Abstract
Objective
Statin intolerance, whether real or perceived, is a growing issue in clinical practice. Our aim was to evaluate the effects of reduced-dose statin therapy complemented with nutraceuticals.
Methods
First phase: Initially, 53 type 2 diabetic statin-treated patients received a supplementation with fish oil (1.7 g EPA + DHA/day), chocolate containing plant sterols (2.2 g/day), and green tea (two sachets/day) for 6 weeks. Second phase: “Good responders” to supplementation were identified after multivariate analysis (n = 10), and recruited for a pilot protocol of statin dose reduction. “Good responders” were then provided with supplementation for 12 weeks: standard statin therapy was kept during the first 6 weeks and reduced by 50% from weeks 6–12.
Results
First phase: After 6 weeks of supplementation, plasma LDL-C (−13.7% ± 3.7, P = .002) and C-reactive protein (−35.5% ± 5.9, P = .03) were reduced. Analysis of lathosterol and campesterol in plasma suggested that intensity of LDL-C reduction was influenced by cholesterol absorption rate rather than its synthesis. Second phase: no difference was observed for plasma lipids, inflammation, cholesterol efflux capacity, or HDL particles after statin dose reduction when compared to standard therapy.
Conclusions
Although limited by the small sample size, our study demonstrates the potential for a new therapeutic approach combining lower statin dose and specific dietary compounds. Further studies should elucidate “good responders” profile as a tool for personalized medicine. This may be particularly helpful in the many patients with or at risk for CVD who cannot tolerate high dose statin therapy.
Trial registration
ClinicalTrials.gov, NCT02732223.
Keywords: Atherosclerosis, Omega-3 fatty acids, Plant sterols, Polyphenols, Responders
Abbreviations: ARA, arachidonic acid; CAT, catalase; CEC, cholesterol efflux capacity; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EGCG, epigalocatechin gallate; EPA, eicosapentaenoic acid; GPx, glutathione peroxidase; GR, glutathione reductase; MDA, malondialdehyde; n-3 FA, omega-3 fatty acids; PPARα, peroxisome proliferator-activated receptor alpha; SOD, superoxide dismutase; SREBP-1c, sterol regulatory element-binding protein-1c; TG, triglycerides
Highlights
-
•
N-3 fatty acids, plant sterols and polyphenols may reduce atherosclerosis burden.
-
•
These nutraceuticals combined to statin therapy significantly reduced LDL-C and CRP.
-
•
Patients with higher sterol absorption rate at baseline had higher LDL-C reduction.
-
•
“Responders” to nutraceuticals had their statin dose effectively reduced by half.
-
•
Identifying “responders” is essential for the personalized treatment of dyslipidemia.
1. Introduction
Cardiovascular disease (CVD), particularly atherosclerosis, is the major cause of morbidity and mortality throughout the world. The main target for CVD prevention is LDL-cholesterol (LDL-C) lowering, usually attained by statins [1]. Although statins are considered a first-line lipid-lowering therapy and have contributed to clinical event reduction, residual cardiovascular risk remains high among statin-treated patients [2]. The most recent study with PCSK9 antibodies (FOURIER trial) supported the LDL-C hypothesis, which is “the lower the better”, showing that this new class of drugs can further decrease the rate of clinical events [3]. However, results have also shown that despite extreme LDL-C reduction, absolute outcomes (MI) were still high after PCSK9 inhibition atop statin therapy [3]. Furthermore, atherosclerotic plaque regression with PCSK9 antibody was previously shown to be only 1% [4], highlighting the need to address risk factors beyond lipids, such as inflammation and oxidative stress. This approach is especially important under diabetic conditions, in which plaque regression is impaired in pre-clinical models even when LDL-C is normalized [5].
