A phenotypic screen identifies drugs, including the endocrine disruptor ethynylestradiol that trigger oil accumulation in the diatom Phaeodactylum, as well as found genes and pathways for metabolic engineering.
Abstract
Microalgae are a promising feedstock for the production of triacylglycerol (TAG) for a variety of potential applications, ranging from food and human health to biofuels and green chemistry. However, obtaining high TAG yields is challenging. A phenotypic assay for the accumulation of oil droplets was developed to screen a library of 1,200 drugs, annotated with pharmacology information, to select compounds that trigger TAG accumulation in the diatom Phaeodactylum tricornutum. Using this screen, we identified 34 molecules acting in a dose-dependent manner. Previously characterized targets of these compounds include cell division and cell signaling effectors, membrane receptors and transporters, and sterol metabolism. Among the five compounds possibly acting on sterol metabolism, we focused our study on ethynylestradiol, a synthetic form of estrogen that is used in contraceptive pills and known for its ecological impact as an endocrine disruptor. Ethynylestradiol impaired the production of very-long-chain polyunsaturated fatty acids, destabilized the galactolipid versus phospholipid balance, and triggered the recycling of fatty acids from membrane lipids to TAG. The P. tricornutum transcriptomic response to treatment with ethynylestradiol was consistent with the reallocation of carbon from sterols to acetyl-coenzyme A and TAG. The mode of action and catabolism of ethynylestradiol are unknown but might involve several up-regulated cytochrome P450 proteins. A fatty acid elongase, Δ6-ELO-B1, might be involved in the impairment of very-long-chain polyunsaturated fatty acids and fatty acid turnover. This phenotypic screen opens new perspectives for the exploration of novel bioactive molecules, potential target genes, and pathways controlling TAG biosynthesis. It also unraveled the sensitivity of diatoms to endocrine disruptors, highlighting an impact of anthropogenic pollution on phytoplankton.
Photosynthetic algae are promising systems for the development of cell factories, since they are able to capture CO2 and produce valuable biomolecules such as triacylglycerols (TAGs). Oils made of TAGs have a broad range of applications, from feed, food, and health to commodity products complementing fossil hydrocarbons and chemistry (Lupette and Maréchal, 2018). TAGs contain three fatty acids (FAs) esterified to a glycerol backbone, and they accumulate inside cells in the form of lipid droplets (Maeda et al., 2017). The potentiality of algal oil lies in the molecular variety of FAs esterified to TAGs, with chain lengths ranging from ∼14 to ∼22 carbons and harboring from zero to six double bonds (Dolch and Maréchal, 2015). Some examples of FAs are palmitic acid (16:0, with 16 carbons and no double bond), oleic acid (18:1), linolenic acid (18:3), eicosapentaenoic acid (EPA; 20:5), etc. Very-long-chain polyunsaturated fatty acids (VLC-PUFAs) like EPA have a higher added value for food or health applications than shorter chained and less saturated FAs, such as 16:0, which makes them usable for biofuel applications (Lupette and Maréchal, 2018). Increasing the productivity and quality of alga-based oil are critical bottlenecks that need to be overcome (Pulz and Gross, 2004; Spolaore et al., 2006; Chisti, 2013; Klein-Marcuschamer et al., 2013; Ruiz et al., 2016; Arbenz et al., 2018).
The biodiversity of algae occupies very distant branches of the tree of life (Brodie et al., 2017), ranging from prokaryotes (i.e. cyanobacteria) to a multitude of eukaryote lineages that arose from a primary endosymbiosis, such as unicellular green algae, or from a secondary endosymbiosis, such as diatoms (Bozarth et al., 2009; Levitan et al., 2014). Important eukaryotic models with well-annotated genomes, transformation techniques, and molecular tools for genetic engineering are being developed, like Chlamydomonas reinhardtii for green algae (Merchant et al., 2007; Scaife and Smith, 2016), Phaeodactylum tricornutum for diatoms (Falciatore et al., 1999; Siaut et al., 2007; Bowler et al., 2008; De Riso et al., 2009; Daboussi et al., 2014; Nymark et al., 2016), and Nannochloropsis spp. for eustigmatophytes (Kilian et al., 2011; Anandarajah et al., 2012; Vieler et al., 2012; Corteggiani Carpinelli et al., 2014). The secondary endosymbionts arose from a complex evolutionary history (McFadden, 1999; Stoebe and Maier, 2002; Kutschera and Niklas, 2005; Gould et al., 2008; Keeling, 2009; Botté and Marechal, 2014; Petroutsos et al., 2014; Brodie et al., 2017); their subcellular ultrastructure is extremely sophisticated (Flori et al., 2016), and the annotation of their genome highlights a large proportion of proteins of unknown function compared with other eukaryotes originating from a primary endosymbiosis (Maier et al., 2000; Gardner et al., 2002; Armbrust et al., 2004; Bowler et al., 2008; Deschamps and Moreira, 2012). Species such as P. tricornutum and Nannochloropsis spp. have cultivation and oil productivity performances suitable for the development of production strains for industrial processes (d’Ippolito et al., 2015; Ajjawi et al., 2017; Wang and Seibert, 2017). Some genetic engineering approaches require robust prior knowledge regarding the functions of genes or pathways that might be modified in an attempt to drive carbon flux toward TAG production (Liao et al., 2016; Brodie et al., 2017). We need to advance our knowledge of the specific subcellular compartmentalization of secondary endosymbionts, cell growth and development, photosynthetic efficiency, CO2 capture, carbon partitioning, glycerolipid metabolism, etc., so as to develop strains and processes with higher biomass yields, TAG productivity, and controlled FA contents.
Phenotypic screens involving large collections of compounds (or chemolibraries) have been used successfully to identify molecules that could trigger the accumulation of TAG in various microalgae strains, such as the green algae C. reinhardtii, Chlorella sorokiniana, and Tetrachlorella alterens (Wase et al., 2017), and secondary endosymbionts, such as Nannochloropsis spp. and P. tricornutum (Franz et al., 2013). Having an active molecule in hand, it is then possible to search for the protein target(s) that could be used to develop a genetically engineered strain, modified in the targeted pathway (Mayer et al., 1999; Haggarty et al., 2000; Maréchal, 2008, 2009); however, this molecule-to-target approach is time consuming and uncertain. Some phenotypic screens have allowed the identification of compounds possibly triggering TAG accumulation in diatoms, but with little information on the putative targets that could be impaired by the active compounds (Franz et al., 2013). Here, we describe the screening of a biologically annotated chemolibrary containing drugs previously approved for safety by the U.S. Food and Drug Administration for at least one indication or used in clinical trials with bioavailability, pharmacology, and toxicity information (Prestwick Chemical Library; 1,200 compounds). The screen was developed to select molecules that trigger the accumulation of TAG within P. tricornutum living cells. Theoretically, hit molecules from this repurpose collection should interact with P. tricornutum proteins that share some structural and/or functional features with the original drug targets (Blagosklonny, 2003; Jeong et al., 2015; Coleman et al., 2016). Our objective was then to identify biological processes or pathways affected by the active molecules and propose lines of research for future P. tricornutum strain developments.
RESULTS
Setup of Screening Conditions and Statistical Robustness of the Selected Multiparametric Assay
Our objective was to define a multiparametric assay that allows the robust measurement of cell concentrations, TAG levels, and ideally other parameters that would allow the monitoring of the physiological status of cells. We first optimized culture conditions for P. tricornutum in different multiwell plate systems. We sought a compromise between the miniaturization of the incubation volume, so as to add the smallest possible amounts of compounds per well, and the decreased capacity of liquids to be mixed in such small volumes, even with permanent shaking. After P. tricornutum CCMP632/CCAP 1055/1 wild-type cells were dispensed in 48- or 96-well plates, we observed an excess of cells adhering to the plastic sides in the 96-well plates. In 48-well plates, the addition of 4-mm glass beads helped limit cell adhesion, aided mixing, and allowed us to measure growth and TAG accumulation after 48-h incubations.
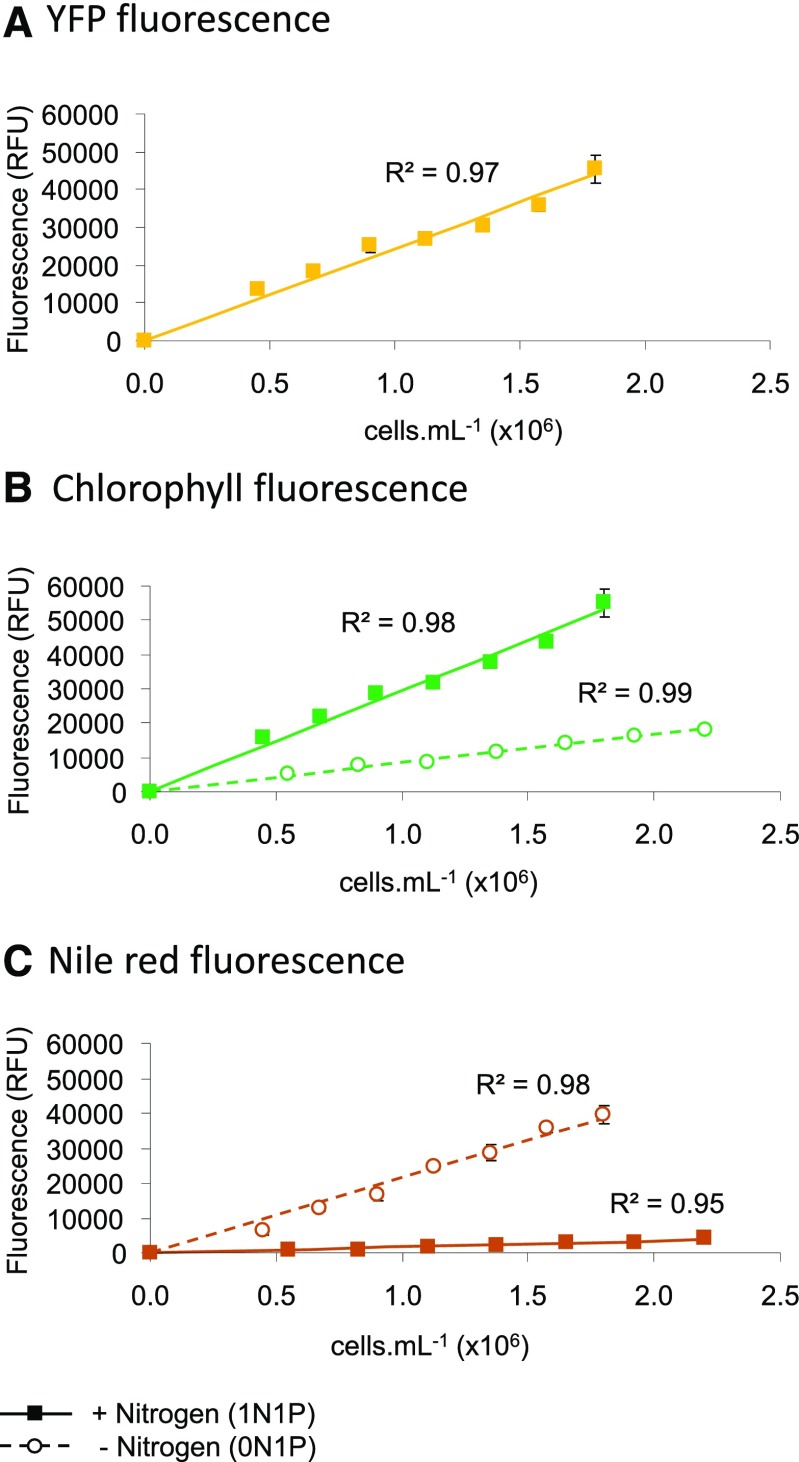
We compared different methods to monitor cell abundance with sufficient high-throughput and statistical robustness. The easiest method would be by deducing cell abundance from light absorbance. We compiled data obtained from 140 independent data points using P. tricornutum cultures grown in plates or in flasks in enriched seawater/artificial water (ESAW) containing or lacking nitrogen (ESAW 1N1P or 0N1P, respectively). Cell concentrations were evaluated using a Malassez counting chamber. In this compiled data set, we detected a linear correlation between cell concentrations and A730 with the following equation: y = 1.834.1−8.x + 0.03758 (where y is the number of cells and x is the absorbance, with a correlation coefficient of 0.9637; Supplemental Fig. S1). Unfortunately, in the conditions used for the screening, the dynamic range (the difference between the highest and lowest values) was not sufficient. We tried to develop 4′,6-diamidino-2-phenylindole or Hoechst staining methods based on the binding of these dyes to nuclear DNA, but the background was too strong to allow a statistically relevant determination of cell abundance. Eventually, we used a strain expressing a Histone H4 fused to Enhanced Yellow Fluorescent Protein (EYFP) at the N terminus (Siaut et al., 2007). Using a calibrated culture with known cell concentrations, the measurement of YFP fluorescence allowed us to evaluate cell abundance with a linear correlation coefficient of 0.97 (Fig. 1A). This correlation is valid from 0.1 to 0.55 mm NaNO3. In the same plates, it was also possible to detect the fluorescence of chlorophylls and of Nile Red, a dye that binds to lipid droplets (Fig. 1, B and C), within a linear range based on positive and negative cell controls (i.e. cells incubated for 48 h in the absence or presence of nitrogen).
Figure 1.
Calibration of the multiparametric assay for the phenotypic screen. The assay was developed using a strain expressing a Histone H4 protein fused to EYFP at the N terminus. Graphs show the correlations between measured fluorescence and cell concentrations after a 48-h incubation in ESAW 1N1P and 0N1P media, respectively. A, Cell abundance. The YFP fluorescence was used to evaluate cell abundance in a calibrated population grown in 1N1P medium by measuring the excitation/emission fluorescence at 515/530 nm. B, Chlorophyll fluorescence. The fluorescence of chlorophyll was used as a basic evaluation of cell physiological status by measuring the excitation/emission fluorescence at 440/680 nm. C, Nile Red fluorescence. The content in TAG was evaluated by staining with Nile Red and measuring the fluorescence at 530/580 nm. Data correspond to biological triplicates ± sd. Correlation coefficients were 0.94 or less. Solid squares represent cells grown in 1N1P medium (+ Nitrogen), and circles represent cells grown in 0N1P medium (− Nitrogen). RFU, Relative fluorescence units.
A statistical analysis classically used to determine the suitability of an assay is the Z′ factor (Zhang et al., 1999) derived from measurements obtained with positive and negative controls. Using our selected assay conditions and after 48-h incubations, a series of low-TAG and high-TAG control cells were analyzed: 17 wells containing cells grown in 1N1P medium (3.3 × 106 cells mL−1) and 17 wells containing cells grown in 0N1P medium (1.6 × 106 cells mL−1), respectively (Supplemental Fig. S2). Based on our results, a Z′ factor was calculated for each parameter: 0.75 for cell abundance measurements (based on YFP fluorescence), 0.64 for TAG content (based on Nile Red fluorescence), and 0.73 for chlorophyll level. These Z′ factors being greater than 0.5, this indicated that the different conditions established during assay optimization led to an accurate standardized assay, suitable for screening the chemical library.
