p-Coumarate acylates the γ-hydroxyls of lignin side chains, particularly on syringyl units, throughout all orders and various families of commelinid monocotyledons.
Abstract
Commelinid monocotyledons are a monophyletic clade differentiated from other monocotyledons by the presence of cell wall-bound ferulate and p-coumarate. The Poaceae, or grass family, is a member of this group, and most of the p-coumarate in the cell walls of this family acylates lignin. Here, we isolated and examined lignified cell wall preparations from 10 species of commelinid monocotyledons from nine families other than Poaceae, including species from all four commelinid monocotyledon orders (Poales, Zingiberales, Commelinales, and Arecales). We showed that, as in the Poaceae, lignin-linked p-coumarate occurs exclusively on the hydroxyl group on the γ-carbon of lignin unit side chains, mostly on syringyl units. Although the mechanism of acylation has not been studied directly in these species, it is likely to be similar to that in the Poaceae and involve BAHD acyl-coenzyme A:monolignol transferases.
The hydroxycinnamates p-coumarate (pCA) and ferulate (FA) occur bound to cell walls of the monocotyledon family Poaceae, which comprises the grasses, including the cereals (Harris and Trethewey, 2010; Hatfield et al., 2017). Most of this pCA acylates the polymer lignin (Ralph, 2010), although modest amounts have been found acylating the major noncellulosic polysaccharides, the glucuronoarabinoxylans (GAXs; Mueller-Harvey et al., 1986; Bartley et al., 2013; Petrik et al., 2014). Lignin is found in most secondary cell walls of vascular plants and provides compressive strength to the walls and to the organs, such as leaves and stems, in which they occur. It also protects cell wall polysaccharides from degradation by invading microbial pathogens. It is quickly becoming a focal point in biorefineries as a key source for the production of valuable coproducts. Lignin is synthesized primarily from three hydroxycinnamyl alcohols (p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol) that are referred to as monolignols (MLs; Freudenberg and Neish, 1968). These MLs are enzymatically oxidized by peroxidases and laccases to form free radicals that then polymerize nonenzymatically to form lignin, with the p-coumaryl, coniferyl, and sinapyl alcohols producing p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) lignin units, respectively (Freudenberg and Neish, 1968). However, there is now abundant evidence that other nontraditional monomers can be incorporated into lignin in both wild-type and genetically modified plants, resulting in a diversity of structural features (Ralph et al., 2004b; Vanholme et al., 2012). MLs acylated by phenolic acids (especially pCA, FA, and p-hydroxybenzoate [pBA]), as well as acetate, are now well-accepted monomers of lignification in various species (see below).
In contrast to pCA, cell wall-bound FA mostly acylates GAXs, although small amounts have recently been found acylating lignin (Karlen et al., 2016). FA (and diferulate) acylating GAXs participate in radical coupling reactions that cross-link these polysaccharides (Ralph et al., 2004a; Hatfield et al., 2017). In addition, they incorporate into lignin and, in doing so, they cross-link GAXs with lignin (Ralph, 2010). This contrasts with pCA, which is pendant on lignin and does not readily participate in radical coupling reactions (Ralph, 2010). Other carboxylic acids also have been found to acylate lignin as pendent units in the Poaceae (grasses) and other taxa. For example, acetate has been shown to acylate lignin in a wide range of taxa, including large amounts in the eudicotyledon kenaf (Hibiscus cannabinus, family Malvaceae; Ralph and Lu, 1998; del Río et al., 2007; Lu and Ralph, 2008) and the monocotyledons abaca (Musa textilis, family Musaceae) and sisal (Agave sisalana, family Asparagaceae; del Río et al., 2007). pBA acylates the lignin of palms in the monocotyledon family Arecaceae (Lu et al., 2015), poplars and aspens (Populus spp.; Morreel et al., 2004), willows (Salix spp., family Salicaceae; Landucci et al., 1992), and Aralia cordata (family Araliaceae; Hibino et al., 1994). Benzoate (BA) and trace amounts of vanillate (VA) also recently were found acylating lignin in leaf bases of Canary Island date palm (Phoenix canariensis; Karlen et al., 2017).
Initial studies on the regiochemistry of pCA acylation on lignins in the family Poaceae suggested that this occurred primarily on the hydroxyl group on the α-carbon of the side chain of lignin units (Nakamura and Higuchi, 1976). However, it has since been shown by NMR spectroscopy to occur exclusively on the hydroxyl group on the γ-carbon (Ralph et al., 1994). Subsequently, the preponderance of S-unit acylation was identified by the derivatization followed by reductive cleavage (DFRC) method that cleaves β-ethers (as well as α-ethers) in lignin but leaves γ-esters intact (Lu and Ralph, 1999). Attachment of pCA to lignin at this particular location has been shown for a range of different grass species in various subfamilies (Soreng et al., 2015) within the Poaceae. These species include maize (Zea mays, subfamily Panicoideae), bromegrass (Bromus spp., subfamily Pooideae), bamboo (Bambusa spp., subfamily Bambusoideae; Lu and Ralph, 1999), sugarcane (Saccharum spp., subfamily Panicoideae; del Río et al., 2015), elephant grass (Pennisetum purpureum, subfamily Panicoideae; del Río et al., 2012), rice (Oryza sativa, subfamily Oryzoideae; Withers et al., 2012; Bartley et al., 2013; Takeda et al., 2017), and the model grasses Brachypodium distachyon (subfamily Pooideae; Petrik et al., 2014) and green foxtail (Setaria viridis, subfamily Panicoideae; de Souza et al., 2018).
The acids, including p-coumaric acid, do not acylate the lignin after polymerization. Instead, acylated ML conjugates are used as nontraditional monomers (Lu and Ralph, 2008). These acylated conjugates are formed from MLs and acyl-CoA cofactors by the action of BAHD acyl-CoA:monolignol transferases (Karlen et al., 2016). (The BAHD acyltransferase family is named after the first letter of each of the first four enzymes characterized [D’Auria, 2006].) The first active p-coumaroyl-CoA:monolignol transferase (PMT) enzyme was identified and characterized in rice (Withers et al., 2012), and homologous enzymes (and genes encoding them) also have been characterized in the model grass B. distachyon (Petrik et al., 2014; Sibout et al., 2016) and maize (Marita et al., 2014; Smith et al., 2017).
