwise comparisons between ARF4m-OE/miR390-OE and miR390-OE or miR390-STTM (**, P < 0.01; n = 5).
The transcript levels of the tasiARF targets ARF3.1, ARF3.2, and ARF4 in poplar tissues were determined. ARF4 was expressed more than the two ARF3 homologs in all the tissues analyzed, except for old leaves (Supplemental Fig. S7). The differences were most noticeable in the roots, in which ARF4 was expressed 14 times more than ARF3.2 and ARF3.1 expression was not detected (Supplemental Fig. S7). Further analyses were focused on ARF4 because it was expressed most strongly in the root tissues. Functional complementation was performed using poplar plants to determine whether ARF4 functions downstream in the miR390/TAS3 context (Fig. 4, E–G). A tasiARF-resistant ARF4 (ARF4m) containing mutated dual tasiARF-binding sites was introduced into the miR390-OE background (Supplemental Fig. S8A). ARF4 was transcribed markedly more in the 35S:ARF4m/miR390-OE plants than in the miR390-OE plants (Supplemental Fig. S8B). Phenotypic analysis indicated that introducing ARF4m compromised LR development of the miR390-OE plants (Fig. 4E), as indicated by LR length and density decreases of 52% and 35%, respectively (Fig. 4, F and G). ARF4m could phenotypically mimic miR390 knockdown, as indicated by the similar phenotypes of the ARF4m-OE and miR390-STTM lines (Fig. 4, E–G). We conclude that ARF4 can mediate miR390-dependent LR development.
ARF4 Negatively Regulates Plant Growth and Salt Tolerance
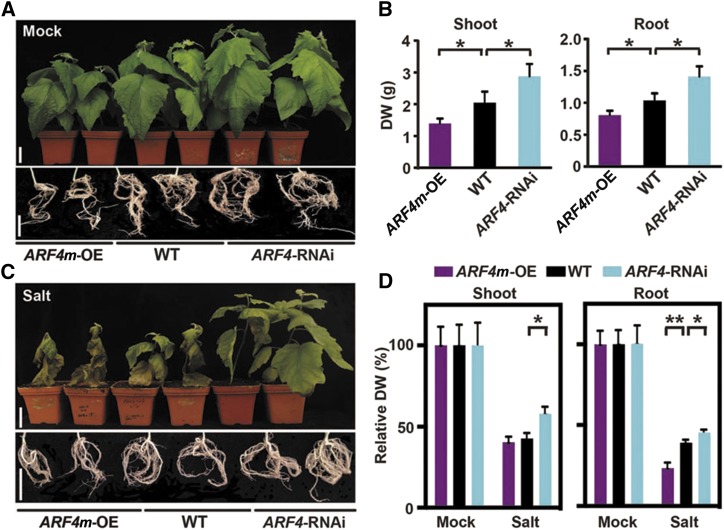
The vectors harboring 35S:ARF4 with the disrupted dual tasiARF-binding sites (ARF4m-OE) and RNA-interfering (RNAi)-based ARF4 knockdown (ARF4-RNAi) were transformed into wild-type poplar plants to evaluate the roles of ARF4 in the developmental phenotypes and salt responses in poplar. ARF4m expression in transgenic plants was determined by RT-qPCR (Supplemental Fig. S9, A and B). The transgenic plants were vegetatively propagated, and lines displaying stable phenotypes in vitro (Supplemental Fig. S9C) and in a greenhouse (Fig. 5A) were selected for further analysis. Under in vitro conditions, LR elongation and density were suppressed markedly by overexpression of the tasiARF-resistant ARF4 (Supplemental Fig. S9, C–E). The dry shoot biomass was 31.9% lower in the ARF4m-OE plants cultivated in soil than in the wild-type plants and up to 40.7% higher in the ARF4-RNAi plants than in the wild-type plants (Fig. 5B). LR growth also was inhibited markedly in the ARF4m-OE lines but enhanced in the ARF4-RNAi lines (Fig. 5, A and B).
Figure 5.
ARF4 negatively regulates plant growth and salt tolerance in poplar. A, Shoot and root phenotypes of wild-type (WT), ARF4m-OE, and ARF4-RNAi plants cultivated in soil for 10 weeks. Bars = 5 cm. B, Quantitative measurements of shoot and root biomass (dry weight [DW]) of wild-type, ARF4m-OE, and ARF4-RNAi plants cultivated in soil for 10 weeks. Error bars represent sd. Asterisks indicate statistically significant differences in one-way ANOVA followed by Dunnett’s test for pairwise comparisons between wild-type and transgenic lines (*, P < 0.05; n = 5). C, Shoot and root phenotypes of wild-type, ARF4m-OE, and ARF4-RNAi plants under salt treatment. Poplar plants were cultivated in soil for 4 weeks and subsequently watered with 200 mm NaCl for 6 weeks. Bars = 5 cm. D, Relative quantification of shoot (E) and root (F) biomass (dry weight) of wild-type, ARF4m-OE, and ARF4-RNAi plants under salt treatment. Poplar plants were cultivated and salinity treated as described in C. The values for each genotype under mock treatment were normalized to 100%. Nonnormalized values are given in Supplemental Figure S10, C and D. Error bars represent sd. Asterisks indicate statistically significant differences in two-way ANOVA followed by Dunnett’s test for pairwise comparisons between wild-type and transgenic lines (*, P < 0.05 and **, P < 0.01; n = 5).
Given that ARF4 expression was suppressed in the presence of salt (Fig. 4D), we explored whether ARF4 contributed to salt tolerance (Fig. 5, C and D; Supplemental Figs. S9 and S10). Wild-type, ARF4m-OE, and ARF4-RNAi seedlings were exposed to NaCl at different concentrations (Supplemental Fig. S9F). After 4 weeks, LR growth was clearly inhibited in all the seedlings exposed to 50 and 75 mm NaCl. The LRs were shorter and there were fewer LRs in the ARF4m-OE than in the wild-type plants (Supplemental Figs. S9, F–H, and S10, A and B). In contrast, markedly less LR growth inhibition by NaCl was found in the ARF4-RNAi plants (Supplemental Fig. S9, F–H). Wild-type and transgenic plants were cultivated in soil and treated with 200 mm NaCl for 6 weeks. The ARF4-RNAi plants were larger and had more salt-tolerant phenotypes than the wild-type plants, but constitutive tasiARF-resistant ARF4 expression led to retarded growth and more severe symptoms of salt toxicity (Fig. 5, C and D; Supplemental Fig. S10, C and D). We conclude that ARF4 negatively regulates biomass accumulation and salt tolerance in poplar, in agreement with the negative regulation of ARF4 expression by miR390.