Novel add-on therapies to maximally tolerated statin have also focused on the atheroprotective properties of HDL, especially its function on reverse cholesterol transport. However, the effectiveness of HDL-targeted agents is still controversial [6]. Although combination therapies have proven necessary [7], and despite increasing efforts in drug development, a critical issue that undermines CVD prevention is the high prevalence of statin undertreatment due to real or perceived adverse effects [8]. Statin intolerance leads to discontinuation or suboptimal statin use; afflicted patients have 50% higher risk of coronary events than those with good statin adherence [9]. Convincing these patients, who claim muscle pain or weakness, to continue the statin therapy is a major challenge in clinical practice.
The combination of nutraceuticals with statin treatment could help overcome these limitations [10], [11]. Bioactive compounds such as omega-3 fatty acids (n-3 FA), plant sterols, and polyphenols are naturally occurring molecules with great potential to reduce atherosclerosis progression by reducing inflammation, LDL-C, and oxidative stress, respectively [12], [13], [14], [15]. Although the biological effects of these single compounds have been extensively evaluated over the last decade [14], [16], [17], studies regarding bioactive compound combinations are still scarce, and the combined action of such agents in atherosclerosis is poorly understood [18]. More interestingly, the potential of reducing statin dose in conjunction with complementary diet therapy has never been investigated and could contribute to reduce adverse effects associated to high statin dosages and increase treatment adherence.
In the present study, we first evaluated the effects of daily consumption of a combined supplementation of n-3 FA (fish oil supplement), plant sterols (enriched chocolate) and polyphenols (green tea) on biomarkers of inflammation, lipidemia and oxidative stress in type 2 diabetic patients treated with statins. We then conducted a pilot study to evaluate – in terms of plasma lipid profile, inflammatory, and oxidative markers – the effects of statin dose reduction by the combined supplementation compared to standard statin therapy (full dose), in a sub-group of patients who better responded to the initial intervention.
2. Methods
2.1. Subjects
Participants with established dyslipidemia and diabetes were recruited from Dante Pazzanese Institute of Cardiology (São Paulo, Brazil). The primary selection criteria were current statin (simvastatin or atorvastatin) and hypoglycemic treatment (metformin and/or gliclazide). Exclusion criteria were patients who were taking n-3 FA, plant sterol, or green tea supplements; patients with poorly controlled diabetes (HbA1c > 7.5%) or dyslipidemia (LDL-C > 100 mg/dL); patients with atherosclerotic cardiovascular disease (ASCVD); pregnant females; patients who presented any congenital cardiac disorders or uncontrolled endocrine, renal; or hepatic disease; patients with excessive alcohol consumption. Based on these criteria, 53 subjects (male n = 19 and female n = 34) were enrolled and completed the first phase of the trial. Patient characteristics are shown in Table 1. The study was approved by the Institutional Review Board of the Dante Pazzanese Institute of Cardiology (CAAE 27349114.5.3001.0067) and all patients provided informed written consent prior to inclusion. ClinicalTrials.gov ID was NCT02732223.
Table 1.
Subjects characteristics.
| n | Mean ± SEM | |
|---|---|---|
| Gender (M/F), n | 19/34 | – |
| Ethnicity (White; Black; Asian) | 42; 10; 1 | – |
| Age (y) | – | 63.8 ± 0.9 |
| Systolic blood pressure (mm Hg) | – | 130.7 ± 2.9 |
| Diastolic blood pressure (mm Hg) | – | 80.2 ± 1.7 |
| Heart rate (bpm) | – | 69.9 ± 1.7 |
| Medical follow up (y) | – | 10.4 ± 0.9 |
| Drugs | ||
| Atorvastatin (20; 40; 80 mg) | 5; 12; 6 | – |
| Sinvastatin (10; 20; 40 mg) | 1; 16; 13 | – |
| Metformin 500–2550 mg (1–3 daily) | 46a | – |
| Gliclazide 30–120 (1–3 daily) | 12a | – |
| Aspirin (100 mg) | 39 | – |
| Diuretic | 42 | – |
| Angiotensin converting enzyme (ACE) inhibitor | 14 | – |
| Angiotensin II receptor antagonist | 26 | – |
| Beta-blocker | 18 | – |
| Calcium channel blocker | 15 | – |
| Alpha2 adrenergic agonist | 2 | – |
| Vasodilator | 2 | – |
| Antiocoagulant | 2 | – |
| Antiarrhythmic agent | 2 | – |
| Thyroid hormone thyroxine | 7 | – |
| Hyporuricemic agent | 6 | – |
| Proton pump inhibitor | 13 | – |
5 subjects were on treatment with both metformin and gliclazide.
