AtVRLK1 functions as a signaling component in coordinating cell elongation and cell wall thickening during growth and development.
Abstract
During the growth and development of land plants, some specialized cells, such as tracheary elements, undergo secondary cell wall thickening. Secondary cell walls contain additional lignin, compared with primary cell walls, thus providing mechanical strength and potentially improving defenses against pathogens. However, the molecular mechanisms that initiate wall thickening are unknown. In this study, we identified an Arabidopsis (Arabidopsis thaliana) leucine-rich repeat receptor-like kinase, encoded by AtVRLK1 (Vascular-Related Receptor-Like Kinase1), that is expressed specifically in cells undergoing secondary cell wall thickening. Suppression of AtVRLK1 expression resulted in a range of phenotypes that included retarded early elongation of the inflorescence stem, shorter fibers, slower root growth, and shorter flower filaments. In contrast, up-regulation of AtVRLK1 led to longer fiber cells, reduced secondary cell wall thickening in fiber and vessel cells, and defects in anther dehiscence. Molecular and cellular analyses showed that down-regulation of AtVRLK1 promoted secondary cell wall thickening and up-regulation of AtVRLK1 enhanced cell elongation and inhibited secondary cell wall thickening. We propose that AtVRLK1 functions as a signaling component in coordinating cell elongation and cell wall thickening during growth and development.
Plant cell walls play crucial roles in many aspects of growth and development, such as providing mechanical strength for maintaining cell shape and upright growth, mediating intercellular communication, and defense against pathogens (Keegstra, 2010). Almost all plant cells have primary walls, but some cells in certain tissues form thickened secondary cell walls. As an example, development of the vascular system, which is composed of wall-thickened cells, starts with cambium cell division followed by differentiation into specialized cells, such as vessel elements and fiber cells. These specialized cells form secondary walls inside of the primary wall upon completion of cell expansion. The deposition of secondary cell walls provides mechanical strength with enhanced water-conducting capabilities (De Rybel et al., 2016).
Specialized cell differentiation is regulated by a range of signals, and several phytohormones have been reported to regulate secondary cell wall formation in association with vascular tissue differentiation. For example, auxin promotes procambial cells to differentiate into thick-walled xylem cells (Milioni et al., 2001; Moyle et al., 2002), while auxin, cytokinins, and brassinosteroids affect the expression of the key secondary cell wall synthesis-associated VASCULAR-RELATED NAC DOMAIN (VND) genes, VND6 and VND7, in Arabidopsis (Arabidopsis thaliana) hypocotyls (Kubo et al., 2005). In addition, peptides and oligosaccharides also have been reported to function as extracellular signals regulating vascular cell differentiation and secondary cell wall thickening. In a zinnia (Zinnia elegans) suspension culture system, a dodecapeptide, TRACHEARY ELEMENT DIFFERENTIATION INHIBITORY FACTOR, from the CLAVATA3/ESR-related gene family, suppresses the differentiation of cells into thick-walled tracheary elements (Ito et al., 2006). Oligosaccharides also have been shown to induce cell expansion and the formation of metaxylem-like tracheary elements (Roberts et al., 1997). In Populus spp., a xylem tissue-specific endo-1,4-β-mannanase, PtrMAN6, catalyzes the hydrolysis of mannan cell wall polysaccharides to produce galactoglucomannan oligosaccharides (GGMOs), which may serve as signaling molecules to suppress cell wall thickening through modulation of a transcriptional regulatory program (Zhao et al., 2013). In addition to developmental regulation, secondary cell wall formation also is regulated by external environmental abiotic and biotic signals. A variety of abiotic stress factors, such as drought, cold, heat, high salinity, and light irradiance, influence the biosynthesis of secondary cell walls (Le Gall et al., 2015). Pathogens also can induce secondary cell wall deposition. N-Acetylchitooligosaccharides from fungal cell walls, which are known to elicit plant defense responses, induce the biosynthesis of characteristic secondary cell wall components in wheat (Triticum aestivum), soybean (Glycine max), and Arabidopsis cell suspensions (Barber et al., 1989; Khan et al., 2003; Cabrera et al., 2006). Finally, in response to cell wall damage during pathogen infection, secondary wall deposition is induced by reactive oxygen species (Denness et al., 2011). However, it is not known how these signal molecules are perceived or how the consequent signals are transmitted to regulate cell wall formation.
Various types of cell surface receptors take part in the perception and transmission of signal molecules. Receptor-like kinase (RLK) proteins are receptors involved in signal pathways that regulate growth and development as well as plant defense responses. Many RLKs are transmembrane proteins with cytoplasmic Ser/Thr kinase domains and divergent extracellular structures. There are more than 400 RLKs with receptor configurations in Arabidopsis that have different extracellular domains for the recognition of diverse ligands (Shiu and Bleecker, 2001). A number of RLKs that are expressed predominantly in the vascular tissue were identified by analysis of promoter:GUS transgenic plants (Wu et al., 2016). Their specific expression patterns suggest a function in vascular tissue development, including specification, differentiation, expansion, and secondary cell wall formation in specialized cells (De Rybel et al., 2016). Among them, PHLOEM INTERCALATED WITH XYLEM (PXY) is a receptor in the signal transduction system that regulates the rate and orientation of vascular cambium cell division and xylem cell differentiation (Fisher and Turner, 2007; Etchells and Turner, 2010). In woody plants, a group of RLKs has been associated with the formation of dynamic cell wall remodeling and secondary cell wall thickening (Song et al., 2011). In this study, we dissected the function of Arabidopsis AtVRLK1 and demonstrate its role in coordinating cell elongation and secondary cell wall thickening during growth and development.
RESULTS
AtVRLK1 Is Highly Expressed in Arabidopsis Vascular Tissue
In a previous study, we detected a group of RLKs from the plasma membrane of differentiating vascular tissue in Populus spp. Among them, one RLK, encoded by Poptr0006s11530 (Ptr351), was associated specifically with xylem cell wall thickening (Song et al., 2011). We identified 13 homologs of Ptr351 from the herbaceous dicot Arabidopsis, the monocot rice (Oryza sativa), and the woody plant poplar (Populus trichocarpa) using a threshold of 50% amino acid sequence identity. A phylogenetic analysis indicated that Ptr351 clustered with Arabidopsis At1g79620, rice LocOs03g21230, and poplar Poptr0016s15080 (Fig. 1A). An analysis of public microarray/RNA sequencing databases revealed that Arabidopsis At1g79620 is highly expressed in the vascular tissue (Supplemental Fig. S1), and it was named Vascular-Related Receptor-Like Kinase1 (AtVRLK1).