Antagonistic Regulation of Auxin Signaling in LRs by miR390 and ARF4
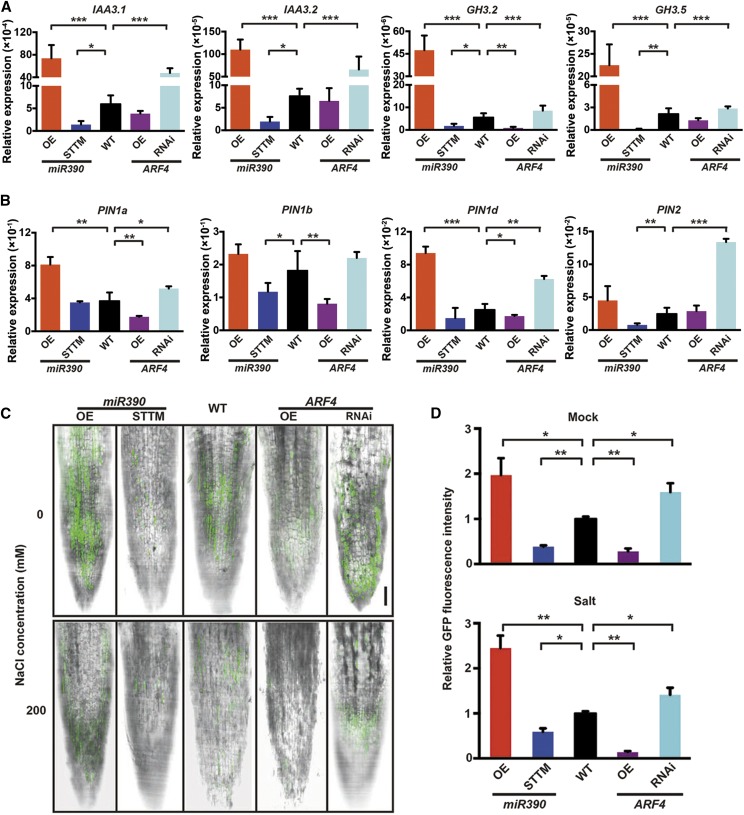
The molecular components involved in the auxin pathway were determined by RT-qPCR to determine the effect of miR390/TAS3/ARF on auxin accumulation and the auxin response. A few typical Aux/IAA (IAA3.1 and IAA3.2) and GH3 (GRETCHEN HAGEN3; GH3.2 and GH3.5) genes (which belong to classic categories of early auxin-responsive genes in various plant species; Guilfoyle, 1999) were transcribed more in the miR390-OE and ARF4-RNAi lines and less in the miR390-STTM and ARF4m-OE lines (Fig. 6A). Similar expression patterns were found in these transgenic lines for several PIN genes (Fig. 6B), which are involved in polar auxin transport in Populus spp. root tissues (Liu et al., 2014).
Figure 6.
Auxin accumulation is altered by miR390 and ARF4 in LR tips. A, Expression levels of typical auxin-responsive genes, including IAA3.1/3.2 and GH3.2/3.5, in LRs of miR390/ARF4 transgenic lines. LRs of 4-week-old seedlings of wild-type (WT), miR390-OE/STTM, and ARF4m-OE/ARF4-RNAi lines cultivated in vitro were collected for RT-qPCR assay. 18S rRNA was used as a reference for normalization. Error bars represent sd. Asterisks indicate statistically significant differences in one-way ANOVA followed by Dunnett’s test for pairwise comparisons between wild-type and transgenic lines (*, P < 0.05; **, P < 0.01; and ***, P < 0.001; n = 3). B, Expression quantification of select PIN genes, including PIN1a, PIN1b, PIN1d, and PIN2, in root tissues of miR390/ARF4 transgenic plants. RT-qPCR assay and statistical analysis were performed as described in A. C, Detection of GFP fluorescence driven by the auxin-responsive DR5 promoter in LR tips of the miR390/ARF4 transgenic lines under salt treatment. Four-week-old seedlings of wild-type, miR390-OE/STTM, and ARF4m-OE/ARF4-RNAi lines harboring the DR5 promoter were subjected to 0 mm (mock) or 200 mm NaCl (salt) for 24 h. LR tips were detected for GFP fluorescence via confocal microscopy. Bar = 10 μm. D, Quantitative measurement of GFP fluorescent signals driven by the auxin-responsive DR5 promoter in LR tips of the miR390/ARF4 transgenic lines under salt treatment. Salt treatment and fluorescence detection were conducted as described in C. Fluorescence was quantified via the software FV10 ASW (Olympus). Error bars represent sd. Asterisks indicate statistically significant differences in one-way ANOVA followed by Dunnett’s test for pairwise comparisons between wild-type and transgenic lines (*, P < 0.05 and **, P < 0.01; n = 5).
Local auxin accumulation is an early signal of LR formation (Dubrovsky et al., 2008); therefore, we investigated whether miR390 and ARF4 affect endogenous auxin signaling in the transgenic lines. The DR5:GFP reporter, a biosensor of in vivo auxin signaling (Ottenschläger et al., 2003), was introduced to indicate miR390/ARF4-induced modifications of the endogenous auxin concentration and signaling in the LR tips (Fig. 6C). The GFP fluorescent signals driven by the auxin-responsive DR5 promoter were stronger in the LR tips of the miR390-OE and ARF4-RNAi plants than in the wild-type plants but were weaker in the miR390-STTM and ARF4m-OE plants than in the wild-type plants (Fig. 6, C and D). Exposure to salt decreased DR5:GFP expression in the wild-type plants, and the effect was much stronger in the miR390-STTM and ARF4m-OE plants than in the wild-type plants (Fig. 6, C and D). Conversely, DR5:GFP expression was less affected in the salt-stressed miR390-OE and ARF4-RNAi plants than in the salt-stressed wild-type plants (Fig. 6, C and D). These results suggest that, in poplar plants, miR390 and ARF4 antagonistically regulate endogenous auxin signaling during LR development in response to salinity.
IAA17 Can Block miR390/ARF4-Mediated LR Development under Salt Stress
As reported in Arabidopsis, salt inhibition of root meristematic activity is caused by modified PIN-mediated auxin accumulation and Aux/IAA-dependent auxin signaling (Liu et al., 2015). Modulated PIN gene expression and auxin signaling as indicated by the DR5 sensor in the poplar LRs (Fig. 6) suggest that the auxin pathway may mediate miR390/ARF4-regulated LR growth and salt tolerance. Firmly establishing auxin-dependent miR390/ARF4 regulation of salt-responsive LR growth required us to investigate what happens to the miR390/ARF4 root phenotypes when auxin signaling is blocked. Nucleus-localized Aux/IAA proteins are key repressors of auxin signaling (Rouse et al., 1998). These proteins usually contain short amino acid sequences (VGWPPV) called Degron motifs, which allow auxin-induced protein turnover via the E3 ubiquitin ligase complex SCFTIR1 (Worley et al., 2000; Tan et al., 2007). Salt stress in Arabidopsis enhances the protein stabilization of IAA17, a canonical Aux/IAA protein that represses auxin signaling (Liu et al., 2015). Mutation of the Degron motif in IAA17, which compromises auxin-dependent protein instability, correspondingly leads to the root tissues becoming insensitive to salt stress (Liu et al., 2015). The poplar genome has two homologous members of IAA17, called IAA17.1 and IAA17.2 (Supplemental Fig. S11A). The protein IAA17.1 is more similar than IAA17.2 to Arabidopsis IAA17 and has an identical Degron motif in domain II to the motif in Arabidopsis IAA17 (Supplemental Fig. S11A). RT-qPCR showed that IAA17.1 is preferentially expressed in the root tissues (Supplemental Fig. S11B). The promoter-driving GUS reporter assays confirmed that IAA17.1 is expressed in the roots, especially in the LR primordia and tips (Supplemental Fig. S11C).
