Upstream open reading frames in the 5′ untranslated region of BOR1, a borate transporter, control boron-dependent translational suppression that, alongside BOR1 degradation, contributes to the avoidance of boron toxicity in plants.
Abstract
Boron (B) is an essential element for plants; however, as high B concentrations are toxic, B transport must be tightly regulated. BOR1 is a borate exporter in Arabidopsis (Arabidopsis thaliana) that facilitates B translocation into shoots under B deficiency conditions. When the B supply is sufficient, BOR1 expression is down-regulated by selective degradation of BOR1 protein, while additional BOR1 regulatory mechanisms are proposed to exist. In this study, we identified a novel B-dependent BOR1 translational suppression mechanism. In vivo and in vitro reporter assays demonstrated that BOR1 translation was reduced in a B-dependent manner and that the 5′-untranslated region was both necessary and sufficient for this process. Mutational analysis revealed that multiple upstream open reading frames in the 5′-untranslated region were required for BOR1 translational suppression, and this process depended on the efficiency of translational reinitiation at the BOR1 open reading frame after translation of the upstream open reading frames. To understand the physiological significance of BOR1 regulation, we characterized transgenic plants defective in either one or both of the BOR1 regulation mechanisms. BOR1 translational suppression was induced at higher B concentrations than those triggering BOR1 degradation. Plants lacking both regulation mechanisms exhibited more severe shoot growth reduction under high-B conditions than did plants lacking BOR1 degradation alone, thus demonstrating the importance of BOR1 translational suppression. This study demonstrates that two mechanisms of posttranscriptional BOR1 regulation, each induced under different B concentrations, contribute to the avoidance of B toxicity in plants.
Regulating the transport of mineral nutrients in plants is critical for maintaining homeostasis (Marschner, 2012). Plants control mineral transporter expression in response to mineral availability at various stages (Aibara and Miwa, 2014). Although transcriptional regulation of such transporters has been studied intensively, there are few reports of posttranscriptional regulation, especially translational regulation.
Boron (B) is an essential micronutrient, as borate cross-links pectic polysaccharide in the primary cell wall (O’Neill et al., 2004; Marschner, 2012); however, it is toxic to plants when present in excess concentrations (Nable et al., 1997; Reid et al., 2004). B concentrations in soil solutions can be changed by natural events, such as leaching out after rainfall and accumulation after drying of topsoil water, and possibly via the decomposition of organic matter (Shorrocks, 1997; Argust, 1998; Park and Schlesinger, 2002). In soil solutions, B exists mainly as uncharged boric acid, which readily permeates cell membranes (Dordas et al., 2000). Under these circumstances, plants use different types of B transporters to cope with the narrow optimum range of B concentrations (Yoshinari and Takano, 2017). Under B deprivation, the NODULIN26-LIKE INTRINSIC PROTEIN5;1 (NIP5;1) and BOR1 genes are expressed in roots to support the effective translocation of B in Arabidopsis (Arabidopsis thaliana). NIP5;1 is a boric acid channel expressed mainly in epidermal cells that facilitates boric acid uptake from soil into root cells (Takano et al., 2006, 2010). BOR1 is an efflux borate transporter expressed in various cell types, including epidermis and endodermis, that exports borate out of cells toward the xylem and contributes to the translocation of B from roots to shoots (Takano et al., 2002, 2010). BOR2, another efflux borate transporter, is important for cross-linking pectins under low-B conditions (Miwa et al., 2013). In the presence of toxic B concentrations, Arabidopsis BOR4, a paralog of BOR1, functions to exclude borate from root cells (Miwa et al., 2014). These B transporters coordinately control B transport in plant bodies in response to external B conditions.
Recent studies have revealed several mechanisms of B-dependent regulation of B transporter gene expression. Under sufficient B, NIP5;1 expression is down-regulated via posttranscriptional regulation (Tanaka et al., 2011). During translation, ribosomes are stalled in its 5′-untranslated region (UTR), which enhances the destabilization of NIP5;1 mRNA (Tanaka et al., 2016). Thus, NIP5;1 mRNA is present at lower levels under sufficient B conditions than under low-B conditions. In contrast to NIP5;1, BOR1 expression is not altered at the mRNA level but is regulated at the protein level (Takano et al., 2005). Under B sufficiency (100 μm boric acid in the medium), BOR1 undergoes ubiquitination followed by endocytic protein degradation (Takano et al., 2005; Kasai et al., 2011). Lys-590 is required for this ubiquitination (Kasai et al., 2011), and three Tyr motifs and a di-Leu motif have been shown to be essential for postendocytic trafficking (Takano et al., 2010; Wakuta et al., 2015). Replacement of these amino acid residues effectively inhibits the reduction of BOR1 under sufficient B concentrations. However, it has been demonstrated that, even if the Tyr motifs are disrupted, BOR1 accumulation decreases when treated with toxic concentrations of B (e.g. 1,000 and 3,000 μm) for a long period (Takano et al., 2010). This suggests the presence of another BOR1 regulatory mechanism that is induced by higher B concentrations.
We aimed to reveal the mechanism that regulates B-dependent BOR1 expression independent of selective protein degradation as well as the biological significance of the two regulatory mechanisms. Here, we describe B-dependent translational suppression mediated by the BOR1 5′-UTR. Translational suppression of BOR1 was induced under higher B concentrations than those required to induce BOR1 degradation, thus revealing that these two regulatory mechanisms depend on different B concentrations. In addition, we demonstrate that this posttranscriptional regulation of BOR1 expression contributes to plant survival under high-B conditions by avoiding excess transport of B.
RESULTS
The BOR1 5′-UTR Is Required for the Translational Suppression of the BOR1 Main Open Reading Frame under High-B Conditions
To examine the mechanism of BOR1 down-regulation in the presence of high B concentrations (more than 100 μm), we quantified mRNA accumulation in roots under low (0.1 μm), sufficient (30 μm), and toxic (3,000 μm) concentrations of B as boric acid (Supplemental Fig. S1). It has been reported that low-B treatment does not affect BOR1 mRNA accumulation (Takano et al., 2005). In this study, even under toxic B concentrations, BOR1 mRNA accumulation was not decreased significantly. This suggested that the regulation mechanism at high B concentrations did not likely involve transcription or mRNA degradation.
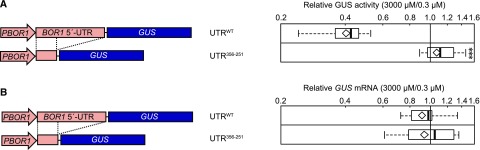
To clarify this posttranscriptional mechanism, we examined the effect of B on the translation of BOR1 mRNA (Fig. 1). In general, 5′-UTRs have an important role in translational regulation (Hinnebusch et al., 2016). The BOR1 5′-UTR is defined as 356 nucleotides (Fig. 2A) based on experimentally obtained full-length cDNA sequences (Seki et al., 2002). First, we investigated the contribution of the 5′-UTR to B-dependent translation in transgenic Arabidopsis carrying a ProBOR1:(BOR1 5′-UTR):GUS construct under low and toxic B concentrations (0.3 and 3,000 μm boric acid, respectively). In the transgenic line carrying the wild-type 5′-UTR (UTRWT), GUS activity decreased to 40% under the toxic B concentration compared with that under the low B concentration (Fig. 1A), whereas removal of 250 nucleotides on the 3′ end of the 5′-UTR (UTR356-251) resulted in the loss of the B-dependent reduction in GUS activity (Fig. 1A). GUS mRNA accumulation was not affected by B concentration (Fig. 1B) in either construct. These results suggested that the 5′-UTR was involved in translational suppression under toxic B conditions and that 250 nucleotides in the 3′ portion were required for this regulation.
Figure 1.
Effects of BOR1 5′-UTR on the expression of the BOR1 ORF. Relative GUS activity (A) and GUS mRNA (B) are shown in roots of transgenic plants under toxic B conditions compared with those under low-B conditions. Schematic representations of the DNA constructs used for plant transformation are shown at left. Eight- or 9-d-old plants were transferred and incubated under 0.3 or 3,000 μm boric acid for 3 d. The values are those relative to the values at 0.3 μm boric acid, which was defined as 1 in each transgenic line. Means and medians were calculated with log-transformed values. Box plots show the distribution among six and four independent lines for UTRWT and UTR356-251, respectively. Diamonds indicate mean values. Asterisks indicate a significant difference compared with that resulting from the wild-type 5′-UTR (***, P < 0.001, Student’s t test).
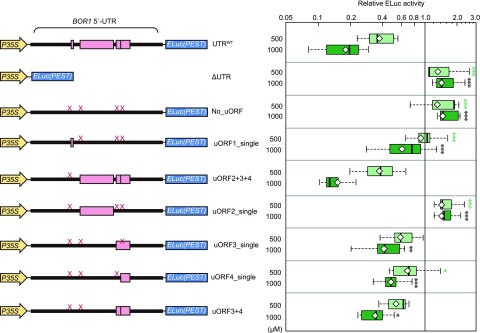
Figure 2.