2.2. Study design
First phase: a crossover intervention was carried out. The protocol was single-blinded once the quantification of ingestion markers in plasma samples was indicative of subject's treatment. Initially, subjects were randomly assigned to receive nutraceuticals or a control treatment for 6 weeks. Daily treatment with nutraceuticals (NTR) consisted of seven fish oil softgels (1.7 g of EPA + DHA), two dark chocolate truffles containing plant sterol esters (2.2 g/day), and two green tea sachets (∼170.8 mg epigalocatechin gallate (EGCG)/day). Control treatment (CON) consisted of seven soy bean oil softgels, two regular dark chocolate truffles, and two anise tea sachets. As green tea and anise tea taste different, participants were informed in the beginning of the protocol that the tea taste would change along the study. The chocolate containing plant sterols was developed with collaboration of Chocolife Indústria e Comércio de Alimentos Funcionais Ltda, São Paulo [19]. The plant sterols PinVitaTMES (70% β-sitosterol) from DuPont™ Danisco® Food Ingredients were purchased from MasterSense Ing. Alim. Ltda. (São Paulo, Brazil). The fish oil and placebo supplements (1 g softgels) were donated by Bionatus Laboratório Botânico Ltda. (São José do Rio Preto, São Paulo). The green tea and anise tea sachets (1.6 and 2.0 g each) were donated by Leão Alimentos e Bebidas (São Paulo, Brazil). Detailed description of chemical composition and oxidative status of food components are available in Appendix S1 (Supporting Information). Participants were instructed to consume 1 softgel after breakfast, 3 softgels, and 1 chocolate truffle twice a day (after main meals) and to drink two cups of tea per day. After a 6-week washout period, the groups were changed following the cross-over design for 6 weeks more. During each step, the subjects maintained their habitual routine and diet. Prescribed drugs were kept without any change throughout the study.
Second phase: Multivariate statistical tools were applied to identify subjects who overall better responded to the combined supplementation provided in the first phase of the trial. Cluster analysis was performed including C-reactive protein, malondialdehyde and LDL-C concentration as active variables. Via this analysis, two sub-groups were identified with the aim of classifying patients that showed greater and lesser degrees of responses to the nutraceuticals intervention. Subjects that made up the so-called responder (“R”; n = 10) and non-responder (“NR”; n = 10) sub-groups were invited to participate in the second phase of the study. A pilot protocol of statin dose reduction by complementary diet therapy was carried out in the “R” sub-group only. Initially, “R” and “NR” subjects were recruited for a first visit, when baseline blood samples were collected and nutraceuticals (NTR) were provided only to responders. The “R” sub-group completed 6 weeks of NTR during which they maintained their prescribed dose of statin. Responders then reduced their statin dose 50%, and completed 6 more weeks of NTR.
2.3. Anthropometric data and biochemical measures
Anthropometrical measures (weight, waist circumference, abdominal circumference, and hip circumference) were recorded at each visit. All blood samples were collected at baseline and after each intervention. Blood was drawn in vacutainer tubes (EDTA for plasma and SST for serum) after at least a 12 h fast. Plasma and serum were separated by centrifugation at 1000 ×g for 5 min at 4 °C (Hitachi, CF-15R, Tokyo, Japan). Total cholesterol, HDL-C, LDL-C, triglycerides, glucose, creatine kinase, urea, alanine transaminase (ALT), aspartate transaminase (AST), glycated hemoglobin (Hb A1c), and high sensitivity C-Reactive Protein (hs-CRP) were performed utilizing commercial kits (Labtest Diagnostica SA, Lagoa Santa, Brazil) with automated system Labmax 240 (Labtest Diagnostica SA, Lagoa Santa, Brazil). Determination of plasma fatty acids (EPA, n-3; DHA, n-3; ARA, n-6), main plant sterols (campesterol and β-sitosterol), and cholesterol precursor (lathosterol) were performed using gas chromatography (GC–MS) [20], [21]. Plasma malondialdehyde (MDA) concentration was assessed using reverse phase HPLC [22]. Cholesterol efflux capacity (CEC) of apolipoprotein-B depleted plasma samples was determined in vitro as total, ABCA1-specific and non-ABCA1-specific efflux of radiolabeled cholesterol from macrophages to the plasma, as described [23]. HDL particle size was determined by ion mobility analysis (IMA) [24]. A detailed description of methodologies is available in Appendix S1 (Supporting Information).