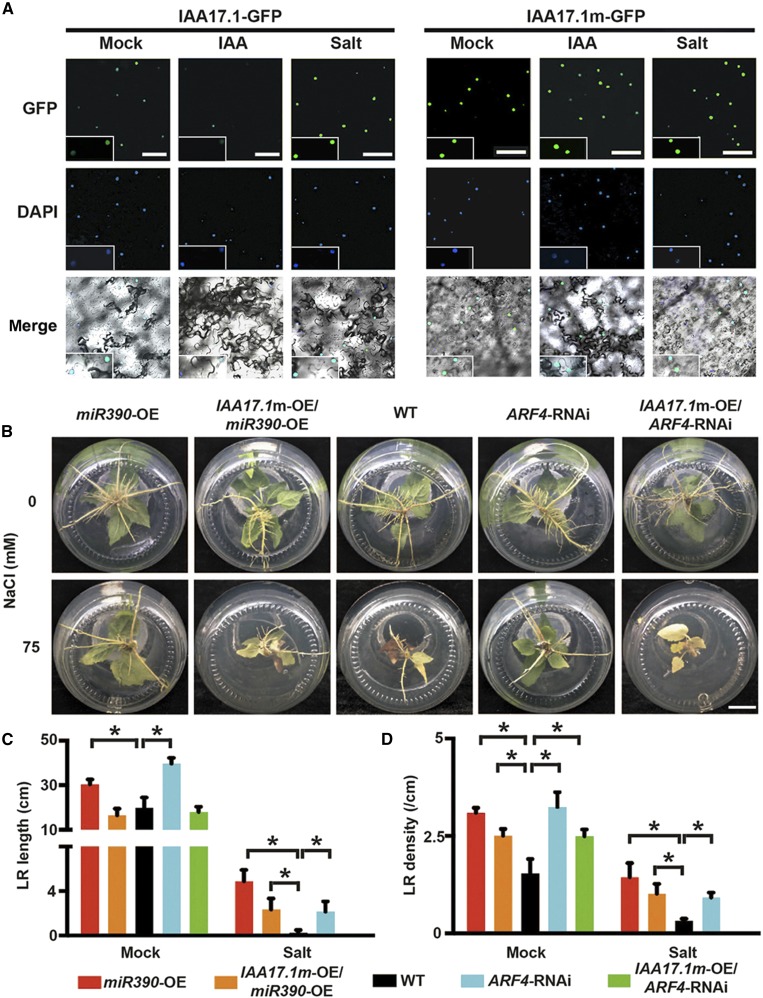
The auxin-dependent regulation of salt-induced root modifications in poplar by miR390/ARF4 was tested using IAA17.1 to block auxin signaling. We first determined whether the stability of the poplar IAA17.1 protein was affected by auxin and salt in the same way as the Arabidopsis IAA17 protein (Fig. 7A). Evolutionary conservation of auxin signaling, including the Aux/IAA protein family (Paponov et al., 2009), means that transient expression in a heterologous system can be used as an efficient method of studying the stability of Aux/IAA proteins in response to auxin (von Behrens et al., 2011; Ludwig et al., 2014). Therefore, auxin/salt-responsive IAA17.1 protein turnover was monitored by fusing the protein with a GFP tag in a transiently expressed Nicotiana benthamiana leaf epidermis. As expected, auxin induced IAA17.1 protein instability, as indicated by decreased GFP fluorescence in leaf epidermal cells exposed to indole-3-acetic acid (Fig. 7A). In contrast, the number of green fluorescent cells and GFP fluorescence increased in the presence of NaCl, indicating that IAA17.1 protein turnover was suppressed (Fig. 7A). To determine whether the Degron motif mediates the change in protein turnover caused by auxin and salt, a Degron-mutated IAA17.1 protein, IAA7.1m, with the first Pro replaced through Ser substitution (Supplemental Fig. S11D), was generated as described previously (Li et al., 2011). GFP fluorescence was stronger in leaf epidermal cells transiently transformed by IAA17.1m with an impaired Degron motif than in the cells transformed by the wild-type IAA17.1 form (Fig. 7A). The fluorescence emitted by IAA17.1m-GFP was affected neither by exogenous auxin nor by the presence of salt (Fig. 7A), indicating that the auxin-responsive IAA17.1 protein was stabilized by salt, depending on the Degron motif. Therefore, the auxin/salt-resistant version of IAA17.1 (IAA17.1m) was introduced into the miR390-OE and ARF4-RNAi lines (Supplemental Fig. S11, E and F) to allow the auxin-dependent machinery of miR390/ARF4-mediated salt tolerance of LR growth to be studied. Transgenic IAA17.1m-OE/miR390-OE and IAA17.1m-OE/ARF4-RNAi plants were more sensitive to salt stress than miR390-OE and ARF4-RNAi plants in terms of LR inhibition (Fig. 7B). The LR lengths and densities in the miR390-OE/ARF4-RNAi plants were at least partly recovered, compared with those in the wild type, by introducing IAA17.1m in the presence of salt (Fig. 7, C and D). These results indicate that miR390/TAS3/ARF-dependent regulation of salt-responsive LR development in poplar plants is mediated by auxin signaling.
Figure 7.
IAA17.1, an Aux/IAA protein, is required for miR390/ARF4-mediated LR development under salt stress. A, Auxin/salt-responsive protein stability of IAA17.1 indicated by fused GFP tag. IAA17.1m, an auxin-resistant form of IAA17.1, was generated by a Ser substitution for the first Pro in the Degron motif of domain II (Supplemental Fig. S11D). GFP-tagged IAA17.1 and IAA17.1m were transiently expressed in epidermal cells of N. benthamiana leaves via Agrobacterium tumefaciens-mediated infiltration. The transformed leaves were treated with auxin or salt for 2 h: 50 μm cycloheximide (CHX, an inhibitor of protein synthesis in eukaryote cells; mock treatment), 50 μm CHX and 20 μm indole-3-acetic acid (IAA; auxin treatment), and 50 μm CHX and 200 mm NaCl (salt treatment). The nuclei of transfected epidermal cells were indicated by 4,6-diamidino-2-phenylindole (DAPI; working solution, 1 μg mL−1). GFP and DAPI fluorescence was determined via confocal microscopy. Bars = 25 μm. B, Root phenotypes of IAA17.1m transgenic seedlings in the miR390-OE or ARF4-RNAi background under mock and salt treatment. The cutting-propagated seedlings of the wild type (WT), miR390-OE, IAA17.1m-OE/miR390-OE, ARF4-RNAi, and IAA17.1m-OE/ARF4-RNAi were cultivated in vitro and subjected to 0 mm (mock) or 75 mm NaCl (salt) for 4 weeks. Bar = 2 cm. C and D, Quantification of total LR length (C) and LR density (D) of IAA17.1m transgenic seedlings in the miR390-OE or ARF4-RNAi background under mock and salt treatment. Salt treatment was performed as described in B. Error bars represent sd. Asterisks indicate statistically significant differences in two-way ANOVA followed by Dunnett’s test for pairwise comparisons between wild-type and transgenic lines (*, P < 0.05; n = 5).
DISCUSSION
Salt stress profoundly affects the architecture of plant roots. Affected plants show symptoms of toxicity, and their root systems display active salt-responsive developmental plasticity (e.g. growth maintenance and halotropism), which strongly facilitates increased salt tolerance (Galvan-Ampudia and Testerink, 2011; Galvan-Ampudia et al., 2013). We found that miR390, an evolutionarily conserved miRNA in plants, is induced by salt during the formation of LR in poplar plants. Phenotypic analysis and functional complementation via stable transformations in poplar suggested that miR390/TAS3/ARF4-dependent salt tolerance occurs via the maintenance of auxin-mediated LR growth. Our results provide evidence that salt-inducible miR390 expression is an adaptive strategy for maintaining growth in poplar plants in saline environments.