Effects of BOR1 5′-UTR nucleotide deletions on B-dependent gene expression in Arabidopsis culture cells. A, Nucleotide sequence of the BOR1 5′-UTR. Sequences shaded in pink represent uORFs. Nucleotides that match the Kozak sequence are highlighted in black. Start codons of uORFs and the main ORF are indicated with boxes. B, Transient expression analysis in Arabidopsis culture cells. Schematic representations of the test DNA constructs (left) and relative reporter activities (right) are shown. The CIPK1 5′-UTR is indicated with a gray box. Thick lines and pink boxes represent the BOR1 5′-UTR and uORFs, respectively. The constructs at bottom represent a BOR1 5′-UTR deletion series. The test and control plasmids were cotransfected into MM2d cells by electroporation. Cells were incubated in liquid medium containing 1, 500, or 750 μm boric acid, and reporter activities were measured by dual luciferase assay. Means ± sd of three independent electroporation samples are shown. The graph area is separated into two parts on different scales. Data for UTR96-10 were obtained in an independent experiment. Asterisks indicate significant differences compared with values at 1 μm in each construct (*, P < 0.05; **, P < 0.01; and ***, P < 0.001, Student’s t test).
The BOR1 5′-UTR Is Sufficient for B-Dependent Gene Expression
To determine whether the BOR1 5′-UTR can confer B-dependent regulation, we performed an expression analysis with a constitutive cauliflower mosaic virus 35S RNA promoter. The ELuc(PEST) reporter gene fused to the BOR1 5′-UTR was transiently expressed in cultured Arabidopsis cells exposed to low (1 μm) and high (500 and 750 μm) concentrations of B as boric acid (Fig. 2B). The B concentrations used were in the range in which control reporter gene activity was not lowered. In the control construct without the 5′-UTR (ΔUTR), reporter activity did not change under different B concentrations, indicating that the enzymatic activity of ELuc(PEST) was not affected by B conditions. In the presence of the BOR1 5′-UTR (UTRWT), reporter activity decreased with increasing B concentrations. Consistent with the results in planta (Fig. 1A), the truncated 5′-UTR (UTR356-251) did not induce B-dependent down-regulation. This demonstrated that B-dependent gene expression mediated by the BOR1 5′-UTR occurred in the transient expression system in Arabidopsis culture cells and that this response was activated in the presence of 500 μm B in the medium. Furthermore, we analyzed the effect of the CBL-INTERACTING PROTEIN KINASE1 (CIPK1) 5′-UTR (CIPK1UTR), which is similar in length (294 nucleotides) to the BOR1 5′-UTR, as an example of genes unrelated to B nutrition. Reporter gene activity with CIPK1UTR did not change significantly in response to different B concentrations. These results showed that the BOR1 5′-UTR sequence was sufficient for B-dependent gene expression and that the down-regulation was specific to the BOR1 5′-UTR.
Two Independent Regions in the BOR1 5′-UTR Induce B-Dependent Gene Expression
The BOR1 5′-UTR harbors four short open reading frames (ORFs; Fig. 2A) called upstream open reading frames (uORFs). The four uORFs were named uORF1 to uORF4 starting from the 5′ end. The uORF1 sequence is AUGUAA, a minimum ORF consisting of a start codon and a stop codon as characterized in NIP5;1 (Tanaka et al., 2016). uORF3 and uORF4 are in-frame ORFs, sharing the same stop codon and possessing one or both of two important nucleotides (A/G at –3, G at +4) in the Kozak consensus sequence for efficient translation (Kozak, 1986; Joshi et al., 1997; Sugio et al., 2010). In eukaryotes, uORFs are known to affect the translation of the downstream ORF (Kozak, 1986; von Arnim et al., 2014; Hinnebusch et al., 2016).
Focusing on the presence of the uORFs, we constructed a series of truncated BOR1 5′-UTR fragments to identify the regions responsible for B-dependent gene expression (Fig. 2B). Portions of the 5′-UTR sequences were deleted from the 3′ end (referred to as UTR356-97, UTR356-136, UTR356-235, and UTR356-251) and from the 5′ end (UTR253-10, UTR239-10, UTR140-10, and UTR96-10). Since uORF3 and uORF4 are in frame, we did not create a construct that deleted only one of them. The effects of the truncated series of 5′-UTRs were analyzed in cultured Arabidopsis cells.
The UTRWT induced a reduction in the relative reporter activity with increasing B concentrations, ultimately decreasing at 750 μm B to 24% of the reporter activity measured at 1 μm B (Fig. 2B). B-dependent reductions in reporter activity of 51% to 65% were observed at 750 μm B for most of the 3′-deleted constructs, including UTR356-97, UTR356-136, and UTR356-235, although the extent of the reduction was weaker compared with that of UTRWT. However, the B-dependent reduction in reporter activity was lost for UTR356-251. These results indicated that the 5′ portion of the 5′-UTR between –356 and –235, but not between –356 to –251, supported B-dependent regulation. This implied that the cis-acting element required for the regulation possibly existed in the 122-nucleotide region between –356 and –235, which included uORF1.
Furthermore, B-dependent gene expression was observed for the three 5′-end truncated constructs (UTR253-10, UTR239-10, and UTR140-10), and the reporter activities were reduced to 25% to 39% at 750 μm B. However, the B-dependent expression was not observed for UTR96-10, which carried no uORF. These results showed that 131 nucleotides in the 3′ portion of the 5′-UTR (–140 to –10) were sufficient for B-dependent regulation, which included uORF3 and uORF4.
Since UTR356-235 and UTR140-10 do not overlap with each other, these results clearly suggested that there were at least two independent regions in the 5′-UTR that were responsible for B-dependent regulation. These results also imply a possible contribution of uORFs in the suppression of a basal expression level of a main ORF, as UTR356-251 and UTR96-10, which contained no uORF, showed higher reporter activities independent of the B conditions.
uORFs Are Necessary for B-Dependent Translation in Planta
To examine the involvement of uORFs in B-dependent expression, we introduced mutations to replace the start codons of the uORFs with an AAG sequence in various combinations (Fig. 3). Among the factors in the 5′-UTR structure that affect translation efficiency, the large mRNA secondary structure was unlikely to be required for B-dependent expression based on the results showing that two relatively short regions, 122 nucleotides in the 5′ portion of the 5′-UTR and 131 nucleotides in the 3′ portion of the 5′-UTR, independently regulated B-dependent expression (Fig. 2B). Moreover, the internal ribosome entry site (Hellen and Sarnow, 2001) was not predicted to be present in the corresponding nucleotides. Therefore, the effects of these mutations likely occurred through the disruption of the uORFs.
Figure 3.
Effects of uORF disruptions on B-dependent gene expression driven by the BOR1 5′-UTR in planta. Schematic representations of the DNA constructs used for plant transformation are shown at left. Thick lines and pink boxes represent the BOR1 5′-UTR and uORFs, respectively. The X marks on the 5′-UTR represent point mutations (AUG to AAG) in the start codons of uORFs. Relative reporter activities in roots under 500 and 1,000 μm boric acid are shown at right. Transgenic plants were grown under 0.3, 500, or 1,000 μm boric acid for 12 d. Roots from 18 to 20 plants from one independent line were harvested as one sample. Reporter activities at 500 and 1,000 μm boric acid are shown relative to that at 0.3 μm boric acid, which was set to 1. Means and medians were calculated with log-transformed values. Box plots show reporter activities among multiple independent transgenic lines, with those resulting from 500 and 1,000 μm in light and dark green, respectively. Diamonds indicate mean values. Asterisks indicate significant differences compared with that resulting from UTRWT under the same B treatment (*, P < 0.05; **, P < 0.01; and ***, P < 0.001, Dunnett’s multiple comparison test). The numbers of tested transgenic line were as follows: UTRWT, six lines; ΔUTR, three lines; No_uORF, five lines; uORF1_single, seven lines; uORF2+3+4, four lines; uORF2_single, five lines; uORF3_single, six lines; uORF4_single, five lines; and uORF3+4, six lines.
We generated transgenic plants carrying the series of constructs shown in Figure 3. Several independent lines were generated for each construct, and lines homozygous for transfer DNA insertion were established. The reporter activities in roots were determined in the transgenic plants grown under low (0.3 μm), high (500 μm), and toxic (1,000 μm) concentrations of B as boric acid. The relative reporter activities at 500 and 1,000 μm B compared with that at 0.3 μm B are shown for the respective independent transgenic lines.
In the wild-type 5′-UTR lines (UTRWT), reporter activity was decreased markedly with increasing B concentrations, whereas for the ΔUTR construct, no such reduction was observed. Since ELuc(PEST) mRNA accumulation was not affected by B concentration, these results indicated that the BOR1 5′-UTR was sufficient for B-dependent translation in planta, consistent with the observations in the transfection (or transient expression) experiments shown in Figure 2B. Disruption of all four uORFs by the AUG-to-AAG mutations (No_uORF) resulted in the complete loss of B-dependent suppression of reporter expression. This result demonstrated that BOR1 uORFs were necessary to elicit a response to B concentration.
Multiple uORFs Control B-Dependent Translation
BOR1 uORF1 (AUGUAA) is a minimum ORF, which has been reported to promote ribosome stalling under high B concentrations, leading to the suppression of main ORF expression in NIP5;1 (Tanaka et al., 2016). To distinguish the effect of uORF1 from the other uORFs, we compared mutated 5′-UTRs carrying only uORF1 (uORF1_single) or the other three uORFs (uORF2+3+4).