2.4. Statistical analysis
Values are expressed as mean ± standard error of mean (SEM). Variance homogeneity and normality were evaluated for all variables. First phase: Percent changes from baseline after each treatment (CTR and NTR) were compared with independent samples T-tests. Data were also compared by dividing the patients into subgroups: patients who presented baseline values above the median and patients who presented baseline values below the median for each biomarker. In these comparisons, the percent of change observed in each subgroup was compared between the two treatments with independent samples T-tests. Second phase: A Principal Component Analysis was applied to separate the patients according to their responses in terms of plasma lipid profile, inflammatory and oxidative stress biomarkers. The first and second components explained 75.38% of the variation. On the basis of the projection of the cases on the factor-plane, 20 patients were randomly recruited from opposite directions and classified as “Responders” and “Non-Responders” to the nutraceuticals. Differences between these two groups were compared by Mann–Whitney U tests. Data obtained only for “Responders” submitted to both treatments (standard statin therapy vs half dose statin) were compared with Wilcoxon-tests. In this last comparison, error type II is also important, since the objective of the study was to confirm the null hypothesis. For this reason, β risk was evaluated estimating a change of 6% for all variables as clinically relevant. The β risk was calculated based on normal distribution and Lehmann method using the software G*Power (Allgemeine Psychologie und Arbeitspsychologie, Dusseldorf, Germany). Spearman correlations were used to assess associations between biomarkers separately within the responder and non-responder groups. Significance was set at P < 0.05. Analyses were performed with the software STATISTICA version 9.0 (StatSoft, Inc., Tulsa, OK, USA).
3. Results
3.1. Nutraceuticals effect on atherosclerosis risk biomarkers
Plasma changes of adherence markers are shown in Figure S1. Mean omega-3/omega-6 ratio (EPA + DHA/ARA) and β-sitosterol/cholesterol ratio increased after treatment with nutraceuticals, indicative of subject compliance. As expected, increases in EPA + DHA concentration after NTR were higher for individuals who presented with lower baseline values (Figure S2).
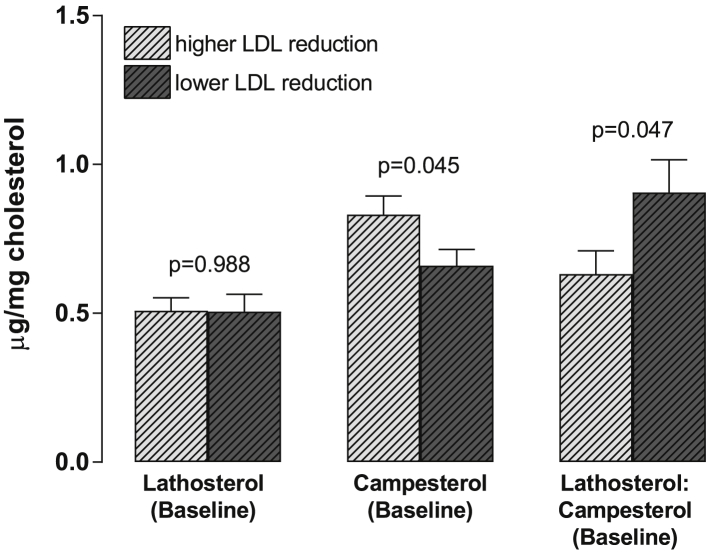
The changes in anthropometric and biochemical parameters of individuals according to the type of intervention are shown in Table 2. Baseline values of all parameters were equal for both treatments, suggesting that the 6-week washout period in our crossover design was sufficient to return the biomarkers to their respective original values. The treatment with nutraceuticals was effective in reducing total cholesterol (−10.1%), LDL-C (−13.7%), non-HDL-C (−12.0%) and hs-CRP (−35.5%). No changes were observed for the other parameters. However, NTR reduced TG (−17%) and MDA (−31%) in a subgroup of patients with baseline values above the median (93 mg/dL and 2.23 umol/L, respectively) (Figure S3). When individuals were grouped according to the intensity of LDL-C reduction after NTR (Figure 1), it was observed that higher LDL-C reduction was independent of baseline lathosterol values (P = 0.988) – a sterol synthesis marker – but was dependent on sterol absorption, represented by campesterol concentration at baseline (P = 0.045), suggesting that individuals characterized as a “sterol absorber” rather than “sterol synthesizer” would respond to plant sterol supplementation with a greater LDL-C reduction.