Primary and Secondary Screens to Identify Molecules Triggering TAG Accumulation in P. tricornutum
The screening of the Prestwick Chemical Library (1,200 compounds) was performed at a final concentration of 10 µm and 0.5% (v/v) dimethyl sulfoxide (DMSO) using the automated multimodule platform from the Centre de Criblage pour des Molecules Bio-actives in Grenoble, France. Thirty plates could be analyzed per week. Combined data are shown in Figure 2.
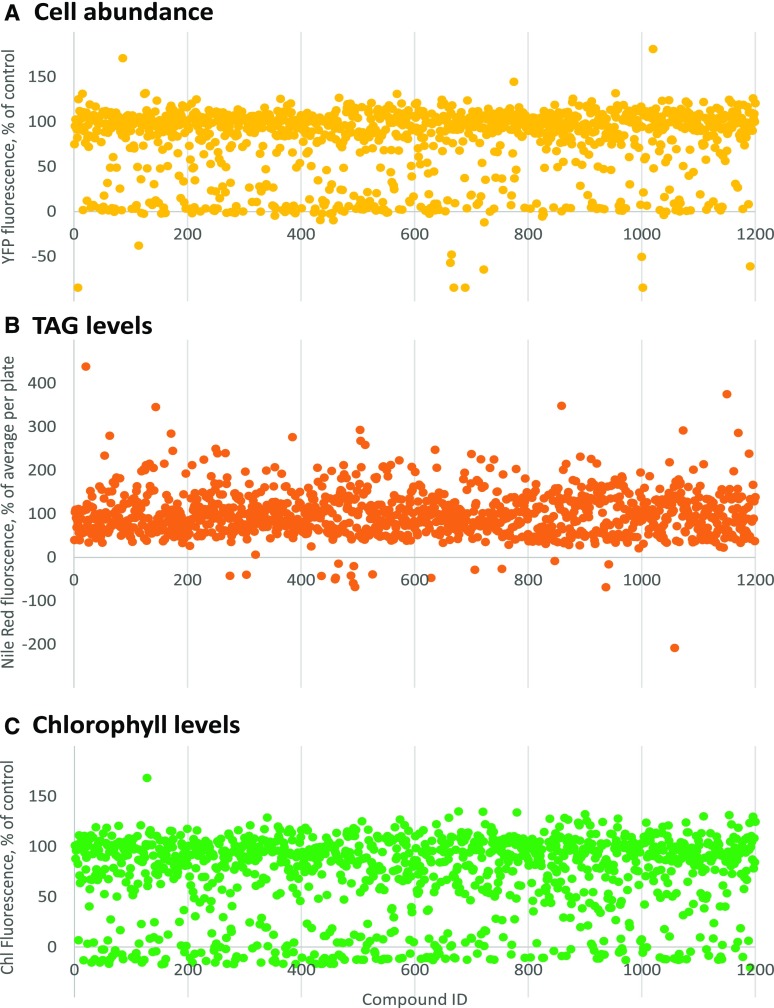
Figure 2.
Multiparametric screen of the Prestwick Chemical Library. Using the developed assay, P. tricornutum cells were incubated for 48 h with compounds of the Prestwick Chemical Library at a final concentration of 10 µm in 0.5% (v/v) DMSO. Multiple parameters were then measured as described in “Materials and Methods.” A, Cell abundance. The concentration of cells was estimated using a strain expressing a Histone H4 protein fused to EYFP based on the fluorescence at 515/530 nm. B, TAG levels. The content of TAG was evaluated by staining with Nile Red and measuring the fluorescence at 530/580 nm. C, Chlorophyll levels. The fluorescence of chlorophyll was measured at 440/680 nm.
Two main criteria were used to select compounds for a secondary screen. The first criterion was a low impact on cell growth, viability, and chlorophyll level. Chlorophyll fluorescence level correlated linearly with cell abundance, with a correlation coefficient of 0.8189 (Fig. 3A). About 20% of the compounds were lethal to P. tricornutum at 10 µm, with YFP fluorescence lower than 10% of that of the control. Some compounds had apparently no impact on cell abundance but led to a strong decrease of chlorophyll, below 50% of the control level. These compounds were not considered further. The second criterion was an increase in TAG level. A total of 160 compounds leading to an increase in Nile Red staining above 120% of the average per plate, while maintaining a cell abundance above 50% of the control (Fig. 3B), were then selected.
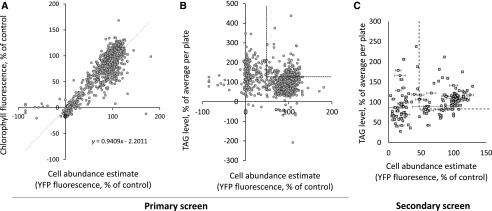
Figure 3.
Selection of compounds based on primary and secondary screens. Based on the primary screen, two criteria were used to select compounds for the secondary screen: (1) low impact on cell abundance and/or chlorophyll level and (2) increase of Nile Red staining level. A, Primary screen, correlation between chlorophyll level and cell abundance detection. Values obtained from chlorophyll fluorescence levels and cell abundance based on YFP fluorescence were plotted. Linear correlation is given by the formula y = 0.9409x – 2.2011 (R2 = 0.8189). B, Primary screen, comparison of Nile Red staining and cell abundance. The dashed line shows spots corresponding to compounds increasing Nile Red staining above 120% of the average per plate while maintaining a cell abundance above 50% of that of the control. C, Secondary screen. Parallel assays were repeated at 10 µm in technical duplicates, using 160 compounds selected following the primary screen. The dashed line shows spots corresponding to compounds increasing Nile Red staining above 120% of the average per plate while maintaining a cell abundance above 50% of that of the control. Error bars indicate sd.
A secondary screen of the 160 compounds consisted of a reevaluation of their effect at 10 µm, in the same conditions and in technical duplicates, so as to discard false positives and rank the compounds (Fig. 3C). We then tested 40 compounds in independent biological triplicates; this resulted in 34 hit molecules showing an increase in Nile Red staining higher than 120% (and up to 385%; Table I).
Table I. Compounds selected after the secondary screen.
Chemical Abstracts Service (CAS) numbers are indicated. Results of the three independent dose-response analyses are compiled, with high Nile Red (NR) dose-dependent detection and toxicity. Previously characterized targets of the selected compounds are classified based on general biological function and/or molecular features.
| Chemical Name | CAS No. | Tertiary Screen Results (Triplicate) and Toxicity (*) in the Tested Concentration Range | Previously Characterized Targets | Known Side Activities on Cytochrome P450 (CYP) Involved in Sterol Metabolism | References |
|---|---|---|---|---|---|
| Nucleic acid biosynthesis and cell division | |||||
| Pentamidine | 140-64-7 | High NR in two of three tests | Nucleic acid-binding activity | Overington et al. (2006) | |
| Oxytetracycline | 6153-64-6 | High NR in two of three tests | Nucleic acid-binding activity | Overington et al. (2006) | |
| Ifosfamide | 3778-73-2 | High NR in two of three tests | Alkylation of DNA | CYP2B6 (steroid hydroxylase) modulator | Furlanut and Franceschi (2003); Chen et al. (2005) |
| Rifaximin | 80621-81-4 | High NR in two of three tests | RNA polymerase-blocking agent | Overington et al. (2006) | |
| Nocodazole | 31430-18-9 | High NR in three of three tests (>120%) | Microtubule-depolymerizing agent | Baas et al. (2016) | |
| Membrane receptors | |||||
| Xylometazoline | 1218-35-5 | High NR in three of three tests (>120%; *) | Adrenergic receptor agonist | Haenisch et al. (2010) | |
| Labetalol | 32780-64-6 | High NR in two of three tests | Adrenergic receptor agonist | CYP2D6 (steroid hydroxylase) inhibitor | Riva et al. (1991); Preissner et al. (2010) |
| Acebutolol | 34381-68-5 | High NR in two of three tests | Adrenergic receptor agonist | CYP2D6 (steroid hydroxylase) inhibitor | van den Meiracker et al. (1988); Preissner et al. (2010) |
| Meclozine | 1104-22-9 | High NR in two of three tests | Dopamine (precursor of adrenaline) antagonist | Haraguchi et al. (1997) | |
| Serotonin | 153-98-0 | High NR in two of three tests | Serotonin receptor ligand | Pytliak et al. (2011) | |
| Alverine | 5560-59-8 | High NR in three of three tests (>120%; *) | Serotonin receptor antagonist | Coelho et al. (2001) | |
| Pirenperone | 75444-65-4 | High NR in two of three tests | Serotonin receptor antagonist | Pawłowski et al. (1985) | |
| Hyoscyamine | 101-31-5 | High NR in two of three tests | Acetylcholine receptor antagonist | Huang et al. (1998) | |
| Pipenzolate | 125-51-9 | High NR in two of three tests | Acetylcholine receptor antagonist | Attwood (1976) | |
| Mecamylamine | 826-39-1 | High NR in two of three tests | Acetylcholine receptor antagonist | Overington et al. (2006) | |
| Bephenium | 3818-50-6 | High NR in two of three tests | Acetylcholine receptor antagonist | Qian et al. (2006) | |
| Dimaprit | 23256-33-9 | High NR in two of three tests | Histamine [2-(1H-imidazol-4-yl)ethanamine] H2 receptor antagonist | Shteinikov et al. (2017) | |
| Membrane transporters | |||||
| Bendroflumethiazide | 73-48-3 | High NR in two of three tests | Solute carrier family 12 member 3 inhibitor/carbonic anhydrase inhibitor | Chen et al. (2002); Tricarico et al. (2006) | |
| Rimantadine | 13392-28-4 | High NR in three of three tests (>120%; *) | Proton pump inhibitor | Imming et al. (2006) | |
| Gaboxadol | 64603-91-4 | Proton-coupled amino acid transporter | Larsen et al. (2010) | ||
| Antimycin A | 1397-94-0 | High NR in three of three tests (>120%) | Mitochondria or chloroplast electron transport chains | Shikanai (2014); Georgakopoulos et al. (2017) | |
| Sterol metabolism | |||||
| Estrone | 53-16-7 | High NR in two of three tests | CYP2B6 (steroid hydroxylase) substrate | Preissner et al. (2010) | |
| Ethynylestradiol | 57-63-6 | High NR in three of three tests (>120%) | CYP2C8 (steroid hydroxylase) inhibitor | Walsky et al. (2005) | |
| Mevastatin | 73573-88-3 | High NR in three of three tests (>120%) | HMG-CoA reductase | CYP2C8 (steroid hydroxylase) inhibitor | Chen et al. (2002); Tornio et al. (2005) |
| Simvastatin | 79902-63-9 | High NR in three of three tests (>120%; *) | HMG-CoA reductase | CYP2C8 (steroid hydroxylase) inhibitor | Liu et al. (2003); Tornio et al. (2005) |
| Ketoconazole | 65277-42-1 | High NR in two of three tests | CYP (sterol 14-α-demethylase) inhibitor | Warrilow et al. (2010) | |
| Cyclic nucleotide signaling | |||||
| Zardaverine | 101975-10-4 | High NR in two of three tests | Cyclic nucleotide phosphodiesterase inhibitor | Schudt et al. (1991) | |
| Trapidil | 15421-84-8 | High NR in two of three tests | Cyclic nucleotide phosphodiesterase inhibitor | Somuncu et al. (2005) | |
| Oxilipin signaling | |||||
| Meloxicam | 71125-38-7 | High NR in two of three tests | Prostanoid signaling modulator/cyclooxygenase COXII/prostaglandin-endoperoxide synthase inhibitor | CYP2C9 (steroid hydroxylase) inhibitor | Chesné et al. (1998); Panara et al. (1999) |
| Azapropazone | 13539-59-8 | High NR in two of three tests | Prostanoid signaling modulator/cyclooxygenase COXII/prostaglandin-endoperoxide synthase inhibitor | Priddy et al. (1990) | |
| Misoprostol | 59122-46-2 | High NR in two of three tests | PG E1 analog/PG receptor agonist | Nataraj et al. (2001) | |
| Enzymes (carbohydrate metabolism, purine catabolism, and/or β-lactam drug resistance) | |||||
| Miglitol | 72432-03-2 | High NR in two of three tests | α-Glycosidase inhibitor | Fukaya et al. (2009) | |
| Flucloxacillin | 1847-24-1 | High NR in two of three tests | Penicillin (β-lactam drug)-binding protein (glycosyltransferase) inhibitor | Mainardi et al. (2008) | |
| Sulbactam | 68373-14-8 | High NR in two of three tests | β-Lactamase inhibitor | Totir et al. (2007) | |
| Allopurinol | 315-30-0 | High NR in three of three tests (>120%) | Inhibition of xanthine (imidazole-derived heterocycle) oxidase | Pacher et al. (2006) | |
Previously characterized targets of the selected compounds were classified based on general biological function and/or molecular features and highlighted seven major categories of targets: (1) nucleic acid biosynthesis and cell division (pentamidine, oxytetracycline, ifosfamide, rifaximin, and nocodazole); (2) membrane receptors (xylometazoline, labetalol, acebutolol, meclizine, serotonin, alverin, pirenperone, hyoscyamine, pipenzolate, mecamylamine, bephenium, and dimaprit); (3) membrane transporters (bendroflumethiazide, rimantadine, gaboxadol, and antimycin A); (4) sterol metabolism (estrone, ethynylestradiol, mevastatin, simvastatin, and ketoconazole); (5) cyclic nucleotide signaling (zardaverine and trapidil); (6) oxylipin signaling (meloxicam, azapropazone, and misoprostol); and (7) unrelated enzymes involved in carbohydrate metabolism, purine catabolism, and/or β-lactam drug resistance (miglitol, flucloxacillin, sulbactam, and allopurinol; Table I). In addition, we examined possible side effects on secondary targets and noticed that six compounds could have pleiotropic effects and act on cytochrome P450 enzymes involved in sterol metabolism (Table I).
Only five compounds triggered an increase in Nile Red staining in all independent biological triplicates and with an apparent low toxicity, nocodazole, antimycin A, ethynylestradiol, mevastatin, and allopurinol.
