The C4 species Gynandropsis gynandra exhibits natural variation in traits important for C4 photosynthesis.
Abstract
Engineering C4 photosynthesis into C3 crops could substantially increase their yield by alleviating photorespiratory losses. This objective is challenging because the C4 pathway involves complex modifications to the biochemistry, cell biology, and anatomy of leaves. Forward genetics has provided limited insight into the mechanistic basis of these properties, and there have been no reports of significant quantitative intraspecific variation of C4 attributes that would allow trait mapping. Here, we show that accessions of the C4 species Gynandropsis gynandra collected from locations across Africa and Asia exhibit natural variation in key characteristics of C4 photosynthesis. Variable traits include bundle sheath size and vein density, gas-exchange parameters, and carbon isotope discrimination associated with the C4 state. The abundance of transcripts encoding core enzymes of the C4 cycle also showed significant variation. Traits relating to water use showed more quantitative variation than those associated with carbon assimilation. We propose that variation in these traits likely adapted the hydraulic system for increased water use efficiency rather than improving carbon fixation, indicating that selection pressure may drive C4 diversity in G. gynandra by modifying water use rather than photosynthesis. The accessions analyzed can be easily crossed and produce fertile offspring. Our findings, therefore, indicate that natural variation within this C4 species is sufficiently large to allow genetic mapping of key C4 traits and regulators.
Plants that use C4 photosynthesis can effectively abolish photorespiratory losses that occur when Rubisco fixes oxygen rather than CO2 (Bowes et al., 1971; Sharkey, 1988). In C4 plants, Rubisco is typically sequestered in bundle sheath (BS) cells that are concentrically arranged around the vasculature. The establishment of a molecular CO2 pump delivers carbon to Rubisco from mesophyll (M) cells via C4 acid intermediates (Garner et al., 2001). C4 photosynthesis relies more heavily on the BS for photosynthesis and less on M cells. It also involves more chloroplasts in BS cells, an increased proliferation of plasmodesmata between M and BS cells, and a higher vein density to increase the volume of the leaf occupied by the BS. These morphological alterations to the leaf that facilitate the C4 cycle are known as Kranz anatomy (Langdale, 2011; Lundgren et al., 2014). Moreover, photosynthesis gene expression is modified such that genes encoding components of the C4 and Calvin-Benson-Bassham cycles are strongly and preferentially expressed in either M or BS cells (Garner et al., 2001; Marshall et al., 2007; Hibberd and Covshoff, 2010).
Despite the complex modifications associated with C4 photosynthesis, current estimates are that the C4 pathway has independently evolved more than 60 times in angiosperms (Sage, 2016), which suggests that a relatively straightforward route may allow the transition from the ancestral C3 state to the derived C4 state. A number of factors appear to have facilitated this apparently complex transition. First, proteins of the C4 pathway operate in the ancestral C3 state (Hibberd and Quick, 2002; Brown et al., 2010; Eastmond et al., 2015). Second, the modifications to gene expression associated with C4 leaves appear to have been built on partly preexisting regulatory architecture found in C3 species (Brown et al., 2011; Kajala et al., 2012; Burgess et al., 2016; Williams et al., 2016; Reyna-Llorens et al., 2018). Moreover, after a binary categorization of phenotypes into either C3 or C4, mathematical modeling indicates that there is likely more than one route from which to acquire the C4 state (Williams et al., 2013) but that subsequent modifications, many of which are associated with rebalancing photorespiration, reinforce these improvements in photosynthesis and fitness (Heckmann et al., 2013; Mallmann et al., 2014; Bräutigam and Gowik, 2016).
Genome-wide analysis of transcript abundance in multiple C3 and C4 species has provided unbiased insight into processes that likely change in C4 compared with C3 leaves (Aubry et al., 2014; Bräutigam et al., 2014; Lauterbach et al., 2017). Furthermore, cis-elements that control the expression of genes encoding components of the C4 cycle have been documented (Akyildiz et al., 2007; Williams et al., 2016; Reyna-Llorens et al., 2018). However, the regulators that recognize these motifs have not been isolated (Reeves et al., 2017). Thus, despite the progress in our understanding of C4 photosynthesis, it is currently not possible to rationally design a C4 pathway in a C3 leaf.
When natural variation is present, the development of a mapping population enables molecular marker-trait associations by methods such as quantitative trait locus mapping and genome-wide association studies. Molecular marker-trait association studies help delineate genomic regions associated with trait variation. Fine genetic mapping of these genomic regions with major effects allows the identification of causal genes underpinning the variation. Such approaches have been used extensively to map loci responsible for numerous complex traits in plants (Mauricio, 2001).
The application of molecular marker-trait associations to study C4 photosynthesis would expedite the discovery of key regulators to engineer increased photosynthetic efficiency in C3 plants. However, to our knowledge, there are currently no examples showing that variation in C4 traits within a single species is sufficient to allow breeding and molecular trait mapping. Interspecific hybrids have been generated between C3 and C4 species of the dicotyledon genus Atriplex (Oakley et al., 2014). Although the progeny possessed variation in C4 phenotypes, specific traits showed limited penetrance, and there were high rates of sterility (Brown and Bouton, 1993). In the grass family, Alloteropsis semialata shows natural variation in C4 parameters and has been classified into C3 or C4 subspecies (Ueno and Sentoku, 2006; Lundgren et al., 2016), but there are currently no reports that these populations have been bred. Therefore, we investigated the extent to which key C4 traits varied in the C4 dicotyledon Gynandropsis gynandra, which is a leafy green vegetable (Sogbohossou et al., 2018) in a clade sister to the Brassicaceae that contains both C3 and C4 species (Brown et al., 2005; Marshall et al., 2007; Feodorova et al., 2010). Here, we show that accessions of G. gynandra show significant variation in both anatomical and physiological traits associated with C4 photosynthesis. These accessions have short generation spans, are sexually compatible, and produce fertile offspring. Our findings indicate that there is sufficient natural variation to allow the use of classical genetics to identify loci controlling the complex C4 phenotype.