Table 2.
Anthropometric and biochemical parameters at baseline and after 6 weeks of nutraceuticals or control treatment.
| Control (CTR) |
Nutraceuticals (NTR) |
p1 | p2 | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | After 6 weeks | % Change | Baseline | After 6 weeks | % Change | |||
| Weight (kg) | 85.9 ± 3.7 | 86.4 ± 3.8 | 0.4 ± 0.2 | 85.6 ± 3.7 | 85.7 ± 3.6 | 0.2 ± 0.3 | 0.948 | 0.616 |
| BMI (kg/m2) | 32.4 ± 1.1 | 32.6 ± 1.1 | 0.4 ± 0.2 | 32.2 ± 1.0 | 32.3 ± 1.0 | 0.2 ± 0.3 | 0.936 | 0.616 |
| Waist circumference (cm) | 100.6 ± 2.5 | 100.4 ± 2.6 | −0.3 ± 0.4 | 100.8 ± 2.4 | 100.1 ± 2.3 | −0.5 ± 0.4 | 0.955 | 0.733 |
| Abdominal circumference (cm) | 104.3 ± 2.7 | 104.4 ± 2.6 | 0.1 ± 0.3 | 105.7 ± 2.5 | 104.4 ± 2.4 | −1.0 ± 0.6 | 0.710 | 0.076 |
| Hip circunference (cm) | 111.4 ± 2.3 | 111.2 ± 2.3 | −0.1 ± 0.4 | 111.1 ± 2.3 | 111.6 ± 2.3 | 0.4 ± 0.4 | 0.946 | 0.271 |
| Glucose (mg/dL) | 102.1 ± 4.7 | 108.0 ± 4.7 | 11.4 ± 5.4 | 100.3 ± 3.4 | 104.7 ± 3.7 | 7.26 ± 3.8 | 0.776 | 0.532 |
| HbA1c (%) | 6.5 ± 0.1 | 6.6 ± 0.1 | 1.7 ± 1.2 | 6.5 ± 0.1 | 6.4 ± 0.1 | −0.6 ± 1.2 | 0.910 | 0.181 |
| Total cholesterol (mg/dL) | 170.9 ± 6.5 | 171.8 ± 5.3 | 5.5 ± 4.0 | 171.9 ± 5.9 | 151.5 ± 5.7 | −10.1 ± 2.7 | 0.913 | 0.002 |
| LDL-C (mg/dL) | 86.1 ± 4.6 | 84.9 ± 3.5 | 7.9 ± 5.7 | 90.1 ± 4.8 | 74.1 ± 3.9 | −13.7 ± 3.7 | 0.543 | 0.002 |
| Non-HDL-C (mg/dL) | 113.3 ± 5.3 | 116.4 ± 3.4 | 10.3 ± 4.8 | 115.4 ± 5.0 | 98.3 ± 4.6 | −12.0 ± 3.4 | 0.776 | 0.000 |
| HDL-C (mg/dL) | 54.8 ± 1.9 | 53.0 ± 1.9 | −0.75 ± 3.6 | 53.7 ± 1.6 | 50.7 ± 1.5 | −3.6 ± 2.7 | 0.634 | 0.527 |
| VLDL-C (mg/dL) | 29.7 ± 2.4 | 33.2 ± 1.8 | 24.9 ± 6.0 | 28.3 ± 2.6 | 25.0 ± 1.7 | 5.3 ± 8.1 | 0.704 | 0.054 |
| TG (mg/dL) | 146.1 ± 13.4 | 150.1 ± .12.6 | 10.7 ± 6.2 | 129.8 ± 14.6 | 125.2 ± 7.8 | 16.7 ± 8.5 | 0.413 | 0.564 |
| CPK | 152.4 ± 15.7 | 129.1 ± 13.3 | −10.4 ± 5.9 | 158.0 ± 16.7 | 139.4 ± 14.0 | −0.1 ± 6.8 | 0.807 | 0.254 |