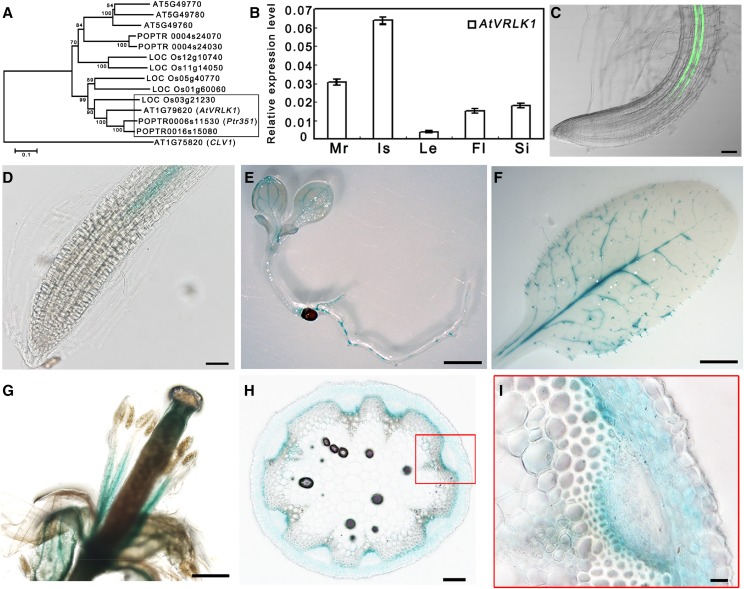
Figure 1.
Phylogenetic and expression analyses of AtVRLK1. A, Phylogenetic analysis of AtVRLK1 homologs from three representative species, poplar, Arabidopsis, and rice. CLV1 from a different subfamily of LRR-RLK genes was used as an outgroup reference. B, Relative expression levels of AtVRLK1 in various organs of 4-week-old Arabidopsis plants. The expression level in each sample was normalized using Arabidopsis ACTIN2 (At3g18780) as an internal control. The values are means ± se; n = 3. Mr, Mature root; Is, inflorescence stem; Le, leaf; Fl, flower; Si, silique. C, Fluorescence in an AtVRLK1pro:GFP transgenic Arabidopsis root. D to I, GUS staining in AtVRLK1pro:GUS transgenic Arabidopsis root (D), seedling (E), rosette leaf (F), flower (G), and vascular bundles of the inflorescence stem (H and I). Bars = 50 µm (C and D), 2 mm (E–G), 100 µm (H), and 20 µm (I).
AtVRLK1 belongs to the leucine-rich repeat (LRR) VIII-1 RLK subfamily, which has eight members in the Arabidopsis genome (Shiu and Bleecker, 2001). Of these, it shares a high sequence similarity with three: At5g49760, At5g49770, and At5g49780 (Fig. 1A). AtVRLK1 contains a presumed extracellular domain, a single transmembrane domain, and a cytoplasmic Ser/Thr kinase domain. The predicted extracellular domain contains 10 LRR structures that are flanked by two Cys pairs. The intracellular domain is composed of an intracellular juxtamembrane domain, a catalytic kinase domain, and a C-terminal domain (Supplemental Fig. S2).
AtVRLK1 expression was detected in Arabidopsis mature roots, inflorescence stems, leaves, flowers, and siliques by reverse transcription quantitative PCR (RT-qPCR), with the highest expression in inflorescence stems (Fig. 1B). We similarly examined the expression of the three AtVRLK1 homologs. Compared with AtVRLK1, At5g49760 was expressed moderately in the inflorescence stem, while the expression of At5g49770 and At5g49780 was barely detected (Supplemental Fig. S3). To further verify AtVRLK1 expression, we analyzed its promoter activity using a promoter-driven GUS reporter system. Analysis of the promoter:GUS transgenic Arabidopsis lines showed that the AtVRLK1 promoter was specifically active in vascular tissues, including vascular bundles in the inflorescence stem, the leaf veins in seedlings and mature plants, and the stele of the root and flower filaments (Fig. 1, D–G). Within the vascular bundle, GUS activity was observed in cambial cells and in early developmental stages of interfascicular fibers (Fig. 1, H and I). In addition, we examined the AtVRLK1 promoter activity in promoter-GFP-overexpressing Arabidopsis. Green fluorescence appeared in thick-walled vascular cells of the roots (Fig. 1C). Together, these results suggested that AtVRLK1 is involved in the process of secondary cell wall thickening.
To investigate the cellular location of AtVRLK1, GFP-tagged AtVRLK1, under the control of a cauliflower mosaic virus 35S promoter, was introduced into Nicotiana benthamiana leaf epidermal cells through Agrobacterium tumefaciens-mediated transformation. Fluorescence analysis revealed that AtVRLK1 was localized in the plasma membrane. Moreover, a GFP-tagged truncated AtVRLK1 protein lacking the cytoplasmic kinase domain also was localized to the plasma membrane (Supplemental Fig. S4).
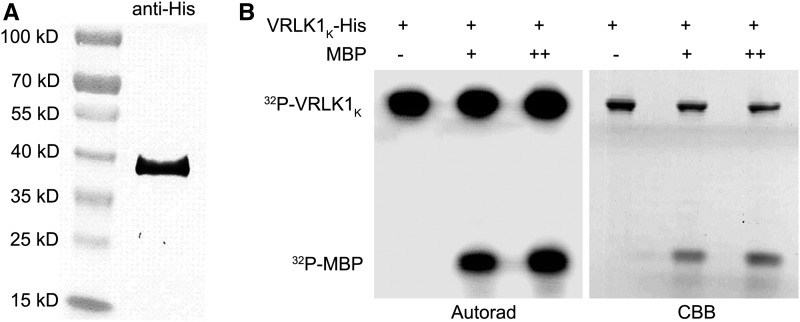
AtVRLK1 kinase activity also was examined in an in vitro kinase activity assay. A recombinant AtVRLK1 intracellular kinase domain (VRLK1K) was produced and purified, and a kinase activity assay indicated that VRLK1K was able to phosphorylate itself and an artificial substrate, myelin basic protein (Fig. 2). These results suggested that AtVRLK1 is a plasma membrane-localized kinase, which may function as a component of a receptor system to phosphorylate other proteins for signal transduction.
Figure 2.
Analysis of AtVRLK1 in vitro kinase activity. A, VRLK1K-His recombinant protein produced in Escherichia coli was detected using anti-His antibodies. B, Coomassie Brilliant Blue (CBB)-stained proteins by SDS-PAGE and autoradiography (Autorad) of the kinase reaction products. MBP, Myelin basic protein. Different amounts of MBP are indicated as + and ++.