Dose-Response Analyses of Hit Molecules Triggering TAG Accumulation in P. tricornutum
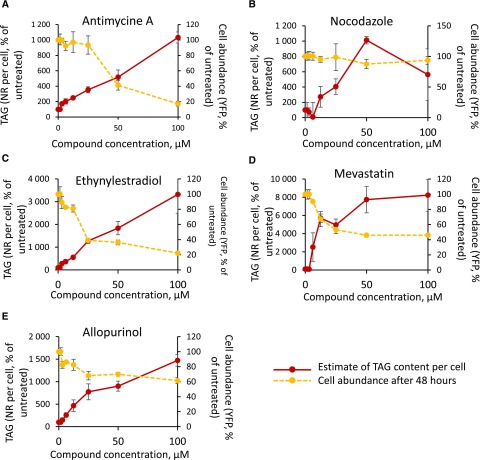
Focusing on the five molecules selected following primary and secondary screens, we performed a tertiary test consisting of dose-response analyses, in triplicate, at compound concentrations of 1.5, 3, 6, 12, 25, 50, and 100 µm (Fig. 4). The first striking observation is that, besides nocodazole, the selected molecules appeared to impair growth at high concentrations, above 25 to 50 µm, based on cell abundance measured via the fluorescence of Histone H4 fused to EYFP. In these conditions, half-maximal inhibitory concentrations are ∼30, ∼20, ∼10, and greater than 100 µm for antimycin A, ethynylestradiol, mevastatin, and allopurinol, respectively. Three compounds triggered a proportional increase in TAG per cell in the tested range (antimycin A, ethynylestradiol, and allopurinol), with half-maximal effective concentrations of ∼50 µm, whereas the increase in Nile Red fluorescence observed with mevastatin and nocodazole reached a limit at 50 µm, with half-maximal effective concentration values of ∼10 and ∼25 µm, respectively.
Figure 4.
Dose-response analyses of secondary screen-selected compounds. Dose-response analyses were performed as described in “Materials and Methods,” in triplicate, at compound concentrations of 1.5, 3, 6, 12, 25, 50, and 100 µm. Cell abundance was evaluated based on the fluorescence of a Histone H4 protein fused to EYFP and expressed as a percentage of the untreated control (100% = YFP level at 0 µm compound). TAG accumulation was evaluated based on Nile Red staining, normalized by cell abundance, and expressed as a percentage of the untreated control (100% = Nile Red level per cell at 0 µm compound).
This dose-response analysis supports the activity of selected compounds triggering TAG accumulation in P. tricornutum, likely via the interference of mitochondria and/or the chloroplast electron transport chain (antimycin A), microtubule dynamics (nocodazole), sterol metabolism (ethynylestradiol and mevastatin), and purine catabolism (allopurinol). The effect of nocodazole, although appearing as very promising, decreased after longer incubations (up to 7 d), suggesting possible degradation of this compound by the diatom. In the following experiments, compound concentrations were adjusted as a compromise between the desired effects on TAG accumulation and the adverse impact on cell growth.
Chemical Interference of Sterol Metabolism
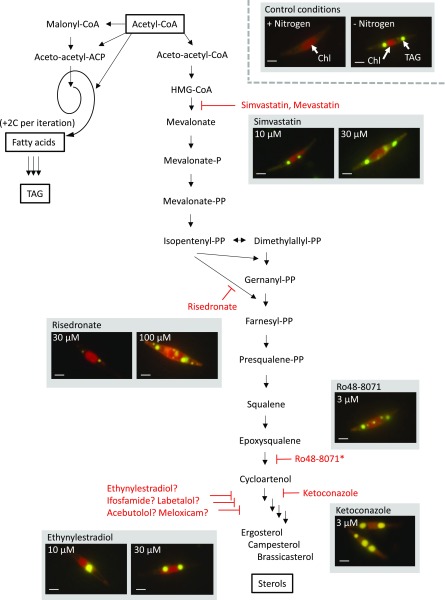
The screen highlighted compounds potentially targeting enzymes of the mevalonate pathway and sterol metabolism from the most upstream enzymes, such as the hydroxymethylglutaryl (HMG)-CoA reductase hit by simvastatin and mevastatin, to downstream enzymatic activities, such as a sterol 14-α-demethylase affected by ketoconazole. This latter compound proved to be toxic at concentrations higher than 30 µm. We analyzed additional molecules known to interfere with this pathway (Fig. 5). The biosynthesis of isopentenyl pyrophosphate and farnesyl pyrophosphate is common to all isoprenoids, whereas the biosynthesis of squalene is specific to sterol biosynthesis. Risedronate, known to inhibit farnesyl pyrophosphate synthase (Bergstrom et al., 2000), triggered an increase in TAG when provided at concentrations higher than 30 µm. We confirmed that Ro48-8071, an oxidosqualene cyclase inhibitor, induced an accumulation of TAG, as described previously (Fabris et al., 2014). The accumulation of TAG was observed when Ro48-8071 was supplied at 3 µm, whereas above 30 µm, this compound was toxic. Figure 5 also shows the possible interference of cytochrome P450 oxidases/hydroxylases acting downstream in the sterol pathway, which could be inhibited by ethynylestradiol, ifosfamide, labetalol, acebutolol, and meloxicam (Table I). Following our screening, the high proportion of inhibitors of sterol metabolism highlights that this pathway represents a strong competitive route, diverting acetyl-CoA from FAs and TAG. This proportion of sterol metabolism antagonists also could reflect a bias in the library of compounds we screened. Interestingly, although high concentrations of molecules impaired growth, lower concentrations triggered an increase in TAG with a low impact on cell proliferation.
Figure 5.
Compounds interfering with sterol metabolism. The pathway is adapted from Fabris et al. (2014). The biosynthesis of isopentenyl pyrophosphate (Isopentenyl-PP) and farnesyl pyrophosphate (Farnesyl-PP), which is common to all isoprenoids, is initiated by the reduction of HMG-CoA into mevalonate. Epoxysqualene is the last noncyclic intermediate in the pathway. The molecular diversity of sterols is then generated downstream of cycloartenol and involves cytochrome P450 cyclooxygenases. The connection with FA and TAG biosynthesis occurs at the level of acetyl-CoA. Sterol and TAG appear as competing sinks for carbon. FA synthesis is an iterative process adding two carbons (2C) per iteration, producing FAs of 16 or 18 carbons. FAs can be elongated further in the cytosol and are used for the biosynthesis of polar and storage glycerolipids, including TAG. Inhibitors are shown in red at the level of their primary enzymatic targets. ?, Putative inhibitors in the downstream part of sterol metabolism acting on cytochrome P450 enzymes; *, the Ro48-8071 effect was described previously by Fabris et al. (2014). Fluorescence imaging of treated wild-type cells highlights chlorophyll fluorescence (440/680 nm), allowing the detection of chloroplasts (Chl) and Nile Red staining (530/580 nm) of oil droplets enriched in TAG. Bars = 5 µm.
Lipidomic Remodeling and Physiological Response Induced by Ethynylestradiol
A previous genetic study demonstrated that the impairment of sterol metabolism following the down-regulation of one of its enzymes, oxidosqualene cyclase, led to an interruption of the sterol biosynthetic pathway and a strong increase in Nile Red fluorescence, suggesting an accumulation of TAG in P. tricornutum (Fabris et al., 2014). However, the precise remodeling of glycerolipids and its mechanism had not been analyzed. The increase in the proportion of TAG was not characterized, and it was not established whether all membrane glycerolipids were altered, regardless of their FA molecular species or their polar heads, or if a specific effect had occurred (Fabris et al., 2014).
We focused our analyses on ethynylestradiol (also named 17-α-ethynylestradiol; Inhoffen and Hohlweg, 1938), since this compound was effective at low concentrations, is supposed to act downstream in the pathway, and is one of the most soluble molecules we identified. Ethynylestradiol is a synthetic estrogen that could mimic potential analogs acting as natural hormones/pheromones/infochemicals. It is a derivative of estrone and is used in birth control pills based on its strong binding affinity to the human estrogen receptor (Jensen and DeSombre, 1973). This compound recently attracted increasing attention because both natural and synthetic estrogens are discharged in the environment by anthropogenic practices, including by humans and domestic animals (Aris et al., 2014; Adeel et al., 2017). Estrogens have demonstrated impacts on the aquatic environment, including a well-studied effect on sex determination in fish (Bhandari et al., 2015; Siegenthaler et al., 2017); however, the effects of these endocrine disruptors on phytoplankton have not been adequately investigated (Liu et al., 2010; Pocock and Falk, 2014).
First, we checked whether the estimate of cell abundance based on Histone H4-YFP fusion was accurate: we repeated the dose-dependent experiment, confirmed that Nile Red staining increased regularly with increasing doses of ethynylestradiol, and reevaluated cell numbers using a Malassez counting chamber (Supplemental Fig. S3), which confirmed that ethynylestradiol had a significant impact on growth at concentrations higher than 8 to 10 µm. The cells did not show any strong defect at the level of photosynthesis, based on PSII efficiency measured by the Fv/Fm ratio, besides a moderate decline above 30 µm (Supplemental Fig. S3). The impact on growth could not be attributed to a transcriptional arrest of nucleotide synthesis (based on the transcriptomic responses of genes annotated in nucleotide biosynthesis that were unchanged [Phatr3_J25204], up-regulated [Phatr3_J14626 and Phatr3_J47067], or slightly down-regulated [Phatr3_EG02569]) or cell division (based on the transcriptomic responses of genes annotated in cell division processes, such as Cdc20 Phatr3_J12783 and APC8 Phatr3_J38886, which appear up-regulated, or CDH1 Phatr3_J2648, which showed no change in expression; Supplemental Table S1).
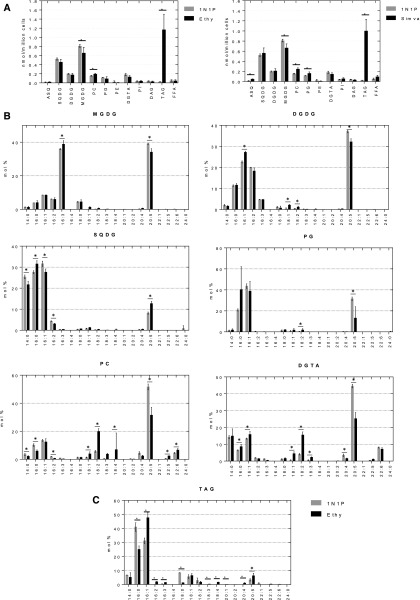
We then sought to confirm whether the increased Nile Red staining level corresponded to a significant change in TAG. To that purpose, we determined the glycerolipid profile of P. tricornutum cells grown in 400 mL of 1N1P medium with 0.5% (v/v) DMSO in the presence or absence of 30 µm ethynylestradiol and at a cell density of 1 × 106 cells mL−1. After a 48-h incubation, glycerolipids were extracted and analyzed as described previously (Abida et al., 2015). Major polar glycerolipids consist of a glycerol backbone to which two FAs are linked by ester bonds and harboring a polar head. In P. tricornutum, chloroplast-specific classes comprise acylsulfoquinovosyldiacylglycerol, sulfoquinovosyldiacylglycerol (SQDG), monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG), and phosphatidylglycerol (PG). Glycerolipids synthesized by the endoplasmic reticulum comprise phosphatidylcholine (PC) and diaclyglycerylhydroxymethyl-N,N,N-trimethyl-β-alanine (DGTA; Abida et al., 2015). Following the treatment with ethynylestradiol, a strong increase in TAG could be observed, confirming the increase in Nile Red staining (Fig. 6A, left). Polar lipids apparently were not strongly affected, besides a specific decrease in MGDG and an increase in PC. Interestingly, similar trends were observed after a treatment with 15 µm simvastatin, another molecule targeting sterol metabolism (Fig. 6A, right).
Figure 6.
Glycerolipid profile following treatment with ethynylestradiol. P. tricornutum cells were cultivated in 1N1P medium until a cell density of 1 × 106 cells mL−1 was reached. Cells were then incubated for 48 h in the presence or absence of compounds, as indicated. Lipids were extracted, separated by thin-layer chromatography (TLC), and analyzed as described in “Materials and Methods.” A, Glycerolipid profiles following treatments with 30 µm ethynylestradiol and simvastatin. Glycerolipids are expressed in nmol per million cells. B, Fatty acid profiles of membrane glycerolipids following treatment with 30 µm ethynylestradiol. C, Fatty acid profile of TAG following treatment with 30 µm ethynylestradiol. Data correspond to biological triplicates ± sd. ASQ, Acylsulfoquinovosyldiacylglycerol; DAG, diacylglycerol; Ethy, ethynylestradiol; FFA, free fatty acid; PE, phosphatidylethanolamine; PI, phosphatidylinositol; Simva, simvastatin. *, P < 0.05 (Student’s t test). Error bars indicate sd.
When analyzing FA profiles within each class of glycerolipids, striking differences could be observed. First, with the noticeable exception of SQDG, the proportion of EPA (20:5) decreased in all major lipid classes (i.e. MGDG, DGDG, PG, PC, and DGTA; Fig. 6B). The synthesis of this VLC-PUFA is still not completely elucidated, but it is now considered that it is elaborated from short-chain FAs, including saturated molecular species such as 16:0 (Dolch et al., 2017b), by a series of elongations and desaturations (Sayanova and Napier, 2004; Dolch and Maréchal, 2015; Sayanova et al., 2017). The elongation occurs on FAs linked to CoA (as acyl-CoAs), whereas desaturations should occur on FAs linked to membrane lipids, possibly PC and/or DGTA, in P. tricornutum (Dolch and Maréchal, 2015; Sayanova et al., 2017). The process is so fast that the unsaturated FA precursors of 20:5 (i.e. 20:4, 20:3, 18:4, 18:3, 18:2, and 18:1) are barely detected in P. tricornutum and represent a low percentage. Following treatment with ethynylestradiol, the decrease in 20:5 coincides with a decrease of 20:4 and an increase of the proportion of their unsaturated FA precursors in both PC (18:4, 18:3, 18:2, and 18:1) and DGTA (18:4, 18:3, and 18:2). Therefore, ethynylestradiol appears to specifically disturb the channeled production of 20:5 via these two lipids, supporting the idea that both PC and DGTA can be platform lipids for the elaboration of EPA in P. tricornutum.
In parallel to this alteration of FA elongation and desaturation generating VLC-PUFAs, TAG content increased (Fig. 6A, left). The TAG profile was modified, with higher proportions in FAs derived from membrane lipids, such as 16:1 and 20:5, and including unsaturated FAs, which are uncommon in P. tricornutum TAG, such as 16:2, 16:3, and 18:4 (Fig. 6C). Altogether, these results indicate that ethynylestradiol impairs EPA elaboration via PC and DGTA, destabilizes the MGDG/PC balance, and triggers a turnover of FAs deriving from membrane lipids to TAG.
Transcriptomic Reprogramming Induced by Ethynylestradiol
The striking modification of the glycerolipid profile, showing an unsuspected disruption of EPA biosynthesis and a strong reallocation of carbon, suggested that P. tricornutum might operate an important transcriptomic reprogramming. P. tricornutum cells were treated for 4 d in independent biological triplicates with 0, 10, and 20 µm ethynylestradiol, conditions known to trigger TAG accumulation with moderate impact on growth (Supplemental Fig. S4). RNA sequencing analysis was performed with an Illumina HiSeq 4000 system, as described in “Materials and Methods.”