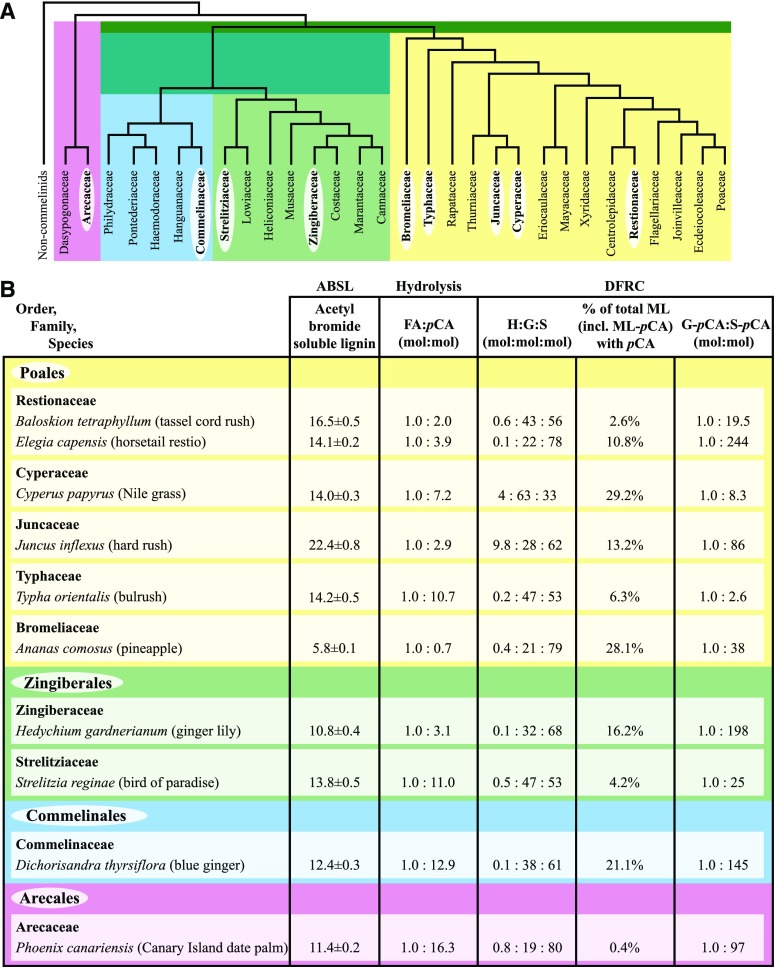
In addition to the family Poaceae, wall-bound pCA and FA occur in a large group of monocotyledons that forms a monophyletic clade and that is now known as the commelinid monocotyledon clade (Harris and Hartley, 1980; Harris and Trethewey, 2010; APG IV, 2016). This clade (Fig. 1A) comprises four orders: the Poales, composed of 14 families (Centrolepidaceae is now placed within the Restionaceae), including the grasses (Poaceae), sedges (Cyperaceae), and bromeliads (Bromeliaceae); the Zingiberales, composed of eight families, including the gingers (Zingiberaceae); the Commelinales, composed of five families, including the spiderworts (Commelinaceae); and the Arecales, composed of two families, including the palms (Arecaceae; APG IV, 2016). The commelinid cell wall preparations analyzed in the original survey contained a mixture of both lignified and nonlignified walls, and all preparations were found to contain both bound FA and pCA (Harris and Hartley, 1980).
Figure 1.
Phylogenetic tree of commelinid monocotyledons and key results from lignified cell wall preparations. A, Phylogenetic tree adapted from Givnish et al. (2010) and Chase et al. (2006). Families sampled in this study are on a white background. In APG IV (2016), Centrolepidaceae is included in Restionaceae. B, Key results from lignified cell wall preparations: ABSL content (percentage dry weight ± se for n = 2), the ratio of FA to pCA as quantified by alkaline hydrolysis, and the proportions of the lignin components as quantified by DFRC.
Here, we have obtained cell wall preparations containing high proportions of lignified cell walls from 10 species of commelinid monocotyledons from nine families outside Poaceae. We determined the proportions of pCA, FA, and pBA released by alkaline hydrolysis and used DFRC (Lu and Ralph, 1999) to determine if they acylate the lignin. We also detected pCA, FA, pBA, BA, and the lignin subunits by using whole-cell wall solution-state NMR spectroscopy (Kim and Ralph, 2010).
RESULTS
Phenolic Acids Released from Cell Wall Preparations by Alkaline Hydrolysis
The content of acetyl bromide soluble lignin (ABSL) in the cell wall preparation containing a high proportion (40% or greater) of walls with a positive phloroglucinol-HCl reaction (lignified cell walls) ranged from ∼11% to 22% (Fig. 1B), with the exception of pineapple (Ananas comosus), which had only 5.8% ABSL, consistent with its having a lower proportion of the cell walls giving a positive lignin reaction (Supplemental Table S1). With the exception of A. comosus, all lignified cell wall preparations, upon alkaline hydrolysis, released much higher total amounts of p-coumaric acid than ferulic acid (Fig. 1B; Table I). The ratio of ferulic to p-coumaric acid ranged from 1:0.7 (for A. comosus) to 1:16.3 (for P. canariensis). Significant amounts of p-hydroxybenzoic acid were released only from P. canariensis, but small amounts also were released from bulrush (Typha orientalis) and bird of paradise (Strelitzia reginae; Table I). No other phenolic acids were detected at significant abundance.
Table I. Amounts of phenolic acids released from lignified cell wall preparations by alkaline hydrolysis and the total amount of DFRC-quantified monomers (including monolignol conjugates).
Means ± from chromatograhy of extracts from duplicate preparations. ND, Not detected.