| AST (U/L) | 28.2 ± 1.7 | 27 ± 1.2 | 4.22 ± 3.4 | 30.8 ± 1.4 | 31.0 ± 1.7 | 2.1 ± 3.8 | 0.240 | 0.678 |
| ALT (U/L) | 23.9 ± 2.0 | 24.3 ± 1.6 | 8.5 ± 7.7 | 26.3 ± 1.6 | 28 ± 2.2 | 7.9 ± 5.0 | 0.356 | 0.946 |
| hs-CRP (mg/L) | 2.1 ± 0.3 | 1.4 ± 0.21 | −10.9 ± 9.3 | 2.1 ± 0.3 | 1.2 ± 0.2 | −35.5 ± 5.9 | 0.912 | 0.027 |
| MDA (umol/L) | 2.4 ± 0.2 | 1.7 ± 0.1 | −1.2 ± 11.8 | 2.2 ± 0.1 | 1.8 ± 0.1 | −1.7 ± 11.1 | 0.479 | 0.976 |
| Glutathione peroxidase (U/mL) | 9.5 ± 0.3 | 9.2 ± 0.3 | 3.9 ± 6.1 | 8.5 ± 0.3 | 8.1 ± 0.2 | 1.7 ± 4.18 | 0.666 | 0.299 |
| Glutathione reductase (U/mL) | 0.081 ± 0.003 | 0.084 ± 0.003 | 10.44 ± 5.53 | 0.082 ± 0.004 | 0.083 ± 0.003 | 6.52 ± 4.32 | 0.844 | 0.577 |
| Superoxide dismutase (U/mL) | 9.6 ± 0.2 | 8.2 ± 0.3 | −12.3 ± 3.6 | 9.4 ± 0.2 | 8.2 ± 0.2 | −10.6 ± 3.4 | 0.510 | 0.726 |
| Catalase (U/mL) | 56.9 ± 1.5 | 63.2 ± 2.2 | 14.9 ± 5.1 | 57.0 ± 1.6 | 70.0 ± 2.4 | 26.1 ± 5.2 | 0.984 | 0.128 |
Values are expressed as mean ± SEM (n = 53). Percent changes from baseline were calculated after each treatment. Treatment with nutraceuticals (NTR) consisted of a combined supplementation of fish oil softgels, plant sterol enriched chocolate truffles and green tea. Control treatment (CTR) consisted of soy bean oil softgels, regular dark chocolate truffles and anise tea sachets. p1: probability value obtained by independent samples T-test for baseline values between NTR and CTR. p2: probability value obtained by independent samples T-test between % change after NTR and CTR.
Bold font indicates statistically significant p values.
Figure 1.
Baseline sterol profile (Mean ± SEM) of subgroups divided according to the intensity of LDL-C reduction (above or below the median; MED = −13%) after treatment with nutraceuticals. P values were obtained by T-test for independent groups. n = 23 in each group.