AtVRLK1 Affects Arabidopsis Growth and Development
To investigate the biological function of AtVRLK1, three Arabidopsis T-DNA insertion lines with insertions predicted to affect AtVRLK1 or its homologs were identified in the Arabidopsis Biological Resource Center mutant collection: SALK_055076 (vrlk1) for AtVRLK1, SALK_71_C11 (m760) for At5g49760, and SAIL_316_D03 (m780) for At5g49780 (Supplemental Fig. S5A). We confirmed that the insertions in each of the three genes resulted in elimination of the corresponding full-length transcript (Supplemental Fig. S5B). None of the mutants showed visible phenotypic abnormalities compared with the wild type. To determine possible functional redundancy, we constructed double mutants of vrlk1 and m760 and of vrlk1 and m780. The vrlk1m760 double mutant was smaller than the wild type at early growth stages; however, the difference in size became insignificant at mature stages (Supplemental Fig. S5, C and D). Since At5g49780 and At5g49760 are in close proximity in the Arabidopsis genome, a triple mutant could not be constructed through genetic crossing. Therefore, we performed targeted mutagenesis of At5g49760 in the vrlk1m780 background using clustered regularly-interspaced short palindromic repeats (CRISPR)/CRISPR-associated nuclease 9 (Cas9) technology (Feng et al., 2014). We examined two independent triple mutant lines (Supplemental Fig. S6, A and B) and observed that both showed weak, but consistent, differences from the wild-type plants, including smaller rosette leaves, shorter inflorescence stems, and shorter first siliques (Supplemental Fig. S6, C–G). Expression of the secondary cell wall formation genes was changed (Supplemental Fig. S6H). Weak abnormal phenotypes of the triple mutants may be due to the possibility that the vrlk1 mutant was complemented by the increased expression of other RLK genes. To circumvent this potential functional redundancy, we used the dominant-negative strategy (Shpak et al., 2003) to dissect AtVRLK1 function. Truncated AtVRLK1 lacking the kinase structure under the control of its native promoter was introduced into Arabidopsis to cause dominant-negative suppression of AtVRLK1 function. For comparison, a full-length AtVRLK1 gene was overexpressed under the control of its own promoter. At least 20 independent transgenic lines were generated from each transformation. After homozygous T3 transgenic lines were screened, the truncated protein in the dominant-negative suppression (DN) lines and the full-size AtVRLK1 protein in the overexpression (OE) lines were examined by western-blot analysis (Fig. 3A). AtVRLK1 suppression line DN6 and overexpression line OE2, which showed the highest levels of transgene expression but did not affect the expression of At5g49760, At5g49770, and At5g49780 (Supplemental Fig. S7), were used for subsequent analyses.
Figure 3.
AtVRLK1 expression regulation affected Arabidopsis phenotypes at different developmental stages. A, Western-blot analysis of AtVRLK1 in inflorescence stems of wild-type (WT), AtVRLK1 OE, and DN Arabidopsis plants. In DN plants, a full size (top band) and a truncated size (bottom band) of AtVRLK1 are detected by anti-AtVRLK1 antibodies. An anti-ACTIN antibody was used as a loading control. B to D, Phenotypes at the age of 2 weeks (B) and 5 weeks (C) and siliques (D). E, Leaf length and width of the plants in B. F, Fertility rates of the wild-type, OE, and DN plants. The values are means ± se; n = 20 in E and 30 in F. Significance was determined by Student’s t test: **, P < 0.01. Bars = 2 cm (B and D) and 5 cm in (C).
The DN and OE plants had distinctly abnormal morphological phenotypes at different growth stages. DN plants had shorter seedling roots, smaller rosette leaves, and shorter inflorescence stems compared with the wild type. In contrast, the OE plants exhibited the opposite phenotypes (Fig. 3, B–D; Supplemental Fig. S8, A–F). This suggested that the suppression of AtVRLK1 inhibited root, leaf, and inflorescence growth, while its up-regulation promoted growth. In addition, both DN and OE plants showed increased sterility. In DN plants, many siliques were shorter and contained fewer seeds than the wild type, while almost all siliques in the OE plants were shorter and 95% had no seeds (Fig. 3, D and F). To investigate the cause of the sterility, we first examined the viability of male and female organs in the OE and DN plants. Fertilizing the OE and DN plants with wild-type pollen, or self-pollinating of the OE and DN plants, resulted in progeny with normal siliques, suggesting that the pistils and pollen from the OE and DN plants were fertile (Supplemental Fig. S9) and that the sterility in both OE and DN plants was caused by a failure of pollination within the stigma.
AtVRLK1 Regulates Cell Wall Thickening in Vascular Tissue of the Inflorescence
AtVRLK1 was highly expressed in the inflorescence stem, and AtVRLK1 suppression led to shorter inflorescences. To investigate how AtVRLK1 affected this process, we monitored inflorescence growth. The stem was marked with two points at the basal stem (Fig. 4A) at the early stage of inflorescence growth, and the distance between the two points was measured every 24 h to determine the elongation rate. As shown in Figure 4, inflorescence elongation was inhibited significantly in DN plants, but was increased slightly in OE plants, compared with the wild type (Fig. 4B). The full plant height of the DN, OE, and wild-type plants also showed differences at the mature stage (Fig. 3C). We also measured fiber cell length in the basal inflorescence stem after elongation had stopped. As shown in Figure 4C, DN plants contained a higher percentage of shorter fibers while OE plants had longer fibers compared with the wild type. During inflorescence growth, the cells of the vascular system, such as fiber cells and vessels, undergo secondary cell wall thickening after cell elongation, and so we looked at cross sections of the vasculature during inflorescence growth. Lignin deposition in the vascular cells was elevated in the DN plants but reduced in OE plants (Fig. 5, A–C). Examination of the vascular cell wall structure by transmission electron microscopy indicated that the secondary cell walls in the OE vascular cells were much thinner than in the wild type. The thickness of the interfascicular fiber, vessel, and xylem fiber cells in the OE plants was decreased by 52%, 18%, and 37%, respectively (Fig. 5, E, H, and J), while the thickness of the interfascicular fiber cells in the DN plants was increased by 14%. No significant change was observed in the thickness of vessel and xylem fiber cells in the DN plants (Fig. 5, F, I, and J). Composition analysis of secondary cell wall components also confirmed that up-regulation of AtVRLK1 resulted in reductions in lignin and crystalline cellulose contents, while AtVRLK1 suppression caused an increase of crystalline cellulose levels (Fig. 5, K and L).
Figure 4.
Effects of AtVRLK1 overexpression and dominant-negative suppression on vascular cell elongation in the inflorescence stem. A, Schematic representation of the measurement scheme to assess inflorescence stem elongation. B, Basal inflorescence stem elongation of wild-type (WT), OE, and DN plants from day 1 to day 6. C, Length distribution of fibers from basal 1-cm inflorescence stems after elongation had stopped. The values are means ± se; n = 5 in B and 800 in C.
Figure 5.
Effects of AtVRLK1 overexpression and dominant-negative suppression on inflorescence stem vascular cell wall thickening in Arabidopsis. A to C, Cross sections of wild-type (WT), OE, and DN inflorescence stems stained with phloroglucinol-HCl. D to F, Transmission electron micrographs of interfascicular fibers in wild-type, OE, and DN inflorescence stems. Inf, Interfascicular fiber. G to I, Transmission electron micrographs of xylem vessels and fibers in wild-type, OE, and DN inflorescence stems. Ve, Vessel; Xf, xylem fiber. J, Quantification of secondary cell wall (SCW) thickness of walls seen in D to I. The values are means ± se of 50 independent cells. K and L, Lignin and crystalline cellulose contents of inflorescence stems from wild-type, OE, and DN plants. M, Expression of secondary cell wall thickening-related genes in inflorescence stems of wild-type, OE, and DN plants. Gene expression was measured in the basal region of the inflorescence stem by RT-qPCR. The expression level in each sample was normalized using Arabidopsis ACTIN2 (At3g18780) as an internal control. The values are means ± se; n = 3. Significance was determined by Student’s t test: *, P < 0.05 and **, P < 0.01. Bars = 50 μm (A–C), 1 μm (D–F), and 2 μm in (G–I).