RESULTS AND DISCUSSION
Accessions of G. gynandra were collected from Africa and Asia (Supplemental Table S1). DNA sequencing and phylogenetic reconstruction generated a taxonomy that was consistent with geographical origin but also indicated that the accession from Benin (West Africa) was more similar to Asian accessions than to those from East Africa (Fig. 1A). These accessions displayed considerable variation in macroscopic characteristics associated with leaf appearance (Fig. 1B; Supplemental Fig. S1A). For example, fully expanded leaflets varied in size and shape, and there was variation in petiole length, the presence of trichomes, and anthocyanin pigmentation. As there was considerable macroscopic variation in leaf characteristics, we then evaluated these accessions for variation in features of Kranz anatomy. Interestingly, there were statistically significant differences in vein density (Fig. 1, C and D; Supplemental Fig. S1B), cross-sectional area of BS strands (Fig. 1, C and E; Supplemental Fig. S1C), size of individual BS cells (Fig. 1F), and stomatal density (Fig. 1, C and G). There was no discernible variation in intervein distance or number of BS cells surrounding each vein (Supplemental Fig. S2). However, on average, East African accessions showed higher vein density, reduced distance between veins, and greater stomatal density than Asian accessions (Supplemental Fig. S3, A–C). Asian accessions typically had larger BS areas and cell sizes than those from the African continent (Supplemental Fig. S3, D and E). Vein density was inversely correlated with BS area and BS cell size but positively correlated with stomatal density (Supplemental Table S2). The average number of BS cells around each vein showed no statistically significant differences between lines (Supplemental Fig. S3F), but the cross-sectional area of the BS and the size of individual BS cells were positively correlated (ρ = 0.8, P < 0.0001). Therefore, we conclude that the area of individual BS cells, rather than the number of these cells per vein bundle, drives the increased BS strand area observed in some accessions. This suggests that genetic determinants of cell size rather than cell proliferation are involved in the variation in BS tissue in G. gynandra. Despite the lower phenotypic variation associated with C4 compared with C3 leaves (Sage and McKown, 2006), our findings demonstrate that flexibility is still possible within individual species that are fully C4.
Figure 1.
Natural variation in Kranz anatomy among a diverse panel of G. gynandra accessions. A, Geographic and phylogenetic relationships for nine accessions from seven countries across Africa and Asia. B, Variation in fully mature whole leaves of 6-week-old plants. C, Traces indicate variation in venation, bundle area, BS cell size, and stomatal density of fully mature leaves for two extreme accessions. D to G, Vein density (D), bundle area (E), BS cell size (F), and stomatal density (G) for all accessions. Asterisks indicate significant differences between accessions (one-way ANOVA: *, P < 0.05; and ****, P < 0.0001). Letters above individual box-scatterplots indicate significant groupings according to Duncan’s multiple range test (α = 0.05). The number of replicates for each accession ranged from three to seven. Bars = 5 cm (B) and 100 µm (C).
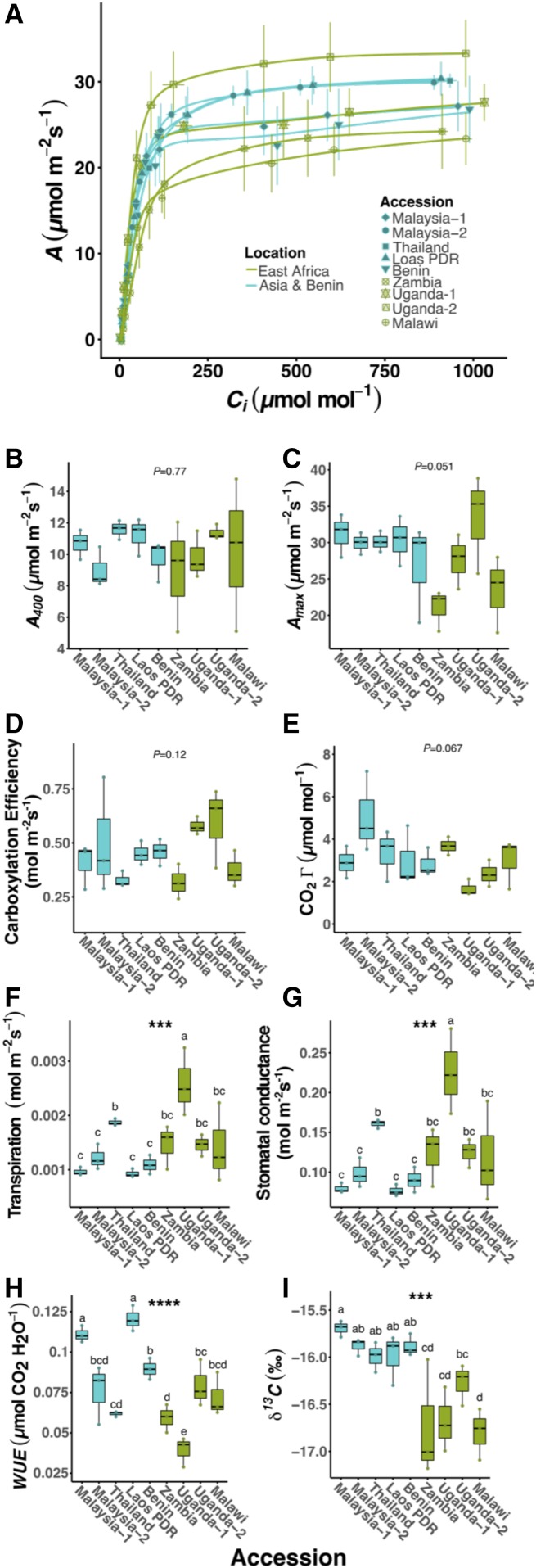
We next investigated whether differences observed in Kranz anatomy affected photosynthetic performance. For all accessions, their CO2 response curves (assimilation [A] and response to the concentration of CO2 inside the leaf [Ci]) were typical of C4 plants, with high carboxylation efficiencies and low CO2 compensation points (Γ; Fig. 2A; Supplemental Fig. S4A). Parameters associated with instantaneous gas exchange, such as maximum rate of photosynthesis (Amax), rate of photosynthesis under the conditions of growth (A400), CO2 carboxylation efficiencies, and Γ, showed little variation between accessions (Fig. 2, B–E; Supplemental Fig. S4, B–E). As C4 species typically grow under relatively high light intensities, variation in these traits may become apparent at higher light. In fact, modifications to stomatal density in Arabidopsis impact assimilation rates at high light but not at lower light intensities (Schlüter et al., 2003). Thus, it is possible that, when grown under different conditions, these G. gynandra accessions would demonstrate additional variation in photosynthetic characteristics.