In in vitro tests, the root systems of 4-week-old poplar seedlings usually have two types of roots: several stem-borne main roots and many LRs emerging from the main roots. The lengths and densities of LRs were both found to be positively regulated by miR390 (Fig. 2, B and C), whereas the lengths and numbers of main roots were not affected (Supplemental Fig. S3, D and E). These results show that miR390 specifically regulates LR phenotypes, probably through expression to different degrees in main roots and LRs (Supplemental Fig. S2B). This accords with the miR390/TAS3/ARF-dependent LR formation in Arabidopsis described previously (Marin et al., 2010; Yoon et al., 2010). Salt-induced miR390 expression also was specific to LR development. Salt-inducible miR390 expression was found in both LR meristems and LR primordia (Fig. 1B; Supplemental Fig. S2D) and accounts for the maintenance of both LR elongation and initiation. Weak induction in stele tissues of main roots was found, but no salt-stimulated miR390 expression occurred in the main root tips (Supplemental Fig. S2D). The variable salt-inducible expression patterns matched the miR390-dependent LR-specific poplar phenotypes. These results suggest that root type-specific mechanisms responsive to salt occur in poplar plants, as proposed for Arabidopsis (Geng et al., 2013).
Auxin is the main triggering signal for root development and plays key roles in salt-induced root morphological and meristematic modulations (Galvan-Ampudia and Testerink, 2011; Liu et al., 2015). The current auxin signaling model suggests that auxin is transduced into nuclei to drive auxin-responsive gene expression (Vanneste and Friml, 2009). IAA3 is a typical early auxin-responsive gene that can affect the sensitivity of LR formation in poplar to auxin (Nilsson et al., 2008). GH3.2 and GH3.5 belong to the primary auxin-responsive GH3 gene family that encodes IAA-amide synthetases, mutations of which increase the resistance of LR formation to exogenous auxin (Staswick et al., 2005). Modulated expression of these primary auxin-responsive genes (Fig. 6A) shows the miR390/ARF4-regulated auxin responses. The distribution of auxin is determined mainly by PIN-mediated auxin transport in poplar plants (Palme and Gälweiler, 1999; Friml et al., 2002). In Arabidopsis, PIN1 displays salt-inhibited protein accumulation in the root meristem (Liu et al., 2015) and PIN2 mediates salt-induced halotropism in the root system (Galvan-Ampudia et al., 2013). The PIN1 and PIN2 members are expressed similarly in different poplar root tissues, including LR tips (Liu et al., 2014). The transcript abundances of several PIN1/PIN2 genes (Fig. 6C) implied that miR390/ARF4-dependent local accumulation of auxin could occur. The subsequent DR5-indicated auxin signaling reveals that miR390/ARF4 orchestrates the accumulation of auxin in LRs under normal and saline conditions (Fig. 6). More importantly, switching off auxin signaling by stably expressing an auxin/salt-resistant version of the IAA17 protein decreased salt-responsive LR growth regulated by miR390/ARF4 to a remarkable degree (Fig. 7). These results together demonstrate that the miR390/ARF4 module maintains salt-tolerant LR growth by attenuating the inhibition of auxin signaling by salt stimuli.
In addition to maintaining LRs in a saline environment, miR390 alleviates salt toxicity to aboveground growing tissues (Fig. 3, D and E). No salt-inducible expression was detected in the leaves (Supplemental Fig. S2D), so the modulation of downstream molecular events mediated by miR390 may not occur in polar shoot tissues. It has been suggested that larger plants are more salt tolerant than smaller plants (Julkowska et al., 2016). Therefore, we speculate that salt-tolerant phenotypes of aboveground growth in miR390-OE lines may be a secondary effect of maintaining root development. Ion exclusion and osmotic tolerance may play roles in these effects (Roy et al., 2014); therefore, further evidence is required to account for salt resistance caused by miR390.
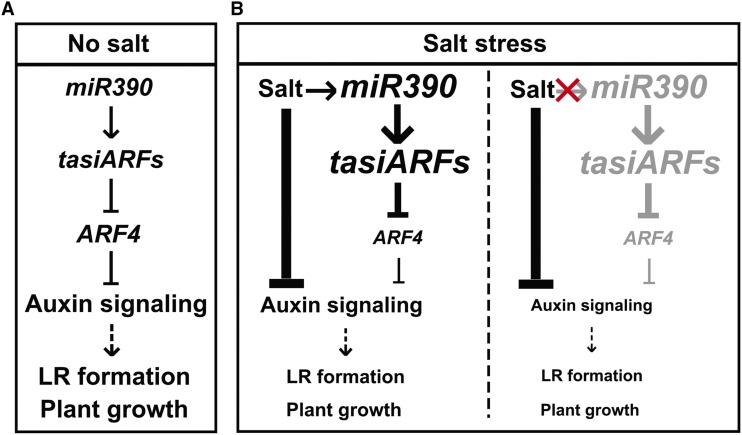
The results of this study allowed a model of miR390/ARF4-dependent LR growth in poplar under normal and saline conditions to be devised (Fig. 8). miR390 produces TAS3-derived tasiARFs that target ARF4 transcripts (Figs. 4 and 8A). The repression of ARF4 releases auxin, which triggers LR initiation and elongation in poplar plants (Figs. 6 and 8A). In highly saline environments, salt markedly inhibits auxin signaling and induces miR390 expression (Figs. 6 and 8B, left). The salt-induced expression of miR390 stimulates the production of tasiARFs for the degradation of ARF4 transcripts (Figs. 1, 4, and 8, left). Attenuated expression of ARF4 facilitates the maintenance of auxin signaling against salt inhibition (Figs. 5, 6, and 8B, left). If the salt induction of miR390 did not occur, auxin signaling and LR formation would be more seriously inhibited by salt (Fig. 8B, right). The miR390/tasiARF/ARF4 module is thereby essential for sustaining LR formation under salinity and salt-tolerant plant growth. In summary, the miR390/TAS3/ARF4 module is involved in LR maintenance against salt toxicity by modulating auxin signaling. This is a molecular strategy for improving the adaptability of poplar plants to saline conditions.
Figure 8.
Proposed model for miR390/ARF4-dependent poplar LR growth under normal and salt conditions. A, miR390 produces TAS3-derived tasiARFs that target ARF4 transcripts via direct binding. The repression of ARF4 releases auxin signaling for LR initiation and elongation. B, Left, Salt remarkably inhibits auxin signaling and induces miR390 expression. The salt-induced expression of miR390 stimulates the production of tasiARFs for the degradation of ARF4 transcripts. Attenuated expression of ARF4 facilitates the maintenance of auxin signaling against salt inhibition. Right, If the salt induction of miR390 does not occur, auxin signaling and LR formation are more seriously inhibited by salt. The miR390/tasiARF/ARF4 module is thereby essential for sustaining LR formation under salinity and salt-tolerant plant growth. Arrows represent a positive regulatory action of one component on another. Lines ending with a trait represents a negative regulatory action. The font size of words and the thickness of arrows/lines in B indicate positive (larger/thicker) or negative (smaller/thinner) regulation compared with that under the normal condition (no salt). The red cross and gray words (right) indicate the hypothetical absence of salt induction of the miR390/tasiARFs/ARF4 module.