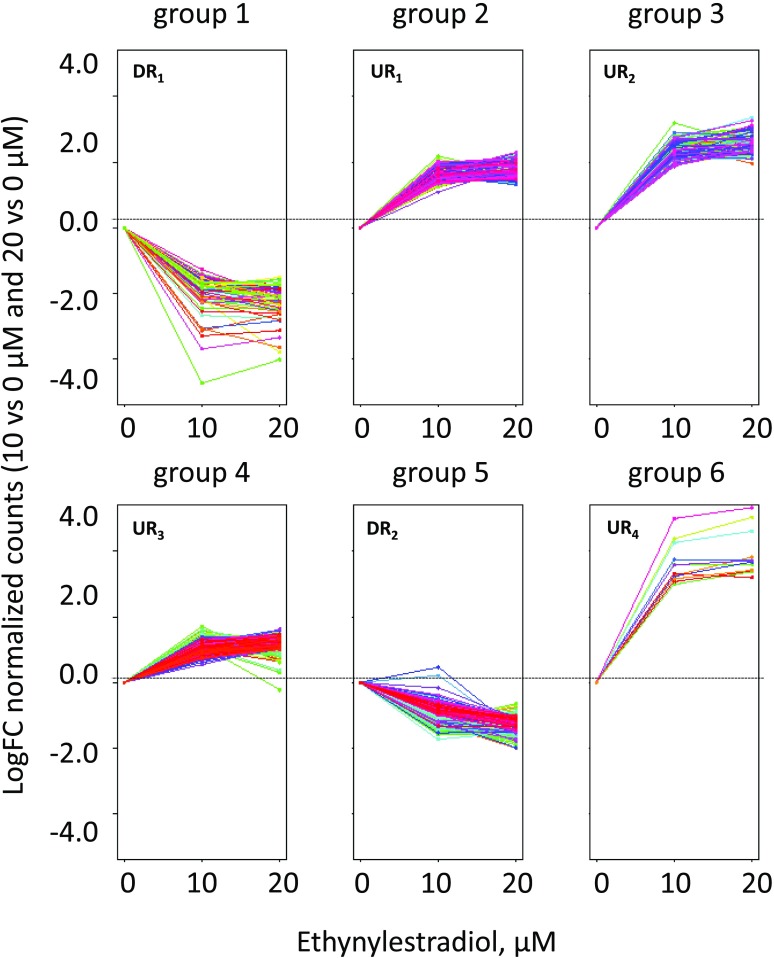
We found 962 genes differentially expressed with a log2 fold change (Log2FC) > 1 in at least one of the comparisons (i.e. comparing ethynylestradiol supplies at 0 versus 10 µm, 0 versus 20 µm, or 10 versus 20 µm) and with P values lower than 0.05 (Supplemental Table S1). A partition of differentially expressed genes was performed using the k-means method, with the number of partitions set to six (Liu et al., 2014; Dolch et al., 2017a). Each group or cluster consisted of genes with similar expression profiles following treatments with ethynylestradiol (Fig. 7). Two clusters comprised genes down-regulated following treatments, group 1 (DR1) containing genes with the strongest magnitude in expression decline (in the −2 to −4 Log2FC range) and group 5 (DR2) with moderate but significant expression decline (Log2FC ∼ −1 to −2). Four clusters comprised genes up-regulated following ethynylestradiol treatments, groups 2, 3, 4, and 6 (UR1, UR2, UR3, and UR4, respectively); UR1 and UR3 showed the most moderate change levels (Log2FC ∼ 1), and UR2 and UR4 showed the highest variation magnitude (Log2FC ∼ 2–4).
Figure 7.
K-means clustering of P. tricornutum differentially expressed genes in response to increasing doses of ethynylestradiol. Only differentially expressed genes with a Log2FC > 1 in at least one of the contrasts (i.e. comparing ethynylestradiol supplies at 10 versus 0 µm, 20 versus 0 µm, or 20 versus 10 µm) and with P < 0.05 (Wald test) were considered for further analyses. A partition of differentially expressed genes was performed using the k-means method, with the number of partitions set to six and clustering based on a Euclidian distance (Liu et al., 2014). Two clusters comprise genes down-regulated following treatments: group 1 (DR1), containing genes with the strongest magnitude in expression decline (in the −2 to −4 Log2FC range); and group 5 (DR2), with moderate but significant expression decline (Log2FC ∼ −1). Four clusters comprise genes up-regulated following ethynylestradiol treatments: groups 2, 3, 4, and 6 (UR1, UR2, UR3, and UR4, respectively).
For each group, we next asked whether Gene Ontology (GO) terms were enriched, using the GOseq R package, with P values lower than 0.05 or 0.1 (Young et al., 2010; Supplemental Table S2). We then mined the results, focusing on carbon metabolism: the putative enrichment of GO terms corresponding to glycolysis, phosphoenolpyruvate production, pyruvate production, acetyl-CoA production, mevalonate pathway, and glycerolipid production (Supplemental Table S2).
UR1 was characterized by a moderate enrichment in genes involved in lipid biosynthesis (P < 0.1). A GO enrichment in terms corresponding to the mevalonate pathway was clearly observed in the UR2 cluster, both at the molecular function (diphosphomevalonate decarboxylase activity; P < 0.05) and biological process (isopentenyl diphosphate biosynthetic process, mevalonate pathway; P < 0.05) levels. The UR2 cluster also was enriched in GO terms corresponding to glycolysis and pyruvate metabolism with P < 0.1. UR3 was not enriched in GO terms corresponding to carbon metabolism. The UR4 cluster also was enriched in GO terms corresponding to acetyl-CoA biosynthesis from pyruvate (P < 0.05). In down-regulated clusters, DR1 did not show any clear enrichment in GO terms linked to carbon metabolism, whereas DR2 showed an enrichment in terms corresponding to gluconeogenesis (P < 0.05).
This analysis of the function of differentially expressed genes highlights that glycolysis was likely stimulated, whereas gluconeogenesis was arrested, and that the mevalonate pathway was up-regulated, consistent with a response to an agent that blocks sterol metabolism. Some genes involved in carbon metabolism also appear tuned toward the production of acetyl-CoA from pyruvate, which was then directed toward FAs and lipids. One has to keep in mind that GO term analyses depends on the extent of GO annotation in the P. tricornutum genome, which has not been completed.
We examined more carefully the corresponding metabolic pathways. It has been demonstrated that the isopentenyl-diphosphate precursor used for sterol biosynthesis derives from the cytosolic mevalonate pathway rather than the plastid-localized 1-desoxy-d-xylulose-5-phosphate pathway (Cvejić and Rohmer, 2000). Supplemental Table S3 shows the dose-dependent response of genes involved in sterol biosynthesis, first via the mevalonate route: in the most upstream section of the pathway, the expression of the HMG-CoA synthase gene, HMGS (Phatr3_J16649), increased in a dose-dependent manner, whereas the expression of the mevalonate kinase gene, MK (Phatr3_J53929), was strongly attenuated. One gene coding for a putative geranyl-diphosphate synthase (Phatr3_J47271) was strongly down-regulated, whereas a geranyl-diphosphate/farnesyl-diphosphate synthase (Phatr3_J49325) was up-regulated, illustrating the fine-tuning of different redundant enzymes within the sterol pathway likely involved in specific physiological or developmental processes. In the most downstream part of the pathway, the genes coding for the putative 14-α-demethylase, CYP51 (Phatr3_J31339), 3-β-hydroxysteroid-Δ(8), Δ-7-isomerase (Phatr3_J36801), Δ-7-sterol Δ-5-dehydrogenase (Phatr3_J14208), Δ-7-sterol reductase (Phatr3_J30461), and sterol C-22 desaturase, CYP710 (Phatr3_J51757), were strongly up-regulated in a dose-dependent manner by the treatment with ethynylestradiol. Interestingly, genes coding for cytochrome P450 enzymes, possibly targeted by ethynylestradiol (most importantly CYP710), were strongly activated. We wondered whether the expression of genes involved in the production of isopentenyl diphosphate via the 1-desoxy-d-xylulose-5-phosphate pathway also could be altered by the treatment, but no specific change could be detected (Supplemental Table S3). Therefore, the reprogramming of sterol biosynthesis genes combines an activation of genes of the CYP family possibly compensating for the inhibition by ethynylestradiol, whereas others, most importantly MK, act like general bottlenecks and possibly reduce the flux of carbon through this pathway.
In Supplemental Table S4, we analyzed genes involved in glycerolipid metabolism, keeping in mind that mechanisms other than the control of steady-state levels of mRNA might occur, including posttranslational modifications and regulation of the stability of proteins. Surprisingly, only a few genes involved in FA and TAG biosynthesis were up-regulated. Consistent with an increase in acetyl-CoA production for FA synthesis, we noticed, for instance, that genes coding for dihydrolipoamide acetyltransferase components of the pyruvate dehydrogenase complex (Phatr3_J23850, Phatr3_EG02309, and Phatr3_J30113) and for the biotin carboxylase subunit of heteromeric acetyl-CoA carboxylase (Phatr3_J49339) were up-regulated. At the level of TAG biosynthesis, only one of the diacylglycerol acyltransferase (DGAT2C) genes was up-regulated (Phatr3_J31662). This, combined with possible variations of other DGAT protein levels and enzymatic activities, could support an activated synthesis of TAG. By contrast, at the level of TAG degradation, the expression of lipases was strikingly down-regulated: MAGL1 (Phatr3_J43352), SDP1-Like (Phatr3_J45518), TAGL-Like-A (Phatr3_J1971), PLA-A (Phatr3_J44005), PLA-B (Phatr3_J44066), and Patatin-Like-A (Phatr3_J43678). Likewise, genes involved in FA β-oxidation in both the peroxisome (Phatr3_J19979, Phatr3_J41969, Phatr3_J45947, Phatr3_J11319, Phatr3_J37372, and Phatr3_J31367) and the mitochondrion (Phatr3_J20310, Phatr3_J35240, Phatr3_J40988, Phatr3_J11811, Phatr3_J54494, and Phatr3_J18064) were down-regulated. Altogether, the transcriptomic reprogramming in response to ethynylestradiol highlights a down-regulation of genes involved in TAG catabolism rather than a strongly enhanced TAG biosynthesis, suggesting that TAG turnover could be slowed down in response to the chemical treatment.
Focusing on VLC-PUFAs, most genes involved in the biosynthesis of EPA (Δ0-ELO-A, Phatr3_J49867; Δ0-ELO-B, Phatr3_J16376; Δ5-ELO-B, Phatr3_J34485; Δ5-ELO-A, Phatr3_J9255; Δ6-ELO-B2, Phatr3_J20508; HACD, Phatr3_J44831; ER-Δ5-FAD-A, Phatr3_J46830; ER-Δ5-FAD-B, Phatr3_J22459; and ER-Δ6-FAD, Phatr3_J29488) were up-regulated. Only one of the genes was down-regulated, coding for Δ6-ELO-B1 (Phatr3_J22274; Supplemental Table S4). This disruption of VLC-PUFA coincides with the up-regulation of the betaine lipid synthase, BTA-Like (Phatr3_J42872), involved in the biosynthesis of DGTA (Supplemental Table S4).
Taken together, and considering that posttranslational modifications and the control of protein levels also might occur, the transcriptomic response is consistent with the observed phenotype and highlights possible genes whose coordinated regulation could lead to a strong modification of the FA profiles in important membrane lipids (PC and DGTA), a modulation of the MGDG/PC ratio, and a carbon reallocation toward TAG by preventing TAG catabolism. This scheme is very different from the transcriptomic reprogramming and lipid remodeling following nitrogen or phosphorus starvation, considered as a reference for industrial developments, in which TAG accumulates mainly following an orchestrated induction of FA synthesis genes and of multiple enzymes involved in TAG biosynthesis (Abida et al., 2015; Alipanah et al., 2015; Levitan et al., 2015).
DISCUSSION
Consistency of the Screen and Identification of Candidate Biological Processes Influencing TAG Levels in P. tricornutum
The use of an annotated library of compounds (i.e. containing small molecules characterized previously on known targets) allows an analysis of screening outputs and a more rapid deduction of target candidates compared with screenings of large-scale libraries containing tens of thousands of unannotated molecules. Here, we screened 1,200 compounds of the Prestwick Chemical Library using a multiparameter assay allowing the evaluation of cell abundance, TAG level, and chlorophyll fluorescence (Fig. 1) with appropriate statistical robustness based on Z′ values. Possible biases or conditions leading to false positive or negative results have to be considered. An important bias lies in the choice of the library of compounds, which could be enriched in drugs developed to act on specific human pathogens, such as molecules binding to neurotransmitter receptors, being effective on metabolic disorders, on cancer, or on specific infectious diseases. This bias is a limitation on the number of target classes we can explore, but it also can be considered an advantage to identify molecules hitting a pathway at the level of multiple target enzymes. The miniaturized assay developed to measure whole-cell responses, allowing a selection of compounds that are active on the phenotype, also can produce false positive or negative results. The parameter used to estimate cell abundance was based on the fluorescence of Histone H4 fused to YFP, correlating linearly with cell counts from 0.1 to 0.55 mm NaNO3 (Fig. 1; Supplemental Fig. S1). Therefore, cell abundance could be underestimated in conditions limiting protein biosynthesis. We confirmed variations of cell abundance following treatments with selected molecules using a Malassez counting chamber (Supplemental Fig. S3). Another important source of erroneous results lies in the use of Nile Red to stain TAG droplets. Nile Red staining allows relative comparisons within a batch of experiments and can react with other nonpolar lipids. Therefore, we confirmed the observed increase of Nile Red staining obtained following selected chemical treatments by direct analysis of glycerolipids.
Our primary screen of 1,200 compounds was performed by incubating P. tricornutum in the presence of molecules supplied at 10 µm for 48 h (Fig. 2). We selected 160 compounds leading to an increase in Nile Red staining above 120% of the average per plate, while maintaining a cell abundance above 50% of that of the control (Fig. 3), and we repeated the screen to discard false positives. A list of 40 molecules was then selected and tested in independent triplicates, and we selected the most active molecules in at least two of the three independent assays. This secondary screen allowed the final selection of 34 hit molecules (Table I). The success of a screen can be evaluated if some of the selected molecules have close chemical structures (showing consistency in the detection of an active chemical scaffold) or if distinct molecules hitting the same biological pathway are selected. Here, the screen pointed to a limited number of biological processes or pathways (i.e. nucleic acid biosynthesis and cell division, membrane receptors and transporters, cyclic nucleotide or oxylipin signaling, and sterol metabolism; Table I). The consistency of the screen was further illustrated by the presence of similar chemical structures: simvastatin and mevastatin share a common statin scaffold, estrone and ethynylestradiol are both estrogens, serotonin is the ligand of serotonin receptors, and alverin and pipenperone are both antagonists of the same receptor. The results of this screen pointed toward sterol metabolism.