| Taxon | Alkaline Hydrolysis-Released Phenolic Acids | DFRC-Quantified Monomers | ||||
|---|---|---|---|---|---|---|
| Order, Family, Species | pCA | FA | pBA | Total H | Total G (Percentage G-pCA of Total G) | Total S (Percentage S-pCA of Total S) |
| mg g−1 dry wt | µmol g−1 ABSL | |||||
| Poales | ||||||
| Restionaceae | ||||||
| Baloskion tetraphyllum | 4.55 ± 0.15 | 2.27 ± 0.04 | ND | 7.3 ± 0.3 | 539 ± 13 (0.3%) | 702 ± 20 (4.4%) |
| Elegia capensis | 14.92 ± 0.17 | 3.85 ± 0.04 | ND | 1.3 ± 0.1 | 287 ± 34 (0.2%) | 1,038 ± 102 (13.8%) |
| Cyperaceae | ||||||
| Cyperus papyrus | 22.81 ± 0.01 | 3.18 ± 0.06 | ND | 0.28 ± 0.01 | 4 ± 0 (4.9%) | 2 ± 0 (80.2%) |
| Juncaceae | ||||||
| Juncus inflexus | 17.19 ± 0.65 | 6.00 ± 0.25 | ND | 4.6 ± 0.0 | 13.3 ± 0.2 (0.5%) | 29 ± 3 (21.0%) |
| Typhaceae | ||||||
| Typha orientalis | 13.73 ± 0.06 | 1.28 ± 0.04 | 0.04 ± 0.00 | 1.7 ± 0.0 | 401 ± 11 (3.8%) | 457 ± 6 (8.5%) |
| Bromeliaceae | ||||||
| Ananas comosus | 4.80 ± 0.19 | 6.84 ± 0.12 | ND | 0.21 ± 0.02 | 10.4 ± 0.3 (3.5%) | 40 ± 4 (34.8%) |
| Zingiberales | ||||||
| Zingiberaceae | ||||||
| Hedychium gardnerianum | 10.54 ± 0.61 | 3.43 ± 0.21 | ND | 0.13 ± 0.02 | 39 ± 0 (0.3%) | 81 ± 2 (23.9%) |
| Strelitziaceae | ||||||
| Strelitzia reginae | 8.05 ± 0.26 | 0.73 ± 0.05 | 0.05 ± 0.00 | 8.1 ± 0.2 | 759 ± 43 (0.4%) | 849 ± 28 (7.8%) |
| Commelinales | ||||||
| Commelinaceae | ||||||
| Dichorisandra thyrsiflora | 13.74 ± 2.87 | 1.07 ± 0.21 | ND | 0.68 ± 0.05 | 217 ± 23 (0.4%) | 348 ± 7 (34.1%) |
| Arecales | ||||||
| Arecaceae | ||||||
| Phoenix canariensis | 2.80 ± 0.16 | 0.17 ± 0.01 | 1.08 ± 0.07 | 38.6 ± 1.1 | 894 ± 82 (<0.1%) | 3,793 ± 77 (0.3%) |
For six of the species, a cell wall preparation containing a lower proportion (20% or less) of walls giving a positive lignin reaction (less-lignified cell wall preparation) also was analyzed (Supplemental Table S2). For these cell wall preparations, the amounts of p-coumaric acid released were substantially lower than the amounts released from the lignified cell wall preparations and ranged from 6.3% in ginger lily (Hedychium gardnerianum) to 28.5% in S. reginae (Supplemental Table S3). In contrast, the amount of ferulic acid released was greater, ranging from 139% in blue ginger (Dichorisandra thyrsiflora) to 600% in P. canariensis. These differences were reflected in much lower ratios of ferulic acid to p-coumaric acid; for example, the FA:pCA ratio of the less-lignified cell wall preparations of A. comosus was 1:0.06 and that of D. thyrsiflora was 1:2.07, as compared with the ratio in the highly lignified cell wall preparations, 1:0.7 (A. comosus) and 1:12.9 (D. thyrsiflora). The amount of p-hydroxybenzoic acid released from the less-lignified cell wall preparation from P. canariensis was 8% of that released from the lignified cell wall preparation. As in the lignified cell wall preparations, small amounts of p-hydroxybenzoic acid also were found in hydrolysates of the less-lignified cell wall preparations of T. orientalis and S. reginae. However, the amounts were only somewhat reduced: they were 61% and 83%, respectively, of those released from the lignified cell wall preparations.
DFRC of Lignified Cell Wall Preparations
ML-pCA conjugates were recovered following DFRC from all the lignified cell wall preparations examined (Table II). The proportion of these conjugates as a percentage of the total DFRC monomers (including ML conjugates) varied from 0.4% for P. canariensis to 29.2% for Nile grass (Cyperus papyrus; Fig. 1B). pCA acylated S more than G units, with the ratio of released G-pCA to S-pCA varying from 1:2.6 for T. orientalis to 1:244 for horsetail restio (Elegia capensis). The percentage of S units acylated by pCA varied from 0.3% in P. canariensis to 80.2% in C. papyrus, whereas the percentage of G units acylated by pCA varied from less than 0.1% in P. canariensis to 4.9% in C. papyrus (Table I). Evidence for the presence of pCA acylating H units was found for T. orientalis and D. thyrsiflora by the detection of the H-monomer conjugates (Table II). Similarly, evidence for the presence of pBA acylating lignins was found only in P. canariensis, in which more (94%) were found on S units than on G units (6%). ML-BA and ML-VA, in which BA and VA acylated predominantly S units, also were found only in this species but were not quantified here. However, proportions have been reported previously from lignin isolated from leaf bases of the same species (Karlen et al., 2017). ML-FAs predominantly acylated S units and were present in all of the species assayed, but these were a very minor component (less than 0.1%) of the lignin. However, because FA, unlike pCA and pBA, participates readily in radical coupling reactions, the amounts released do not reflect the amounts incorporated.
Table II. Individual lignin components as quantified by DFRC from lignified cell wall preparations and reported as µmol g−1 ABSL.
ND, Not detected; trace, compound detected but the signal was too weak to quantify; ± = /SE.
| Order, Family, Species | H-OH/Ac | H-pCA | G-OH/Ac | G-pBA | G-pCA | G-FA | S-OH/Ac | S-pCA | S-pBA | S-FA |
|---|---|---|---|---|---|---|---|---|---|---|
| Poales | ||||||||||
| Restionaceae | ||||||||||
| Baloskion tetraphyllum | 7.3 ± 0.3 | ND | 537 ± 13 | ND | 1.58 ± 0.11 | trace | 670 ± 18 | 31 ± 2 | ND | 1.42 ± 0.16 |
| Elegia capensis | 1.3 ± 0.1 | ND | 286 ± 34 | ND | 0.59 ± 0.03 | 0.20 ± 0.01 | 894 ± 89 | 143 ± 13 | ND | 1.15 ± 0.01 |
| Cyperaceae | ||||||||||
| Cyperus papyrus | 0.28 ± 0.01 | ND | 4.3 ± 0.5 | ND | 0.22 ± 0.01 | ND | 0.45 ± 0.01 | 1.85 ± 0.01 | ND | trace |
| Juncaceae | ND | |||||||||
| Juncus inflexus | 4.6 ± 0.0 | ND | 13.2 ± 0.2 | ND | 0.07 ± 0.00 | ND | 23 ± 3 | 6.1 ± 0.2 | 0.06 ± 0.00 | |
| Typhaceae | ||||||||||
| Typha orientalis | 1.6 ± 0.0 | trace | 386 ± 10 | ND | 15.1 ± 0.7 | trace | 419 ± 3 | 39 ± 3 | ND | 0.10 ± 0.02 |
| Bromeliaceae | ||||||||||
| Ananas comosus | 0.21 ± 0.02 | ND | 10.1 ± 0.3 | ND | 0.36 ± 0.06 | ND | 26 ± 1 | 14 ± 2 | ND | 0.13 ± 0.01 |
| Zingiberales | ||||||||||
| Zingiberaceae | ||||||||||
| Hedychium gardnerianum | 0.13 ± 0.02 | ND | 39 ± 0 | ND | 0.10 ± 0.00 | ND | 61 ± 1 | 19 ± 1 | ND | trace |
| Strelitziaceae | ||||||||||
| Strelitzia reginae | 8.1 ± 0.2 | ND | 756 ± 43 | ND | 2.67 ± 0.03 | 0.06 ± 0.00 | 783 ± 27 | 66 ± 1 | ND | 0.22 ± 0.00 |
| Commelinales | ||||||||||
| Commelinaceae | ||||||||||
| Dichorisandra thyrsiflora | 0.66 ± 0.04 | trace | 217 ± 23 | ND | 0.82 ± 0.07 | ND | 229 ± 3 | 118 ± 4 | ND | 0.22 ± 0.01 |
| Arecales | ||||||||||
| Arecaceae | ||||||||||
| Phoenix canariensisa | 38.6 ± 1.1 | ND | 818 ± 81 | 59 ± 3 | 0.12 ± 0.02 | 0.03 ± 0.03 | 2,317 ± 77 | 11.5 ± 0.1 | 996 ± 17 | 0.25 ± 0.03 |
ML-BA and ML-VA also were detected in the DFRC analysis, predominantly as S-BA and S-VA. Their proportions are reported by Karlen et al. (2017).