Unexpectedly, the extent of B-dependent suppression of reporter expression was markedly weaker in the uORF1_single plants than in the UTRWT plants. The mean value of the reporter activity in the UTRWT plants was 0.17 at 1,000 μm B when the reporter activity at 0.3 μm was set to 1, whereas the uORF1_single plants showed only a modest reduction, reaching 0.65 at 1,000 μm B, and the uORF2+3+4 plants, with a reporter activity of 0.14 at 1,000 μm B, induced B-dependent regulation comparable to the level observed in the UTRWT plants. The occurrence of the B response in the uORF1_single and uORF2+3+4 plants was in agreement with the observation that two independent regions caused B-dependent suppression in cultured Arabidopsis cells, as shown in Figure 2. As observed in planta, these results indicated that uORF1 (AUGUAA) was functional but not necessarily required, and the other three uORFs were the pivotal cis-element for B-dependent regulation of translation. This demonstrates the presence of a regulation mechanism that differs from that present in NIP5;1, which requires AUGUAA for regulation.
Next, we examined the effects of uORF2, uORF3, and uORF4 independently using the constructs uORF2_single, uORF3_single, and uORF4_single. The presence of uORF2 alone did not induce B-dependent expression suppression, similar to the ΔUTR and No_uORF constructs. In contrast, uORF3_single and uORF4_single induced B-dependent expression, although the extent of the suppression was less than that of UTRWT. The mean suppression values of uORF3_single and uORF4_single at 1,000 μm B were 0.42 and 0.49, respectively.
Finally, we examined the effect of the combination of uORF3 and uORF4 (uORF3+4). The mean value of uORF3+4 under 1,000 μm B was 0.34, indicating that uORF3 and uORF4 together did not show a strong additive effect. However, the mean value of 0.34 was higher than those of UTRWT and uORF2+3+4. From these results, uORF3 and uORF4 appeared to be indispensable factors in B-dependent down-regulation, which was enhanced by the coexistence of uORF2.
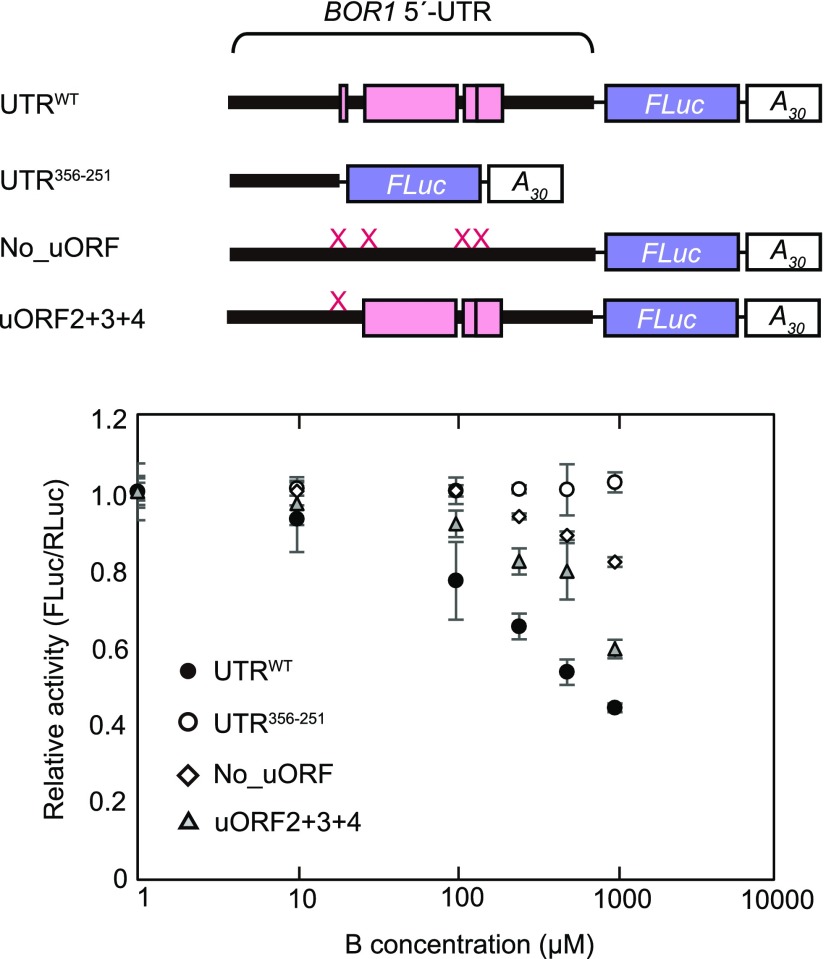
B-Dependent Translation Mediated by the BOR1 5′-UTR Is Recapitulated in Vitro
To obtain further insights into the regulatory mechanism, we examined whether B-dependent translation mediated by the BOR1 5′-UTR was reproducible in vitro. RNA carrying a BOR1 5′-UTR:FLuc-A30 sequence was synthesized via in vitro transcription and subjected to the wheat germ extract (WGE)-based in vitro translation system in the presence of additional boric acid. Relative reporter activity was determined by co-translating control RNA carrying RLuc sequences. The relative reporter activity from the synthetic RNA carrying the native BOR1 5′-UTR (UTRWT) demonstrated a dose-dependent reduction in reporter activity in the presence of 10 to 1,000 μm B (Fig. 4). Conversely, in a truncated-type BOR1 5′-UTR (UTR356-251) containing no uORFs, no dose-dependent reduction in reporter activity was detected. This showed that the BOR1 5′-UTR controlled B-dependent translation in a cell-free translation system. Furthermore, disruption of all uORFs (No_uORF) markedly weakened B-dependent translational suppression, since the reporter activity was decreased only in the presence of at least 500 μm B and the extent of suppression at 1,000 μm was only 20%, whereas a 50% reduction was observed in the UTRWT (Fig. 4). These results provide evidence that the uORF-mediated regulation is, at least in part, reproducible in a cell-free translation system.
Figure 4.
Translation mediated by BOR1 5′-UTR under different B concentrations in vitro. Synthesized test RNA and control RNA were subjected to the WGE-based translation system in the presence of additional 1, 10, 100, 250, 500, and 1,000 μm boric acid. After 120 min, reporter activities were measured by dual luciferase assay. The top shows schematic representations of the synthetic test RNAs. Thick lines and pink boxes represent the BOR1 5′-UTR and uORFs, respectively. The X marks on the 5′-UTR represent point mutations (AUG to AAG) in the start codons of uORFs. The bottom shows reporter activities, which are relative to reporter activity values measured at 1 μm boric acid. Means ± sd are shown (n = 3).
Translational Reinitiation Is Involved in B-Dependent Translation
Since B-dependent translational suppression by the BOR1 5′-UTR was mediated mainly by uORF2, uORF3, and uORF4 in planta (Fig. 3), we further examined the mechanism underlying the down-regulation by uORF2, uORF3, and uORF4. Analysis of uORF2+3+4 confirmed its ability to induce B-dependent translational suppression in vitro (Fig. 4). Three possible mechanisms to reduce the translation of the main ORF (i.e. BOR1 ORF) under high B concentrations were hypothesized: (1) ribosomes stall during the translation of uORF2, uORF3, and uORF4 under high-B conditions; (2) the translation efficiency of uORF2, uORF3, and uORF4 is elevated under high-B conditions; or (3) the reinitiation efficiency of translation after translating uORF2, uORF3, and uORF4 is decreased under high B concentrations.
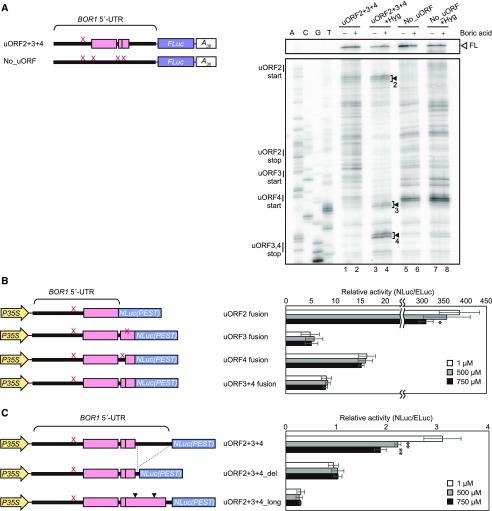
First, we assessed the potential for ribosome stalling during uORF translation using a primer extension inhibition (toeprinting) assay. After translation of the in vitro-transcribed RNAs uORF2+3+4 and No_uORF in vitro, the ribosome-associated RNAs were subjected to reverse transcription with 32P-labeled primer. The resulting cDNA products were separated on sequencing gels. Among the bands specific to uORF2+3+4 (lanes 1 and 2 compared with lanes 5 and 6 in Fig. 5A), no B-dependent increase in signal intensity was observed, suggesting that B-dependent ribosome stalling was unlikely to be a basal mechanism.
Figure 5.
Examination of possible mechanisms of uORF-mediated translational regulation. A, Toeprint analysis. Synthetic RNAs were translated for 30 min in the presence (+) or absence (−) of additional boric acid (1,000 μm). +Hyg indicates that hygromycin B was added at 0 min of translation. Schematic representations of synthetic test RNAs are shown at left. Thick lines and gray boxes represent the BOR1 5′-UTR and uORFs, respectively. The X marks on the 5′-UTR represent point mutations (AUG to AAG) in the start codons of uORFs. The gels at right show the images of nucleotide markers and toeprint signals. Full-length primer extension products (FL) are indicated with the white arrowhead. The numbered black arrowheads (2–4) represent hygromycin B-dependent toeprint signals derived from uORF2, uORF3, and uORF4, respectively. B, Reporter expression in Arabidopsis cell cultures driven by BOR1 5′-UTR uORFs. Schematic models of the DNA constructs used for transformation (left) and relative reporter activities (right) are shown. Reporter gene NLuc(PEST) was fused directly to uORFs. C, Reporter expression in Arabidopsis cell cultures driven by BOR1 5′-UTR mutated to inhibit reinitiation. Schematic models of the DNA constructs used for transformation (left) and relative reporter activities (right) are shown. In uORF2+3+4_del, 84 nucleotides (−90 to −7) were removed from downstream regions of uORF3 and uORF4. In uORF2+3+4_long, two point mutations were introduced to prevent the formation of a stop codon, resulting in the elongation of uORF3 and uORF4. Black arrowheads indicate the positions of nucleotide substitutions. Asterisks indicate significant differences compared with reporter activity values measured at 1 μm boric acid for each construct (*, P < 0.05 and **, P < 0.01, Student’s t test).