Five molecules were selected based on the detection of their bioactivity in all of the tests, likely interference with mitochondria and/or the chloroplast electron transport chain (antimycin A), microtubule dynamics (nocodazole), sterol metabolism (ethynylestradiol and mevastatin), and purine catabolism (allopurinol). We confirmed that the increase in Nile Red staining was dose dependent (Fig. 4), with an arrest of growth induced at concentrations higher that 10 to 50 µm, depending on the molecules. This indicates that the molecules might hit vital targets and/or that high doses increase unspecific toxicity. The advantage of using chemicals over conventional genetics is well known when addressing the question of essential proteins: one can study a phenotypic response at sublethal concentrations, decrease the activity of the target of interest with different magnitudes, and trigger the phenotypic response at any developmental stage by simply adding the compound and eventually combining with other molecules or genetic backgrounds (Soleilhac et al., 2010). In addition, the chemical structures of selected molecules might be close enough to those of natural molecules to suggest a mimicry of a natural signaling process.
The output of the screen also was consistent with some previous studies, showing an increase of TAG (1) after the impairment of microtubule dynamics (using taxol) and cell division in Arabidopsis (Arabidopsis thaliana) cells (Meï et al., 2017), (2) after the chemical inhibition or genetic knockdown of sterol metabolism enzymes in P. tricornutum (Fabris et al., 2014), or (3) after incubation with nitric oxide (Dolch et al., 2017a), a known activator of nucleotide cyclases, opposing cyclic nucleotide phosphodiesterases (Francis et al., 2010). Focusing on compounds acting on sterol metabolism, we expanded our tests to additional molecules that were not screened initially but are known to act at the levels of other enzymes of the pathway (Fig. 5) and confirmed that carbon partitioning was balanced between this pathway and TAG net production. The consistency of the screen suggests that all of the biological processes we highlighted are promising for more in-depth investigations.
Focused Analysis of the P. tricornutum Response to Ethynylestradiol
Ethynylestradiol is a synthetic derivative of the natural hormones estrone and estradiol (Inhoffen and Hohlweg, 1938). The presence of the ethynyl group makes this estrogenic steroid more resistant to oxidation and stable in the environment, with a half-life higher than 100 d in water under aerobic conditions (Adeel et al., 2017). The water solubility of ethynylestradiol is 16 mm at 20°C, and the logarithm of its n-octanol/water partition coefficient (or logP) is 3.63 (Adeel et al., 2017), indicating a strong efficiency in crossing cell barriers (Lipinski et al., 2001). Used in birth control pills (Jensen and DeSombre, 1973), ethynylestradiol’s binding affinity to the estrogen receptor is 2 times higher in humans and up to 5 times higher in some fish species compared with natural hormones (Aris et al., 2014). Both natural and synthetic estrogens are endocrine disruptors and pose ecological hazards following their discharge in the environment by anthropogenic practices (Aris et al., 2014; Adeel et al., 2017). High ethynylestradiol concentrations have an impact on sex determination in some fish species (Bhandari et al., 2015; Siegenthaler et al., 2017). Our knowledge of the effects of estrogen on phytoplankton is very scarce, with only two reports regarding a negative impact on growth in the green alga C. reinhardtii (Pocock and Falk, 2014) and in the pennate diatom Navicula incerta (Liu et al., 2010). Interestingly, treatment with 10 µm ethynylestradiol triggered an increase in total acyl lipids in N. incerta (Liu et al., 2010), possibly due to the increase in TAG observed here in P. tricornutum. In addition, a bioaccumulation of ethynylestradiol was observed in N. incerta (Liu et al., 2010, 2012), supporting that diatoms could concentrate this molecule over time. The study of the effect of high concentrations of molecules (i.e. an acute exposure) could mimic the physiological situation after a long-term exposure to low doses.
The mode of action of ethynylestradiol in P. tricornutum is unknown; however, past reports of the inhibition of cytochrome P450 enzymes involved in sterol metabolism (Table I; Fig. 5) and the up-regulation of CYP genes (Supplemental Table S3) suggest that one or more CYP proteins might be targeted. The biodegradation of ethynylestradiol by N. incerta (Liu et al., 2010) also suggests that some CYP enzyme(s) might eventually use this estrogen as a substrate, leading to its bioconversion into a nonactive compound. Thus, diatoms might contribute to reducing the environmental concentration of this endocrine disruptor and decrease the detrimental impact on other aquatic species feeding on diatoms (Liu et al., 2012).
The phenotypic response to ethynylestradiol is marked by a strong accumulation of TAG, which does not seem to balance a dramatic decline of polar lipid quantities, besides a moderate decrease in MGDG (Fig. 6). This response suggests that TAG might simply accumulate following the disturbance of sterol biosynthesis, leading to an accumulation of acetyl-CoA diverted toward FA and TAG biosynthesis. This quantitative reallocation of carbon from one sink (sterols) to another (TAG) is supported by the up-regulation of genes involved in acetyl-CoA biosynthesis and the down-regulation of genes involved in TAG degradation (Supplemental Table S4). In addition, gene expression profiles support a modification of fluxes within the central carbon metabolism, as reported previously after chemical treatment performed on C. reinhardtii (Wase et al., 2017). In the current status of P. tricornutum genome annotation, and without precise subcellular compartmentalization of central carbon metabolism genes, the fluxes are difficult to finely assess. Nevertheless, ethynylestradiol treatment seems to induce an arrest of gluconeogenesis, also observed when P. tricornutum is subjected to nitrogen starvation (Alipanah et al., 2015), and an increased expression of genes involved in glycolysis and acetyl-CoA production. Chrysolaminarin is a polysaccharide that can be an alternative form of carbon storage competing with TAG. Whereas two independent studies have shown that the knockout of the UDP-Glc-pyrophosphorylase/phosphoglucomutase gene (Phatr3_EG02613) led to an increase in TAG (Daboussi et al., 2014; Zhu et al., 2016), this gene was up-regulated following treatment with ethynylestradiol (Supplemental Table S1), suggesting that the interruption in the flux of carbon toward sterols might be diverted toward the two forms of carbon storage, chrysolaminarin and TAG. We did not analyze the metabolomic status of carbohydrates. This should be investigated in the future.
Following treatment with ethynylestradiol, the analysis of FAs within each class of glycerolipids showed striking differences, including in membrane glycerolipids, in particular a decrease in the proportion of 20:5 FAs in all major lipid classes (i.e. MGDG, DGDG, PG, PC, and DGTA; Fig. 6B). The synthesis of 20:5 FAs starts in the endoplasmic reticulum from short-chain acyl-CoAs, including saturated molecular species such as 16:0 (Dolch et al., 2017b). It proceeds by a series of elongations of acyl-CoAs and of desaturation on FAs linked to membrane lipids, possibly PC and/or DGTA (Sayanova and Napier, 2004; Dolch and Maréchal, 2015; Sayanova et al., 2017). Elongation includes Δ0-ELOs (16:0→18:0) and Δ6-ELOs (18:3→20:3; Dolch et al., 2017b). Ethynylestradiol treatment induced an increase of the proportion of FA precursors for VLC-PUFAs, in both PC (18:4, 18:3, 18:2, and 18:1) and DGTA (18:4, 18:3, and 18:2), before elongation by a Δ6-ELO. Consistently, although most genes involved in 20:5 FA biosynthesis were up-regulated, including the gene coding for the betaine lipid platform on which desaturation occurs, Δ6-ELO-B1 (Phatr3_J22274) was strikingly down-regulated (Supplemental Table S4). This result suggests that transcriptomic control of a Δ6-ELO might occur when increasing doses of ethynylestradiol are provided. Based on these experiments, it is impossible to assess whether this down-regulation is due to an indirect or direct effect of ethynylestradiol. Although posttranslational regulation might occur in combination with the transcriptomic changes observed here, an interruption of 20:5 FA biosynthesis at the level of a Δ6-ELO is expected based on lipid profiles, and this impairment coincides with a decrease of Δ6-ELO-B1 expression. This might be an additional mechanism altering glycerolipids following treatments with estrogens.
CONCLUSION
This study identified molecules that could be of interest for direct implementation in an industrial process aiming at triggering the accumulation of TAG in diatoms, which would be distinct from established methods such as nutrient starvation (Abida et al., 2015). One advantage of this approach is that it allowed us to determine cultivation conditions permitting the accumulation of TAG with lower impact on growth. The use of an annotated library of compounds allowed for the identification of some metabolic pathways that might be scrutinized in more detail for genetic engineering strategies in the future. Some of the identified pathways confirm past studies, such as nucleotide syntheses and cell division processes (Kim et al., 2017; Meï et al., 2017; Prioretti et al., 2017), sterol metabolism (Fabris et al., 2014; Gallo et al., 2017), or, indirectly, nitric oxide signaling (Dolch et al., 2017a). Other remarkable biological processes highlighted here, involving membrane receptors and transporters or oxylipin signaling, need to be investigated. The identification of compounds interfering with catecholamine metabolism, known to interfere with lipolysis in humans (Camell et al., 2017), raises the question of an analogous system in diatoms.
The discovery of two endocrine disruptors (ethynylestradiol and estrone) among the five more active molecules is one of the major results of this study. Ethynylestradiol has been reported to be used in aquaculture to develop single-sex populations of fish (Aris et al., 2014). Our study shows that these compounds potentially have a strong impact on phytoplankton, which needs to be addressed in environmental contexts. On the one hand, the mode of action likely implies interference with the metabolism in P. tricornutum, possibly via the inhibition of one or more CYP enzyme(s) and the reallocation of carbon from one sink (sterols) to TAG. This putative mechanism should be investigated in the future. On the other hand, VLC-PUFA biosynthesis is strikingly impaired, with an accumulation of intermediates upstream of 18- to 20-carbon acyl elongation, and coincides with the down-regulation of a gene involved in this specific elongation. This latter effect indicates that a specific transcriptomic control of VLC-PUFA biosynthesis might be activated directly or indirectly by estrogens. Treatment with ethynylestradiol might interfere with a process occurring in response to a natural sterol, either in an intracellular or a cell-to-cell signaling process. Although natural doses of ethynylestradiol have not been measured systematically in the past, with some reports indicating ∼15 pm in seawater (Adeel et al., 2017), the capacity of pennate diatoms to accumulate estrogens (Liu et al., 2010, 2012) shows the relevance of studying these organisms following acute exposures. Future work is needed to address the question of the physiological processes chemically impaired by synthetic estrogens and to address the environmental consequences of the response of diatoms detailed here. Possible effects of the active compounds described here on other phytoplanktonic species also should be investigated.
MATERIALS AND METHODS
Chemicals and the Library of Small Molecules
Chemicals used in the composition of growth media and solvents were obtained from Sigma-Aldrich. The Prestwick Chemical Library of 1,200 compounds was obtained from Prestwick Chemical and consists exclusively of U.S. Food and Drug Administration-approved compounds. The chemical library was stored at −20°C at a 10 mm concentration in 100% DMSO, and 50-µL working solutions were prepared in 48-well plates before screening.
Cultivation of Phaeodactylum tricornutum
Experiments were performed with the P. tricornutum Bohlin Strain 8.6 CCMP2561 (Culture Collection of Marine Phytoplankton, now known as NCMA: National Center for Marine Algae and Microbiota) and with PtYFP, a strain expressing a Histone H4 fused to EYFP at the N terminus, kindly provided by Angela Falciatore (Université Pierre et Marie Curie; Siaut et al., 2007). P. tricornutum cells were maintained and grown in 50-mL cultures at 20°C in 250-mL flasks in a modified ESAW medium [NaCl 362.7 mm, Na2SO4 25 mm, KCl 8.03 mm, NaHCO3 2.067 mm, KBr 0.725 mm, H3BO3 0.372 mm, NaF 0.0657 mm, MgCl2 47.18 mm, CaCl2 9.134 mm, SrCl2 0.082 mm, Na2-glycerophosphate 21.8 µm, Na2SiO3 105.6 µm, Na2EDTA 14.86 µm, Fe(NH4)2(SO4)2 5.97 µm, FeCl3 0.592 µm, MnSO4 2.42 µm, ZnSO4 0.254 µm, CoSO4 0.0569 µm, Na2MoO4 0.52 µm, H3BO3 61.46 µm, Na2SeO3 10 nm, biotin (vitamin H) 8.18 nm, cobalamin (vitamin B12) 2.94 nm, and thiamine (vitamin B1) 0.594 µm; Falciatore et al., 2000], using either 0.55 mm NaNO3 and 2.2 × 10−2 mm NaH3PO4 or 10 times enriched nitrogen and phosphate sources (ESAW 10N10P, containing 5.5 mm NaNO3 and 0.22 mm NaH3PO4; Abida et al., 2015). Cells were grown on a 12-h/12-h light (50 μmol m−2 s−1)/dark cycle. Cells were subcultured twice per week by inoculating 106 cells mL−1 with fresh medium. Growth was evaluated using a Malassez cell counting chamber or by the A730 using a TECAN plate reader.
Automated High-Throughput Screening
The screening of small molecules was performed using the automated multimodule platform from the Centre de Criblage pour des Molecules Bio-actives. An 800-mL culture of PtYFP was grown in ESAW 1N1P medium until the cell concentration reached 2.5 × 106 cells mL−1. Cells were centrifuged at 3,500 rpm for 5 min, and the supernatant was gently discarded. Cells were then resuspended in fresh ESAW 0N1P (without nitrogen and containing 2.2 × 10−2 mm NaH3PO4) and adjusted to a final concentration of 106 cells mL−1. Clear 48-well plates (NUNC; 055431) were prepared by adding a 4-mm glass bead (Roth; HH55.1) in each well. First, 450-µL aliquots of PtYFP cells were dispensed into the wells corresponding to high-TAG controls, completed with 50 µL of 0N1P medium (cells incubated in 500 µL of low-nitrogen medium). NaNO3 was then added to the remaining PtYFP cell suspension (1 × 106 cells mL−1) and mixed gently to obtain a nitrogen-rich medium (ESAW 1N1P containing 0.55 mm NaNO3 and 2.2 × 10−2 mm NaH3PO4). Compounds from the Prestwick Chemical Library were then added in each well to reach final incubation conditions of 10 µm of compounds, 0.5% (v/v) DMSO, and 1 × 106 cells mL−1. Glass beads aided culture growth in the 48-well plates. The cultures were incubated for 48 h at 100 rpm under a 12-h/12-h light (50 µmol photons m−2 s−1)/dark cycle at 20°C in an artificial climate incubator (HT multitron; Infors). Control conditions included cells in ESAW 1N1P (low-TAG controls) and 0N1P (high-TAG controls) grown without any added drug. After 48 h, 150 µL was transferred from the 48-well plate to a black 96-well plate (Greiner; 655086), and YFP and chlorophyll fluorescence levels were measured at excitation/emission wavelengths of 515/530 nm and 440/680 nm, respectively, using the TECAN Infinite M1000 plate reader. To assess the accumulation of neutral lipids, 40 µL of Nile Red (2.5 µg mL−1 9-diethylamino-5-benzo[α]phenoxazinone in DMSO) was added per well and incubated for 20 min at room temperature. The concentration of Nile Red was defined based on optimal staining of lipid droplets with lower background detection. Although a smaller volume of Nile Red could be used (Wase et al., 2015), a 40-µL volume was defined to prevent automated pipetting variations. Nile Red fluorescence was measured at excitation/emission wavelengths of 530/580 nm. Compounds triggering an increase in Nile Red fluorescence levels were selected for a secondary screen, with priority given to hit molecules triggering both high Nile Red and high YFP fluorescence. A total of 160 hit molecules were reevaluated at 10 µm and, based on the obtained results, 40 compounds were selected for a tertiary screen.