Figure 2.
Physiological variation for photosynthetic gas-exchange parameters among a diverse panel of G. gynandra accessions. A, A versus Ci response curve. B, A400 (400 µL L−1 atmospheric CO2 concentration [Ca] and photosynthetic photon flux density [PPFD] of 350 µmol m−2 s−1). C, Amax (1,200 µL L−1 Ca and PPFD of 2,000 µmol m−2 s−1). D, Γ. E, Carboxylation efficiency. F, Transpiration. G, Stomatal conductance. H, WUE. I, δ13C. Asterisks indicate significant differences between accessions (one-way ANOVA, n = 3: ***, P < 0.001; and ****, P < 0.0001). Letters above individual box-scatterplots indicate significant groupings according to Duncan’s multiple range test (α = 0.05).
There were statistically significant differences in transpiration (Fig. 2F), stomatal conductance (Fig. 2G), and water use efficiency (WUE; Fig. 2H). Furthermore, there was also significant variation in the carbon isotope discrimination against 13C (δ13C) in leaf dry matter (Fig. 2I), which is a measure of the efficiency of the C4 carbon pump over the lifetime of the leaf. Accessions from Asia-Benin showed reduced discrimination against δ13C compared with the East African lines (Supplemental Fig. S4I). Therefore, these data indicate that accessions of G. gynandra possess significant variation in parameters linked to the balance between water use and photosynthesis that influenced the efficiency of the C4 cycle over their lifetimes.
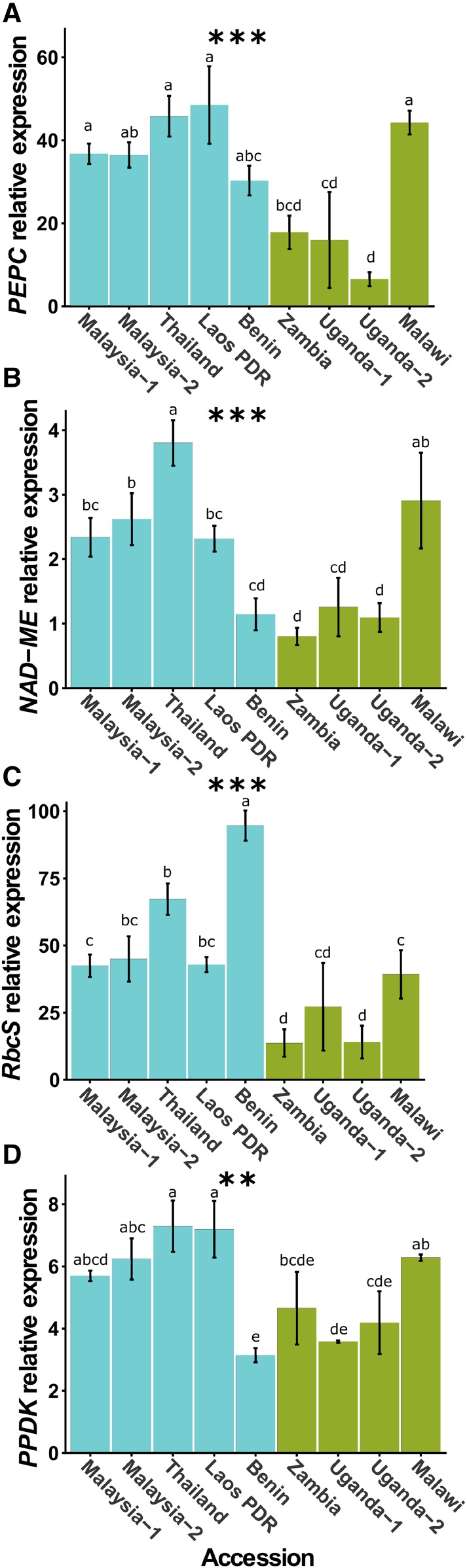
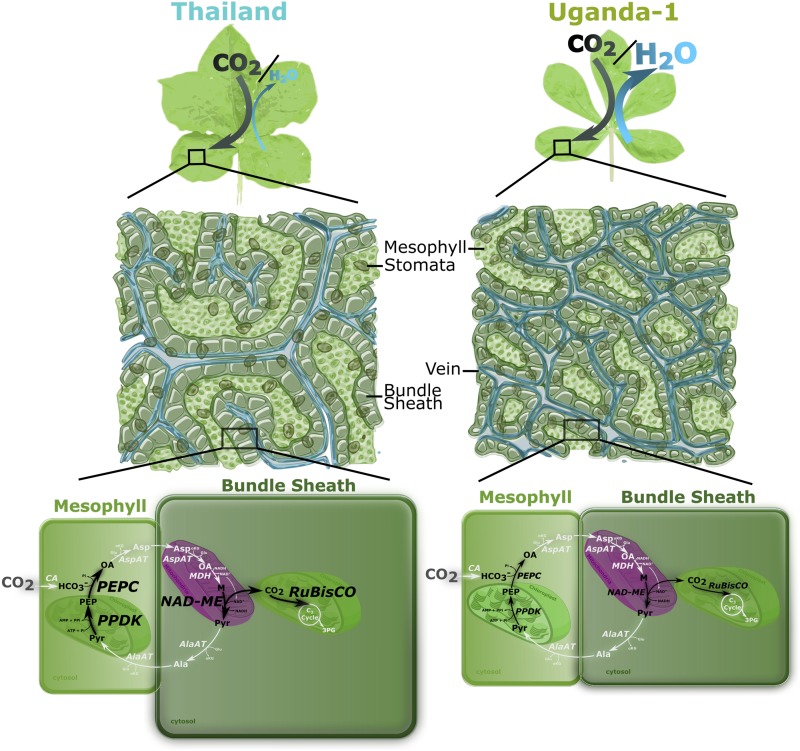
We next sought to investigate the extent to which the transcript abundance of core genes in the C4 cycle differed between the accessions. Interestingly, there were statistically significant differences in the abundance of transcripts encoding phosphoenolpyruvate carboxylase (PEPC), which catalyzes the first committed step of the C4 cycle, the BS-specific decarboxylase NAD-dependent malic enzyme (NAD-ME), the small subunit of Rubisco (RbcS), and pyruvate,orthophosphate dikinase (PPDK) that regenerates phosphoenolpyruvate, the primary acceptor of HCO3− (Fig. 3). These differences in C4-related transcript abundance were associated with the geographical location and phylogeny of the accessions, with the accessions from Asia-Benin accumulating greater levels of C4-related transcripts than those from East Africa (Supplemental Fig. S5, A–D). Understanding how the genes encoding photosynthetic enzymes become strongly expressed and patterned to either M or BS cells of C4 species is a longstanding area of research. Although progress has been made in identifying the cis-elements responsible for this phenomenon, little is known about the transcription factors involved. Therefore, the intraspecific variation in expression of genes encoding enzymes of the C4 cycle in G. gynandra provides an opportunity to identify trans-factors important for C4 photosynthesis. Despite accessions functioning with similar photosynthetic efficiencies under ambient CO2 and light conditions, when assessed by phylogenetic grouping, those with more pronounced Kranz traits (e.g. larger BS tissues and lower vein densities) exhibited increased Amax, WUE, and δ13C (Supplemental Fig. S4, C, H, and I) and stronger expression of the core genes of the C4 cycle (Supplemental Fig. S5). To summarize, there were striking differences between accessions, such that some possessed higher WUE, lower density of stomata and veins, larger BS areas and cell sizes, higher δ13C (indicative of a stronger C4 cycle), and increased expression of genes encoding C4 enzymes (Fig. 4).