We also observed that the expression levels of genes involved in secondary cell wall formation, including the transcription factors SND1, VND6, and MYB46, as well as the cellulose and lignin biosynthetic genes CesA4, CesA7, PAL, and 4CL1, were higher in DN plants but lower in OE plants (Fig. 5M; Supplemental Fig. S8G). The change of expression of these genes in DN plants is consistent with that in the triple mutants (Supplemental Fig. S6H). Taken together, these results suggest that AtVRLK1 plays a role in enhancing cell elongation while inhibiting secondary cell wall biosynthesis in the vascular cells of the inflorescence.
AtVRLK1 Expression Affects Filament Elongation and Secondary Thickening of the Endothecium
Stamens and pistils are important components of floral organs, and precise coordination between stamen and pistil development is essential to ensure efficient pollination (Smyth et al., 1990). To investigate why both the suppression and up-regulation of AtVRLK1 resulted in sterility, we examined the floral development of DN and OE plants at different developmental stages. Based on an established staging system for Arabidopsis flower development (Smyth et al., 1990), the flowers shown in Figure 6, A and B, are at stages 13 to 15. In the wild type, flowers at stage 14 had stamens that extended above the stigma to release pollen grains for pollination (Fig. 6D). However, in the DN plants, the stamens did not elongate above the stigma, suggesting that pollen would not be delivered to the stigma for subsequent pollination (Fig. 6G). DN flowers showed significantly shorter stamens at stages 14 and 15 (Fig. 6I) as well as reduced cell elongation of stamen filaments (Fig. 6, J–L). We hypothesized that, due to the incompatible stamen length, pollen failed to reach the stigma, resulting in sterility in the DN plants. These results suggest that the suppression of AtVRLK1 inhibited cell elongation in the filaments, thereby affecting pollination.
Figure 6.
Effects of AtVRLK1 dominant-negative suppression on filament elongation. A, Inflorescences of wild-type and DN plants. Red arrows indicate the flowers used for observation. B, Flowers at stage 13 (S13), stage 14 (S14), and stage 15 (S15) from wild-type and DN plants. C to E, Anatomy and micrographs of flowers from S13 to S15 from an inflorescence of a wild-type plant. F to H, Anatomy and micrographs of flowers from S13 to S15 from an inflorescence of a DN plant. I, Ratios of stamen length (the average length of four high stamens in a flower) to pistil length of wild-type (WT) and DN plants. The values are means ± se of 10 independent flowers. J and K, Micrographs of filament epidermal cells of wild-type and DN plants. L, Quantification of the lengths of filament epidermal cells in J and K. The values are means ± se of 30 cells from three independent fully elongated filaments. Significance was determined by Student’s t test: **, P < 0.01. Bars = 2 mm (A and B), 500 µm (C–H), and 20 µm (J and K).
The OE plants also showed severe sterility (Fig. 3, D and F), and an examination of the floral structures revealed that the anthers failed to dehisce. In Arabidopsis, anther dehiscence is necessary for mature pollen to be released, as shown in the images of a wild-type plant in Figure 7A. In comparison, the anthers of the OE lines were smooth, and no pollen grains were observed on the stigma (Fig. 7B). Many studies have shown that failure of anther dehiscence can be caused by an inhibition of secondary cell wall thickening in the anther endothecium (Wilson et al., 2011). To further examine the secondary cell wall thickening in the anther endothecium, transverse sections of anthers were examined at different flower developmental stages. Prior to stage 11, no obvious difference between wild-type and OE plants was observed. From stage 11 to stage 13, the wild type showed secondary wall thickening, or fibrous bands, in the endothecium and the anthers opened along the stomium, releasing the pollen grains (Fig. 7, C and E). However, the OE plants did not show secondary wall thickening in the endothecium and the anthers failed to open, so the mature pollen grains were retained in the anthers (Fig. 7, D and F). The secondary cell wall of the anther endothecium consists mainly of cellulose and lignin (Wilson et al., 2011). Chromogenic staining revealed secondary cell wall lignin in anthers of the wild type but not in the OE plants (Fig. 7, G and H). Moreover, the expression of transcription factors that regulate secondary cell wall thickening (MYB26, NST1, and NST2) and secondary cell wall biosynthesis genes (CesA4, CesA8, PAL, 4CL, C4H, F5H, and CCR) was down-regulated in the OE lines (Fig. 7I). These results suggest that up-regulation of AtVRLK1 inhibited secondary wall thickening in endothecium cells, similar to the inhibition of secondary wall thickening in the vascular cells of the inflorescence.
Figure 7.
Effects of AtVRLK1 overexpression on anther dehiscence in Arabidopsis. A, Wild-type anthers dehisced, and pollen grains were released to the stigma. B, OE line anthers failed to dehisce, and no pollen grains were released. The insets in the top right corner show magnified images of the boxed anthers in A and B. C and E, Transverse sections of wild-type anthers at stages 11 and 13, respectively. D and F, Transverse sections of OE anthers at stages 11 and 13, respectively. C, Connective; En, endothecium; Fb, fibrous bands; PG, pollen grains; St, stomium; T, tapetum; V, vascular tissue. G and H, Phloroglucinol-HCl staining of wild-type and OE inflorescences. I, Expression of secondary wall thickening-related genes in flowers of wild-type (WT) and OE plants. Gene expression was measured in the flowers at anther stages 11 to 13 by RT-qPCR. The expression level in each sample was normalized using Arabidopsis ACTIN2 (At3g18780) as an internal control. The values are means ± se; n = 3. Significance was determined by Student’s t test: **, P < 0.01. Bars = 0.2 mm (A and B), 100 μm (C–F), and 2 mm (G and H).
DISCUSSION
AtVRLK1 Is an RLK Involved in Secondary Cell Wall Formation during Arabidopsis Development
Secondary cell wall thickening is one of the most important events in vascular tissue development, providing plants with mechanical strength and transportation routes necessary for their growth and development (De Rybel et al., 2016), but its regulation is not well understood. Many RLK genes are expressed specifically in the vascular tissue of various species, indicating an involvement in the development of vascular wall-thickened cells (Song et al., 2010, 2011; Wu et al., 2016).The Arabidopsis genome encodes more than 600 RLKs, which are thought to play a role in receiving and transmitting signals in various physiological processes (Shiu and Bleecker, 2001). However, the functions of most RLKs remain to be determined.