Next, we investigated the translation efficiency of uORF2, uORF3, and uORF4 under different B concentrations. Addition of hygromycin B, which inhibits translation elongation, to the in vitro translation system enabled the detection of the translation initiation site. In the presence of hygromycin B, doublet bands appeared 12 to 15 nucleotides downstream of each uORF start codon (Fig. 5A, lanes 3 and 4), whereas no bands or only obscure bands were observed at the corresponding positions without hygromycin B (Fig. 5A, lanes 1 and 2) as well as in the RNA without uORFs (Fig. 5A, lanes 5–8). The intensities of the doublet bands were not changed appreciably by the addition of B. The detection of doublet bands in these positions indicated that ribosomes had been arrested at the start codons by hygromycin B, providing evidence of the translation of these uORFs. Translation of uORF2, uORF3, and uORF4 was further confirmed in transient expression experiments using cultured Arabidopsis cells. NLuc reporter without its own start codon was translationally fused to uORF2, uORF3, uORF4, or uORF3+4, and the reporter activity was detected (Fig. 5B). The reporter gene expression indicated that the start codons of uORF2, uORF3, and uORF4 were functional in terms of translation initiation. The reporter activities in uORF3 fusion, uORF4 fusion, and uORF3+4 fusion were much lower than in uORF2 fusion, probably because uORF3 and/or uORF4 were translated only by the ribosomes that did not translate the uORF2 in these constructs. The reporter activity was not changed by B concentration, although the uORF2-fused reporter decreased under high B concentrations to 79% at 750 μm. The in vitro and in vivo analyses confirmed that uORF2, uORF3, and uORF4 were translated, and the translational efficiency was not increased under high-B conditions. Therefore, mechanism 2 is unlikely.
Finally, we examined the possible involvement of translational reinitiation. In a general model of eukaryotic translation, ribosomes dissociate from mRNA after translation of an ORF. In some cases, the small subunit of the ribosome resumes scanning and subsequently initiates translation of a downstream ORF after the reacquisition of translation initiation factors and the recruitment of a large subunit of ribosome. This is called translational reinitiation. Here, it was hypothesized that B concentrations would change the efficiency of translational reinitiation after the translation of uORF3 or uORF4, since uORF3 and uORF4 are basal factors for B-dependent translation in planta (Fig. 3). By truncation of the sequence downstream of uORF3 and uORF4 (uORF2+3+4_del) or expansion of the uORF length (uORF2+3+4_long), the distance between the uORFs and the main ORF was shortened to inhibit translational reinitiation. In such constructs, the small ribosome is not expected to have sufficient time to prepare for the next translation after the translation of uORF3 and uORF4 and, thus, fails to reinitiate translation at the start codon of the main ORF (Kozak, 1987; Roy et al., 2010). The reporter activities of uORF2+3+4_del and uORF2+3+4_long did not change significantly under different B concentrations (Fig. 5C), suggesting the involvement of reinitiation in B-dependent translation. In these constructs, the reporter activity was relatively lower than that of uORF2+3+4. These results suggested that reinitiation after translation of uORF3 and uORF4 supported a basal translation level of the main ORF under low B concentrations and that the reinitiation efficiency was reduced under high B concentrations.
Translational Suppression of BOR1 Is Induced under Higher B Concentrations Than Are Required to Induce BOR1 Degradation
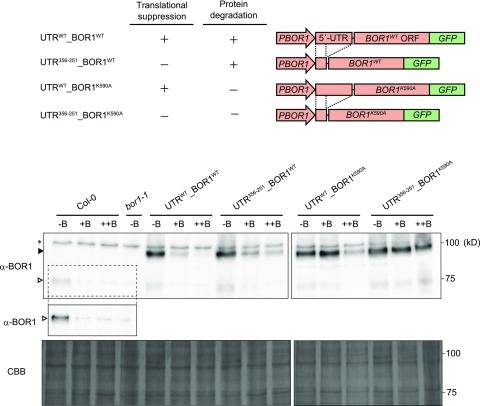
In the following three sections, we describe our investigation of the physiological impacts of the two BOR1 regulation mechanisms in planta by generating transgenic Arabidopsis plants lacking the B-dependent translational suppression characterized in this study and/or protein degradation described by Takano et al. (2005, 2010; Fig. 6). Translational suppression and protein degradation were disrupted by a truncated 5′-UTR (UTR356-251; Fig. 1) and by substitution of Lys-590 with Ala (K590A) in BOR1 (Kasai et al., 2011). The four DNA constructs were introduced into bor1-1, a loss-of-function mutant of BOR1. Multiple independent lines exhibiting similar levels of mRNA accumulation were selected for each construct for subsequent analysis.
Figure 6.
BOR1-GFP accumulation under different boric acid concentrations in transgenic plants with various disruptions in BOR1 regulation mechanisms. The top shows schematic representations of DNA constructs. The presence and absence of posttranscriptional regulations are indicated with + and −, respectively. GFP was fused to the C terminus of BOR1WT/BOR1K590A. The DNA constructs were introduced into bor1-1, a BOR1 loss-of-function mutant. The bottom shows immunoblot analysis of the microsome fraction from plant roots. Plants were grown for 12 d on solid medium containing 0.3 μm (−B), 100 μm (+B), and 1,000 μm (++B) boric acid. A representative accumulation pattern is shown. White and black arrowheads indicate the positions of protein bands corresponding to native BOR1 and BOR1-GFP, respectively. The asterisk indicates nonspecific bands. The protein bands that resemble that of native BOR1 in the bor1-1 transformed lines could be degradation products of BOR1-GFP. Images of two different membranes are shown. For Columbia-0 (Col-0) and bor1-1, the protein bands corresponding to native BOR1 (dashed box) are shown in higher contrast in the cropped image. Membrane staining with Coomassie Brilliant Blue (CBB) is depicted below as a loading control.
The accumulation of BOR1-GFP in the roots of the transgenic plants was examined when they were continuously grown under low (–B; 0.3 μm boric acid), sufficient (+B; 100 μm), and toxic (++B; 1,000 μm) B conditions in solid medium (Fig. 6). BOR1-GFP levels under 0.3 μm (–B) were not appreciably different in all the transgenic lines, although UTR356-251 increased basal expression levels in a transient assay in Arabidopsis culture cells (Fig. 2B). Similar levels of luciferase protein also were observed in the transgenic plants in UTRWT and No_uORF used in the experiment in Figure 3. Taken together, the effects of the elimination of uORFs on basal expression level are considered to be much smaller in planta than those observed in transient expression in Arabidopsis culture cells, possibly due to lower translational efficiency of uORFs and higher efficiency of reinitiation in BOR1 5′-UTR in planta.
In wild-type Col-0 plants, native BOR1 accumulated under 0.3 μm (–B) and was undetectable under 100 μm (+B) and 1,000 μm (++B). The accumulation pattern of BOR1WT-GFP in the control transgenic plants UTRWT_BOR1WT was similar to that of native BOR1. However, in the transgenic plants defective in both translational suppression and protein degradation (UTR356-251_BOR1K590A), BOR1K590A-GFP accumulated consistently in all of the tested B conditions (Fig. 6). This clearly demonstrated that translational suppression and protein degradation were the predominant mechanisms governing the reduction of BOR1 accumulation with this construct under sufficient and toxic B conditions.
In the UTR356-251_BOR1WT line lacking translational suppression but harboring selective protein degradation, BOR1WT-GFP accumulated at 0.3 μm (–B) but decreased at 100 μm (+B) and was reduced further at 1,000 μm (++B). This result demonstrated the induction of protein degradation at 100 μm (+B), consistent with the results of Takano et al. (2010) and the dose dependency of the effect between 100 μm (+B) and 1,000 μm (++B). The intensities of the bands at 100 μm (+B) and 1,000 μm (++B) were slightly higher than those in the control UTRWT_BOR1WT, suggestive of the minor contribution of translational suppression. In the UTRWT_BOR1K590A line harboring only translational suppression, no difference in BOR1K590A-GFP accumulation was detected between 0.3 μm (–B) and 100 μm (+B), but accumulation was reduced significantly at 1,000 μm (++B). This demonstrated that translational suppression was not strongly induced at 100 μm but was induced substantially at 1,000 μm (++B), confirming that B-dependent translational suppression was induced at a higher B concentration range than was required to induce protein degradation. These observations suggested that BOR1 expression was down-regulated mainly by protein degradation under sufficient B conditions and was decreased further by both translational suppression and protein degradation at higher B concentrations.