Figure 3.
Transcript abundance for key enzymes in the C4 cycle among diverse G. gynandra accessions. Differences in transcript abundance were determined by reverse transcription-quantitative PCR (RT-qPCR) relative to the ACTIN7 gene. Asterisks indicate significant differences between accessions (one-way ANOVA, n = 3: **, P < 0.01; and ***, P < 0.001). Letters above individual bar charts indicate significant groupings among accessions according to Duncan’s multiple range test (α = 0.05).
Figure 4.
Summary of differences in anatomy, physiology, and C4-related transcript abundance exhibited by extreme G. gynandra accessions. The accessions Thailand and Uganda-1 had larger BS cells, lower vein and stomatal density, and higher WUE, δ13C, and transcript abundance of core C4 cycle enzymes. Differential transcript abundance among accessions is indicated by black arrows, and larger font represents higher relative transcript abundance. Enzymatic steps in white were not investigated.
The considerable variation reported in this study offers a valuable germplasm resource to identify regulators of the C4 pathway and Kranz anatomy through genetic mapping. All accessions in this study hybridize easily. Emasculation and pollination need only take 15 to 30 s per flower. For example, the most divergent accessions regarding anatomy, Malaysia-1 × Malawi, Malaysia-2 × Malawi, and their reciprocal crosses, produce an average 52 ± 11 seeds per silique (n = 6), whose offspring are fully fertile. The F1 hybrids displayed intermediate characteristics, differing in leaflet number, presence of trichomes on the petiole, and leaf size and shape (Supplemental Fig. S6). Therefore, these hybrid populations provide a breeding foundation to delineate regulatory mechanisms. More broadly, the discovery of intraspecific variation in a C4 grass would be particularly useful in mapping traits relevant to improving photosynthesis in crops and, thus, introduce C4 photosynthesis into C3 cereals.
While our understanding of the regulatory mechanisms underlying C4 metabolism is growing, there is still a significant gap in tools to expand our understanding of the regulation behind Kranz anatomy and the C4 biochemical cycle. Methods such as quantitative trait locus mapping or genome-wide association studies in G. gynandra or an equally diverse C4 species may provide insights into the regulation of Kranz development. In this study, most trait variation in G. gynandra was associated with characteristics relating to water use that impact carbon capture. It is noteworthy that modifications to C3 leaves considered to represent early steps on the path toward the C4 phenotype also are associated with water use rather than CO2 fixation (Sage, 2004; Williams et al., 2013). As natural vegetation is not considered to be under strong selection pressure to optimize photosynthesis (Long et al., 2015; Ort et al., 2015), it seems likely that C4 trait variation continues to be driven at least partially through the optimization of water use rather than photosynthesis per se.