MATERIALS AND METHODS
Gene Cloning and Vector Construction
The precursor of miR390a was amplified using the primers Pre-miR390a-OE-fw/rv (Supplemental Table S1) from genomic DNA of Populus trichocarpa and ligated via TA-cloning into the pCXSN vector (Chen et al., 2009) under the control of the cauliflower mosaic virus 35S promoter. The STTM-based knockdown construct of miR390 was generated according to a previous report (Yan et al., 2012). Briefly, a pair of primers mimicking the sequences of the miR390-binding site with an insertion of AGA (miR390-STTM-BamHI-fw/rv; Supplemental Table S1) was used for the amplification of the 88-bp spacer (Supplemental Fig. S3B) from a de novo synthesized oligonucleotide fragment containing the spacer sequence. The PCR product was then constructed into the pCXSN vector via BamHI restriction sites (Supplemental Fig. S3B). A promoter fragment of 1,509 bp upstream of the miR390 precursor was amplified from the genomic DNA of Populus tomentosa using the primers Pro-miR390-BamHI-fw/rv (Supplemental Table S1) and constructed via BamHI restriction sites into pXGUS-P to drive the expression of GUS (Chen et al., 2009). The full-length coding sequence of ARF4 was amplified from the cDNA of P. trichocarpa using the primers ARF4-CDS-fw/rv (Supplemental Table S1) and constructed into pCXSN for sequencing via homologous recombination. Due to the presence of dual tasiARF-binding sites, overlap PCR was conducted twice to generate tasiARF-resistant ARF4 (ARF4m) using the primer pairs ARF4-BglII-fw/ARF4m-1-rv, ARF4m-1-fw/ARF4-BglII-rv, ARF4-BglII-fw/ARF4m-2-rv, and ARF4m-2-fw/ARF4-BglII-rv (Supplemental Table S1). The resulting ARF4m fragment was constructed into modified pCAMBIA1305 with the replacement of hygromycin by kanamycin for positive selection of transgenic plants via BglII restriction sites. The ARF4-RNAi vector was constructed via intron-containing hairpin RNA silencing (Wesley et al., 2001). Briefly, a 223-bp sequence of ARF4 was amplified using the primer pair ARF4-RNAi-BamHI-fw/ARF4-RNAi-rv (Supplemental Table S1). Meanwhile, for a spacer, a 388-bp fragment of the GUS gene was prepared using the primers Spacer-RNAi-fw/rv (Supplemental Table S1), both of which contain the reverse complementary sequence of the primer ARF4-RNAi-rv at the 5′ end. Subsequently, the hairpin structure harboring sense/antisense arms separated by an oligonucleotide fragment of GUS was generated via overlap PCR using ARF4-RNAi-BamHI-fw and cloned into pCXSN via BamHI restriction sites. The full-length coding sequence of IAA17.1 was amplified from cDNA using the primers IAA17.1-stop-BamHI-fw/rv (Supplemental Table S1) and constructed into modified pCAMBIA1305 with the kanamycin-resistant gene under the control of the 35S promoter. The mutation of its Degron motif (IAA17.1m; Supplemental Fig. S11B) was introduced via overlap PCR using the primer pairs IAA17.1-stop-BamHI-fw/IAA17.1m-rv and IAA17.1m-fw/IAA17.1-stop-BamHI-rv (Supplemental Table S1). The sequences of IAA17.1 and IAA17.1m without the TGA stop codon were subcloned into pCAMBIA1300-GFP to fuse with the GFP tag using the primers IAA17.1-non-stop-BamHI-fw/rv (Supplemental Table S1). A promoter region of 1,809 bp upstream of the ATG start codon of IAA17.1 was amplified using the primers Pro-IAA17.1-SalI-fw/PstI-rv (Supplemental Table S1) and cloned into pXGUS-P via TA-cloning to drive GUS expression. Multiple sequence alignments were conducted using DNAMAN8 (Lynnon Biosoft), and phylogenetic construction was performed with MEGA6.06 (Tamura et al., 2013).
Generation of Transgenic Poplar Lines
For functional characterization, the pCXSN vectors for the overexpression of miR390a, ARF4m, and IAA17.1m, miR390-STTM and ARF4-RNAi, were stably transformed into P. tomentosa (clone 741) plants. For functional complementation, 35S:ARF4m was transformed into the miR390-OE line while 35S:IAA17.1m was transformed into the miR390-OE or ARF4-RNAi transgenic line. For promoter analyses, the pXGUS-P vectors harboring the GUS gene driven by the promoter of miR390a or IAA17.1 were transformed into wild-type plants. For the detection of auxin signaling, the DR5-GFP plasmid was transformed into the wild-type, miR390-OE/STTM, and ARF4m-OE/ARF4-RNAi lines, respectively. Genetic transformation of poplar was conducted via Agrobacterium tumefaciens-mediated infiltration of leaf discs as described previously (Jia et al., 2010). PCR genotyping for positive transgenic lines was performed through amplifying hygromycin- or kanamycin-resistant genes from genomic DNA (with primers Hyg or Kana-fw/rv; Supplemental Table S1).
Growth Conditions and Salt Treatment of Poplar
Transgenic and wild-type poplar plants were propagated via in vitro microcutting. Shoot segments of 3 to 4 cm with two to three young leaves were cut from sterilized seedlings and cultivated on sterile WPM (Jia et al., 2010) for 4 weeks at 25°C with 16 h of light of 5,000 lx and 8 h of dark. For salt stress, shoot microcuttings were cultivated for 4 weeks on the WPM supplemented with 25, 50, and 75 mm NaCl in parallel with the salt-free mock treatment. A digital camera (EOS 550D; Canon) was used to take photographs of root systems, and ImageJ (https://imagej.nih.gov/ij/) was used to quantify root parameters. The experiments under in vitro conditions were conducted using three batches of plant materials including five biological replicates (an individual plant represents a biological replicate) each time.
For physiological experiments in soil, 4-week-old microcutting-propagated plants of wild-type and transgenic lines were transferred to soil in pots and cultivated for 4 weeks in a greenhouse at 23°C to 25°C with light of 10,000 lx under a 16/8-h day/night cycle. The plants were irrigated subsequently with 0 (mock treatment) or 200 mm NaCl (salt treatment) every 2 d for 6 weeks. Afterward, root and aboveground tissues were collected for dry weight measurement. The experiments in soil were performed using two batches of plant materials including five biological replicates (an individual plant represents a biological replicate) each time.