Dose-Response Assays
A double-strength concentration range of selected compounds was obtained by dilution in ESAW 1N1P medium with 1% (v/v) DMSO, and 250 µL was dispensed in triplicate in 48-well plates (NUNC; 055431). The same volume (250 µL) of a P. tricornutum culture at a cell concentration of 2 × 106 cells mL−1 was added in each well to reach a final condition of 1 × 106 cells mL−1 and 0.5% (v/v) DMSO in ESAW medium. A 4-mm glass bead (Roth; HH55.1) was added in each well to aid culture growth in the 48-well plates. Plates were then incubated for 48 h at 100 rpm under a 12-h/12-h light (50 µmol photons m−2 s−1)/dark cycle at 20°C in an artificial climate incubator (HT multitron; Infors). Control conditions included untreated cells with 0.5% (v/v) DMSO and cells in ESAW medium without NaNO3. After 48 h, 300 µL was transferred from the 48-well plate to a clear 96-well plate (NUNC; 55260). The A730 was measured using the TECAN Infinite M1000 plate reader. The concentration of each culture was calculated using the linear relationship between cells mL−1 counting assessed with a Malassez chamber and A730. A 160-µL aliquot was transferred to a black 96-well plate (Greiner; 655086), and chlorophyll fluorescence was measured at excitation and emission wavelengths of 440 and 680 nm, respectively. Then, 40 µL of Nile Red (2.5 µg mL−1 in DMSO) was added in each well, and cells were incubated for 20 min at room temperature. Nile Red fluorescence was measured at excitation and emission wavelengths of 530 and 580 nm, respectively. The relative fluorescence values of Nile Red and chlorophyll were normalized per million cells and compared as a percentage of the untreated control. The volume of culture remaining in the NUNC clear 96-well plates (140 µL) was used to measure PSII efficiency, represented by the Fv/Fm value.
Photosynthetic Parameters
To determine photosynthesis parameters in cell cultures, room temperature fast chlorophyll fluorescence kinetics were measured using a Speedzen MX fluorescence imaging system (JBeamBio) with settings described previously (Johnson et al., 2009; Allorent et al., 2013). A 140-µL volume of P. tricornutum culture was transferred to a transparent 96-well plate and dark incubated for 15 to 30 min before measurements. Excitation was performed in the blue range (λ = 450 nm; F0). F0 is the steady-state fluorescence in dark-adapted cultures, Fm is the maximal fluorescence after a saturating light pulse with green light (520 nm) of dark-adapted cultures, Fm′ is the maximal fluorescence after a saturating light pulse with green light (520 nm) of light-adapted cultures, and Fv is the difference between Fm and F0. With these parameters, the maximum efficiency of energy conversion of PSII can be calculated as Fv/Fm (Butler and Kitajima, 1975; Misra et al., 2012).
Glycerolipid Analyses
For lipid analysis, P. tricornutum CCMP632/CCAP 1055/1 cultures were initiated at a 1 × 106 cells mL−1 concentration and incubated at 20°C with 100 rpm under a 12-h/12-h light (50 µmol photons m−2 s−1)/dark cycle. Depending on the treatment, cells were collected at day 2, 4, or 7 in 50-mL Falcon tubes via centrifugation at 3,500 rpm for 10 min. Pellets were immediately snap frozen by transfer in liquid nitrogen and stored at −80°C until lipid extraction. After freeze drying, pellets were suspended in 4 mL of boiling ethanol for 5 min to prevent lipid degradation, and lipids were extracted as described earlier (Abida et al., 2015) by the addition of 2 mL of methanol and 8 mL of chloroform at room temperature. The mixture was then saturated with argon and stirred for 1 h at room temperature. After filtration through glass wool, cell remains were rinsed with 3 mL of chloroform:methanol (2:1, v/v), and 5 mL of 1% (w/v) NaCl was then added to the filtrate to initiate biphase formation. The chloroform phase was dried under argon before solubilizing the lipid extract in pure chloroform. Total glycerolipids were quantified from their FAs: in an aliquot fraction, a known quantity of the 15:0 FA standard was added, and the FAs present were transformed as methyl esters (FAME) by a 1-h incubation in 3 mL of 2.5% (v/v) H2SO4 in pure methanol at 100°C. The reaction was stopped by the addition of 3 mL of water and 3 mL of hexane. The hexane phase was analyzed by gas chromatography-flame ionization detection (Perkin Elmer) on a BPX70 (SGE) column. FAMEs were identified by comparison of their retention times with those of standards (Sigma-Aldrich) and quantified by the surface peak method using the 15:0 standard for calibration. To quantify the various classes of neutral and polar glycerolipids, lipids were separated by TLC onto glass-backed silica gel plates (Merck) using two distinct resolving systems. To isolate neutral lipids including TAG and free FAs, lipids were resolved by TLC run in one dimension with hexane:diethylether:acetic acid (70:30:1, v/v). To isolate membrane glycerolipids, lipids were resolved by two-dimensional TLC. The first solvent was chloroform:methanol:water (65:25:4, v/v) and the second was chloroform:acetone:methanol:acetic acid:water (50:20:10:10:5, v/v). Lipids were then visualized under UV light, after spraying with 2% (w/v) 8-anilino-1-naphthalenesulfonic acid in methanol, and scraped off the plate. FAMEs were then prepared by methanolysis directly from the silica and quantified by gas chromatography-flame ionization detection as described above.
Screening Statistics
The quality of the screening assay was assessed based on the calculation of the Z′ factor (Zhang et al., 1999) with the following equation:
where σc+ and σc− are the sd of readout values obtained with positive and negative controls and μc+ and μc− are the means of readout values obtained with positive and negative controls (i.e. high-TAG and low-TAG controls, respectively).
Transcriptomic Analyses
P. tricornutum cells were grown in 50 mL of ESAW medium in 100-mL flasks until they reached a minimum concentration of 6 × 106 cells mL−1. Cells were then incubated 4 d with increasing concentrations of 17α-ethynylestradiol [19-nor-17α-pregna-1,3,5(10)-trien-20-yne-3,17-diol] provided at 0, 10, and 20 µm, with a final concentration of 0.5% (v/v) DMSO. Following treatment, frozen cells (2 × 108 cells) were sent to BGI for RNA extraction using the TRIzol reagent (Chomczynski and Sacchi, 1987), allowing the collection of 10 to 20 µg of RNA per sample. Quality was assessed based on the 28S/18S ratio and RNA integrity number tests using an Agilent 2100 Bioanalyzer. Libraries were prepared for stranded 100-bp paired-end sequencing according to BGI procedures. In brief, double-stranded and single-stranded DNA were degraded using DNase I; poly(A)-containing mRNA molecules were purified from total RNA using poly(T) oligo-attached magnetic beads; and mRNAs were fragmented into small pieces using a Covaris M220 focused ultrasonicator. First-strand cDNA was then generated using random hexamer-primed reverse transcription. This was followed by a second-strand cDNA synthesis, and cDNAs were purified. The synthesized cDNAs were subjected to end repair and then were 3′ adenylated. BGI-designed adaptors were ligated to the ends of these 3′ adenylated cDNA fragments. The products of the ligation reaction were purified on Tris-acetate EDTA agarose gels and amplified by PCR. Obtained libraries were validated using the Agilent 2100 Bio-analyzer and ABI StepOnePlus Real-Time PCR analysis. Samples were analyzed independently using the Illumina HiSeq 4000 system (20 m reads), generating on average 22,555,637 raw sequencing reads and then 22,368,247 clean reads after the removal of adaptor sequences, high content of unknown bases, and low-quality reads (BGI proprietary pipeline). After filtering, clean reads were first mapped on the most recent genome version of P. tricornutum (http://protists.ensembl.org/Phaeodactylum_tricornutum/Info/Index) using the HISAT (Kim et al., 2015)/Bowtie2 (Langmead et al., 2009) tool for a primary read counting. The average mapping ratio with reference genes was 77.54%. The gene expression level was quantified using RSEM (Li and Dewey, 2011). For each sample, the number of identified expressed genes was counted, and the proportion to total gene number in the database was calculated (Supplemental Table S5). For gene expression comparisons, data were filtered based on the detection of one read in at least one sample per treatment or genomic mutation and then normalized using the DESseq2 method (Varet et al., 2016). Clustering was achieved based on expression profiles as described previously (Dolch et al., 2017a).
Accession Numbers
Sequences from the P. tricornutum genome analyzed more carefully in this study can be found via Ensembl! under the following accession numbers: Phatr3_EG01524, Phatr3_EG01947, Phatr3_EG01955, Phatr3_EG02208, Phatr3_EG02286, Phatr3_EG02309, Phatr3_EG02454, Phatr3_EG02496, Phatr3_EG02521, Phatr3_EG02619, Phatr3_J10068, Phatr3_J10497, Phatr3_J11319, Phatr3_J11390, Phatr3_J11811, Phatr3_J11916, Phatr3_J12420, Phatr3_J12420, Phatr3_J12431, Phatr3_J12642, Phatr3_J12740, Phatr3_J12884, Phatr3_J12902, Phatr3_J13073, Phatr3_J13076, Phatr3_J14202, Phatr3_J15031, Phatr3_J15211, Phatr3_J15730, Phatr3_J16364, Phatr3_J16376, Phatr3_J17086, Phatr3_J17720, Phatr3_J17720, Phatr3_J18029, Phatr3_J18064, Phatr3_J18940, Phatr3_J18940, Phatr3_J1971, Phatr3_J19979, Phatr3_J20143, Phatr3_J20143, Phatr3_J20310, Phatr3_J20342, Phatr3_J20460, Phatr3_J20460, Phatr3_J20508, Phatr3_J21116, Phatr3_J21201, Phatr3_J21988, Phatr3_J22122, Phatr3_J2215, Phatr3_J22274, Phatr3_J22357, Phatr3_J22404, Phatr3_J22459, Phatr3_J22510, Phatr3_J22554, Phatr3_J22677, Phatr3_J22803, Phatr3_J23639, Phatr3_J23850, Phatr3_J23913, Phatr3_J24195, Phatr3_J24610, Phatr3_J24739, Phatr3_J25769, Phatr3_J25932, Phatr3_J26029, Phatr3_J26714, Phatr3_J28009, Phatr3_J28068, Phatr3_J28652, Phatr3_J28797, Phatr3_J29157, Phatr3_J29488, Phatr3_J29702, Phatr3_J30113, Phatr3_J30145, Phatr3_J30282, Phatr3_J30514, Phatr3_J31367, Phatr3_J31440, Phatr3_J31492, Phatr3_J31662, Phatr3_J31994, Phatr3_J31994, Phatr3_J32083, Phatr3_J3262, Phatr3_J32902, Phatr3_J33198, Phatr3_J33435, Phatr3_J33720, Phatr3_J33864, Phatr3_J33864, Phatr3_J34485, Phatr3_J34526, Phatr3_J35240, Phatr3_J36821, Phatr3_J37086, Phatr3_J37086, Phatr3_J37367, Phatr3_J37372, Phatr3_J37652, Phatr3_J38509, Phatr3_J39710, Phatr3_J39949, Phatr3_J40163, Phatr3_J40200, Phatr3_J40261, Phatr3_J40880, Phatr3_J40988, Phatr3_J41423, Phatr3_J41423, Phatr3_J41515, Phatr3_J41570, Phatr3_J41886, Phatr3_J41969, Phatr3_J42398, Phatr3_J42683, Phatr3_J42872, Phatr3_J43010, Phatr3_J43099, Phatr3_J43116, Phatr3_J43320, Phatr3_J43352, Phatr3_J43463, Phatr3_J43469, Phatr3_J43678, Phatr3_J43773, Phatr3_J43773, Phatr3_J43773, Phatr3_J44005, Phatr3_J44028, Phatr3_J44066, Phatr3_J44231, Phatr3_J44401, Phatr3_J44584, Phatr3_J44645, Phatr3_J44806, Phatr3_J44831, Phatr3_J45223, Phatr3_J45510, Phatr3_J45510, Phatr3_J45518, Phatr3_J45551, Phatr3_J45758, Phatr3_J45947, Phatr3_J46175, Phatr3_J46453, Phatr3_J46570, Phatr3_J46595, Phatr3_J46830, Phatr3_J47389, Phatr3_J48423, Phatr3_J48664, Phatr3_J48778, Phatr3_J48778, Phatr3_J48799, Phatr3_J48859, Phatr3_J48977, Phatr3_J49163, Phatr3_J4918, Phatr3_J49339, Phatr3_J49462, Phatr3_J49524, Phatr3_J49544, Phatr3_J49771, Phatr3_J49867, Phatr3_J50356, Phatr3_J50742, Phatr3_J50770, Phatr3_J51214, Phatr3_J51454, Phatr3_J51519, Phatr3_J52268, Phatr3_J52368, Phatr3_J52648, Phatr3_J5271, Phatr3_J54017, Phatr3_J54068, Phatr3_J54151, Phatr3_J54168, Phatr3_J54222, Phatr3_J54494, Phatr3_J54528, Phatr3_J54709, Phatr3_J54709, Phatr3_J54974, Phatr3_J54983, Phatr3_J55069, Phatr3_J55111, Phatr3_J55153, Phatr3_J55157, Phatr3_J55192, Phatr3_J55209, Phatr3_J5527, Phatr3_J7164, Phatr3_J7678, Phatr3_J8663, Phatr3_J8860, Phatr3_J8975, Phatr3_J9255, Phatr3_J9316, Phatr3_J9538, Phatr3_J9619, Phatr3_J9709, Phatr3_J9794, Phatr3_Jdraft559, Phatr3_J23913, Phatr3_J1664 , Phatr3_J16870, Phatr3_J53929, Phatr3_EG02641, Phatr3_EG02359, Phatr3_EG00741, Phatr3_J12533, Phatr3_EG02290, Phatr3_J47271, Phatr3_J49325, Phatr3_EG02293, Phatr3_J10824, Phatr3_J49447, Phatr3_J31339, Phatr3_J10852, Phatr3_J48864, Phatr3_J36801, Phatr3_J14208, Phatr3_J30461, Phatr3_J51757, Phatr3_J1689, Phatr3_J9258, Phatr3_J12330, Phatr3_EG02383, Phatr3_J12330, Phatr3_J44955, and Phatr3_J41845.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Measurement of P. tricornutum cell abundance.
Supplemental Figure S2. Evaluation of miniature assay robustness.
Supplemental Figure S3. Response of P. tricornutum cells to increasing doses of ethynylestradiol.
Supplemental Figure S4. Growth of P. tricornutum cells used for RNAseq analyses.
Supplemental Table S1. Whole-gene expression profiles following incubation of P. tricornutum cells with increasing doses of ethynylestradiol.
Supplemental Table S2. GOseq analysis of GO enrichment in P. tricornutum gene clusters differentially expressed following ethynylestradiol treatments.