We observed that AtVRLK1 is expressed in Arabidopsis vascular tissue in roots, hypocotyls, leaves, inflorescence stems, flowers, and siliques, and particularly highly in inflorescence stems, as shown by RT-qPCR and GUS staining analysis (Fig. 1, B–I). Expression pattern analysis in rosette leaves of 14-d-old seedlings also showed a vascular expression pattern (Wu et al., 2016). The location of AtVRLK1 expression indicates its involvement in secondary cell wall thickening in these cells. However, an AtVRLK1 single mutant showed no obvious phenotypic changes, and double and triple mutants of its homologs showed weak phenotypes, suggesting the possible existence of other RLKs functionally redundant with AtVRLK1. Using dominant-negative suppression to perturb AtVRLK1 function, we showed that AtVRLK1-suppressed plants had a variety of phenotypic changes in primary root elongation, rosette leaf size, inflorescence stem length, and fertility. These changes were associated with abnormal secondary cell wall thickening in different stages of development during vegetative and reproductive growth, suggesting a broad role for AtVRLK1 in secondary cell wall thickening. It is possible that the dominant-negative suppression interfered with other homologous proteins and gave rise to more significant phenotypic alternations than we observed in the mutants. This suggests the involvement of other homologs that may play a role in the AtVRLK1-related pathway.
AtVRLK1 Contributes to Cell Elongation and Secondary Cell Wall Thickening in Arabidopsis
Plant vascular tissues result from procambium cell division, which gives rise to an organization of different types of vascular cells, including xylem and phloem, followed by secondary wall thickening (De Rybel et al., 2016). In such specialized cells, secondary wall formation occurs after the termination of cell elongation, but the molecular mechanism that triggers the transition from cell elongation to secondary cell wall thickening is still largely unknown. Our data indicate that AtVRLK1 plays a role in regulating cell elongation and secondary cell wall thickening of wall-thickened cells in Arabidopsis.
In the inflorescence stem of Arabidopsis, suppression of AtVRLK1 resulted in short vascular wall-thickened cells, such as fibers, while overexpression of AtVRLK1 had the opposite effect. Moreover, secondary cell wall thickening was enhanced in AtVRLK1-suppressed plants and suppressed in AtVRLK1-overexpressing lines. Transcriptional factors that regulate secondary cell wall formation have been identified, such as SND1, VND6, and MYB46 (Zhong et al., 2010), while CesA4 and CesA7 are cellulose synthases that are required for secondary cell wall synthesis (McFarlane et al., 2014), and PAL and 4CL1 are key enzymes in lignin synthesis (Vanholme et al., 2010). Expression of these genes was up-regulated in AtVRLK1-suppressed plants and down-regulated in AtVRLK1-overexpressing plants. This cellular and molecular evidence suggests that AtVRLK1 promotes cell elongation and restrains secondary cell wall thickening.
In addition to its expression in the inflorescence stem, AtVRLK1 is expressed in the filament stele, as shown by promoter-GUS staining. The filament stele is a tube in the vascular bundle composed of continuous vessel cells with secondary cell wall thickening that anchors the stamen to the flower and conducts water and nutrients (Goldberg et al., 1993). We found that dominant-negative suppression of AtVRLK1 reduced the fertility rate because of the suppressed elongation of filaments during stamen development, leading to the failure of mature pollen grains to reach the stigma to complete fertilization. We hypothesize that if the secondary cell wall of the vascular cells in the filament is precociously deposited, the elongation of the filament will be restrained, resulting in a shorter filament. The phenotype of suppressed filament elongation and the expression pattern in the wall-thickened vascular cells indicate that AtVRLK1 has a role in the regulation of cell elongation and thickening of filament vascular cells. In late development of the stamen of wild-type Arabidopsis, pollen maturation and septum degeneration occur, together with the deposition of lignocellulosic materials that form localized fibrous bands in endothecial cells. This is followed by stomium cell breakage and pollen release ( Wilson et al., 2011). Overexpression of AtVRLK1 under the control of its native promoter induced severe sterility, and a combination of chromogenic staining for lignin gene expression analysis revealed that the lignocellulosic thickening in the endothecial cells was suppressed. We conclude that AtVRLK1 plays a role in suppressing anther endothecium secondary wall thickening prior to pollen grain maturation.
AtVRLK1 May Mediate Signals Needed for Secondary Cell Wall Thickening
The formation of cell walls involves a complex of signals in the various processes of cell wall deposition and remodeling (Höfte, 2015). For example, WAKs (Wall-Associated Kinases), CrRLK1Ls (Catharanthus roseus RLK1-Like Kinases), and LRR-RLKs act in signaling pathways that regulate cell wall remodeling (Steinwand and Kieber, 2010; Wolf, 2017). Arabidopsis WAK1 and WAK2 can bind to pectin and oligogalacturonides and may play a role in the remodeling of primary cell walls during cell expansion and in response to environmental factors (Decreux and Messiaen, 2005; Kohorn et al., 2009; Kohorn, 2016). A mutant of the CrRLK1L family gene FERONIA (FER), fer, has a short hypocotyl and a dwarf phenotype, while THESEUS1 (THE1) is genetically redundant with two other members of the CrRLK1L gene family, HERCULES1/2 (HERK1/2), as the the1herk1 and the1herk2 genotypes were reported to have the same cell elongation phenotype as fer (Guo et al., 2009). These CrRLK1Ls function as regulators of cell expansion, involving the spatial and temporal control of cell wall extensibility (Nissen et al., 2016). In addition, two LRR-RLK genes, FEI1 and FEI2, were shown to play a role in the regulation of cell wall function, since the fei1fei2 double mutant displayed anisotropic expansion of root cells and ectopic lignin deposition (Xu et al., 2008). FEI2 also plays a role in the regulation of cellulose synthesis in the seed coat mucilage structure in Arabidopsis (Harpaz-Saad et al., 2011). RLK proteins have been proposed to act as sensors of cell wall integrity that regulate cell expansion (Engelsdorf and Hamann, 2014). It is not clear whether the initiation of secondary cell wall thickening requires signal reception, but we provide evidence that AtVRLK1 may mediate the transduction of a signal that promotes secondary cell wall deposition.
As a receptor-like kinase, AtVRLK1 recognizes its ligand and transfers the signal through its kinase activity. It has been reported that, in poplar, GGMOs produced by the endo-1,4-β-mannanase, PtrMAN6, serve as signal molecules to modulate the transcriptional program of cell wall thickening in xylem vessel cells (Zhao et al., 2013). GGMOs and other oligosaccharides generated as a result of primary cell wall remodeling during cell expansion are the likely ligands for receptors controlling the switch from primary cell wall to secondary cell wall deposition. Regarding the downstream components of the AtVRLK1 signal transduction pathway, a phylogenetic analysis of the Arabidopsis RLK family based on the kinase domain sequences showed that the LRR VIII-1 subfamily, to which AtVRLK1 belongs, is closely related to the WAK and CrRLK1L subfamilies (Shiu and Bleecker, 2001). Therefore, similar downstream responses, such as RAC/ROP GTPases and MPK signaling pathways, may be common to the three subfamilies (Engelsdorf and Hamann, 2014). AtVRLK1 also has the potential to directly regulate secondary cell wall-related transcription factors through their phosphorylation. For example, we suggest that VND7, a master switch that initiates secondary cell wall formation in vessels, is a likely downstream factor regulated by AtVRLK1, as it shows a similar expression pattern in roots (Yamaguchi et al., 2008).