MATERIALS AND METHODS
Plant Accessions and Growth Conditions
A selection of nine diverse accessions of Gynandropsis gynandra was made from a larger germplasm collection based on initial phenotypic and genetic screening. Five accessions were from Africa and four from Asia (Supplemental Table S1; materials available on request from M.E.S.). Plants from all G. gynandra accessions were grown under identical conditions prior to sampling. After germination, all seeds were planted in 5:1 F2 compost (Levington Advance):fine vermiculite premixed with 0.17 g L−1 insecticide (Imidasect 5GR; Fargro) in 5-cm3 cells. Seedlings were kept in a growth chamber at 350 µmol photons m−2 s−1 PPFD, with a 16-h photoperiod, at 25°C, 60% relative humidity (RH), and ambient [CO2]. A single dose of 3 mL L−1 slow-release 17N-9P-11K fertilizer (All Purpose Continuous Release Plant Food; Miracle-Gro) was applied after 1.5 weeks. Plants for physiological measurements were grown under identical conditions to those for Kranz measurements for the first 3 weeks, after which the plants for Kranz measurements were assayed and the plants for physiological measurements were replanted in 13-cm3 pots with a 5:1 M3 soil (Levington Advance):medium vermiculite soil mixture and moved to a growth room set to 23°C, 60% RH, ambient [CO2], and 350 µmol photons m−2 s−1 PPFD with a 16-h photoperiod. All plants were watered by an automated system whereby the bottom of the trays containing the 13 cm wide pots was flooded to a depth of 4 cm every 48 h for 10 min, following which the irrigation water was drained.
DNA Sequencing and Phylogenetic Analyses
Genomic DNA was extracted using a cetyltrimethylammonium bromide extraction method. Briefly, leaf samples were ground uniformly in 300 µL of cetyltrimethylammonium bromide extraction buffer containing 2% (w/v) polyvinylpyrrolidone-40 and 1.7% (v/v) β-mercaptoethanol. After vortexing, samples were incubated at 65°C for 30 min, and 300 µL of 24:1 chloroform:isoamyl alcohol was then added. After vortexing and centrifugation, 300 µL of supernatant was removed and placed in 300 µL of isopropanol. DNA was precipitated overnight at −20°C and then centrifuged at full speed for 15 min. The supernatant was removed, the pellet was washed with 200 µL of 96% (v/v) ethanol prior to drying, and the DNA was resuspended in nuclease-free water.
To generate a phylogeny for the accessions, internal transcribed spacer (ITS) regions were sequenced using universal primers ITS 1 and ITS 4, following a protocol published previously (White et al., 1990). In short, ITS regions I and II and the 5.8 S polycistronic rRNA precursor transcribed region were amplified by PCR. These PCR products were purified, and fragments were sequenced. Sequences were then aligned using the MAFFT online server (MAFFT version 7; https://mafft.cbrc.jp/alignment/server/), and a best scoring maximum likelihood tree was generated with RAxML version 8.0.0 on the CIPRES science gateway (Miller et al., 2010).
Preparation of Leaf Tissue Sections for Kranz Measurements
Three weeks after germination, tissue was harvested from healthy plants from the center trifoliate leaves of the second pair of fully expanded true leaves. A 3-mm2 rectangle was cut from the leaf adjacent to the midvein with a razor blade for transverse sections. Two slightly larger rectangles were cut from identical regions for paradermal sectioning and RT-qPCR analysis.
For transverse sections, leaf tissue in plastic cuvettes was submerged in a 4% (v/v) paraformaldehyde in PBS solution (Sigma-Aldrich), placed in a vacuum chamber for 1 h, and incubated at 4°C overnight for fixing. Cuvettes then underwent an ethanol dehydration series from 30% to 90% (v/v) ethanol solutions (Thermo Fisher Scientific) in 10% (v/v) increments for 45 min each at 4°C with a final overnight treatment at 4°C in 95% (v/v) ethanol with 0.1% (w/v) eosin dye solution (Sigma-Aldrich). The dye solution was washed three times with 100% (v/v) ethanol at room temperature. The samples were embedded in resin according to the Technovit 7100 (Kulzer) manufacturer’s protocol. Hardened resin blocks were cut with a manual rotary microtome (Thermo Fisher Scientific). Sections were placed on microscope slides and stained with 0.1% (w/v) Toluidine Blue solution (Sigma-Aldrich) prior to imaging on a light microscope.
For paradermal sections, fresh tissue samples were placed in plastic cuvettes and incubated in 3:1 100% (v/v) ethanol:acetic acid solution before treatment with 70% (v/v) ethanol solution (refreshed once) at 37°C overnight. To clear the samples, cuvettes were submerged in 5% NaOH solution for 3 h at 37°C, rinsed twice with 70% (v/v) ethanol, and stored at 4°C. After storage, the samples were stained with a 0.1% (v/v) eosin dye in 95% (v/v) ethanol solution. Samples were stored overnight at 4°C and washed with 70% (v/v) ethanol three times before transfer to slides for imaging. To determine stomatal density, impressions of the abaxial epidermis of each central leaflet were generated by applying a thin coat of transparent nail varnish (Boots). After drying, the varnish was peeled off and mounted onto a glass slide for imaging.