RT-qPCR
Root tissues of 4-week-old seedlings of wild-type and transgenic lines cultivated under in vitro conditions were frozen in liquid nitrogen for gene expression assays via RT-qPCR. Four-week-old wild-type seedlings were subjected to 200 mm NaCl for 6, 12, and 24 h, and their root tissues were collected in liquid nitrogen for RNA isolation. The RNAs for tissue-specific expression quantification were prepared from 10-week-old plants grown in soil, including the tissues of roots, stem, the first two leaves on the top (young leaves), the fifth and sixth leaves (middle leaves), and the two lowest leaves aboveground (old leaves). Trizol reagent was used for total RNA extraction from the tissues frozen in liquid nitrogen according to the product manual. cDNA synthesis and RT-qPCR were performed using the PrimeScript RT Reagent Kit (Takara) in a TP800 Real-Time PCR machine (Takara) as described by the instructions. The poplar 18S rRNA was used as a reference gene for normalization of the expression data. The transcript levels of TAS3.1/3.2, ARF4/3.1/3.2/2.1/2.2/2.3/2.4/2.5, IAA17.1/3.1/3.2, GH3.2/3.5, and PIN1a/1b/1d/2 were determined using gene-specific primer pairs (Supplemental Table S1). The expression levels of mature miR390 and tasiARFs were assayed via stem-loop RT-qPCR, a method for the sensitive and specific quantification of small RNAs (Varkonyi-Gasic et al., 2007). The U6 snRNA was used for data normalization of miR390 and tasiARF expression. The primers for stem-loop reverse transcription, the specific forward primers, and the universal reverse primer are listed in Supplemental Table S1. Due to nucleotide variations at the 3′ end of tasiARF1, tasiARF2, and tasiARF3, degenerate primers were designed for stem-loop reverse transcription of tasiARFs (Supplemental Table S1). The expression data were calculated with the ΔΔCt method. The RT-qPCR assays were performed in three biological replicates (a mixture of at least two individual plants represents a biological replicate) together with three technical replicates for each gene.
GUS Staining
The positive GUS reporter lines driven by the promoter of miR390a and IAA17.1 were cultivated on WPM for 4 weeks and then histologically stained. For the detection of salt-inducible expression, the miR390a-promoter-GUS seedlings were subjected to 200 mm NaCl for 6 h before histological staining. The seedlings were incubated in staining buffer (0.5 m Tris, pH 7, and 10% (v/v) Triton X-100) with 1 mm 5-bromo-4-chloro-3-indolyl-d-glucuronide at 37°C for 6 h in dark. Chlorophyll was removed using an ethanol series: 20%, 35%, and 50% (v/v) ethanol at room temperature for 30 min each. The chlorophyll-free stained seedlings were observed with an Olympus SZX16 microscope and documented with a camera (Olympus DP73).
DR5-GFP Detection
The DR5-GFP plasmid (Ottenschläger et al., 2003) was stably transformed into the positive poplar transgenic lines of the wild type, miR390-OE/STTM, and ARF4m-OE/RNAi using A. tumefaciens-mediated infiltration of leaf discs as described previously (Jia et al., 2010). Four-week-old DR5-GFP reporter plants cultivated on WPM solid medium were carefully shifted to liquid WPM containing 0 mm (mock) or 200 mm NaCl (salt) and treated for 24 h. LR tips were detected afterward for the DR5-driving GFP fluorescence via a confocal microscope (Olympus FV1200). GFP was excited at 488 nm by an argon laser, and the emission was collected with a 505- to 530-nm bandpass filter. The fluorescence was quantified via the software FV10 ASW (Olympus).
Protein Stability Assay of IAA17.1
The constructs of IAA17.1 and IAA17.1m fused with GFP were transiently transformed into the leaves of 4-week-old Nicotiana benthamiana via A. tumefaciens-mediated infiltration. The transformed N. benthamiana leaves were treated with auxin or salt for 2 h as follows: 50 μm CHX (mock treatment), 50 μm CHX and 20 μm IAA (auxin treatment), and 50 μm CHX and 200 mm NaCl (salt treatment). The nuclei of transfected epidermal cells were stained by DAPI (working solution, 1 μg mL−1). Fluorescent signals of GFP and DAPI were documented using a confocal laser microscope (Olympus FV1200).
GFP fluorescence was viewed as described above. The DAPI signal was excited at 405 nm, and the emission was collected with a 450- to 480-nm bandpass filter.
Statistical Analyses
Quantitative data for expression analyses, GUS activities, fluorescence intensities, and physiological measurements were determined for statistical significance using Student’s t test and one-way or two-way ANOVA as described in the corresponding figure legends. For ANOVA, Dunnett’s test was performed to distinguish significant differences between pairwise samples (*, P < 0.05; **, P < 0.01; and ***, P < 0.001).
Accession Numbers
The sequences used in this study are available in Phytozome (version 11.0) under the following numbers: miR390a (MI0002305), miR390b (MI0002306), miR390c (MI000230), miR390d (MI0002308), ARF2.1 (Potri.012G106100.1), ARF2.2 (Potri.015G105300.1), ARF2.3 (Potri.002G207100.1), ARF2.4 (Potri.001G066200.1) ARF2.5 (Potri.003G163600.1), ARF3.1 (Potri.004G050200.1), ARF3.2 (Potri.011G059300.1), ARF3.3 (Potri.011G059100.1), ARF4 (Potri.009G011800.1), IAA3.1 (Potri.005G053800.1), IAA3.2 (Potri.013G041300.1), GH3.2 (Potri.001G298300.1), GH3.5 (Potri.001G410400.1), PIN1a (Potri.012G047200.1), PIN1b (Potri.015G038700.1), PIN1c (Potri.006G037000.1), and PIN2 (Potri.018G139400.1).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Sequence analysis and secondary structure prediction of poplar miR390s.
Supplemental Figure S2. Tissue-specific expression and promoter analyses of miR390s in poplar.
Supplemental Figure S3. Generation of miR390-OE and -STTM transgenic poplar lines.
Supplemental Figure S4. miR390 target sites and tasiARFs harbored in the poplar TAS3 genes.
Supplemental Figure S5. Phylogenetic analysis of ARF2/3/4 and the binding sites for tasiARFs within their transcripts.
Supplemental Figure S6. Expression levels of tasiARFs and ARF2s in miR390-OE and -STTM transgenic lines.
Supplemental Figure S7. Tissue-specific expression levels of ARF3.1, ARF3.2, and ARF4 in poplar tissues determined by RT-qPCR.
Supplemental Figure S8. Overexpression of ARF4 with disrupted binding sites of tasiARFs in the miR390-OE lines.
Supplemental Figure S9. Root phenotypes of ARF4m-OE and -RNAi transgenic seedlings exposed to mock and salt conditions.
Supplemental Figure S10. Nonnormalized data for root phenotypes of ARF4m-OE and -RNAi transgenic seedlings subjected to mock and salt conditions.
Supplemental Figure S11. Constitutive expression of IAA17.1m in the miR390-OE or ARF4-RNAi transgenic lines.
Supplemental Table S1. Sequences of oligonucleotide primers used in this study.
Acknowledgments
We thank Dr. Bangjun Wang (Southwest University) for kindly providing the DR5-GFP plasmid.
Footnotes
This work was supported by grants from the National Key Research and Development Program (2016YFD0600105 to K.L.), by the National Natural Science Foundation of China (31370672 to K.L. and 31500216 to C.X.), by Fundamental Research Funds for the Central Universities (XDJK2016B032 to C.X.), and by the National Undergraduate Innovation and Entrepreneurship Training Program of China (201601635021 to M.L.).