S units also were the predominant units making up lignin, except in C. papyrus, where there were more G units (63%; Fig. 1B). The highest proportions of S units (78%–80%) were in the lignins of E. capensis, A. comosus, and P. canariensis. With the exception of hard rush (Juncus inflexus) and C. papyrus, which had lignins with 9.8% and 4% H units, respectively, the content of H units ranged between only 0.1% and 0.8%.
NMR Spectroscopy of the Lignified Cell Wall Preparations
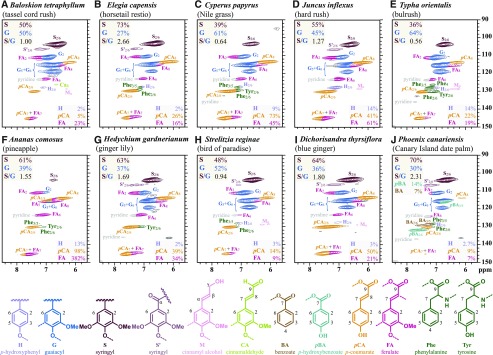
The aromatic regions of 2D heteronuclear single-quantum coherence (HSQC) NMR spectra of lignified cell walls were consistent with lignins comprising predominantly S and G units with little to no H units (Fig. 2). Most of the species had higher or similar proportions of S to G units, except for T. orientalis and C. papyrus, which indicated more G (64% and 61%, respectively) than S units (36% and 39%, respectively). The highest proportions of H units were found in J. inflexus (14%) and T. orientalis (14%, with a substantial amount of protein); this signal can overlap with BA (Karlen et al., 2017) and protein signals from Phe (Kim et al., 2017), complicating signal detection and assignment.
Figure 2.
Aromatic regions of 2D 1H-13C correlation (HSQC) spectra. Results were obtained from gels of lignified cell walls of commelinid monocotyledon species in DMSO-d6/pyridine-d5 (4:1): Baloskion tetraphyllum (A), Elegia capensis (B), Cyperus papyrus (C), Juncus inflexus (D), Typha orientalis (E), Ananas comosus (F), Hedychium gardnerianum (G), Strelitzia reginae (H), Dichorisandra thyrsiflora (I), and Phoenix canariensis (J). Structures of lignin subunits, phenolic esters, and amino acids that are known to be present in plant cell walls are shown below. The substructure units are color coded to match their assignments in spectra A to J.
Also identifiable were FA, pCA, and pBA. The weakest pCA signal was observed for the P. canariensis cell wall preparation, consistent with the lowest amount of pCA released in this species upon alkaline hydrolysis. The spectra from all cell wall preparations from all species also showed correlation signals for FA. The spectrum from the P. canariensis cell wall preparation was the only one with signals for pBA and BA. The levels of the cinnamic (pCA and FA) and benzoic (BA and pBA) acids also can be assessed via their NMR volume integrals; however, because of the mobility of these end units, their integral volumes severely overrepresent their real quantities and should be used only as relative measures (Mansfield et al., 2012). Quantification from the NMR spectra (Fig. 2) was consistent with the alkaline hydrolysis data (Table I) in identifying the species with high and low proportions of FA, pCA, and pBA.
DISCUSSION
Our results show that lignified cell wall preparations from all the species examined, representing all four orders of the commelinid monocotyledons, had pCA bound to their cell walls. The much lower amounts of pCA released by alkaline hydrolysis from less-lignified cell wall preparations from the same species indicate that the bound pCA is concentrated in lignified cell walls, consistent with the notion that most of the pCA acylates the lignin, as has been demonstrated previously in the Poaceae. Indeed, as in the grasses, our DFRC results showed that pCA acylates the γ-OH of the lignin unit side chains, predominantly the S units. However, as in the grasses, at least some of the pCA also may acylate the GAXs, but this was not investigated in this study. GAXs are known to occur in the cell walls, probably both the secondary and primary walls, in other commelinid monocotyledon families, although only in small proportions in the primary walls of palms (Arecaceae; Smith and Harris, 1995; Harris et al., 1997; Carnachan and Harris, 2000a; Peña et al., 2016).
We chose two powerful approaches to examine acylation by pCA. The first, whole-cell wall gel-NMR, allows profiling of the cell wall (lignins and polysaccharides except for the crystalline cellulose) without requiring its degradation (except that incurred in the ball-milling step) or fractionation (Kim and Ralph, 2010; Mansfield et al., 2012). NMR readily reveals pCA (and other aromatics associated with the wall) but does not differentiate between those that acylate the lignin versus polysaccharide components. Due to the nature of the HSQC pulse sequence and the difference in the spin-spin and spin-lattice relaxation behavior of protons and carbons in mobile end units and appendages versus those from the less mobile backbone of the polymer, such acylating components are vastly overrepresented, as described previously (Kim and Ralph, 2010; Mansfield et al., 2012). That said, relative comparisons are valid, provided they are not overinterpreted as being quantitatively accurate. The DFRC method is arguably the best quantitative method to unambiguously determine if pCA acylates lignin (Regner et al., 2018). However, as with other degradative methods that release normal lignin monomers, DFRC is limited to examining only those moieties that are released by cleaving β-ether units and does not allow the determination of partitioning between lignin and polysaccharides; there are emerging methods attempting to address partitioning, but not in a truly quantitative way (Petrik et al., 2014).
Our results are consistent with an earlier DFRC study of lignins isolated from the cell walls of two commelinid monocotyledons: abaca (Musaceae, Zingiberales) and curaua (Ananas lucidus [syn. A. erectifolius], Bromeliaceae, Poales; del Río et al., 2008). In both species, pCA (as well as acetate) acylated the γ-OH of the lignin unit side chains, predominantly the S units. Earlier studies on two Cyperaceae (Poales) species also are consistent with the observation here that the main source of the cell wall-bound pCA is from acylated lignin. The walls of the outer cell layers of the corm of Chinese water chestnut (Eleocharis dulcis; Grassby et al., 2013) and of the tubers of chufa (tiger nut [Cyperus esculentus]; Parker et al., 2000) were shown to be lignified and to contain high proportions of pCA, in contrast to the walls of the inner, nonlignified cells that contained only low levels of pCA. Although we did not report the information, in a previous study (Karlen et al., 2016) we monitored (by DFRC) lignin-linked pCA in cell wall preparations of the following noncommelinid monocotyledons: sisal (Asparagaceae, Asparagales), orange lily (Lilium henryi, Liliaceae, Liliales), cv Stargazer lily (Lilium orientalis, Liliaceae, Liliales), Veitchii screw pine (Pandanus tectorius, Pandanaceae, Pandales), voodoo lily (Amorphophallus bulbifer, Araceae, Alismatales), and sweet flag (Acorus calamus, Acoraceae, Acorales). No evidence of lignin-linked pCA was observed in the cell wall preparations of these species.