Regulation via Translational Suppression Causes a More Gradual Decline of BOR1 Than Does Protein Degradation
To investigate the time dependence of the two BOR1 regulation mechanisms, BOR1-GFP was observed in the roots of the transgenic plants transferred from 0.3 μm (–B) to 1,000 μm (++B) in solid medium (Supplemental Fig. S2). In the UTRWT_BOR1WT and UTR356-251_BOR1WT lines harboring selective protein degradation, GFP intensity was decreased within 5 h, as reported previously by Takano et al. (2010). In the UTRWT_BOR1K590A line harboring only translational suppression, GFP intensity was reduced gradually within 24 h. In the UTR356-251_BOR1K590A line lacking both regulation mechanisms, GFP intensity was not decreased. These findings indicate that translational suppression results in a more gradual decline of BOR1 than selective protein degradation.
Two Posttranscriptional BOR1 Regulation Mechanisms Contribute to High B Tolerance
We characterized the growth of regulation-disrupted plants under low (10 μm), sufficient (100 μm), high (250 μm), or toxic (500 μm) B conditions in hydroponic cultures (Fig. 7). Since BOR1 has an important role in the translocation of B from roots to shoots, we observed shoot growth and B accumulation in shoots. The boric acid concentration for toxic B conditions (500 μm) was set lower than that for the solid medium system (1,000 μm) because the transpiration stream is facilitated in hydroponic culture, enabling more B to accumulate in shoots.
Figure 7.
Growth and B concentration in hydroponic culture-grown shoots of transgenic plants with various disruptions in BOR1 regulation mechanisms. A, Shoot growth of the transgenic plants. After germination, seedlings were transferred to liquid medium containing 10, 100, 250, or 500 μm boric acid and incubated for 19 d. B and C, Shoot dry weight (B) and B concentration (C). Means ± sd are shown (n = 3–7). Different letters indicate significant differences among plants under the same B treatment (P < 0.05, Tukey-Kramer test).
Under low-B conditions (10 μm), the shoot growth of bor1-1 was less than that of Col-0 and showed a dark green color and shrinkage of rosette leaves due to the decrease in B translocation, as reported previously (Noguchi et al., 1997). Such leaf phenotypes were not observed in all transgenic plants, indicating that BOR1WT-GFP or BOR1K590A-GFP, driven by the promoter with a truncated 5′-UTR, was able to complement the loss-of-function mutation of BOR1.
When exposed to 500 μm boric acid, the plants lacking both BOR1 translational regulation and BOR1 degradation (UTR356-251_BOR1K590A) exhibited severe growth defects compared with Col-0 and the other transgenic plants (Fig. 7, A and B). This suggested that BOR1 regulation was required for normal growth under high-B conditions. A growth reduction was not observed in plants lacking only translational suppression (UTR356-251_BOR1WT) compared with the control transgenic plants (UTRWT_BOR1WT). The growth of plants lacking only protein degradation (UTRWT_BOR1K590A) decreased, except for line 2, but the effect was not as severe as that observed in UTR356-251_BOR1K590A.
Higher B concentrations in shoots were observed in the plants with defective protein degradation (UTR356-251_BOR1K590A and UTRWT_BOR1K590A, except for line 2) at or above 100 μm (Fig. 7C). This suggested that the growth reduction of these lines under high-B conditions was caused by higher accumulation of B in shoots due to overloading of B by BOR1, indicating that protein degradation is the major mechanism that prevents B overaccumulation.
To clarify the effects of translational suppression in the absence of protein degradation, we compared UTR356-251_BOR1K590A and UTRWT_BOR1K590A. Under 250 and 500 μm boric acid, the B concentration in shoots was higher in UTR356-251_BOR1K590A than in UTRWT_BOR1K590A. Since the B concentrations in these plants did not differ significantly under low-B conditions (10 μm), the difference in B concentrations between UTR356-251_BOR1K590A and UTRWT_BOR1K590A was likely attributable to the contribution of high B-induced translational suppression. Together, these results indicate that two posttranscriptional regulatory mechanisms induced under different ranges of boric acid concentrations contribute to reducing BOR1 accumulation and preventing the overloading of B under high-B conditions.
DISCUSSION
This study revealed that BOR1 expression is regulated by translational suppression, which, together with the selective degradation of BOR1, contributes to reducing the accumulation of BOR1 under high-B conditions. Furthermore, it was confirmed that the two posttranscriptional regulatory mechanisms are induced under different ranges of B concentrations. Here, the conservation and possible mechanisms of this uORF-mediated translational regulation and the biological roles of the two-step regulation are discussed.
Conservation of Multiple uORFs among BOR1 Orthologs
This study demonstrated that BOR1 5′-UTR was sufficient for the B-dependent translational suppression of the main BOR1 ORF (Figs. 2 and 3). Furthermore, the loss of B dependency by the removal of the four uORFs, including uORF1 (AUGUAA), suggested that these uORFs are essential cis-elements for the B-dependent translational suppression (Figs. 2 and 3). In NIP5;1, the AUGUAA sequence is required for B-dependent ribosome stalling, and the upstream AU-rich sequence enhances mRNA degradation (Tanaka et al., 2016). The fact that BOR1 mRNA levels did not change was consistent with the fact that the BOR1 5′-UTR does not carry AU-rich sequences. In the BOR1 5′-UTR, uORF1 (AUGUAA) induced B-dependent suppression in cultured Arabidopsis cells and to a lesser extent in planta (Figs. 2 and 3). However, even in the absence of uORF1, B-dependent expression was observed in both systems and was fully observed in planta. These results suggest that uORF1 is not a major element required for BOR1 translational regulation and that this process is controlled mainly by the other uORFs, which differs from the mechanism of NIP5;1.
To assess the conservation of uORFs among BOR1 homologs, we characterized the 5′-UTRs of BOR1 homologs in vascular plants using experimentally obtained full-length cDNAs (Supplemental Fig. S3). BOR genes were divided into two clades based on their amino acid sequences (Wakuta et al., 2015): clade I as AtBOR1 type for efficient B transport under B-limited conditions and clade II as AtBOR4 type to confer high B tolerance. The functions of several of the listed genes in B homeostasis have already been characterized, and the results support the validity of this classification (Nakagawa et al., 2007; Reid, 2007; Sutton et al., 2007; Miwa et al., 2013; Chatterjee et al., 2014). Orthologs of AtBOR1 in clade I in vascular plants contain multiple uORFs in the 5′-UTR. Conversely, the genes belonging to clade II contain no uORFs in the 5′-UTR (Supplemental Fig. S3B). These observations establish a clear link between the presence of uORFs and B transporter function, supporting the hypothesis that uORFs have a key role in down-regulating the expression of AtBOR1-type BORs under high B concentrations.
Although multiple uORFs have been found commonly in BOR1 orthologs, the peptide sequences encoded by the uORFs do not seem to be conserved. The known models of effector molecule-dependent translational regulation by uORFs in plants are highly dependent on conserved peptide sequences, for example, in the translational regulation of AtAdoMetDC1 by polyamine and bZIP11 by Suc (Hanfrey et al., 2005; Rahmani et al., 2009; Uchiyama-Kadokura et al., 2014; Yamashita et al., 2017). In this regard, it seems unlikely that BOR1 translation is regulated in a uORF peptide-dependent manner.
Regarding BOR1 degradation, the acidic di-Leu motif and Lys residue required for selective protein degradation were found in proteins in clade I (Supplemental Fig. S3B), consistent with the results of Wakuta et al. (2015). This further supports the possibility that these genes undergo two regulation steps, uORF-mediated translational suppression and protein degradation, and this two-step regulation appears to be conserved in a wide range of plant species.
Possible Mechanisms of BOR1 Translational Regulation
The B-dependent translation by the BOR1 5′-UTR was reproduced in vitro (Fig. 4), suggesting that the response does not require the cell membrane or cell wall. It also suggests that boric acid or borate acts as an effector molecule that directly controls the event.
The analysis shown in Figure 5 suggests the possibility that the B-dependent translation of the main BOR1 ORF is controlled by the efficiency of translation reinitiation. The reinitiation efficiency can be changed by the cellular concentration of ternary complex (TC), a complex composed of Met-tRNAiMet and GTP-bound eukaryotic initiation factor 2 (eIF2). Ribosomes can initiate translation only after the acquisition of TCs. As studied in GCN4 in yeast (Hinnebusch, 2005), after the translation of a uORF, high TC concentrations in cells allow ribosomes to reacquire TC rapidly so that the ribosome can translate the downstream ORF. However, when TC concentrations are low, most ribosomes cannot rebind to TC before reaching the start codon of the downstream ORF, causing them to bypass the ORF (Dever et al., 1992). A study in yeast suggested that toxic levels of boric acid inhibited TC formation via the phosphorylation of the α-subunit of eIF2 (Uluisik et al., 2011). In Arabidopsis BOR1, we hypothesize that the reinitiation efficiency after the translation of uORF3 and uORF4 decreases due to low TC levels under high-B conditions, suppressing the translation of the main ORF.