References
- Adenot X, Elmayan T, Lauressergues D, Boutet S, Bouché N, Gasciolli V, Vaucheret H (2006) DRB4-dependent TAS3 trans-acting siRNAs control leaf morphology through AGO7. Curr Biol 16: 927–932 [DOI] [PubMed] [Google Scholar]
- Allen E, Xie Z, Gustafson AM, Carrington JC (2005) MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121: 207–221 [DOI] [PubMed] [Google Scholar]
- Axtell MJ, Jan C, Rajagopalan R, Bartel DP (2006) A two-hit trigger for siRNA biogenesis in plants. Cell 127: 565–577 [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Cabrera J, Barcala M, García A, Rio-Machín A, Medina C, Jaubert-Possamai S, Favery B, Maizel A, Ruiz-Ferrer V, Fenoll C, et al. (2016) Differentially expressed small RNAs in Arabidopsis galls formed by Meloidogyne javanica: a functional role for miR390 and its TAS3-derived tasiRNAs. New Phytol 209: 1625–1640 [DOI] [PubMed] [Google Scholar]
- Chen S, Polle A (2010) Salinity tolerance of Populus. Plant Biol (Stuttg) 12: 317–333 [DOI] [PubMed] [Google Scholar]
- Chen S, Songkumarn P, Liu J, Wang GL (2009) A versatile zero background T-vector system for gene cloning and functional genomics. Plant Physiol 150: 1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luis A, Markmann K, Cognat V, Holt DB, Charpentier M, Parniske M, Stougaard J, Voinnet O (2012) Two microRNAs linked to nodule infection and nitrogen-fixing ability in the legume Lotus japonicus. Plant Physiol 160: 2137–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M (2005) Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9: 109–119 [DOI] [PubMed] [Google Scholar]
- Dickmann DI. (2006) Silviculture and biology of short-rotation woody crops in temperate regions: then and now. Biomass Bioenergy 30: 696–705 [Google Scholar]
- Duan L, Dietrich D, Ng CH, Chan PM, Bhalerao R, Bennett MJ, Dinneny JR (2013) Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell 25: 324–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benková E (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci USA 105: 8790–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlgren N, Montgomery TA, Howell MD, Allen E, Dvorak SK, Alexander AL, Carrington JC (2006) Regulation of AUXIN RESPONSE FACTOR3 by TAS3 ta-siRNA affects developmental timing and patterning in Arabidopsis. Curr Biol 16: 939–944 [DOI] [PubMed] [Google Scholar]
- Friml J, Benková E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jürgens G, et al. (2002) AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108: 661–673 [DOI] [PubMed] [Google Scholar]
- Galvan-Ampudia CS, Testerink C (2011) Salt stress signals shape the plant root. Curr Opin Plant Biol 14: 296–302 [DOI] [PubMed] [Google Scholar]
- Galvan-Ampudia CS, Julkowska MM, Darwish E, Gandullo J, Korver RA, Brunoud G, Haring MA, Munnik T, Vernoux T, Testerink C (2013) Halotropism is a response of plant roots to avoid a saline environment. Curr Biol 23: 2044–2050 [DOI] [PubMed] [Google Scholar]
- Garcia D, Collier SA, Byrne ME, Martienssen RA (2006) Specification of leaf polarity in Arabidopsis via the trans-acting siRNA pathway. Curr Biol 16: 933–938 [DOI] [PubMed] [Google Scholar]
- Geng Y, Wu R, Wee CW, Xie F, Wei X, Chan PM, Tham C, Duan L, Dinneny JR (2013) A spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. Plant Cell 25: 2132–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AR, Lorenz R, Bernhart SH, Neuböck R, Hofacker IL (2008) The Vienna RNA websuite. Nucleic Acids Res 36: W70–W74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle TJ. (1999) Auxin-regulated genes and promoters. In Hooykaas PJJ, Hall M, Libbenga KL, eds, Biochemistry and Molecular Biology of Plant Hormones. Elsevier, Leiden, The Netherlands, pp 423–459 [Google Scholar]
- Guilfoyle TJ, Hagen G (2007) Auxin response factors. Curr Opin Plant Biol 10: 453–460 [DOI] [PubMed] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51: 463–499 [DOI] [PubMed] [Google Scholar]
- Hobecker KV, Reynoso MA, Bustos-Sanmamed P, Wen J, Mysore KS, Crespi M, Blanco FA, Zanetti ME (2017) The microRNA390/TAS3 pathway mediates symbiotic nodulation and lateral root growth. Plant Physiol 174: 2469–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias MJ, Terrile MC, Windels D, Lombardo MC, Bartoli CG, Vazquez F, Estelle M, Casalongué CA (2014) MiR393 regulation of auxin signaling and redox-related components during acclimation to salinity in Arabidopsis. PLoS ONE 9: e107678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Pardo JM, Batelli G, Van Oosten MJ, Bressan RA, Li X (2013) The Salt Overly Sensitive (SOS) pathway: established and emerging roles. Mol Plant 6: 275–286 [DOI] [PubMed] [Google Scholar]
- Jia Z, Sun Y, Yuan L, Tian Q, Luo K (2010) The chitinase gene (Bbchit1) from Beauveria bassiana enhances resistance to Cytospora chrysosperma in Populus tomentosa Carr. Biotechnol Lett 32: 1325–1332 [DOI] [PubMed] [Google Scholar]
- Julkowska MM, Klei K, Fokkens L, Haring MA, Schranz ME, Testerink C (2016) Natural variation in rosette size under salt stress conditions corresponds to developmental differences between Arabidopsis accessions and allelic variation in the LRR-KISS gene. J Exp Bot 67: 2127–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K. (2013) Auxin and the integration of environmental signals into plant root development. Ann Bot 112: 1655–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Q, Kim HS, Wang Z, Ji CY, Jeong JC, Lee HS, Choi YI, Xu B, Deng X, Yun DJ, et al. (2017) Down-regulation of GIGANTEA-like genes increases plant growth and salt stress tolerance in poplar. Plant Biotechnol J 15: 331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser O (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435: 446–451 [DOI] [PubMed] [Google Scholar]
- Li H, Tiwari SB, Hagen G, Guilfoyle TJ (2011) Identical amino acid substitutions in the repression domain of auxin/indole-3-acetic acid proteins have contrasting effects on auxin signaling. Plant Physiol 155: 1252–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lei M, Yan Z, Wang Q, Chen A, Sun J, Luo D, Wang Y (2014) The REL3-mediated TAS3 ta-siRNA pathway integrates auxin and ethylene signaling to regulate nodulation in Lotus japonicus. New Phytol 201: 531–544 [DOI] [PubMed] [Google Scholar]
- Liu B, Zhang J, Wang L, Li J, Zheng H, Chen J, Lu M (2014) A survey of Populus PIN-FORMED family genes reveals their diversified expression patterns. J Exp Bot 65: 2437–2448 [DOI] [PubMed] [Google Scholar]
- Liu W, Li RJ, Han TT, Cai W, Fu ZW, Lu YT (2015) Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol 168: 343–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig Y, Berendzen KW, Xu C, Piepho HP, Hochholdinger F (2014) Diversity of stability, localization, interaction and control of downstream gene activity in the maize Aux/IAA protein family. PLoS ONE 9: e107346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HS, Liang D, Shuai P, Xia XL, Yin WL (2010) The salt- and drought-inducible poplar GRAS protein SCL7 confers salt and drought tolerance in Arabidopsis thaliana. J Exp Bot 61: 4011–4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin E, Jouannet V, Herz A, Lokerse AS, Weijers D, Vaucheret H, Nussaume L, Crespi MD, Maizel A (2010) miR390, Arabidopsis TAS3 tasiRNAs, and their AUXIN RESPONSE FACTOR targets define an autoregulatory network quantitatively regulating lateral root growth. Plant Cell 22: 1104–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651–681 [DOI] [PubMed] [Google Scholar]