In addition to pCA and FA, the lignified cell walls of the palm species P. canariensis contained significant proportions of pBA. Wall-bound pBA has been reported previously from species across the palm family (Arecaceae; Pearl et al., 1959). In our study here, we did not detect any evidence of pBA acylating lignin in the wall preparations of the other commelinid monocotyledons examined. Furthermore, no evidence was found for lignin-linked pBA in the cell wall preparations of noncommelinid monocotyledons in the study by Karlen et al. (2016) (see above), indicating that such acylation is a trait only of the Arecaceae. pBA acylates lignin on the γ-OH of the side chains, mostly on S units, in coconut (Cocos nucifera) coir fiber (Rencoret et al., 2013), African oil palm (Elaeis guineensis) empty fruit bunch fibers (Lu et al., 2015), and the leaf-base tissues of P. canariensis (Karlen et al., 2017). Like pCA, pBA is pendant on lignin and does not readily participate in radical coupling reactions (Ralph, 2010). In all three of these studies, there was a notable lack of pCA acylation of the lignin in some or all the tissues studied. Thus, although pCA acylates lignin throughout the commelinid monocotyledon clade, at the base of the clade the pCA may, in some tissue regions, be absent.
In conclusion, our results showed that pCA acylates lignin in the lignified cell wall preparations we isolated from all 10 species of commelinid monocotyledons examined representing nine families outside the Poaceae and all four commelinid monocotyledon orders (Poales, Zingiberales, Commelinales, and Arecales). As in Poaceae, lignin-linked pCA occurs exclusively on the hydroxyl group on the γ-carbon side chains of lignin units, mostly on S units. Furthermore the mechanism of acylation is likely to be similar to that in the Poaceae and to involve BAHD acyl-CoA:ML transferases. However, the exact identities of these enzymes and the genes encoding them are unknown.
MATERIALS AND METHODS
Microscopy
Bright-field and fluorescence microscopy were carried out using a Zeiss Axioplan 2 microscope fitted with a 12-V, 100-W halogen lamp, a mercury vapor lamp (HBO 100), and an incidence illuminator fitted with a G 365 nm excitation filter, FT395 chromatic beam splitter, and LP420 barrier filter. Images were captured with a Zeiss AxioCam HR digital camera.
Plant Material and Cell Wall Preparations
The sources of the plant material and organs used in the studies are described in Supplemental Table S1. Transverse sections of organs were cut by hand using a razor blade. Tissues containing cells with high proportions of lignified walls were identified histochemically using phloroglucinol-HCl, the Wiesner reagent (Adler et al., 1948), with which lignified walls give a red coloration (Harris et al., 1982), and by UV fluorescence microscopy of sections mounted in water and in 0.1 m ammonium hydroxide solution, in which lignified walls fluoresce blue (Harris and Hartley, 1976, 1980; Smith and Harris, 1995). Cell wall preparations from such tissues contained high proportions of walls (40% or greater) giving a positive phloroglucinol-HCl reaction for lignin and are referred to as lignified cell wall preparations (Supplemental Table S1). For comparison, in six of the 10 species used, tissues were used containing low proportions of lignified walls and gave cell wall preparations containing low proportions (20% or less) of lignified walls (Supplemental Table S2). These are referred to as less-lignified cell wall preparations, and only the amounts of phenolic acids released by alkaline hydrolysis were measured.
The selected tissues were homogenized using a Benchtop Ring Mill (Rocklabs) in MOPS-KOH buffer (20 mm, pH 6.8). The homogenates were centrifuged (1,000g, 10 min), and the pellets were resuspended in buffer, filtered onto nylon mesh (11-µm pore size), washed successively with buffer, ethanol, methanol, and n-pentane, and then air dried. Starch grains were detected histochemically using iodine in potassium iodide (0.2 g of iodine and 2 g of potassium iodide in 100 mL of water) with starch staining blue-black (Carnachan and Harris, 2000a). Starch was detected in cell wall preparations from Cyperus papyrus (lignified cell wall preparation), Hedychium gardnerianum (both types of cell wall preparations), and Phoenix canariensis (both types of cell wall preparations) and were destarched using porcine pancreatic α-amylase as described by Carnachan and Harris (2000a).
Alkaline Hydrolysis, Extraction, and HPLC Analysis of the Phenolic Acids
Cell wall preparations (5 mg in duplicate) were suspended in 2 m sodium hydroxide (1.25 mL) with the internal standard 2-hydroxycinnamic acid (10 µg; Sigma-Aldrich). The suspension was placed under nitrogen and shaken at 200 rpm, in the dark, for 20 h at room temperature. The suspensions were filtered (glass microfiber filter, type GF/C; Whatman), and the filtrate was acidified to pH < 2 with 12 m HCl. The phenolic acids were extracted with diethyl ether (3 × 1 mL). The diethyl ether extracts were combined, evaporated to dryness under nitrogen, redissolved in tetrahydrofuran:water (1 mL, 1:1, v/v), and analyzed on an Agilent 1000 series HPLC device (Agilent Technologies). Chromatography of extracts from each duplicate preparation was carried out using an adaptation of the method of Dobberstein and Bunzel (2010) on a Luna phenyl hexyl column (250 mm × 4.6 mm i.d., 5-μm particle size, with a 3-mm × 4.6-mm i.d. guard column; Phenomenex). The sample volume was 20 µL, and the column was held at 45°C. Ternary solvent systems were made up from 0.1% (v/v) aqueous formic acid (A), acetonitrile (B), and methanol (C) at a flow rate of 1 mL min−1. Separations were carried out as follows: initially 88.3% A, 11.7% (v/v) B, and 0% (v/v) C, held for 10 min, then linear over 10 min to 79.3% (v/v) A, 18% (v/v) B, and 2.7% (v/v) C, linear over 5 min to 73% (v/v) A, 22.5% (v/v) B, and 4.5% (v/v) C, linear over 5 min to 32.5% (v/v) A, 45% (v/v) B, and 22.5% (v/v) C, then linear over 3 min to the initial mixture and held for 10 min. Chromatograms were monitored at 280 nm. All operations were carried out under illumination from a UV stop lamp (12 V, 50 W; Sylvania Australasia) to avoid UV radiation, which causes cis-trans-isomerization (Hartley and Jones, 1975). The response factors were determined using pure phenolic acids (Sigma-Aldrich); the cis-isomers of ferulic and p-coumaric acids were quantified using the response factors of their respective trans-isomers (Carnachan and Harris, 2000b).