Measurement of Kranz Anatomy Traits
Slides of all leaf sections were imaged with an Olympus BX41 light microscope with a mounted Micropublisher 3.3 RTV camera (Q Imaging). Images were captured with Q-Capture Pro 7 software, and measurements were analyzed with the software ImageJ (Schneider et al., 2012). To maximize comparability, strict criteria were applied for all image analyses. Microscopy of transverse leaf sections was used to quantify the BS in terms of average BS tissue area (the total cross-sectional area of all BS cells immediately surrounding a vein) and BS cell size (the average cross-sectional area of individual BS cells around the vein). To quantify BS tissue area, the freehand selection tool was used to subtract the integrated area of each vein from the integrated area of all BS cells in direct contact with the vein on images with 200× total magnification. This value was divided by the number of BS cells in each vein bundle to obtain the average BS cell size. For intervein distance (the distance between the centers of adjacent veins in transverse sections), only vein bundles were measured for which the following criteria did not apply: wide (indicates branching) or extremely large veins, veins with distorted BS cells due to contact with adjacent BS tissues (indicates merging), and veins with damaged BS cells. The line selection tool was used to measure the linear distance between the centers of adjacent veins on images with 40× total magnification. Vein density (vein length per unit area of leaf) was quantified on paradermal sections on images with 100× total magnification. Slides were imaged with the same microscopy equipment as transverse sections but set to Ph3 (phase contrast). Three images (from three different leaves per plant) were randomly selected for measurement. The freehand line tool was used to trace all veins (both major and minor) along their center. As it was not possible to trace all veins in an image simultaneously, individual vein sections were measured progressively without overlap and the individual lengths summed. The total vein length was divided by the image area to obtain the density. Stomatal density (the number of stomata per unit area of leaf) was measured on three subsampled leaves from three random plants on images with 200× total magnification. The total number of stomata was divided by the image area to obtain the density.
Photosynthetic Performance
An LI-6800 portable photosynthesis infrared gas analyzer system (LI-COR) equipped with a multiphase flash fluorimeter was used to assess physiological differences for photosynthetic parameters between G. gynandra accessions. All physiological measurements were performed on the central leaflet of three independent 5-week-old plants, grown in separate pots. For stomatal conductance, transpiration (E), and A400, measurements were taken during ambient conditions of growth (400 µL L−1
Ca and PPFD of 350 µmol m−2 s−1). WUE was defined as A400/E. A combination chlorophyll fluorescence and A/Ci curve was measured for three plants from each accession. Ca reference values were 400, 400, 300, 200, 100, 50, 25, 400, 400, 400, 600, 800, 1,000, 1,200, and 400 µL L−1, with a saturating rectangular pulse of 12,000 µmol m−2 s−1 at each reference point. Otherwise, measurements were made at a PPFD of 2,000 µmol m−2 s−1, 23°C, and 60% RH at each reference point. All leaves covered the full area of the cuvette. Measurements were carried out on consecutive days between 1 and 8 h postdawn, measuring one plant selected at random from each accession per day. Rates of gas exchange including Amax at 1,200 µL L−1
Ca represent the measurements made, but a line of best fit also was plotted to fit the A/Ci response data to a logarithmic trend using the generalized additive model feature of the tidyverse package in R. Γ was calculated using linear regression of A and Ci measurements made between Ca of 25 to 200 µL L−1 (Sharkey, 1988). Adjusted R2 values for all regression lines ranged between 0.993 and 0.997. Carboxylation efficiency was calculated as the partial derivative 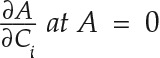 . δ13C analysis was performed according to methods described previously (Royles et al., 2016) on three biological replicates per accession with 500 µg of dried leaf tissue.
. δ13C analysis was performed according to methods described previously (Royles et al., 2016) on three biological replicates per accession with 500 µg of dried leaf tissue.
Statistical Analysis
For all tests, individual plants were considered experimental units in a complete randomized design. Data were analyzed in SAS (University Version; SAS Institute) and in R (version 3.4.2; R Studio). The R packages agricolae, car, cowplot, dplyr, ggplot2, multcompView, and tidyverse were used for data analysis and downloaded via the Install Packages Tool in RStudio (version 1.0.143; R Studio). A one-way ANOVA (α = 0.05) with type III sums of squares, which accounts for unequal replication, compared all means from anatomical and physiological measurements among G. gynandra accessions, and Student’s t test (α = 0.05) was used to compare means of accessions by phylogenetic proximity, using the PROC GLM and PROC TTEST functions in SAS, respectively. Null hypotheses were rejected for specific ANOVAs or Student’s t tests for any population with P ≤ 0.05. Levene’s test was used to evaluate homoscedasticity and was centered at the median (Levene, 1960) using the car package in R. All analyses showed equal variance (α = 0.05), except for the number of BS cells (Supplemental Table S3). Duncan’s multiple range posthoc test was used for mean separation on accessions (α = 0.05) with statistically significant ANOVAs in the R package agricolae (Duncan, 1955). To provide conservative lower type I experiment error rates, Tukey’s studentized range test (HSD) also was used (α = 0.05) with statistically significant ANOVAs in SAS and was plotted in R with the packages dplyr and multcompView (Supplemental Fig. S7). Pearson product-moment correlation coefficients were calculated to find associations among features of Kranz traits (Pearson, 1895) using the PROC CORR function in SAS. Linear regression was performed using the PROC REG function in SAS to calculate Γ and carboxylation efficiency. The total number of replicates used in each experiment is summarized in Supplemental Table S4.
Analysis of Transcript Abundance
Leaf tissue samples for RNA extraction were harvested simultaneously with samples for Kranz trait measurements after 3 weeks of growth using the opposite paired trifoliate leaf to that harvested for sectioning and imaging. These fresh leaf samples were immediately flash frozen with liquid nitrogen and stored at −80°C. Total RNA was extracted from three tissue samples per accession with an RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. An on-column DNase digestion protocol was applied to remove genomic DNA contamination (Qiagen) before cDNA was synthesized with Invitrogen SuperScript II reverse transcriptase enzyme according to the manufacturer’s instructions (Thermo Fisher Scientific). All cDNA samples were stored at −20°C before RT-qPCR. Primers were designed for quantitative PCR of the C4 cycle genes PEPC, NAD-ME, RbcS, and PPDK (Supplemental Table S5), and reactions were carried out as reported previously (Burgess et al., 2016) on three biological and three technical replicates.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers MH188307 to MH188315.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Representative images for macroscopic and microscopic variation in leaf anatomy across a panel of G. gynandra accessions.