Taken together, these results support the model presented in Figure 8. During cell expansion, unknown signaling molecules function as ligands to be recognized by the extracellular domain of AtVRLK1. Ligand recognition activates a downstream signaling pathway that inhibits secondary cell wall formation through critical NAC and MYB transcription factors. When specialized cells expand to a certain size, the inhibition of secondary cell wall formation is relieved due to reduced ligand levels or the participation of other regulatory factors. In this study, AtVRLK1 dominant-negative suppression caused precocious secondary cell wall thickening and reduced cell elongation. Overexpression of AtVRLK1 delayed the secondary cell wall thickening of vascular cells and inhibited lignocellulosic wall thickening in the anther endothecium wall. Whether in the vascular tissue of different organs or in the endothecium of the anther, AtVRLK1 performs a similar function in coordinating cell elongation and secondary cell wall formation. Although more studies are needed to elucidate the mechanistic details, our data provide new insights into the signal transduction pathway used to switch from cell elongation to secondary cell wall formation.
Figure 8.
Proposed model for the role of AtVRLK1 in the coordination of cell elongation and secondary cell wall formation. During growth and development, following expansion to a certain size, specialized cells, such as fibers and vessels, activate the program of secondary cell wall (brown) deposition inside the primary cell wall (pink). The plasma membrane-localized RLK, AtVRLK1, recognizes ligands (blue dots), which may be generated during cell expansion and inhibit secondary cell wall component deposition in both vascular cells and anther endothecial cells through a transcriptional regulatory network.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) Columbia (Col-0) plants were used as the wild type. Three T-DNA insertion mutants in the Col-0 background, vrlk1 (SALK_055076), m760 (SALK_71_C11), and m780 (SAIL_316_D03), were obtained from the Salk Institute Genomic Analysis Laboratory (http://signal.salk.edu) and confirmed by PCR and RT-qPCR using the primer sets listed in Supplemental Table S1. Double mutants were generated from crossing the relevant single mutants. Arabidopsis and Nicotiana benthamiana plants were grown in a phytotron under a light and dark cycle of 16 and 8 h, respectively, at 22°C. Arabidopsis seeds were sterilized with 3% sodium hypochlorite for 15 min, rinsed with sterile water three times, and placed on solid plates containing Murashige and Skoog medium at 4°C for 2 d. The seeds were grown in the phytotron for 7 d before being transplanted to soil.
Sequence Analysis of AtVRLK1 and Its Homologs
The nucleic acid sequences of Arabidopsis, poplar (Populus trichocarpa), and rice (Oryza sativa) genes were identified from the Arabidopsis Biological Resource Center (http://www.arabidopsis.org/abrc/) , poplar genome (http://www.phytozome.net/poplar), and The Institute for Genomic Research (http://rice.plantbiology.msu.edu/) databases, respectively. Their deduced protein sequences were analyzed using ClustalW (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Phylogenetic relationships between AtVRLK1 and homologous genes were analyzed using the MEGA4.0 program and the neighbor-joining method (Tamura et al., 2007). Bootstrap values were calculated from 1,000 trials. The conserved domains of proteins were predicted using the Conserved Domain Search Service (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
Gene Cloning and Construct Preparation
Two XbaI-PatI restriction fragments containing GFP from the PA7 plasmid and GUS from the pBI121 plasmid were inserted between the XbaI and PatI sites of the binary pCAMBIA2300 plasmid to create two modified binary vectors. The 760-bp promoter sequence corresponding to the region upstream of the start site (ATG) of the AtVRLK1 gene to the next gene termination site (TGA) was amplified from Arabidopsis genomic DNA and cloned into the modified pCAMBIA2300 vectors between the EcoRI and XbaI sites to create two constructs for gene expression pattern analysis. The 3-kb full-coding sequence and the 1.8-kb truncated-coding sequence of AtVRLK1 were amplified by PCR (cycling conditions: 98°C for 5 min; 35 cycles of 98°C for 15 s, 55°C for 15 s, and 72°C for 2 min; and 72°C for 5 min) from cDNA derived from Arabidopsis inflorescence stems. To investigate the genetic function of AtVRLK1, the 3-kb fragment was cloned into AtVRLK1pro::GUS to replace the GUS coding sequence for overexpression and the 1.8-kb fragment was cloned into AtVRLK1p::GFP between the SmaI and XbaI sites for dominant-negative suppression. Each of the above constructs was introduced into Col-0 through Agrobacterium tumefaciens-mediated transformation using the floral dip method (Clough and Bent, 1998). The generated transformants were selected on solid plates containing one-half-strength Murashige and Skoog medium supplemented with 50 mg L−1 kanamycin. Independent T1 lines were selected, and homozygous T3 lines were isolated. The primers used in this study are listed in Supplemental Table S1.
In Vitro Kinase Activity Assays
An AtVRLK1 sequence encoding the intracellular region and kinase domain (1,762–2,916 bp) was amplified by PCR (cycling conditions: 98°C for 5 min; 35 cycles of 98°C for 15 s, 55°C for 15 s, and 72°C for 1 min; and 72°C for 5 min) using the VRLK-KF and VRLK-KR primers. The amplified fragment was cloned into the NdeI and HindIII sites of pET-26b(+) to generate a C-terminal His-tagged fusion protein. The constructs were transformed into the Escherichia coli BL21 (DE3) strain for recombinant protein expression and purification according to previous methods (Song et al., 2010). Recombinant protein expression was induced with 0.5 m isopropylthio-β-galactoside at 18°C overnight, and the recombinant proteins were purified from the cell lysate using Ni-NTA agarose (Qiagen). In vitro phosphorylation assays were performed as described previously (Gui et al., 2016), and the reaction products were separated by SDS-PAGE and visualized by Coomassie Brilliant Blue staining and autoradiography using a Typhoon FLA 9000 phosphor imager (Amersham).
GUS Staining and Analysis
AtVRLK1pro:GUS transgenic Arabidopsis plants were examined for GUS activity. Hand-cut sections of roots, seedlings, rosette leaves, opened flowers, and inflorescence stems (1 cm from the base) were incubated in 90% (v/v) acetone for 10 min, rinsed with distilled water three times, then incubated in GUS staining solution (100 mm sodium phosphate [pH 7], 10 mm EDTA, 0.5 mm ferricyanide, 0.5 mm ferrocyanide, 0.1% [v/v] Triton X-100, 20% [v/v] methanol, and 2 mm X-Gluc) at 37°C until the GUS signal was visible. Following staining, sections were cleared by 75% ethanol, then observed and photographed using a dissecting microscope (SZX7; Olympus).
Gene Expression Analysis
Samples from mature roots, inflorescence stems, leaves, flowers, and siliques were collected and ground into a powder in liquid nitrogen (Tissuelyser-48; JingXin). Total RNA was isolated using the E.Z.N.A Plant RNA Kit (R6827; Omega Bio-tek). cDNA was synthesized using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (AT311; TransGen Biotech). Gene-specific primers were designed to amplify a specific fragment of the detected genes (Supplemental Table S1) and verified for their specificity and amplification efficiency. Using the cDNA as template, quantitative PCR (cycling conditions: 95°C for 5 min; 40 cycles of 95°C for 15 s, 56°C for 15 s, and 72°C for 15 s; and 72°C for 5 min) was performed using SYBR Green Supermix (Bio-Rad), UNICONTM qPCR SYBR Green Master Mix (Yeasen Biotech), and an iQ5 Real-Time PCR Detection System (Bio-Rad). The gene expression level in each sample was normalized to the expression level of AtACTIN2 (At3g18780).