Determination of ABSL Content
The lignified cell wall preparations (2.5−5 mg in duplicate) were dissolved in 25% (v/v) acetyl bromide in 17.4 m acetic acid (0.2 mL) and heated at 50°C for 2 h with occasional mixing (Hatfield et al., 1999; Fukushima and Hatfield, 2001). After cooling, 2 m NaOH (1 mL) and 0.5 m hydroxylamine hydrochloride (0.175 mL) were added and made up to 10 mL with 17.4 m acetic acid, and the A280 was measured with a Shimadzu UV-1800 spectrometer (model UV-1800; Shimadzu Scientific Instruments). An extinction coefficient of 20 L g−1 cm−1 was used to calculate the content of ABSL.
DFRC
Incorporation of monolignol conjugates (ML-X, where X is the γ-acylating carboxylic acid) into the lignin was determined using the ether-cleaving ester-retaining DFRC method established previously for ML-OH/Ac, ML-pBA, ML-pCA, ML-FA, and ML-BA conjugates (Lu and Ralph, 1999; Lu et al., 2015; Karlen et al., 2017; Smith et al., 2017). The DFRC protocol used here was as follows.
The lignified cell wall preparations (6−50 mg in duplicate) were stirred in 2-dram vials fitted with polytetrafluoroethylene pressure-release caps in acetyl bromide:acetic acid (1:4 [v/v], 3 mL). After heating for 2 h at 50°C, the solvents were removed on a SpeedVac (Thermo Scientific SPD131DDA; 50°C, 35 min, 35 torr min−1 ramp down to 0.1 torr). Crude films were suspended in absolute ethanol (0.5 mL), dried on the SpeedVac (50°C, 15 min, 35 torr min−1 ramp down to 1 torr), and then suspended in dioxane:acetic acid:water (5:4:1 [v/v/v], 5 mL) with nano-powdered zinc (250 mg). The vials were then sealed and sonicated, to ensure the suspension of solids, in the dark at room temperature for 16 to 20 h. The reaction mixtures were then quantitatively transferred with dichloromethane (DCM; 2 × 2 mL) into separatory funnels charged with saturated ammonium chloride (10 mL) and the isotopically labeled internal standards. Organics were extracted with DCM (3 × 10 mL), combined, dried over anhydrous sodium sulfate, and filtered, and the solvents were removed via rotary evaporation (water bath at 50°C). Free hydroxyl groups on DFRC products were then acetylated using a solution of pyridine and acetic anhydride (1:1 [v/v], 4 mL), after which the solvents were removed on a rotary evaporator to yield crude oily films. To remove most of the polysaccharide-derived products, acetylated DFRC products were loaded onto solid-phase extraction cartridges (Supelco Supelclean LC-Si solid-phase extraction tube; 3 mL; P/N: 505048) with DCM (2 × 1 mL). After elution with hexane:ethyl acetate (1:1 [v/v], 8 mL), the eluted organics were combined and the solvents were removed by rotary evaporation. The products were transferred with DCM to gas chromatography vials containing a 300-μL insert, with the final sample volumes of 200 μL. Samples were analyzed by triple-quadrupole gas chromatography-tandem mass spectrometry (GC-MS/MS; Shimadzu GCMS-TQ8030) in multiple reaction monitoring mode using synthetic standards for authentication and isotopically labeled internal standards for quantitation. The gas chromatography program and acquisition parameters are listed in Supplemental Tables S4 and S5, respectively.
NMR Spectroscopy
Lignified cell wall preparations (∼120 mg) of each species were finely milled, in duplicate, in a Retsch (Haan) PM100 planetary ball mill in a 20-mL zirconium dioxide grinding bowl, with 10- × 10-mm zirconium dioxide ball bearings at 600 rpm, for six cycles of 5 min of grinding and a 5-min break (Kim and Ralph, 2010). Ball-milled material (∼60 mg) was transferred to a 5-mm NMR tube and a premixed solution of DMSO-d6 (99.9% D; Sigma-Aldrich) and pyridine-d5 (99.96% D; Sigma-Aldrich; 4:1 [v/v], 565 μL) was added and the contents mixed using a Teflon-lined magnet placed inside the NMR tube until they appeared homogenous. Spectra of the cell wall gels were acquired at the University of Auckland NMR Centre on a Bruker 600-MHz UltraShield spectrometer equipped with a 5-mm QNP 1H/13C/31P/19F z-gradient cryoprobe (Bruker). The 1H-13C correlation experiment was an adiabatic HSQC variant (Bruker standard pulse sequence hsqcetgpsisp2.2) typically with the following parameters: spectra were acquired from −1 to 11 ppm in F2 (1H) using 2,048 data points for an acquisition time of 136 ms, an interscan relaxation delay (D1) of 1 s, and 0 to 210 ppm in F1 (13C) using 512 data points for an F1 acquisition time of 8.1 ms, using 16 or 32 scans per increment, with a total acquisition time of 5 h 20 min. The DMSO solvent peak was used as an internal reference (δC 39.51, δH 2.5 ppm). The distribution of the various components in the whole cell walls was calculated by manually volume integrating the contours in HSQC plots using Bruker’s TopSpin 3.5 software (Mac version). The 1H-13C correlation signals selected for relatively quantifying the aromatic units were H2/6, G2, S2/6, and the oxidized S′2/6 and are reported on a G2 + 1/2(S2/6 + S′2/6) = 100% basis. The correlation signals for pCA (pCA2/6), FA (FA2), BA (BA2/6), and pBA (pBA2/6) are reported on the same total aromatic G2 + 1/2(S2/6 + S′2/6) = 100% basis. Where correlation signals overlapped with other components (e.g. G2, H2/6, FA2, and pCA2/6), the region integrated was selected to best correlate the integral volume to match the results from the DFRC and base hydrolysis data. NMR analysis was carried out on replicate gels obtained from the cell wall preparation of each species.
Accession Numbers
Sequence data referred to in this article can be found in the GenBank/EMBL data libraries under accession numbers LOC_Os01g18744 (OsPMT), Bradi2g36910.1 (BdPMT1), Bradi1g36980.1 (BdPMT2), and GRMZM2G028104_P01 (ZmPMT).
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. Sources of plant species and organs used to produce lignified cell wall preparations.
Supplemental Table S2. Plant species and organs used to produce less-lignified cell wall preparations.
Supplemental Table S3. Amounts of phenolic acids released from less-lignified cell wall preparations.
Supplemental Table S4. Chromatographic parameters for GC-MS/MS characterization of the DFRC product mix.
Supplemental Table S5. Multiple reaction monitoring parameters for GC-MS/MS characterization of the DFRC product mix.
Acknowledgments
We thank the University of Auckland’s NMR laboratory manager, Dr. M. Schmitz, for providing NMR facilities and Dr. R.A. Smith for providing the phloroglucinol-HCl image used in the TOC graphic.
Footnotes
This research was supported by a Specific Cooperative Agreement (58-3655-8-19f) with the U.S. Dairy Forage Research Center, USDA Agricultural Research Service, via funding from the U.S. Department of Energy Biosciences Program [DF-A102-06ER64299 (2006)]; by the U.S. Department of Energy Great Lakes Bioenergy Research Center (DOE Office of Science BER DE-FC02-07ER64494); and by the University of Auckland.