As shown in Figure 2A, BOR1 uORF3 and uORF4 carry one or both of the two important nucleotides of the Kozak sequence. It is likely that the Kozak sequence ensures a high frequency of translation initiation of these uORFs. After translation of uORF3 or uORF4, the characteristics of the BOR1 5′-UTR structure may enhance reinitiation. In general, the reinitiation efficiency after the translation of a uORF is affected by several cis-determinants, including the nucleotide sequence surrounding the uORF, the distance between the uORF and the downstream ORF (where longer distances are better), and the time required for translation of the uORF, which is determined by the length of the uORF (where shorter lengths are better) and the translational elongation rate (Kozak, 1987; Gunišová et al., 2016; Hinnebusch et al., 2016). In the case of BOR1, the requirement of the appropriate distance between the uORF and the downstream ORF for B-dependent expression was demonstrated by the results shown in Figure 5C. Because of the frequent translation of uORF3 and uORF4 and the subsequent reinitiation ensured by the characteristics of the BOR1 5′-UTR structure, changes in the reinitiation efficiency after the translation of uORF3 or uORF4 likely result in the B-dependent translation of the main ORF. Although it remains unclear how BOR1 uORF2 enhances B-dependent translational suppression, the multiple uORFs in the BOR1 5′-UTR seem to be coordinated for the regulation of the main ORF translation.
Biological Significance of the Two-Step Regulation of BOR1
We identified nutrient-dependent translation, which has received little attention among the gene expression steps in nutrient transporters. It is reasonable to assume that plants possess multiple regulatory mechanisms that tightly control the expression of transporters. To date, several multistep nutrient-dependent regulatory mechanisms in plant nutrient transporter genes have been reported. For example, for Arabidopsis PHOSPHATE TRANSPORTER1;1, which encodes a phosphate transporter for phosphate uptake in roots, phosphorus deficiency increases mRNA accumulation (Muchhal et al., 1996) and enhances the exit of the protein from the endoplasmic reticulum (González et al., 2005; Bayle et al., 2011), whereas protein degradation is induced by phosphorus repletion (Bayle et al., 2011). In the case of Arabidopsis IRON-REGULATED TRANSPORTER1, which encodes a metal transporter, mRNA levels are up-regulated under low-iron conditions (Eide et al., 1996; Connolly et al., 2002), and the protein is targeted from early endosomes/trans-Golgi network to the plasma membrane under low concentrations of non-iron metals (Barberon et al., 2014).
Notably, the two steps of posttranscriptional regulation of BOR1 are induced at different B concentration ranges (Fig. 6). Most studies on nutrient transporters have focused on one nutrient-dependent regulatory mechanism under two nutrient conditions in each experimental system, making it difficult to directly compare differences in the concentration range required to induce the respective mechanisms. The physiological role of BOR1 two-step regulation in directly controlling protein quantity can be considered as follows. Under sufficient B conditions, the accumulation of BOR1 is regulated mostly by protein degradation; therefore, it is rapidly (Supplemental Fig. S2) and precisely altered in response to changes in external B concentrations. On the other hand, under continuous toxic B concentrations, BOR1 is not required and can be harmful; therefore, the synthesis of BOR1 is stopped via translation suppression. This two-step regulation under different B concentrations is likely beneficial for the cost-effective regulation of BOR1 expression. The presence of multiple regulatory mechanisms induced under different nutrient concentrations suggests that plants use independent regulatory mechanisms to fulfill the demands of fine-tuning nutrient transport.
MATERIALS AND METHODS
Plant Growth and Transformation
Arabidopsis (Arabidopsis thaliana) ecotype Col-0 was used as a wild-type strain. Col-0 and bor1-1 were derived from our laboratory stock. Plants were grown in solid or liquid medium (Fujiwara et al., 1992) in which B concentration was adjusted with boric acid. Boric acid concentrations in media were set in the range where Col-0 plant growth was not impaired. The boric acid concentration for toxic B conditions in hydroponic cultures was set lower than in the solid medium system (approximately millimolar levels) due to the high transpiration stream. Solid medium additionally contained 1% (w/v) Suc and 1% (w/v) gellan gum (Wako Pure Chemicals). Plants were incubated at 22°C in a growth chamber. Light conditions were a 16-h-light/8-h-dark cycle.
For transformation, Col-0 was used for pIA and pTF series and bor1-1 for pTH series. Transformation was conducted with Agrobacterium tumefaciens strain GV3101 (C58C1Rifr) pMP90 (Gmr), according to the floral dip method. Transformants were selected on solid medium containing 1% (w/v) Suc, 0.15% (w/v) gellan gum, and hygromycin B (20 mg L−1) for pIA and pTH series and 1% (w/v) Suc, 0.8% (w/v) agar, and kanamycin (50 mg L−1) for pTF series.
Plasmid Construction
Plasmid construction is described in Supplemental Text S1. Plasmids used in this study and primers for construction are listed in Supplemental Table S1, and the sequences of the primers are shown in Supplemental Table S2. Plasmids carrying point-mutated BOR1 5′-UTR are listed in Supplemental Table S3.
mRNA Quantification
Eight-day-old plants grown under 30 μm boric acid were transferred to the solid medium containing different concentrations of boric acid and then incubated for 3 d. Total RNA extraction from roots by the RNeasy Plant Mini kit (Qiagen), reverse transcription, and quantitative real-time PCR were performed as described (Miwa et al., 2014). The sequences of primers for BOR1 and elongation factor 1α (EF1α) were described by Takano et al. (2005), and the primers for GUS and β-tubulin were described by Miwa et al. (2014). BOR1 and GUS transcript levels were standardized to the levels of β-tubulin and EF1α, respectively. The values of BOR1/β-tubulin are those relative to the mean value at 30 μm boric acid, which was defined as 1.
Measurement of GUS Activity
Plants were first grown under 30 μm boric acid for 8 to 9 d and then exposed to 0.3 or 3,000 μm boric acid for 3 d. The bulk of 30 to 40 T2 plants in one independent line were harvested as one sample. Protein extraction from roots, measurement of GUS activity, and measurement of total protein concentration by the Bradford assay were conducted as described (Miwa et al., 2014). Relative values of GUS mRNA (GUS/EF1α) and GUS activity (production of 4-methylumbelliferone in μmol g−1 protein min−1) under 3,000 μm were determined when the value at 0.3 μm boric acid was set to 1 in each of the transgenic lines.
Transient Expression Analysis in Cultured Arabidopsis Cells
Transient expression analysis was basically performed as described by Ebina et al. (2015). The test plasmids containing ELuc(PEST) reporter were cotransfected with the internal control plasmid Pro-35S:Renilla luciferase (pKM75; Tanaka et al., 2016). When NLuc(PEST) was used in the test plasmids, Pro-35S:ELuc(PEST) was used as a control plasmid. Protoplasts of cultured Arabidopsis MM2d cells (Menges and Murray, 2002) were transformed with plasmid DNAs by electroporation and incubated under different concentrations of boric acid. For the preparation of protoplasts, MM2d cells in log phase were collected by centrifugation and then washed by 0.4 m mannitol solution. The cells were suspended in enzyme solution (PCM medium containing 1% [w/v] cellulase RS [Yakult] and 0.1% [w/v] pectolyase Y-23 [Kyowa Chemicals]) and incubated for 3 h at 26°C under darkness with gentle shaking. PCM medium is LS medium containing 0.4 m mannitol. The protoplasts were collected by centrifugation and washed by wash buffer (0.4 m mannitol, 5 mm CaCl2, and 12.5 mm sodium acetate [pH 5.8]). Protoplasts were suspended in 0.4 m mannitol.
DNA solution, containing 10 µg of test plasmids and 5 µg of control plasmid, was mixed with 2 × 106 protoplasts in 500 µL of electroporation buffer (5 mm 2-morpholinoethanesulfonic acid, 70 mm potassium chloride, and 0.3 m mannitol [pH 5.8]). Samples were subjected to electroporation by ECM630 (BTM) at 190 V, capacitance 100 µF, and resistance 475 Ω. Protoplasts were incubated at 25°C for 5 min. Protoplasts were collected and suspended in 1.6 mL of PCM medium (prepared without boric acid) and dispensed to three equivalent portions (500 µL each). A total of 500 µL of PCM medium, containing different concentrations of boric acid, was added to protoplasts to expose the cells under 1, 500 and 750 μm boric acid. Protoplasts were incubated at 22°C for 40 to 45 h under continuous darkness. After incubation, the protoplasts were homogenized in 100 µL of protein extraction solution (100 mm sodium phosphate [pH 7] and 5 mm DTT), and cell debris were removed by centrifugation. The extracts were subjected to luciferase assay. To measure ELuc and RLuc activities, the PicaGene Dual Sea Pansy Luminescence kit (Toyo Ink) was used. For the combination of NLuc and ELuc, the Nano-Glo Luciferase Assay System (Promega) and the PicaGene Luminescence kit (Toyo Ink) were used to measure each activity separately.
Luciferase Assay in Transgenic Plants
T3 homozygous plants were grown continuously on solid medium containing 0.3, 500, or 1,000 μm boric acid for 12 d. Roots were harvested from 18 to 20 plants from one independent line as one sample and homogenized in protein extraction buffer (50 mm sodium phosphate [pH 7], 5 mm DTT, and 2 mm EDTA). Cell debris were removed by centrifugation, and the supernatant was subjected to luciferase assay. ELuc activities were measured by the PicaGene Luminescence kit (Toyo Ink). To normalize the ELuc activity, total protein concentrations were measured by Bradford assay using the Quick Start Bradford Protein Assay (Bio-Rad). Reporter activities at 500 and 1,000 μm of each independent line were calculated as relative values when the value at 0.3 μm was set to 1.