Microscopic and Cell Wall Thickness Analysis
Arabidopsis inflorescence stems (1 cm from the base) were sectioned by hand. The cross sections were stained with 0.5% (w/v) phloroglucinol in 12% HCl for 3 min and immediately observed with a bright-field microscope (BX51; Olympus). To examine the structure of the anthers, Arabidopsis flowers at different developmental stages were collected and fixed, dehydrated, embedded, sectioned, stained, and observed according to a previously described protocol (Yu et al., 2013). To observe cell wall thickness, Arabidopsis inflorescence stems (1 cm from the base) at different growth times were cut into 2-mm pieces for ultrathin sectioning and transmission electron microscopy, which was performed as described previously (Song et al., 2010). Cell wall thickness was measured from transmission electron micrographs using ImageJ software (Zhao et al., 2014).
Antibody Production and Western-Blot Analysis
The AtVRLK1 antibody was produced commercially (YouKe Biotech). A region encoding a polypeptide from amino acids 351 to 530 of AtVRLK1 was subcloned into the prokaryotic expression vector pET28a (Yu et al., 2013). The polypeptide was expressed, purified, and used as an antigen to raise polyclonal antibodies in rabbits. Anti-ACTIN monoclonal antibodies were purchased from Abmart.
For western-blot analysis, inflorescence stems of 3-week-old transgenic Arabidopsis plants were collected and ground into a powder with liquid nitrogen and then mixed with an equal volume of 2× loading buffer (0.1 m Tris-HCl [pH 6.8], 4% SDS, 0.2% bromophenol blue, and 20% glycerol). As described in an established method (Song et al., 2010), proteins were electrotransferred onto a polyvinylidene difluoride membrane and incubated with anti-AtVRLK1 (1:500 dilution) and anti-ACTIN (1:2,000 dilution) antibodies, followed by a washing step.
Chemical Analysis of Secondary Cell Wall Components
Inflorescence stem segments (10 cm from the base) of 3- and 5-week-old Arabidopsis plants were collected and ground into a powder in liquid nitrogen to prepare alcohol-insoluble residues according to Song et al. (2016). After destarching with 10 μL of amylase (50 µg mL−1 water, from a Bacillus spp.; Sigma) and 5 μL of pullulanase (18.7 units, from Bacillus acidopullulyticus; Sigma), the crystalline cellulose and lignin contents were analyzed as described previously (Foster et al., 2010a,b).
Histochemical Lignin Assay and Fiber Length Measurement
Inflorescences from wild-type and overexpressing transgenic plants were collected and stained with phloroglucinol-HCl as described previously (Mizuno et al., 2007). The inflorescence stem segments (1 cm from the base) of 3- and 5-week-old Arabidopsis plants were collected. The fiber cells were separated, stained, examined, and measured as described previously (Yu et al., 2013).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative under locus identifiers At1g79620 (AtVRLK1), At5g61480 (PXY), At1g32770 (SND1), At2g46770 (NST1), At3g61910 (NST2), At5g62380 (VND6), At1g71930 (VND7), At3g13890 (MYB26), At5g12870 (MYB46), At5g44030 (CesA4), At5g17420 (CesA7), At4g18780 (CesA8), At2g37040 (PAL), and At1g51680 (4CL1) and in the GenBank data library under the accession numbers XM_002323644 and JX840449 (PtrMAN6).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Expression profile of AtVRLK1 (At1g79620).
Supplemental Figure S2. Structure prediction of AtVRLK1 and homologs.
Supplemental Figure S3. Expression of the three AtVRLK1 homologs At5g49760, At5g49770, and At5g49780 in various tissues.
Supplemental Figure S4. Localization of AtVRLK1 in the plasma membrane.
Supplemental Figure S5. Analysis of Arabidopsis T-DNA insert mutants.
Supplemental Figure S6. Construction and analysis of triple mutants.
Supplemental Figure S7. Transcript levels of At5g49760, At5g49770, and At5g49780 in inflorescence stems of wild-type and transgenic Arabidopsis.
Supplemental Figure S8. Phenotypes of AtVRLK1 OE and DN lines.
Supplemental Figure S9. Artificial pollination between transgenic plants and wild-type Arabidopsis.
Supplemental Table S1. List of primers used in this study.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Xiaoyan Gao, Zhiping Zhang, and Jiqin Li (Shanghai Institute of Plant Physiology and Ecology) for assistance with transmission electron microscopy and Xiaoshu Gao (Shanghai Institute of Plant Physiology and Ecology) for assistance with confocal laser scanning microscopy. We thank Dr. Jian-Kang Zhu for providing the 18T-dCas9-chimer Arabidopsis vector.
Footnotes
This research was supported by the National Natural Science Foundation of China (31630014), the Ministry of Science and Technology of China (2016YFD0600104), and the Chinese Academy of Sciences (CEMPS2016009).
Articles can be viewed without a subscription.