Articles can be viewed without a subscription.
References
- Adler E, Björkquist KJ, Häggroth S (1948) Über die Ursache der Farbreaktionen des Holzes. Acta Chem Scand 2: 93–94 [Google Scholar]
- APG IV (2016) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot J Linn Soc 181: 1–20 [Google Scholar]
- Bartley LE, Peck ML, Kim SR, Ebert B, Manisseri C, Chiniquy DM, Sykes R, Gao L, Rautengarten C, Vega-Sánchez ME, et al. (2013) Overexpression of a BAHD acyltransferase, OsAt10, alters rice cell wall hydroxycinnamic acid content and saccharification. Plant Physiol 161: 1615–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnachan SM, Harris PJ (2000a) Polysaccharide compositions of primary cell walls of the palms Phoenix canariensis and Rhopalostylis sapida. Plant Physiol Biochem 38: 699–708 [Google Scholar]
- Carnachan SM, Harris PJ (2000b) Ferulic acid is bound to the primary cell walls of all gymnosperm families. Biochem Syst Ecol 28: 865–879 [DOI] [PubMed] [Google Scholar]
- Chase MW, Fay MF, Devey DS, Maurin O, Ronsted N, Davies TJ, Pillon Y, Petersen G, Seberg O, Tamura MN, et al. (2006) Multigene analyses of monocot relationships: a summary. Aliso 22: 63–75 [Google Scholar]
- D’Auria JC. (2006) Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 9: 331–340 [DOI] [PubMed] [Google Scholar]
- del Río JC, Marques G, Rencoret J, Martínez AT, Gutiérrez A (2007) Occurrence of naturally acetylated lignin units. J Agric Food Chem 55: 5461–5468 [DOI] [PubMed] [Google Scholar]
- del Río JC, Rencoret J, Marques G, Gutiérrez A, Ibarra D, Santos JI, Jiménez-Barbero J, Zhang L, Martínez AT (2008) Highly acylated (acetylated and/or p-coumaroylated) native lignins from diverse herbaceous plants. J Agric Food Chem 56: 9525–9534 [DOI] [PubMed] [Google Scholar]
- del Río JC, Prinsen P, Rencoret J, Nieto L, Jiménez-Barbero J, Ralph J, Martínez AT, Gutiérrez A (2012) Structural characterization of the lignin in the cortex and pith of elephant grass (Pennisetum purpureum) stems. J Agric Food Chem 60: 3619–3634 [DOI] [PubMed] [Google Scholar]
- del Río JC, Lino AG, Colodette JL, Lima CF, Gutiérrez A, Martínez AT, Lu F, Ralph J, Rencoret J (2015) Differences in the chemical structure of the lignins from sugarcane bagasse and straw. Biomass Bioenergy 81: 322–338 [Google Scholar]
- de Souza WR, Martins PK, Freeman J, Pellny TK, Michaelson LV, Sampaio BL, Vinecky F, Ribeiro AP, de Cunha BADB, Kobayashi AK, et al. (2018) Suppression of a single BAHD gene in Setaria viridis causes large, stable decreases in cell wall feruloylation and increases biomass digestibility. New Phytol 218: 81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein D, Bunzel M (2010) Separation and detection of cell wall-bound ferulic acid dehydrodimers and dehydrotrimers in cereals and other plant materials by reversed phase high-performance liquid chromatography with ultraviolet detection. J Agric Food Chem 58: 8927–8935 [DOI] [PubMed] [Google Scholar]
- Freudenberg K, Neish AC (1968) Constitution and Biosynthesis of Lignin. Springer-Verlag, Berlin [Google Scholar]
- Fukushima RS, Hatfield RD (2001) Extraction and isolation of lignin for utilization as a standard to determine lignin concentration using the acetyl bromide spectrophotometric method. J Agric Food Chem 49: 3133–3139 [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Ames M, McNeal JR, Mckain MR, Steele PR, dePamphilis CW, Graham SW, Pires JC, Stevenson DW, Zomlefer WB, et al. (2010) Assembling the tree of the monocotyledons: plastome sequence phylogeny and evolution of Poales. Ann Mo Bot Gard 97: 584–616 [Google Scholar]
- Grassby T, Jay AJ, Merali Z, Parker ML, Parr AJ, Faulds CB, Waldron KW (2013) Compositional analysis of Chinese water chestnut (Eleocharis dulcis) cell-wall material from parenchyma, epidermis, and subepidermal tissues. J Agric Food Chem 61: 9680–9688 [DOI] [PubMed] [Google Scholar]
- Harris PJ, Hartley RD (1976) Detection of bound ferulic acid in cell walls of the Gramineae by ultraviolet fluorescence microscopy. Nature 259: 508–510 [Google Scholar]
- Harris PJ, Hartley RD (1980) Phenolic constituents of the cell walls of monocotyledons. Biochem Syst Ecol 8: 153–160 [Google Scholar]
- Harris PJ, Trethewey JAK (2010) The distribution of ester-linked ferulic acid in the cell walls of angiosperms. Phytochem Rev 9: 19–33 [Google Scholar]
- Harris PJ, Hartley RD, Barton GE (1982) Evaluation of stabilized diazonium salts for the detection of phenolic constituents of plant cell walls. J Sci Food Agric 33: 516–520 [Google Scholar]
- Harris PJ, Kelderman MR, Kendon MF, McKenzie RJ (1997) Monosaccharide compositions of unlignified cell walls of monocotyledons in relation to the occurrence of wall-bound ferulic acid. Biochem Syst Ecol 25: 167–179 [Google Scholar]
- Hartley RD, Jones EC (1975) Effect of ultraviolet light on substituted cinnamic acids and the estimation of their cis and trans isomers by gas chromatography. J Chromatogr A 107: 213–218 [Google Scholar]
- Hatfield RD, Grabber J, Ralph J, Brei K (1999) Using the acetyl bromide assay to determine lignin concentrations in herbaceous plants: some cautionary notes. J Agric Food Chem 47: 628–632 [DOI] [PubMed] [Google Scholar]
- Hatfield RD, Rancour DM, Marita JM (2017) Grass cell walls: a story of cross-linking. Front Plant Sci 7: 2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino T, Shibata D, Ito T, Tsuchiya D, Higuchi T, Pollet B, Lapierre C (1994) Chemical properties of lignin from Aralia cordata. Phytochemistry 37: 445–448 [Google Scholar]