In Vitro Translation and Toeprint Assay
For in vitro translation, RNAs carrying poly(A) sequence were synthesized in in vitro transcription. Plasmids UTRWT (pIA24), UTR356-251 (pIA28), No_uORF (pIA50), and uORF2+3+4 (pIA36) were used. For control (RLuc-A30), pMI27 (Chiba et al., 2003) carrying an RLuc gene in pSP64 poly(A) vector was used. Plasmids were linearized by digestion with EcoRI. Linearized DNA was purified by the FastGene Gel/PCR Extraction kit (Nippon Genetics) and dissolved in RNase/DNase-free water. For each construct, 1 µg of DNA was subjected to in vitro transcription by the AmpliCap SP6 High Yield Message Maker kit (CellScript) according to the manufacturer’s instruction. Synthesized RNA was purified by the RNeasy Plant Mini kit (Qiagen) and dissolved in RNase-free water. Then, poly(A)-RNA was isolated by binding to oligo(dT) beads using the GenElute mRNA Miniprep Kit (Sigma-Aldrich).
Synthesized RNAs, test RNA (BOR1 5′-UTR-LUC-A30), and control RNA (RLuc-A30) were subjected to in vitro translation under various boric acid concentrations. In vitro translation was carried out following the protocol of WGE (Promega). WGE, amino acid solution, and RNase inhibitor (RNasin; Promega) were mixed on ice and divided into aliquots. Filtered boric acid was added to 1, 10, 100, 250, 500, and 1,000 μm as final concentrations. The boric acid concentrations were set in the range where control reporter activities were not lowered. Synthesized RNAs, 20 fmol of test RNA, and 2 fmol of the control RNA were added. After incubation for 2 h at 25°C, samples were transferred on ice and diluted 50-fold by ice-cold water. The diluted samples were subjected to FLuc-RLuc dual luciferase assay using the PicaGene Dual Sea Pansy Luminescence kit (Toyo Ink).
Toeprint assay was carried out as described by Hayashi et al. (2017). Oligonucleotide primer 416 (Supplemental Table S2) was labeled at its 5′ terminus with T4 polynucleotide kinase (Takara) and [γ-32P]ATP (110 TBq mmol−1; Perkin-Elmer). In vitro translation of 200 fmol of synthetic RNA (BOR1 5′ -UTR-LUC-A30) was performed with the addition of 0 or 1,000 μm boric acid and 0 or 2 mm hygromycin B as final concentrations. Translation samples were subjected to reverse transcription by the SuperScript III First-Strand Synthesis System (ThermoFisher) using the 32P-labeled primer. Reverse transcription samples were separated by electrophoresis on an 8% (w/v) polyacrylamide/7 m urea sequence gel. The DNA sequence ladder was prepared with pIA50 (No_uORF) as a template.
Immunoblotting
Microsome fractions of plant roots were subjected to immunoblotting by BOR1 antibody. Procedures were modified slightly from those described by Takano et al. (2010). The transgenic lines pAT83 (UTRWT-BOR1WT; Takano et al., 2010), pTH3 (UTR356-251_BOR1WT), pKKF065 (UTRWT_BOR1K590A; Kasai et al., 2011), and pTH4 (UTR356-251_BOR1K590A) were used. Homozygous plants were grown in solid medium containing 0.3, 100, and 1,000 μm boric acid for 12 d. Roots (100–300 mg) were harvested and homogenized in 1 mL of homogenization buffer (250 mm Tris [pH 8.5], 290 mm Suc, and 25 mm EDTA) by beads shocker. Protease inhibitor cocktail (Complete mini; Roche) and 75 mm 2-mercaptoethanol were added to homogenization buffer before use. Cell debris were removed by centrifugation, and the supernatant was ultracentrifuged for 30 min at 100,000g to obtain the microsome fraction. The microsome fraction was dissolved in storage buffer (50 mm potassium phosphate [pH 6.3], 1 mm magnesium sulfate, and 20% [v/v] glycerol). The samples (5 μg) were mixed with 4xNuPAGE LDS sample buffer containing 10% (v/v) 2-mercaptoethanol. The samples were incubated for 3 min at 100°C and then put on ice. They were applied to a NuPAGE 4-12% (w/v) Bis-Tris gel (Invitrogen) and subjected to electrophoresis in MOS buffer at 200 V constant. Precision Plus Protein Standard Dual Color (Bio-Rad) was used as a size marker. After electrophoresis, blotting to PVDF membrane was performed with the wet-transfer method. For reaction with antibodies, BOR1 polyclonal antibody was used at a 400-fold dilution in CanGet Signal Solution 1 (Toyobo) and horseradish peroxidase-conjugated anti-rabbit IgG polyclonal antibody (GE Healthcare) was used at a 6,000-fold dilution in Can Get Signal Solution 2 (Toyobo). The recognition site of the BOR1 antibody is VGNSPKPASCGRSPLNQSSSN (target 4), and the BOR1 antibody does not recognize BOR2. For signal detection, Immobilon Western Chemiluminescent HRP substrate (Millipore) was added on the membrane. Chemiluminescence was detected by ImageQuant LAS 4000mini (GE Healthcare). The PVDF membrane was then subjected to 0.25% (w/v) Coomassie Brilliant Blue R250 staining.
Fluorescence Imaging by Confocal Microscopy
The regulation-disrupted transgenic plants were grown on solid medium containing 0.3 μm boric acid for 3 to 4 d and shifted to solid medium with 0.3 or 1,000 μm boric acid. GFP fluorescence was observed after incubation for 0, 5, 7, 10, or 24 h in root tips. Confocal images were acquired with a Leica TCS-SP8 system and a HyD hybrid detector using 20× water-immersion (numerical aperture = 0.75) objective lenses (Leica Microsystems). Pinholes were adjusted to 1 airy unit. Excitation/detection wavelengths were 488 nm/495 to 540 nm. Analysis of images was conducted with ImageJ software.
Measurement of B Concentration
Plants were germinated with a supply of ultrapure water for 6 to 8 d and then transferred to liquid medium containing various concentrations of boric acid. After incubation for 19 d, shoots were harvested and dried at 60°C. After measurement of dry weight, shoots were digested with nitric acid and hydrogen peroxide (Wako Pure Chemicals). Digested shoots were dissociated with 2% (w/v) nitric acid and subjected to inductively coupled plasma mass spectrometry (ELAN DRC-e; Perkin-Elmer).
Statistical Analyses
The following statistical analyses were conducted by R software: Student’s t test for GUS mRNA, GUS activity, and reporter activity measurements (Figs. 1, 2, and 5); Dunnett’s test after ANOVA for reporter activity measurements (Fig. 3); and Tukey-Kramer test for dry weight and B concentration measurements in shoots (Fig. 7).
Accession Numbers
Arabidopsis Genome Initiative locus identifiers for the genes in this article are as follows: BOR1 (At2g47160), NIP5;1 (At4g10380), BOR2 (At3g62270), BOR4 (At1g15460), and CIPK1 (At3g17510). BOR1 cDNA sequence data can be found in the GenBank/EMBL databases under accession number BT000732.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. BOR1 mRNA accumulation under different B conditions.
Supplemental Figure S2. Time-course analysis of BOR1-GFP levels.
Supplemental Figure S3. The presence of uORFs in BOR1 homologs among vascular plants.
Supplemental Table S1. Plasmids and primers used for DNA construction.
Supplemental Table S2. Primers used in this study.
Supplemental Table S3. Plasmids carrying point-mutated BOR1 5′-UTR.
Supplemental Text S1. Plasmid construction.
Acknowledgments
We thank Noriya Hayashi for assistance in the toeprint assay, Michiko Tsukamoto and Chisato Morioka for technical assistance, and Toshihiro Watanabe and members of the Laboratory of Plant Nutrition, Graduate School of Agriculture, Hokkaido University, for assistance in inductively coupled plasma mass spectrometry.
Footnotes
This work was supported in part by Japan Society for the Promotion of Science (JSPS) through a Grant-in-Aid for Young Scientists (A) to K.M. (no. 16H06187) and a Grant-in-Aid for Scientific Research (S) to T.F. (no. 25221202) and by the Hayashi Memorial Foundation for Female Natural Scientists to K.M. I.A. is supported by the Research Fellowship for Young Scientists from JSPS.
Articles can be viewed without a subscription.