- Nagasaki H, Itoh J, Hayashi K, Hibara K, Satoh-Nagasawa N, Nosaka M, Mukouhata M, Ashikari M, Kitano H, Matsuoka M, et al. (2007) The small interfering RNA production pathway is required for shoot meristem initiation in rice. Proc Natl Acad Sci USA 104: 14867–14871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Karlberg A, Antti H, Lopez-Vernaza M, Mellerowicz E, Perrot-Rechenmann C, Sandberg G, Bhalerao RP (2008) Dissecting the molecular basis of the regulation of wood formation by auxin in hybrid aspen. Plant Cell 20: 843–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenschläger I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K (2003) Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100: 2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme K, Gälweiler L (1999) PIN-pointing the molecular basis of auxin transport. Curr Opin Plant Biol 2: 375–381 [DOI] [PubMed] [Google Scholar]
- Paponov IA, Teale W, Lang D, Paponov M, Reski R, Rensing SA, Palme K (2009) The evolution of nuclear auxin signalling. BMC Evol Biol 9: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse D, Mackay P, Stirnberg P, Estelle M, Leyser O (1998) Changes in auxin response from mutations in an AUX/IAA gene. Science 279: 1371–1373 [DOI] [PubMed] [Google Scholar]
- Roy SJ, Negrão S, Tester M (2014) Salt resistant crop plants. Curr Opin Biotechnol 26: 115–124 [DOI] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, et al. (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Si J, Zhou T, Bo W, Xu F, Wu R (2014) Genome-wide analysis of salt-responsive and novel microRNAs in Populus euphratica by deep sequencing. BMC Genet (Suppl 1) 15: S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si-Ammour A, Windels D, Arn-Bouldoires E, Kutter C, Ailhas J, Meins F Jr, Vazquez F (2011) miR393 and secondary siRNAs regulate expression of the TIR1/AFB2 auxin receptor clade and auxin-related development of Arabidopsis leaves. Plant Physiol 157: 683–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W (2005) Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17: 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Zhang W, Hu H, Li B, Wang Y, Zhao Y, Li K, Liu M, Li X (2008) Salt modulates gravity signaling pathway to regulate growth direction of primary roots in Arabidopsis. Plant Physiol 146: 178–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30: 2725–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N (2007) Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446: 640–645 [DOI] [PubMed] [Google Scholar]
- Tang RJ, Liu H, Bao Y, Lv QD, Yang L, Zhang HX (2010) The woody plant poplar has a functionally conserved salt overly sensitive pathway in response to salinity stress. Plant Mol Biol 74: 367–380 [DOI] [PubMed] [Google Scholar]
- Vanneste S, Friml J (2009) Auxin: a trigger for change in plant development. Cell 136: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP (2007) Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Behrens I, Komatsu M, Zhang Y, Berendzen KW, Niu X, Sakai H, Taramino G, Hochholdinger F (2011) Rootless with undetectable meristem 1 encodes a monocot-specific AUX/IAA protein that controls embryonic seminal and post-embryonic lateral root initiation in maize. Plant J 66: 341–353 [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang W, Li K, Sun F, Han C, Wang Y, Li X (2008) Salt-induced plasticity of root hair development is caused by ion disequilibrium in Arabidopsis thaliana. J Plant Res 121: 87–96 [DOI] [PubMed] [Google Scholar]
- Wang Y, Li K, Li X (2009) Auxin redistribution modulates plastic development of root system architecture under salt stress in Arabidopsis thaliana. J Plant Physiol 166: 1637–1645 [DOI] [PubMed] [Google Scholar]
- Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, et al. (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27: 581–590 [DOI] [PubMed] [Google Scholar]
- West G, Inzé D, Beemster GTS (2004) Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol 135: 1050–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley CK, Zenser N, Ramos J, Rouse D, Leyser O, Theologis A, Callis J (2000) Degradation of Aux/IAA proteins is essential for normal auxin signalling. Plant J 21: 553–562 [DOI] [PubMed] [Google Scholar]
- Xia R, Xu J, Meyers BC (2017) The emergence, evolution, and diversification of the miR390-TAS3-ARF pathway in land plants. Plant Cell 29: 1232–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Allen E, Wilken A, Carrington JC (2005) DICER-LIKE 4 functions in trans-acting small interfering RNA biogenesis and vegetative phase change in Arabidopsis thaliana. Proc Natl Acad Sci USA 102: 12984–12989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Gu Y, Jia X, Kang W, Pan S, Tang X, Chen X, Tang G (2012) Effective small RNA destruction by the expression of a short tandem target mimic in Arabidopsis. Plant Cell 24: 415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Tang RJ, Jiang CM, Li B, Kang T, Liu H, Zhao N, Ma XJ, Yang L, Chen SL, et al. (2015) Overexpression of the PtSOS2 gene improves tolerance to salt stress in transgenic poplar plants. Plant Biotechnol J 13: 962–973 [DOI] [PubMed] [Google Scholar]
- Yao W, Wang S, Zhou B, Jiang T (2016) Transgenic poplar overexpressing the endogenous transcription factor ERF76 gene improves salinity tolerance. Tree Physiol 36: 896–908 [DOI] [PubMed] [Google Scholar]
- Yifhar T, Pekker I, Peled D, Friedlander G, Pistunov A, Sabban M, Wachsman G, Alvarez JP, Amsellem Z, Eshed Y (2012) Failure of the tomato trans-acting short interfering RNA program to regulate AUXIN RESPONSE FACTOR3 and ARF4 underlies the wiry leaf syndrome. Plant Cell 24: 3575–3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Han X, Li Y, Wang J, Wang D, Wang S, Fu X, Ye W (2017) Comparative analysis of cotton small RNAs and their target genes in response to salt stress. Genes (Basel) 8: E369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon EK, Yang JH, Lim J, Kim SH, Kim SK, Lee WS (2010) Auxin regulation of the microRNA390-dependent transacting small interfering RNA pathway in Arabidopsis lateral root development. Nucleic Acids Res 38: 1382–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LH, Wu SJ, Peng YS, Liu RN, Chen X, Zhao P, Xu P, Zhu JB, Jiao GL, Pei Y, et al. (2016) Arabidopsis EDT1/HDG11 improves drought and salt tolerance in cotton and poplar and increases cotton yield in the field. Plant Biotechnol J 14: 72–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JL, Shi H (2013) Physiological and molecular mechanisms of plant salt tolerance. Photosynth Res 115: 1–22 [DOI] [PubMed] [Google Scholar]
- Zhao H, Jiang J, Li K, Liu G (2017) Populus simonii × Populus nigra WRKY70 is involved in salt stress and leaf blight disease responses. Tree Physiol 37: 827–844 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang T, Zhang W, Li X (2011) SOS3 mediates lateral root development under low salt stress through regulation of auxin redistribution and maxima in Arabidopsis. New Phytol 189: 1122–1134 [DOI] [PubMed] [Google Scholar]
- Zhou C, Han L, Fu C, Wen J, Cheng X, Nakashima J, Ma J, Tang Y, Tan Y, Tadege M, et al. (2013) The trans-acting short interfering RNA3 pathway and no apical meristem antagonistically regulate leaf margin development and lateral organ separation, as revealed by analysis of an argonaute7/lobed leaflet1 mutant in Medicago truncatula. Plant Cell 25: 4845–4862 [DOI] [PMC free article] [PubMed] [Google Scholar]