- Karlen SD, Zhang C, Peck ML, Smith RA, Padmakshan D, Helmich KE, Free HCA, Lee S, Smith BG, Lu F, et al. (2016) Monolignol ferulate conjugates are naturally incorporated into plant lignins. Sci Adv 2: e1600393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlen SD, Smith RA, Kim H, Padmakshan D, Bartuce A, Mobley JK, Free HCA, Smith BG, Harris PJ, Ralph J (2017) Highly decorated lignins in leaf tissues of the Canary Island date palm Phoenix canariensis. Plant Physiol 175: 1058–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Ralph J (2010) Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d6/pyridine-d5. Org Biomol Chem 8: 576–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Padmakshan D, Li Y, Rencoret J, Hatfield RD, Ralph J (2017) Characterization and elimination of undesirable protein residues in plant cell wall materials for enhancing lignin analysis by solution-state nuclear magnetic resonance spectroscopy. Biomacromolecules 18: 4184–4195 [DOI] [PubMed] [Google Scholar]
- Landucci LL, Deka GC, Roy DNA (1992) 13C NMR study of milled wood lignins from hybrid Salix clones. Holzforschung 46: 505–511 [Google Scholar]
- Lu F, Ralph J (1999) Detection and determination of p-coumaroylated units in lignins. J Agric Food Chem 47: 1988–1992 [DOI] [PubMed] [Google Scholar]
- Lu F, Ralph J (2008) Novel tetrahydrofuran structures derived from β-β-coupling reactions involving sinapyl acetate in Kenaf lignins. Org Biomol Chem 6: 3681–3694 [DOI] [PubMed] [Google Scholar]
- Lu F, Karlen SD, Regner M, Kim H, Ralph SA, Sun RC, Kuroda K, Augustin MA, Mawson R, Sabarez H, et al. (2015) Naturally p-hydroxylated lignins in palms. BioEnergy Res 8: 934–952 [Google Scholar]
- Mansfield SD, Kim H, Lu F, Ralph J (2012) Whole plant cell wall characterization using solution-state 2D NMR. Nat Protoc 7: 1579–1589 [DOI] [PubMed] [Google Scholar]
- Marita JM, Hatfield RD, Rancour DM, Frost KE (2014) Identification and suppression of the p-coumaroyl CoA:hydroxycinnamyl alcohol transferase in Zea mays L. Plant J 78: 850–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreel K, Ralph J, Kim H, Lu F, Goeminne G, Ralph S, Messens E, Boerjan W (2004) Profiling of oligolignols reveals monolignol coupling conditions in lignifying poplar xylem. Plant Physiol 136: 3537–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Harvey I, Hartley RD, Harris PJ, Curzon EH (1986) Linkage of p-coumaroyl and feruloyl groups to cell-wall polysaccharides of barley straw. Carbohydr Res 148: 71–85 [Google Scholar]
- Nakamura Y, Higuchi T (1976) Ester linkage of p-coumaric acid in bamboo lignin. Holzforschung 30: 187–191 [Google Scholar]
- Parker ML, Ng A, Smith AC, Waldron KW (2000) Esterified phenolics of the cell walls of chufa (Cyperus esculentus L.) tubers and their role in texture. J Agric Food Chem 48: 6284–6291 [DOI] [PubMed] [Google Scholar]
- Pearl IA, Beyer DL, Laskowski D (1959) Alkaline hydrolysis of representative palms. TAPPI 42: 779–782 [Google Scholar]
- Peña MJ, Kulkarni AR, Backe J, Boyd M, O’Neill MA, York WS (2016) Structural diversity of xylans in the cell walls of monocots. Planta 244: 589–606 [DOI] [PubMed] [Google Scholar]
- Petrik DL, Karlen SD, Cass CL, Padmakshan D, Lu F, Liu S, Le Bris P, Antelme S, Santoro N, Wilkerson CG, et al. (2014) p-Coumaroyl-CoA:monolignol transferase (PMT) acts specifically in the lignin biosynthetic pathway in Brachypodium distachyon. Plant J 77: 713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph J. (2010) Hydroxycinnamates in lignification. Phytochem Rev 9: 65–83 [Google Scholar]
- Ralph J, Lu F (1998) The DFRC method for lignin analysis. 6. A simple modification for identifying natural acetates on lignins. J Agric Food Chem 46: 4616–4619 [Google Scholar]
- Ralph J, Hatfield RD, Quideau S, Helm RF, Grabber JH, Jung HJG (1994) Pathway of p-coumaric acid incorporation into maize lignin as revealed by NMR. J Am Chem Soc 116: 9448–9456 [Google Scholar]
- Ralph J, Bunzel M, Marita JM, Hatfield RD, Lu F, Kim H, Schatz PF, Grabber JH, Steinhart H (2004a) Peroxidase-dependent cross-linking reactions of p-hydroxycinnamates in plant cell walls. Phytochem Rev 3: 79–96 [Google Scholar]
- Ralph J, Lundquist K, Brunow G, Lu F, Kim H, Schatz PF, Marita JM, Hatfield RD, Ralph SA, Christensen JH, et al. (2004b) Lignins: natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem Rev 3: 29–60 [Google Scholar]
- Regner M, Bartuce A, Padmakshan D, Ralph J, Karlen S (2018) Reductive cleavage method for quantitation of monolignols and low-abundance monolignol conjugates. ChemSusChem (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rencoret J, Ralph J, Marques G, Gutiérrez A, Martínez Á, del Río JC (2013) Structural characterization of lignin isolated from coconut (Cocos nucifera) coir fibers. J Agric Food Chem 61: 2434–2445 [DOI] [PubMed] [Google Scholar]
- Sibout R, Le Bris P, Legée F, Cézard L, Renault H, Lapierre C (2016) Structural redesigning Arabidopsis lignins into alkali-soluble lignins through the expression of p-coumaroyl-CoA:monolignol transferase PMT. Plant Physiol 170: 1358–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BG, Harris PJ (1995) Polysaccharide composition of unlignified cell walls of pineapple [Ananas comosus (L.) Merr.] fruit. Plant Physiol 107: 1399–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Cass CL, Mazaheri M, Sekhon RS, Heckwolf M, Kaeppler H, de Leon N, Mansfield SD, Kaeppler SM, Sedbrook JC, et al. (2017) Suppression of CINNAMOYL-CoA REDUCTASE increases the level of monolignol ferulates incorporated into maize lignins. Biotechnol Biofuels 10: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Zuloaga FO, Judziewicz EJ, Filgueiras TS, Davis JI, Morrone O (2015) A worldwide phylogenetic classification of the Poaceae (Gramineae). J Syst Evol 53: 117–137 [Google Scholar]
- Takeda Y, Koshiba T, Tobimatsu Y, Suzuki S, Murakami S, Yamamura M, Rahman MM, Takano T, Hattori T, Sakamoto M, et al. (2017) Regulation of CONIFERALDEHYDE 5-HYDROXYLASE expression to modulate cell wall lignin structure in rice. Planta 246: 337–349 [DOI] [PubMed] [Google Scholar]
- Vanholme R, Morreel K, Darrah C, Oyarce P, Grabber JH, Ralph J, Boerjan W (2012) Metabolic engineering of novel lignin in biomass crops. New Phytol 196: 978–1000 [DOI] [PubMed] [Google Scholar]
- Withers S, Lu F, Kim H, Zhu Y, Ralph J, Wilkerson CG (2012) Identification of grass-specific enzyme that acylates monolignols with p-coumarate. J Biol Chem 287: 8347–8355 [DOI] [PMC free article] [PubMed] [Google Scholar]