Supplemental Figure S2. Nonvariable features of Kranz anatomy among accessions.
Supplemental Figure S3. Natural variation in features of Kranz anatomy between G. gynandra accessions from Asia-Benin and those from East Africa.
Supplemental Figure S4. Physiological variation for photosynthetic gas-exchange parameters between G. gynandra accessions from Asia-Benin and those from East Africa.
Supplemental Figure S5. Transcript abundance differences for key enzymes in the C4 cycle between G. gynandra phylogenetic clusters.
Supplemental Figure S6. Leaf appearance of F1 hybrids between G. gynandra lines that exhibited the greatest differences in Kranz features.
Supplemental Figure S7. Quantitative natural variation among diverse G. gynandra accessions controlling for lower type I error rates.
Supplemental Table S1. Accessions of G. gynandra investigated and their source regions.
Supplemental Table S2. Pearson product-moment correlation coefficients for Kranz anatomy traits.
Supplemental Table S3. Summary statistics for Levene’s test for homogeneity of variance for individual ANOVAs (α = 0.05).
Supplemental Table S4. The number of replicates for anatomical measurements.
Supplemental Table S5. List of primers for RT-qPCR analyses.
Acknowledgments
We thank Frank Becker and Beatrice Landoni (Wageningen University) for providing ITS sequence data and initial information on the selected lines, Blandine Gilbert for help with image processing, and James Rolfe for carbon isotope analysis.
Footnotes
G.R. was supported by a Gates Cambridge Trust PhD Fellowship and P.S. by Advanced European Research Council grant 694733 Revolution to J.M.H.
[CC-BY]: Article free via Creative Commons CC-BY 4.0 license.
References
- Akyildiz M, Gowik U, Engelmann S, Koczor M, Streubel M, Westhoff P (2007) Evolution and function of a cis-regulatory module for mesophyll-specific gene expression in the C4 dicot Flaveria trinervia. Plant Cell 19: 3391–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry S, Kelly S, Kümpers BMC, Smith-Unna RD, Hibberd JM (2014) Deep evolutionary comparison of gene expression identifies parallel recruitment of trans-factors in two independent origins of C4 photosynthesis. PLoS Genet 10: e1004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G, Ogren WL, Hageman RH (1971) Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun 45: 716–722 [DOI] [PubMed] [Google Scholar]
- Bräutigam A, Gowik U (2016) Photorespiration connects C3 and C4 photosynthesis. J Exp Bot 67: 2953–2962 [DOI] [PubMed] [Google Scholar]
- Bräutigam A, Schliesky S, Külahoglu C, Osborne CP, Weber APM (2014) Towards an integrative model of C4 photosynthetic subtypes: insights from comparative transcriptome analysis of NAD-ME, NADP-ME, and PEP-CK C4 species. J Exp Bot 65: 3579–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HR, Bouton JH (1993) Physiology and genetics of interspecific hybrids between photosynthetic types. Annu Rev Plant Physiol Plant Mol Biol 44: 435–456 [Google Scholar]
- Brown NJ, Parsley K, Hibberd JM (2005) The future of C4 research: maize, Flaveria or Cleome? Trends Plant Sci 10: 215–221 [DOI] [PubMed] [Google Scholar]
- Brown NJ, Palmer BG, Stanley S, Hajaji H, Janacek SH, Astley HM, Parsley K, Kajala K, Quick WP, Trenkamp S, et al. (2010) C4 acid decarboxylases required for C4 photosynthesis are active in the mid-vein of the C3 species Arabidopsis thaliana, and are important in sugar and amino acid metabolism. Plant J 61: 122–133 [DOI] [PubMed] [Google Scholar]
- Brown NJ, Newell CA, Stanley S, Chen JE, Perrin AJ, Kajala K, Hibberd JM (2011) Independent and parallel recruitment of preexisting mechanisms underlying C4 photosynthesis. Science 331: 1436–1439 [DOI] [PubMed] [Google Scholar]
- Burgess SJ, Granero-Moya I, Grangé-Guermente MJ, Boursnell C, Terry MJ, Hibberd JM (2016) Ancestral light and chloroplast regulation form the foundations for C4 gene expression. Nat Plants 2: 16161. [DOI] [PubMed] [Google Scholar]
- Duncan DB. (1955) Multiple range and multiple F tests. Biometrics 11: 1–42 [Google Scholar]
- Eastmond PJ, Astley HM, Parsley K, Aubry S, Williams BP, Menard GN, Craddock CP, Nunes-Nesi A, Fernie AR, Hibberd JM (2015) Arabidopsis uses two gluconeogenic gateways for organic acids to fuel seedling establishment. Nat Commun 6: 6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feodorova TA, Voznesenskaya EV, Edwards GE, Roalson EH (2010) Biogeographic patterns of diversification and the origins of C4 in Cleome (Cleomaceae). Syst Bot 35: 811–826 [Google Scholar]
- Garner DMG, Mure CM, Yerramsetty P, Berry JO (2001) Kranz anatomy and the C4 pathway. eLS
- Heckmann D, Schulze S, Denton A, Gowik U, Westhoff P, Weber APM, Lercher MJ (2013) Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153: 1579–1588 [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Covshoff S (2010) The regulation of gene expression required for C4 photosynthesis. Annu Rev Plant Biol 61: 181–207 [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Quick WP (2002) Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 415: 451–454 [DOI] [PubMed] [Google Scholar]
- Kajala K, Brown NJ, Williams BP, Borrill P, Taylor LE, Hibberd JM (2012) Multiple Arabidopsis genes primed for recruitment into C4 photosynthesis. Plant J 69: 47–56 [DOI] [PubMed] [Google Scholar]