References
- Barber MS, Bertram RE, Ride JP (1989) Chitin oligosaccharides elicit lignification in wounded wheat leaves. Physiol Mol Plant Pathol 34: 3–12 [Google Scholar]
- Cabrera JC, Messiaen J, Cambier P, Van Cutsem P (2006) Size, acetylation and concentration of chitooligosaccharide elicitors determine the switch from defence involving PAL activation to cell death and water peroxide production in Arabidopsis cell suspensions. Physiol Plant 127: 44–56 [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Decreux A, Messiaen J (2005) Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol 46: 268–278 [DOI] [PubMed] [Google Scholar]
- Denness L, McKenna JF, Segonzac C, Wormit A, Madhou P, Bennett M, Mansfield J, Zipfel C, Hamann T (2011) Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol 156: 1364–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, Mähönen AP, Helariutta Y, Weijers D (2016) Plant vascular development: from early specification to differentiation. Nat Rev Mol Cell Biol 17: 30–40 [DOI] [PubMed] [Google Scholar]
- Engelsdorf T, Hamann T (2014) An update on receptor-like kinase involvement in the maintenance of plant cell wall integrity. Ann Bot 114: 1339–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells JP, Turner SR (2010) The PXY-CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Development 137: 767–774 [DOI] [PubMed] [Google Scholar]
- Feng Z, Mao Y, Xu N, Zhang B, Wei P, Yang DL, Wang Z, Zhang Z, Zheng R, Yang L, et al. (2014) Multigeneration analysis reveals the inheritance, specificity, and patterns of CRISPR/Cas-induced gene modifications in Arabidopsis. Proc Natl Acad Sci USA 111: 4632–4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K, Turner S (2007) PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Curr Biol 17: 1061–1066 [DOI] [PubMed] [Google Scholar]
- Foster CE, Martin TM, Pauly M (2010a) Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass). Part I. Lignin. J Vis Exp (37): 1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CE, Martin TM, Pauly M (2010b) Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass). Part II. Carbohydrates. J Vis Exp (37): 1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RB, Beals TP, Sanders PM (1993) Anther development: basic principles and practical applications. Plant Cell 5: 1217–1229 10.1105/tpc.5.10.1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui J, Zheng S, Liu C, Shen J, Li J, Li L (2016) OsREM4.1 interacts with OsSERK1 to coordinate the interlinking between abscisic acid and brassinosteroid signaling in rice. Dev Cell 38: 201–213 [DOI] [PubMed] [Google Scholar]
- Guo H, Li L, Ye H, Yu X, Algreen A, Yin Y (2009) Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc Natl Acad Sci USA 106: 7648–7653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz-Saad S, McFarlane HE, Xu S, Divi UK, Forward B, Western TL, Kieber JJ (2011) Cellulose synthesis via the FEI2 RLK/SOS5 pathway and cellulose synthase 5 is required for the structure of seed coat mucilage in Arabidopsis. Plant J 68: 941–953 [DOI] [PubMed] [Google Scholar]
- Höfte H. (2015) The yin and yang of cell wall integrity control: brassinosteroid and FERONIA signaling. Plant Cell Physiol 56: 224–231 [DOI] [PubMed] [Google Scholar]
- Ito Y, Nakanomyo I, Motose H, Iwamoto K, Sawa S, Dohmae N, Fukuda H (2006) Dodeca-CLE peptides as suppressors of plant stem cell differentiation. Science 313: 842–845 [DOI] [PubMed] [Google Scholar]
- Keegstra K. (2010) Plant cell walls. Plant Physiol 154: 483–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W, Prithiviraj B, Smith DL (2003) Chitosan and chitin oligomers increase phenylalanine ammonia-lyase and tyrosine ammonia-lyase activities in soybean leaves. J Plant Physiol 160: 859–863 [DOI] [PubMed] [Google Scholar]
- Kohorn BD. (2016) Cell wall-associated kinases and pectin perception. J Exp Bot 67: 489–494 [DOI] [PubMed] [Google Scholar]
- Kohorn BD, Johansen S, Shishido A, Todorova T, Martinez R, Defeo E, Obregon P (2009) Pectin activation of MAP kinase and gene expression is WAK2 dependent. Plant J 60: 974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M, Udagawa M, Nishikubo N, Horiguchi G, Yamaguchi M, Ito J, Mimura T, Fukuda H, Demura T (2005) Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev 19: 1855–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall H, Philippe F, Domon JM, Gillet F, Pelloux J, Rayon C (2015) Cell wall metabolism in response to abiotic stress. Plants (Basel) 4: 112–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HE, Döring A, Persson S (2014) The cell biology of cellulose synthesis. Annu Rev Plant Biol 65: 69–94 [DOI] [PubMed] [Google Scholar]
- Milioni D, Sado PE, Stacey NJ, Domingo C, Roberts K, McCann MC (2001) Differential expression of cell-wall-related genes during the formation of tracheary elements in the Zinnia mesophyll cell system. Plant Mol Biol 47: 221–238 [PubMed] [Google Scholar]
- Mizuno S, Osakabe Y, Maruyama K, Ito T, Osakabe K, Sato T, Shinozaki K, Yamaguchi-Shinozaki K (2007) Receptor-like protein kinase 2 (RPK 2) is a novel factor controlling anther development in Arabidopsis thaliana. Plant J 50: 751–766 [DOI] [PubMed] [Google Scholar]
- Moyle R, Schrader J, Stenberg A, Olsson O, Saxena S, Sandberg G, Bhalerao RP (2002) Environmental and auxin regulation of wood formation involves members of the Aux/IAA gene family in hybrid aspen. Plant J 31: 675–685 [DOI] [PubMed] [Google Scholar]
- Nissen KS, Willats WGT, Malinovsky FG (2016) Understanding CrRLK1L function: cell walls and growth control. Trends Plant Sci 21: 516–527 [DOI] [PubMed] [Google Scholar]
- Roberts AW, Donovan SG, Haigler CH (1997) A secreted factor induces cell expansion and formation of metaxylem-like tracheary elements in xylogenic suspension cultures of Zinnia. Plant Physiol 115: 683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB (2001) Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci USA 98: 10763–10768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED, Lakeman MB, Torii KU (2003) Dominant-negative receptor uncovers redundancy in the Arabidopsis ERECTA leucine-rich repeat receptor-like kinase signaling pathway that regulates organ shape. Plant Cell 15: 1095–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell 2: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Shen J, Li L (2010) Characterization of cellulose synthase complexes in Populus xylem differentiation. New Phytol 187: 777–790 [DOI] [PubMed] [Google Scholar]
- Song D, Xi W, Shen J, Bi T, Li L (2011) Characterization of the plasma membrane proteins and receptor-like kinases associated with secondary vascular differentiation in poplar. Plant Mol Biol 76: 97–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Gui J, Liu C, Sun J, Li L (2016) Suppression of PtrDUF579-3 expression causes structural changes of the glucuronoxylan in Populus. Front Plant Sci 7: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinwand BJ, Kieber JJ (2010) The role of receptor-like kinases in regulating cell wall function. Plant Physiol 153: 479–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W (2010) Lignin biosynthesis and structure. Plant Physiol 153: 895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ZA, Song J, Taylor B, Yang C (2011) The final split: the regulation of anther dehiscence. J Exp Bot 62: 1633–1649 [DOI] [PubMed] [Google Scholar]
- Wolf S. (2017) Plant cell wall signalling and receptor-like kinases. Biochem J 474: 471–492 [DOI] [PubMed] [Google Scholar]
- Wu Y, Xun Q, Guo Y, Zhang J, Cheng K, Shi T, He K, Hou S, Gou X, Li J (2016) Genome-wide expression pattern analyses of the Arabidopsis leucine-rich repeat receptor-like kinases. Mol Plant 9: 289–300 [DOI] [PubMed] [Google Scholar]
- Xu SL, Rahman A, Baskin TI, Kieber JJ (2008) Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell 20: 3065–3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Kubo M, Fukuda H, Demura T (2008) Vascular-related NAC-DOMAIN7 is involved in the differentiation of all types of xylem vessels in Arabidopsis roots and shoots. Plant J 55: 652–664 [DOI] [PubMed] [Google Scholar]
- Yu L, Sun J, Li L (2013) PtrCel9A6, an endo-1,4-β-glucanase, is required for cell wall formation during xylem differentiation in Populus. Mol Plant 6: 1904–1917 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Song D, Sun J, Li L (2013) Populus endo-beta-mannanase PtrMAN6 plays a role in coordinating cell wall remodeling with suppression of secondary wall thickening through generation of oligosaccharide signals. Plant J 74: 473–485 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Sun J, Xu P, Zhang R, Li L (2014) Intron-mediated alternative splicing of WOOD-ASSOCIATED NAC TRANSCRIPTION FACTOR1B regulates cell wall thickening during fiber development in Populus species. Plant Physiol 164: 765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Lee C, Ye ZH (2010) Evolutionary conservation of the transcriptional network regulating secondary cell wall biosynthesis. Trends Plant Sci 15: 625–632 [DOI] [PubMed] [Google Scholar]