References
- Aibara I, Miwa K (2014) Strategies for optimization of mineral nutrient transport in plants: multilevel regulation of nutrient-dependent dynamics of root architecture and transporter activity. Plant Cell Physiol 55: 2027–2036 [DOI] [PubMed] [Google Scholar]
- Argust P. (1998) Distribution of boron in the environment. Biol Trace Elem Res 66: 131–143 [DOI] [PubMed] [Google Scholar]
- Barberon M, Dubeaux G, Kolb C, Isono E, Zelazny E, Vert G (2014) Polarization of IRON-REGULATED TRANSPORTER 1 (IRT1) to the plant-soil interface plays crucial role in metal homeostasis. Proc Natl Acad Sci USA 111: 8293–8298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayle V, Arrighi JF, Creff A, Nespoulous C, Vialaret J, Rossignol M, Gonzalez E, Paz-Ares J, Nussaume L (2011) Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation. Plant Cell 23: 1523–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Tabi Z, Galli M, Malcomber S, Buck A, Muszynski M, Gallavotti A (2014) The boron efflux transporter ROTTEN EAR is required for maize inflorescence development and fertility. Plant Cell 26: 2962–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Sakurai R, Yoshino M, Ominato K, Ishikawa M, Onouchi H, Naito S (2003) S-Adenosyl-L-methionine is an effector in the posttranscriptional autoregulation of the cystathionine γ-synthase gene in Arabidopsis. Proc Natl Acad Sci USA 100: 10225–10230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly EL, Fett JP, Guerinot ML (2002) Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 14: 1347–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG (1992) Phosphorylation of initiation factor 2α by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 68: 585–596 [DOI] [PubMed] [Google Scholar]
- Dordas C, Chrispeels MJ, Brown PH (2000) Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol 124: 1349–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina I, Takemoto-Tsutsumi M, Watanabe S, Koyama H, Endo Y, Kimata K, Igarashi T, Murakami K, Kudo R, Ohsumi A, et al. (2015) Identification of novel Arabidopsis thaliana upstream open reading frames that control expression of the main coding sequences in a peptide sequence-dependent manner. Nucleic Acids Res 43: 1562–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA 93: 5624–5628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Hirai MY, Chino M, Komeda Y, Naito S (1992) Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol 99: 263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González E, Solano R, Rubio V, Leyva A, Paz-Ares J (2005) PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 is a plant-specific SEC12-related protein that enables the endoplasmic reticulum exit of a high-affinity phosphate transporter in Arabidopsis. Plant Cell 17: 3500–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunišová S, Beznosková P, Mohammad MP, Vlčková V, Valášek LS (2016) In-depth analysis of cis-determinants that either promote or inhibit reinitiation on GCN4 mRNA after translation of its four short uORFs. RNA 22: 542–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanfrey C, Elliott KA, Franceschetti M, Mayer MJ, Illingworth C, Michael AJ (2005) A dual upstream open reading frame-based autoregulatory circuit controlling polyamine-responsive translation. J Biol Chem 280: 39229–39237 [DOI] [PubMed] [Google Scholar]
- Hayashi N, Sasaki S, Takahashi H, Yamashita Y, Naito S, Onouchi H (2017) Identification of Arabidopsis thaliana upstream open reading frames encoding peptide sequences that cause ribosomal arrest. Nucleic Acids Res 45: 8844–8858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellen CU, Sarnow P (2001) Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev 15: 1593–1612 [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG. (2005) Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol 59: 407–450 [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG, Ivanov IP, Sonenberg N (2016) Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science 352: 1413–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi CP, Zhou H, Huang X, Chiang VL (1997) Context sequences of translation initiation codon in plants. Plant Mol Biol 35: 993–1001 [DOI] [PubMed] [Google Scholar]
- Kasai K, Takano J, Miwa K, Toyoda A, Fujiwara T (2011) High boron-induced ubiquitination regulates vacuolar sorting of the BOR1 borate transporter in Arabidopsis thaliana. J Biol Chem 286: 6175–6183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. (1986) Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell 44: 283–292 [DOI] [PubMed] [Google Scholar]
- Kozak M. (1987) Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol Cell Biol 7: 3438–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. (2012) Mineral Nutrition of Higher Plants, Ed 3 Academic Press, San Diego [Google Scholar]
- Menges M, Murray JA (2002) Synchronous Arabidopsis suspension cultures for analysis of cell-cycle gene activity. Plant J 30: 203–212 [DOI] [PubMed] [Google Scholar]
- Miwa K, Wakuta S, Takada S, Ide K, Takano J, Naito S, Omori H, Matsunaga T, Fujiwara T (2013) Roles of BOR2, a boron exporter, in cross linking of rhamnogalacturonan II and root elongation under boron limitation in Arabidopsis. Plant Physiol 163: 1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K, Aibara I, Fujiwara T (2014) Arabidopsis thaliana BOR4 is upregulated under high boron conditions and confers tolerance to high boron. Soil Sci Plant Nutr 60: 349–355 [Google Scholar]
- Muchhal US, Pardo JM, Raghothama KG (1996) Phosphate transporters from the higher plant Arabidopsis thaliana. Proc Natl Acad Sci USA 93: 10519–10523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nable RO, Banuelos GS, Paull JG (1997) Boron toxicity. Plant Soil 193: 181–198 [Google Scholar]
- Nakagawa Y, Hanaoka H, Kobayashi M, Miyoshi K, Miwa K, Fujiwara T (2007) Cell-type specificity of the expression of Os BOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. Plant Cell 19: 2624–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Yasumori M, Imai T, Naito S, Matsunaga T, Oda H, Hayashi H, Chino M, Fujiwara T (1997) bor1-1, an Arabidopsis thaliana mutant that requires a high level of boron. Plant Physiol 115: 901–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MA, Ishii T, Albersheim P, Darvill AG (2004) Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu Rev Plant Biol 55: 109–139 [DOI] [PubMed] [Google Scholar]
- Park H, Schlesinger WH (2002) Global biogeochemical cycle of boron. Global Biogeochem Cycles 16: 1072 [Google Scholar]
- Rahmani F, Hummel M, Schuurmans J, Wiese-Klinkenberg A, Smeekens S, Hanson J (2009) Sucrose control of translation mediated by an upstream open reading frame-encoded peptide. Plant Physiol 150: 1356–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid R. (2007) Identification of boron transporter genes likely to be responsible for tolerance to boron toxicity in wheat and barley. Plant Cell Physiol 48: 1673–1678 [DOI] [PubMed] [Google Scholar]
- Reid RJ, Hayes JE, Post A, Stangoulis JCR, Graham RD (2004) A critical analysis of the causes of boron toxicity in plants. Plant Cell Environ 27: 1405–1414 [Google Scholar]
- Roy B, Vaughn JN, Kim BH, Zhou F, Gilchrist MA, Von Arnim AG (2010) The h subunit of eIF3 promotes reinitiation competence during translation of mRNAs harboring upstream open reading frames. RNA 16: 748–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Kamiya A, Ishida J, Satou M, Sakurai T, Nakajima M, Enju A, Akiyama K, Oono Y, et al. (2002) Functional annotation of a full-length Arabidopsis cDNA collection. Science 296: 141–145 [DOI] [PubMed] [Google Scholar]
- Shorrocks VM. (1997) The occurrence and correction of boron deficiency. Plant Soil 193: 121–148 [Google Scholar]
- Sugio T, Matsuura H, Matsui T, Matsunaga M, Nosho T, Kanaya S, Shinmyo A, Kato K (2010) Effect of the sequence context of the AUG initiation codon on the rate of translation in dicotyledonous and monocotyledonous plant cells. J Biosci Bioeng 109: 170–173 [DOI] [PubMed] [Google Scholar]
- Sutton T, Baumann U, Hayes J, Collins NC, Shi BJ, Schnurbusch T, Hay A, Mayo G, Pallotta M, Tester M, et al. (2007) Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science 318: 1446–1449 [DOI] [PubMed] [Google Scholar]
- Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, Hayashi H, Yoneyama T, Fujiwara T (2002) Arabidopsis boron transporter for xylem loading. Nature 420: 337–340 [DOI] [PubMed] [Google Scholar]
- Takano J, Miwa K, Yuan L, von Wirén N, Fujiwara T (2005) Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc Natl Acad Sci USA 102: 12276–12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Wada M, Ludewig U, Schaaf G, von Wirén N, Fujiwara T (2006) The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18: 1498–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Tanaka M, Toyoda A, Miwa K, Kasai K, Fuji K, Onouchi H, Naito S, Fujiwara T (2010) Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc Natl Acad Sci USA 107: 5220–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Takano J, Chiba Y, Lombardo F, Ogasawara Y, Onouchi H, Naito S, Fujiwara T (2011) Boron-dependent degradation of NIP5;1 mRNA for acclimation to excess boron conditions in Arabidopsis. Plant Cell 23: 3547–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Sotta N, Yamazumi Y, Yamashita Y, Miwa K, Murota K, Chiba Y, Hirai MY, Akiyama T, Onouchi H, et al. (2016) The minimum open reading frame, AUG-Stop, induces boron-dependent ribosome stalling and mRNA degradation. Plant Cell 28: 2830–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama-Kadokura N, Murakami K, Takemoto M, Koyanagi N, Murota K, Naito S, Onouchi H (2014) Polyamine-responsive ribosomal arrest at the stop codon of an upstream open reading frame of the AdoMetDC1 gene triggers nonsense-mediated mRNA decay in Arabidopsis thaliana. Plant Cell Physiol 55: 1556–1567 [DOI] [PubMed] [Google Scholar]
- Uluisik I, Kaya A, Fomenko DE, Karakaya HC, Carlson BA, Gladyshev VN, Koc A (2011) Boron stress activates the general amino acid control mechanism and inhibits protein synthesis. PLoS ONE 6: e27772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arnim AG, Jia Q, Vaughn JN (2014) Regulation of plant translation by upstream open reading frames. Plant Sci 214: 1–12 [DOI] [PubMed] [Google Scholar]
- Wakuta S, Mineta K, Amano T, Toyoda A, Fujiwara T, Naito S, Takano J (2015) Evolutionary divergence of plant borate exporters and critical amino acid residues for the polar localization and boron-dependent vacuolar sorting of AtBOR1. Plant Cell Physiol 56: 852–862 [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Takamatsu S, Glasbrenner M, Becker T, Naito S, Beckmann R (2017) Sucrose sensing through nascent peptide-meditated ribosome stalling at the stop codon of Arabidopsis bZIP11 uORF2. FEBS Lett 591: 1266–1277 [DOI] [PubMed] [Google Scholar]
- Yoshinari A, Takano J (2017) Insights into the mechanisms underlying boron homeostasis in plants. Front Plant Sci 8: 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]