Supplemental Table S3. Sterol metabolism gene expression following ethynylestradiol treatment.
Supplemental Table S4. Lipid gene expression following ethynylestradiol treatment.
Supplemental Table S5. Whole-gene expression profiles following incubation of P. tricornutum cells with increasing doses of ethynylestradiol.
Acknowledgments
We thank Séverine Collin, Marie-Odile Fauvarque, Michel Ferrand, Giovanni Finazzi, and Dimitris Petroutsos for fruitful discussions and advice.
Footnotes
This work was supported by grants from Agence Nationale de la Recherche (DiaDomOil), Commissariat à l’Energie Atomique (Irtelis and Flagship PhD grant programs), and Agence Nationale de la Recherche Programme Investissement d’Avenir (Océanomics).
References
- Abida H, Dolch LJ, Meï C, Villanova V, Conte M, Block MA, Finazzi G, Bastien O, Tirichine L, Bowler C, et al. (2015) Membrane glycerolipid remodeling triggered by nitrogen and phosphorus starvation in Phaeodactylum tricornutum. Plant Physiol 167: 118–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeel M, Song X, Wang Y, Francis D, Yang Y (2017) Environmental impact of estrogens on human, animal and plant life: a critical review. Environ Int 99: 107–119 [DOI] [PubMed] [Google Scholar]
- Ajjawi I, Verruto J, Aqui M, Soriaga LB, Coppersmith J, Kwok K, Peach L, Orchard E, Kalb R, Xu W, et al. (2017) Lipid production in Nannochloropsis gaditana is doubled by decreasing expression of a single transcriptional regulator. Nat Biotechnol 35: 647–652 [DOI] [PubMed] [Google Scholar]
- Alipanah L, Rohloff J, Winge P, Bones AM, Brembu T (2015) Whole-cell response to nitrogen deprivation in the diatom Phaeodactylum tricornutum. J Exp Bot 66: 6281–6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allorent G, Courtois F, Chevalier F, Lerbs-Mache S (2013) Plastid gene expression during chloroplast differentiation and dedifferentiation into non-photosynthetic plastids during seed formation. Plant Mol Biol 82: 59–70 [DOI] [PubMed] [Google Scholar]
- Anandarajah K, Mahendraperumal G, Sommerfeld M, Hu Q (2012) Characterization of microalga Nannochloropsis sp mutants for improved production of biofuels. Appl Energy 96: 371–377 [Google Scholar]
- Arbenz A, Perrin R, Avérous L (2018) Elaboration and properties of innovative biobased PUIR foams from microalgae. J Polym Environ 26: 254–262 [Google Scholar]
- Aris AZ, Shamsuddin AS, Praveena SM (2014) Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: a review. Environ Int 69: 104–119 [DOI] [PubMed] [Google Scholar]
- Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH, Zhou S, Allen AE, Apt KE, Bechner M, et al. (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306: 79–86 [DOI] [PubMed] [Google Scholar]
- Attwood D. (1976) Aggregation of antiacetylcholine drugs in aqueous solution: micellar properties of some diphenylmethane derivatives. J Pharm Pharmacol 28: 407–409 [DOI] [PubMed] [Google Scholar]
- Baas PW, Rao AN, Matamoros AJ, Leo L (2016) Stability properties of neuronal microtubules. Cytoskeleton (Hoboken) 73: 442–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom JD, Bostedor RG, Masarachia PJ, Reszka AA, Rodan G (2000) Alendronate is a specific, nanomolar inhibitor of farnesyl diphosphate synthase. Arch Biochem Biophys 373: 231–241 [DOI] [PubMed] [Google Scholar]
- Bhandari RK, Deem SL, Holliday DK, Jandegian CM, Kassotis CD, Nagel SC, Tillitt DE, Vom Saal FS, Rosenfeld CS (2015) Effects of the environmental estrogenic contaminants bisphenol A and 17α-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. Gen Comp Endocrinol 214: 195–219 [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. (2003) A new science-business paradigm in anticancer drug development. Trends Biotechnol 21: 103–106 [DOI] [PubMed] [Google Scholar]
- Botté CY, Maréchal E (2014) Plastids with or without galactoglycerolipids. Trends Plant Sci 19: 71–78 [DOI] [PubMed] [Google Scholar]
- Bowler C, Allen AE, Badger JH, Grimwood J, Jabbari K, Kuo A, Maheswari U, Martens C, Maumus F, Otillar RP, et al. (2008) The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456: 239–244 [DOI] [PubMed] [Google Scholar]
- Bozarth A, Maier UG, Zauner S (2009) Diatoms in biotechnology: modern tools and applications. Appl Microbiol Biotechnol 82: 195–201 [DOI] [PubMed] [Google Scholar]
- Brodie J, Chan CX, De Clerck O, Cock JM, Coelho SM, Gachon C, Grossman AR, Mock T, Raven JA, Smith AG, et al. (2017) The algal revolution. Trends Plant Sci 22: 726–738 [DOI] [PubMed] [Google Scholar]
- Butler WL, Kitajima M (1975) Fluorescence quenching in photosystem II of chloroplasts. Biochim Biophys Acta 376: 116–125 [DOI] [PubMed] [Google Scholar]
- Camell CD, Sander J, Spadaro O, Lee A, Nguyen KY, Wing A, Goldberg EL, Youm YH, Brown CW, Elsworth J, et al. (2017) Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550: 119–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Jounaidi Y, Waxman DJ (2005) Enantioselective metabolism and cytotoxicity of R-ifosfamide and S-ifosfamide by tumor cell-expressed cytochromes P450. Drug Metab Dispos 33: 1261–1267 [DOI] [PubMed] [Google Scholar]
- Chen X, Ji ZL, Chen YZ (2002) TTD: Therapeutic Target Database. Nucleic Acids Res 30: 412–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesné C, Guyomard C, Guillouzo A, Schmid J, Ludwig E, Sauter T (1998) Metabolism of meloxicam in human liver involves cytochromes P4502C9 and 3A4. Xenobiotica 28: 1–13 [DOI] [PubMed] [Google Scholar]
- Chisti Y. (2013) Constraints to commercialization of algal fuels. J Biotechnol 167: 201–214 [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159 [DOI] [PubMed] [Google Scholar]
- Coelho AM, Jacob L, Fioramonti J, Bueno L (2001) Rectal antinociceptive properties of alverine citrate are linked to antagonism at the 5-HT1A receptor subtype. J Pharm Pharmacol 53: 1419–1426 [DOI] [PubMed] [Google Scholar]
- Coleman DT, Gray AL, Stephens CA, Scott ML, Cardelli JA (2016) Repurposed drug screen identifies cardiac glycosides as inhibitors of TGF-β-induced cancer-associated fibroblast differentiation. Oncotarget 7: 32200–32209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corteggiani Carpinelli E, Telatin A, Vitulo N, Forcato C, D’Angelo M, Schiavon R, Vezzi A, Giacometti GM, Morosinotto T, Valle G (2014) Chromosome scale genome assembly and transcriptome profiling of Nannochloropsis gaditana in nitrogen depletion. Mol Plant 7: 323–335 [DOI] [PubMed] [Google Scholar]
- Cvejić JH, Rohmer M (2000) CO2 as main carbon source for isoprenoid biosynthesis via the mevalonate-independent methylerythritol 4-phosphate route in the marine diatoms Phaeodactylum tricornutum and Nitzschia ovalis. Phytochemistry 53: 21–28 [DOI] [PubMed] [Google Scholar]
- Daboussi F, Leduc S, Maréchal A, Dubois G, Guyot V, Perez-Michaut C, Amato A, Falciatore A, Juillerat A, Beurdeley M, et al. (2014) Genome engineering empowers the diatom Phaeodactylum tricornutum for biotechnology. Nat Commun 5: 3831. [DOI] [PubMed] [Google Scholar]
- De Riso V, Raniello R, Maumus F, Rogato A, Bowler C, Falciatore A (2009) Gene silencing in the marine diatom Phaeodactylum tricornutum. Nucleic Acids Res 37: e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps P, Moreira D (2012) Reevaluating the green contribution to diatom genomes. Genome Biol Evol 4: 683–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Ippolito G, Sardo A, Paris D, Vella FM, Adelfi MG, Botte P, Gallo C, Fontana A (2015) Potential of lipid metabolism in marine diatoms for biofuel production. Biotechnol Biofuels 8: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolch LJ, Lupette J, Tourcier G, Bedhomme M, Collin S, Magneschi L, Conte M, Seddiki K, Richard C, Corre E, et al. (2017a) Nitric oxide mediates nitrite-sensing and adaptation and triggers a remodeling of lipids. Plant Physiol 175: 1407–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolch LJ, Maréchal E (2015) Inventory of fatty acid desaturases in the pennate diatom Phaeodactylum tricornutum. Mar Drugs 13: 1317–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolch LJ, Rak C, Perin G, Tourcier G, Broughton R, Leterrier M, Morosinotto T, Tellier F, Faure JD, Falconet D, et al. (2017b) A palmitic acid elongase affects eicosapentaenoic acid and plastidial monogalactosyldiacylglycerol levels in Nannochloropsis. Plant Physiol 173: 742–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabris M, Matthijs M, Carbonelle S, Moses T, Pollier J, Dasseville R, Baart GJ, Vyverman W, Goossens A (2014) Tracking the sterol biosynthesis pathway of the diatom Phaeodactylum tricornutum. New Phytol 204: 521–535 [DOI] [PubMed] [Google Scholar]
- Falciatore A, Casotti R, Leblanc C, Abrescia C, Bowler C (1999) Transformation of nonselectable reporter genes in marine diatoms. Mar Biotechnol (NY) 1: 239–251 [DOI] [PubMed] [Google Scholar]
- Falciatore A, d’Alcalà MR, Croot P, Bowler C (2000) Perception of environmental signals by a marine diatom. Science 288: 2363–2366 [DOI] [PubMed] [Google Scholar]
- Flori S, Jouneau PH, Finazzi G, Maréchal E, Falconet D (2016) Ultrastructure of the periplastidial compartment of the diatom Phaeodactylum tricornutum. Protist 167: 254–267 [DOI] [PubMed] [Google Scholar]
- Francis SH, Busch JL, Corbin JD, Sibley D (2010) cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 62: 525–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz AK, Danielewicz MA, Wong DM, Anderson LA, Boothe JR (2013) Phenotypic screening with oleaginous microalgae reveals modulators of lipid productivity. ACS Chem Biol 8: 1053–1062 [DOI] [PubMed] [Google Scholar]
- Fukaya N, Mochizuki K, Tanaka Y, Kumazawa T, Jiuxin Z, Fuchigami M, Goda T (2009) The alpha-glucosidase inhibitor miglitol delays the development of diabetes and dysfunctional insulin secretion in pancreatic beta-cells in OLETF rats. Eur J Pharmacol 624: 51–57 [DOI] [PubMed] [Google Scholar]
- Furlanut M, Franceschi L (2003) Pharmacology of ifosfamide. Oncology (Suppl 2) 65: 2–6 [DOI] [PubMed] [Google Scholar]
- Gallo C, d’Ippolito G, Nuzzo G, Sardo A, Fontana A (2017) Autoinhibitory sterol sulfates mediate programmed cell death in a bloom-forming marine diatom. Nat Commun 8: 1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, et al. (2002) Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419: 498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulos ND, Wells G, Campanella M (2017) The pharmacological regulation of cellular mitophagy. Nat Chem Biol 13: 136–146 [DOI] [PubMed] [Google Scholar]
- Gould SB, Waller RF, McFadden GI (2008) Plastid evolution. Annu Rev Plant Biol 59: 491–517 [DOI] [PubMed] [Google Scholar]
- Haenisch B, Walstab J, Herberhold S, Bootz F, Tschaikin M, Ramseger R, Bönisch H (2010) Alpha-adrenoceptor agonistic activity of oxymetazoline and xylometazoline. Fundam Clin Pharmacol 24: 729–739 [DOI] [PubMed] [Google Scholar]
- Haggarty SJ, Mayer TU, Miyamoto DT, Fathi R, King RW, Mitchison TJ, Schreiber SL (2000) Dissecting cellular processes using small molecules: identification of colchicine-like, taxol-like and other small molecules that perturb mitosis. Chem Biol 7: 275–286 [DOI] [PubMed] [Google Scholar]
- Haraguchi K, Ito K, Kotaki H, Sawada Y, Iga T (1997) Prediction of drug-induced catalepsy based on dopamine D1, D2, and muscarinic acetylcholine receptor occupancies. Drug Metab Dispos 25: 675–684 [PubMed] [Google Scholar]
- Huang XP, Williams FE, Peseckis SM, Messer WS Jr (1998) Pharmacological characterization of human m1 muscarinic acetylcholine receptors with double mutations at the junction of TM VI and the third extracellular domain. J Pharmacol Exp Ther 286: 1129–1139 [PubMed] [Google Scholar]
- Imming P, Sinning C, Meyer A (2006) Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov 5: 821–834 [DOI] [PubMed] [Google Scholar]
- Inhoffen HH, Hohlweg W (1938) Neue per os-wirksame weibliche Keimdrüsenhormon-Derivate: 17-Aethinyl-oestradiol und Pregnen-in-on-3-ol-17. Naturwissenschaften 26: 96 [Google Scholar]
- Jensen EV, DeSombre ER (1973) Estrogen-receptor interaction. Science 182: 126–134 [DOI] [PubMed] [Google Scholar]
- Jeong DE, Song HJ, Lim S, Lee SJ, Lim JE, Nam DH, Joo KM, Jeong BC, Jeon SS, Choi HY, et al. (2015) Repurposing the anti-malarial drug artesunate as a novel therapeutic agent for metastatic renal cell carcinoma due to its attenuation of tumor growth, metastasis, and angiogenesis. Oncotarget 6: 33046–33064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson X, Vandystadt G, Bujaldon S, Wollman FA, Dubois R, Roussel P, Alric J, Béal D (2009) A new setup for in vivo fluorescence imaging of photosynthetic activity. Photosynth Res 102: 85–93 [DOI] [PubMed] [Google Scholar]
- Keeling PJ. (2009) Chromalveolates and the evolution of plastids by secondary endosymbiosis. J Eukaryot Microbiol 56: 1–8 [DOI] [PubMed] [Google Scholar]
- Kilian O, Benemann CS, Niyogi KK, Vick B (2011) High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc Natl Acad Sci USA 108: 21265–21269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12: 357–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Brown CM, Kim MK, Burrows EH, Bach S, Lun DS, Falkowski PG (2017) Effect of cell cycle arrest on intermediate metabolism in the marine diatom Phaeodactylum tricornutum. Proc Natl Acad Sci USA 114: E8007–E8016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Marcuschamer D, Chisti Y, Benemann JR, Lewis D (2013) A matter of detail: assessing the true potential of microalgal biofuels. Biotechnol Bioeng 110: 2317–2322 [DOI] [PubMed] [Google Scholar]