- Langdale JA. (2011) C4 cycles: past, present, and future research on C4 photosynthesis. Plant Cell 23: 3879–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach M, Schmidt H, Billakurthi K, Hankeln T, Westhoff P, Gowik U, Kadereit G (2017) De novo transcriptome assembly and comparison of C3, C3-C4, and C4 species of tribe Salsoleae (Chenopodiaceae). Front Plant Sci 8: 1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene H. (1960) Robust tests for equality of variances. In Ingram O, Harold H, eds, Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling. Stanford University Press, Stanford, CA, pp 278–292 [Google Scholar]
- Long SP, Marshall-Colon A, Zhu XG (2015) Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161: 56–66 [DOI] [PubMed] [Google Scholar]
- Lundgren MR, Osborne CP, Christin PA (2014) Deconstructing Kranz anatomy to understand C4 evolution. J Exp Bot 65: 3357–3369 [DOI] [PubMed] [Google Scholar]
- Lundgren MR, Christin PA, Escobar EG, Ripley BS, Besnard G, Long CM, Hattersley PW, Ellis RP, Leegood RC, Osborne CP (2016) Evolutionary implications of C3-C4 intermediates in the grass Alloteropsis semialata. Plant Cell Environ 39: 1874–1885 [DOI] [PubMed] [Google Scholar]
- Mallmann J, Heckmann D, Bräutigam A, Lercher MJ, Weber APM (2014) The role of photorespiration during the evolution of C4 photosynthesis in the genus Flaveria. eLife 3: e02478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DM, Muhaidat R, Brown NJ, Liu Z, Stanley S, Griffiths H, Sage RF, Hibberd JM (2007) Cleome, a genus closely related to Arabidopsis, contains species spanning a developmental progression from C3 to C4 photosynthesis. Plant J 51: 886–896 [DOI] [PubMed] [Google Scholar]
- Mauricio R. (2001) Mapping quantitative trait loci in plants: uses and caveats for evolutionary biology. Nat Rev Genet 2: 370–381 [DOI] [PubMed] [Google Scholar]
- Miller M, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In 2010 Gateway Computing Environments Workshop. GCE, New Orleans, LA, pp 1–8 [Google Scholar]
- Oakley JC, Sultmanis S, Stinson CR, Sage TL, Sage RF (2014) Comparative studies of C3 and C4 Atriplex hybrids in the genomics era: physiological assessments. J Exp Bot 65: 3637–3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Merchant SS, Alric J, Barkan A, Blankenship RE, Bock R, Croce R, Hanson MR, Hibberd JM, Long SP, et al. (2015) Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc Natl Acad Sci USA 112: 8529–8536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson K. (1895) Notes on regression and inheritance in the case of two parents. Proc R Soc Lond 58: 240–242 [Google Scholar]
- Reeves G, Grangé-Guermente MJ, Hibberd JM (2017) Regulatory gateways for cell-specific gene expression in C4 leaves with Kranz anatomy. J Exp Bot 68: 107–116 [DOI] [PubMed] [Google Scholar]
- Reyna-Llorens I, Burgess SJ, Reeves G, Singh P, Stevenson SR, Williams BP, Stanley S, Hibberd JM (2018) Ancient duons may underpin spatial patterning of gene expression in C4 leaves. Proc Natl Acad Sci USA 115: 1931–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royles J, Amesbury MJ, Roland TP, Jones GD, Convey P, Griffiths H, Hodgson DA, Charman DJ (2016) Moss stable isotopes (carbon-13, oxygen-18) and testate amoebae reflect environmental inputs and microclimate along a latitudinal gradient on the Antarctic Peninsula. Oecologia 181: 931–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF. (2004) Tansley review. The evolution of C4 photosynthesis. New Phytol 161: 30. [DOI] [PubMed] [Google Scholar]
- Sage RF. (2016) A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: species number, evolutionary lineages, and Hall of Fame. J Exp Bot 67: 4039–4056 [DOI] [PubMed] [Google Scholar]
- Sage RF, McKown AD (2006) Is C4 photosynthesis less phenotypically plastic than C3 photosynthesis? J Exp Bot 57: 303–317 [DOI] [PubMed] [Google Scholar]
- Schlüter U, Muschak M, Berger D, Altmann T (2003) Photosynthetic performance of an Arabidopsis mutant with elevated stomatal density (sdd1-1) under different light regimes. J Exp Bot 54: 867–874 [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. (1988) Estimating the rate of photorespiration in leaves. Physiol Plant 73: 147–152 [Google Scholar]
- Sogbohossou EOD, Achigan-Dako EG, Maundu P, Solberg S, Deguenon EMS, Mumm RH, Hale I, Van Deynze A, Schranz ME (2018) A roadmap for breeding orphan leafy vegetable species: a case study of Gynandropsis gynandra (Cleomaceae). Hortic Res 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno O, Sentoku N (2006) Comparison of leaf structure and photosynthetic characteristics of C3 and C4 Alloteropsis semialata subspecies. Plant Cell Environ 29: 257–268 [DOI] [PubMed] [Google Scholar]
- White T, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc 18: 315–322 [Google Scholar]
- Williams BP, Johnston IG, Covshoff S, Hibberd JM (2013) Phenotypic landscape inference reveals multiple evolutionary paths to C4 photosynthesis. eLife 2: e00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BP, Burgess SJ, Reyna-Llorens I, Knerova J, Aubry S, Stanley S, Hibberd JM (2016) An untranslated cis-element regulates the accumulation of multiple C4 enzymes in Gynandropsis gynandra mesophyll cells. Plant Cell 28: 454–465 [DOI] [PMC free article] [PubMed] [Google Scholar]