- Kutschera U, Niklas KJ (2005) Endosymbiosis, cell evolution, and speciation. Theory Biosci 124: 1–24 [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M, Holm R, Jensen KG, Sveigaard C, Brodin B, Nielsen CU (2010) 5-Hydroxy-L-tryptophan alters gaboxadol pharmacokinetics in rats: involvement of PAT1 and rOat1 in gaboxadol absorption and elimination. Eur J Pharm Sci 39: 68–75 [DOI] [PubMed] [Google Scholar]
- Levitan O, Dinamarca J, Hochman G, Falkowski PG (2014) Diatoms: a fossil fuel of the future. Trends Biotechnol 32: 117–124 [DOI] [PubMed] [Google Scholar]
- Levitan O, Dinamarca J, Zelzion E, Lun DS, Guerra LT, Kim MK, Kim J, Van Mooy BA, Bhattacharya D, Falkowski PG (2015) Remodeling of intermediate metabolism in the diatom Phaeodactylum tricornutum under nitrogen stress. Proc Natl Acad Sci USA 112: 412–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Dewey CN (2011) RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 12: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JC, Mi L, Pontrelli S, Luo S (2016) Fuelling the future: microbial engineering for the production of sustainable biofuels. Nat Rev Microbiol 14: 288–304 [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46: 3–26 [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang R, Zhao JJ, Rogers JD, Hsieh JY, Fang W, Matuszewski BK, Dobrinska MR (2003) Determination of simvastatin-derived HMG-CoA reductase inhibitors in biomatrices using an automated enzyme inhibition assay with radioactivity detection. J Pharm Biomed Anal 32: 107–123 [DOI] [PubMed] [Google Scholar]
- Liu T, Zhang S, Chen J, Jiang K, Zhang Q, Guo K, Liu Y (2014) The transcriptional profiling of glycogenes associated with hepatocellular carcinoma metastasis. PLoS ONE 9: e107941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Guan Y, Gao Q, Tam NF, Zhu W (2010) Cellular responses, biodegradation and bioaccumulation of endocrine disrupting chemicals in marine diatom Navicula incerta. Chemosphere 80: 592–599 [DOI] [PubMed] [Google Scholar]
- Liu Y, Tam NFY, Guan YT, Gao BY (2012) Influence of a marine diatom on the embryonic toxicity of 17 alpha-ethynylestradiol to the abalone Haliotis diversicolor supertexta. Water Air Soil Pollut 223: 4383–4395 [Google Scholar]
- Lupette J, Maréchal E (2018) Phytoplankton glycerolipids, challenging but promising prospects from biomedicine to green chemistry and biofuels. In La Barre S, Bates SS, eds, Blue Technologies: Production and Use of Marine Molecules. Wiley VCH, Weinheim, Germany, pp. 191–215 [Google Scholar]
- Maeda Y, Nojima D, Yoshino T, Tanaka T (2017) Structure and properties of oil bodies in diatoms. Philos Trans R Soc Lond B Biol Sci 372: 20160408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier UG, Douglas SE, Cavalier-Smith T (2000) The nucleo morph genomes of cryptophytes and chlorarachniophytes. Protist 151: 103–109 [DOI] [PubMed] [Google Scholar]
- Mainardi JL, Villet R, Bugg TD, Mayer C, Arthur M (2008) Evolution of peptidoglycan biosynthesis under the selective pressure of antibiotics in Gram-positive bacteria. FEMS Microbiol Rev 32: 386–408 [DOI] [PubMed] [Google Scholar]
- Maréchal E. (2008) Chemogenomics: a discipline at the crossroad of high throughput technologies, biomarker research, combinatorial chemistry, genomics, cheminformatics, bioinformatics and artificial intelligence. Comb Chem High Throughput Screen 11: 582. [DOI] [PubMed] [Google Scholar]
- Marechal E. (2009) In silico strategies for target discovery. Infect Genet Evol 9: 370–371 [DOI] [PubMed] [Google Scholar]
- Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ (1999) Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286: 971–974 [DOI] [PubMed] [Google Scholar]
- McFadden GI. (1999) Endosymbiosis and evolution of the plant cell. Curr Opin Plant Biol 2: 513–519 [DOI] [PubMed] [Google Scholar]
- Meï CE, Cussac M, Haslam RP, Beaudoin F, Wong YS, Marechal E, Rébeillé F (2017) C1 metabolism inhibition and nitrogen deprivation trigger triacylglycerol accumulation in Arabidopsis thaliana cell cultures and highlight a role of NPC in phosphatidylcholine-to-triacylglycerol pathway. Front Plant Sci 7: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz-Laylin LK, Maréchal-Drouard L, et al. (2007) The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318: 245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra AN, Misra M, Singh R (2012) Chlorophyll fluorescence in plant biology. In Misra AN, ed, Biophysics. InTech, pp 171–192 [Google Scholar]
- Nataraj C, Thomas DW, Tilley SL, Nguyen MT, Mannon R, Koller BH, Coffman TM (2001) Receptors for prostaglandin E(2) that regulate cellular immune responses in the mouse. J Clin Invest 108: 1229–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nymark M, Sharma AK, Sparstad T, Bones AM, Winge P (2016) A CRISPR/Cas9 system adapted for gene editing in marine algae. Sci Rep 6: 24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL (2006) How many drug targets are there? Nat Rev Drug Discov 5: 993–996 [DOI] [PubMed] [Google Scholar]
- Pacher P, Nivorozhkin A, Szabó C (2006) Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 58: 87–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panara MR, Renda G, Sciulli MG, Santini G, Di Giamberardino M, Rotondo MT, Tacconelli S, Seta F, Patrono C, Patrignani P (1999) Dose-dependent inhibition of platelet cyclooxygenase-1 and monocyte cyclooxygenase-2 by meloxicam in healthy subjects. J Pharmacol Exp Ther 290: 276–280 [PubMed] [Google Scholar]
- Pawłowski L, Siwanowicz J, Bigajska K, Przegaliński E (1985) Central antiserotonergic and antidopaminergic action of pirenperone, a putative 5-HT2 receptor antagonist. Pol J Pharmacol Pharm 37: 179–196 [PubMed] [Google Scholar]
- Petroutsos D, Amiar S, Abida H, Dolch LJ, Bastien O, Rébeillé F, Jouhet J, Falconet D, Block MA, McFadden GI, et al. (2014) Evolution of galactoglycerolipid biosynthetic pathways: from cyanobacteria to primary plastids and from primary to secondary plastids. Prog Lipid Res 54: 68–85 [DOI] [PubMed] [Google Scholar]
- Pocock T, Falk S (2014) Negative impact on growth and photosynthesis in the green alga Chlamydomonas reinhardtii in the presence of the estrogen 17α-ethynylestradiol. PLoS ONE 9: e109289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissner S, Kroll K, Dunkel M, Senger C, Goldsobel G, Kuzman D, Guenther S, Winnenburg R, Schroeder M, Preissner R (2010) SuperCYP: a comprehensive database on cytochrome P450 enzymes including a tool for analysis of CYP-drug interactions. Nucleic Acids Res 38: D237–D243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddy AR, Killick SR, Elstein M, Morris J, Sullivan M, Patel L, Elder M (1990) The effect of prostaglandin synthetase inhibitors on human preovulatory follicular fluid prostaglandin, thromboxane, and leukotriene concentrations. J Clin Endocrinol Metab 71: 235–242 [DOI] [PubMed] [Google Scholar]
- Prioretti L, Avilan L, Carriere F, Montane MH, Field B, Gregori G, Menand B, Gontero B (2017) The inhibition of TOR in the model diatom Phaeodactylum tricornutum promotes a get-fat growth regime. Algal Res 26: 265–274 [Google Scholar]
- Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65: 635–648 [DOI] [PubMed] [Google Scholar]
- Pytliak M, Vargová V, Mechírová V, Felšöci M (2011) Serotonin receptors: from molecular biology to clinical applications. Physiol Res 60: 15–25 [DOI] [PubMed] [Google Scholar]
- Qian H, Martin RJ, Robertson AP (2006) Pharmacology of N-, L-, and B-subtypes of nematode nAChR resolved at the single-channel level in Ascaris suum. FASEB J 20: 2606–2608 [DOI] [PubMed] [Google Scholar]
- Riva E, Mennini T, Latini R (1991) The alpha- and beta-adrenoceptor blocking activities of labetalol and its RR-SR (50:50) stereoisomers. Br J Pharmacol 104: 823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J, Olivieri G, de Vree J, Bosma R, Willems P, Reith JH, Eppink MHM, Kleinegris DMM, Wijffels RH, Barbosa MJ (2016) Towards industrial products from microalgae. Energy Environ Sci 9: 3036–3043 [Google Scholar]
- Sayanova O, Mimouni V, Ulmann L, Morant-Manceau A, Pasquet V, Schoefs B, Napier JA (2017) Modulation of lipid biosynthesis by stress in diatoms. Philos Trans R Soc Lond B Biol Sci 372: 20160407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayanova OV, Napier JA (2004) Eicosapentaenoic acid: biosynthetic routes and the potential for synthesis in transgenic plants. Phytochemistry 65: 147–158 [DOI] [PubMed] [Google Scholar]
- Scaife MA, Smith AG (2016) Towards developing algal synthetic biology. Biochem Soc Trans 44: 716–722 [DOI] [PubMed] [Google Scholar]
- Schudt C, Winder S, Eltze M, Kilian U, Beume R (1991) Zardaverine: a cyclic AMP specific PDE III/IV inhibitor. Agents Actions Suppl 34: 379–402 [PubMed] [Google Scholar]
- Shikanai T. (2014) Central role of cyclic electron transport around photosystem I in the regulation of photosynthesis. Curr Opin Biotechnol 26: 25–30 [DOI] [PubMed] [Google Scholar]
- Shteinikov VY, Korosteleva AS, Tikhonova TB, Potapieva NN, Tikhonov DB (2017) Ligands of histamine receptors modulate acid-sensing ion channels. Biochem Biophys Res Commun 490: 1314–1318 [DOI] [PubMed] [Google Scholar]
- Siaut M, Heijde M, Mangogna M, Montsant A, Coesel S, Allen A, Manfredonia A, Falciatore A, Bowler C (2007) Molecular toolbox for studying diatom biology in Phaeodactylum tricornutum. Gene 406: 23–35 [DOI] [PubMed] [Google Scholar]
- Siegenthaler PF, Bain P, Riva F, Fent K (2017) Effects of antiandrogenic progestins, chlormadinone and cyproterone acetate, and the estrogen 17α-ethinylestradiol (EE2), and their mixtures: transactivation with human and rainbowfish hormone receptors and transcriptional effects in zebrafish (Danio rerio) eleuthero-embryos. Aquat Toxicol 182: 142–162 [DOI] [PubMed] [Google Scholar]
- Soleilhac E, Nadon R, Lafanechere L (2010) High-content screening for the discovery of pharmacological compounds: advantages, challenges and potential benefits of recent technological developments. Expert Opin Drug Discov 5: 135–144 [DOI] [PubMed] [Google Scholar]
- Somuncu S, Cakmak M, Erdogan S, Caglayan O, Akman H, Kaya M (2005) Trapidil, an inhibitor for phosphodiesterase and platelet-derived-growth factor, ameliorates corrosive esophageal burn in rats. Tohoku J Exp Med 207: 203–208 [DOI] [PubMed] [Google Scholar]
- Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101: 87–96 [DOI] [PubMed] [Google Scholar]
- Stoebe B, Maier UG (2002) One, two, three: nature’s tool box for building plastids. Protoplasma 219: 123–130 [DOI] [PubMed] [Google Scholar]
- Tornio A, Pasanen MK, Laitila J, Neuvonen PJ, Backman JT (2005) Comparison of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) as inhibitors of cytochrome P450 2C8. Basic Clin Pharmacol Toxicol 97: 104–108 [DOI] [PubMed] [Google Scholar]
- Totir MA, Helfand MS, Carey MP, Sheri A, Buynak JD, Bonomo RA, Carey PR (2007) Sulbactam forms only minimal amounts of irreversible acrylate-enzyme with SHV-1 beta-lactamase. Biochemistry 46: 8980–8987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricarico D, Mele A, Conte Camerino D (2006) Carbonic anhydrase inhibitors ameliorate the symptoms of hypokalaemic periodic paralysis in rats by opening the muscular Ca2+-activated-K+ channels. Neuromuscul Disord 16: 39–45 [DOI] [PubMed] [Google Scholar]
- van den Meiracker AH, Man in’t Veld AJ, Fischberg DJ, Molinoff PB, van Eck HJ, Boomsma F, Derkx FH, Schalekamp MA (1988) Acute and long-term effects of acebutolol on systemic and renal hemodynamics, body fluid volumes, catecholamines, active renin, aldosterone, and lymphocyte beta-adrenoceptor density. J Cardiovasc Pharmacol 11: 413–423 [DOI] [PubMed] [Google Scholar]
- Varet H, Brillet-Guéguen L, Coppée JY, Dillies MA (2016) SARTools: a DESeq2- and EdgeR-based R pipeline for comprehensive differential analysis of RNA-Seq data. PLoS ONE 11: e0157022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieler A, Wu G, Tsai CH, Bullard B, Cornish AJ, Harvey C, Reca IB, Thornburg C, Achawanantakun R, Buehl CJ, et al. (2012) Genome, functional gene annotation, and nuclear transformation of the heterokont oleaginous alga Nannochloropsis oceanica CCMP1779. PLoS Genet 8: e1003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsky RL, Gaman EA, Obach RS (2005) Examination of 209 drugs for inhibition of cytochrome P450 2C8. J Clin Pharmacol 45: 68–78 [DOI] [PubMed] [Google Scholar]
- Wang JK, Seibert M (2017) Prospects for commercial production of diatoms. Biotechnol Biofuels 10: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrilow AG, Martel CM, Parker JE, Melo N, Lamb DC, Nes WD, Kelly DE, Kelly SL (2010) Azole binding properties of Candida albicans sterol 14-alpha demethylase (CaCYP51). Antimicrob Agents Chemother 54: 4235–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wase N, Tu B, Allen JW, Black PN, DiRusso CC (2017) Identification and metabolite profiling of chemical activators of lipid accumulation in green algae. Plant Physiol 174: 2146–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wase N, Tu B, Black PN, DiRusso CC (2015) Phenotypic screening identifies brefeldin A/ascotoxin as an inducer of lipid storage in the algae Chlamydomonas reinhardtii. Algal Res 11: 74–84 [Google Scholar]
- Young MD, Wakefield MJ, Smyth GK, Oshlack A (2010) Gene Ontology analysis for RNA-seq: accounting for selection bias. Genome Biol 11: R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Chung TD, Oldenburg KR (1999) A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4: 67–73 [DOI] [PubMed] [Google Scholar]
- Zhu BH, Shi HP, Yang GP, Lv NN, Yang M, Pan KH (2016) Silencing UDP-glucose pyrophosphorylase gene in Phaeodactylum tricornutum affects carbon allocation. New Biotechnol 33: 237–244 [DOI] [PubMed] [Google Scholar]