3.2. Pilot study of statin dose reduction
Results obtained in the first phase of the study showed that combined intake of n-3 FA, plant sterols, and polyphenols reduced lipemia and markers of inflammation and oxidative stress in some Type 2 diabetic, statin-treated patients. Based on these results, a cluster analysis was performed aiming to group patients with greater and lesser degrees of responses to the nutraceuticals. Ten patients from each cluster were invited to participate of the second phase. Figure S4 shows the percent change of LDL and hs-CRP for “R (Responders)” and “NR (Non-Responders),” measured at the end of the first phase. No difference was observed in MDA between the groups (P = 0.910). At the beginning of the second phase (pilot study), “R” and “NR” groups characteristics were compared (Table 3). We observed that “R” exhibited higher concentration of HDL-C (P = 0.031) and lower small HDL particle concentration (P = 0.045) than “NR.” Although not significant, “R” showed a trend toward higher concentration of large HDL particles (P = 0.063) and non-ABCA1 specific cholesterol efflux capacity (P = 0.075) than “NR.”
Table 3.
Anthropometric and biochemical parameters of Non-Responders (NR) and Responders (R) at the second phase of the study.
|
NR (n = 10) Baseline |
R (n = 10) Baseline/SST |
R HDS |
p1 | p2 | |
|---|---|---|---|---|---|
| Age(y) | 62.7 ± 2.0 | 62.1 ± 2.4 | – | – | – |
| Gender (M/F), n | 4/6 | 3/7 | – | – | – |
| Atorvastatin (20; 40; 80 mg) | 1; 2;0 | 1; 1;1 | – | – | – |
| Sinvastatin (20; 40) | 3; 4 | 4; 3 | – | – | – |
| Weight (kg) | 86.0 ± 11.0 | 75.9 ± 5.6 | 76.9 ± 5.8 | 0.526 | 0.939 |
| BMI (kg/m2) | 32.0 ± 2.5 | 29.5 ± 1.8 | 29.9 ± 1.8 | 0.526 | 0.879 |
| Waist circunference (cm) | 99.7 ± 6.6 | 91.9 ± 4.3 | 93.0 ± 4.3 | 0.407 | 0.879 |
| Abdominal circunference (cm) | 106.3 ± 5.6 | 98.9 ± 3.6 | 96.8 ± 3.5 | 0.661 | 0.650 |
| Hip circunference (cm) | 114.0 ± 5.8 | 104.8 ± 3.4 | 106.0 ± 3.5 | 0.306 | 0.677 |
| Glucose (mg/dL) | 115.8 ± 11.9 | 121.5 ± 17.1 | 121.6 ± 6.5 | 0.910 | 0.174 |
| Total cholesterol (mg/dL) | 178.4 ± 9.9 | 210.7 ± 19.6 | 196.3 ± 12.7 | 0.241 | 0.597 |
| LDL-C (mg/dL) | 101.9 ± 8.7 | 117.0 ± 14.7 | 109.0 ± 9.5 | 0.762 | 0.791 |
| HDL-C (mg/dL) | 43.4 ± 3.2 | 61.3 ± 6.1 | 62.6 ± 5.6 | 0.031 | 0.939 |
| VLDL-C (mg/dL) | 33.1 ± 4.9 | 32.4 ± 6.6 | 24.7 ± 2.0 | 0.850 | 0.427 |
| TG (mg/dL) | 215.8 ± 45.2 | 165.3 ± 50.2 | 114.7 ± 14.6 | 0.162 | 0.623 |
| hs-CRP (mg/L) | 2.2 ± 0.4 | 3.4 ± 0.91 | 2.6 ± 0.8 | 0.521 | 0.791 |
| Total HDL particle (μM) | 19.5 ± 0.8 | 22.6 ± 1.4 | 23.0 ± 1.8 | 0.104 | 0.850 |
| Extra Small HDL particle (μM) | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.2 | 0.910 | 0.969 |
| Small HDL particle (μM) | 6.6 ± 0.8 | 4.7 ± 0.8 | 4.4 ± 0.7 | 0.045 | 0.733 |
| Total small (xs + s) HDL particle (μM) | 7.9 ± 0.7 | 6.2 ± 0.7 | 5.9 ± 0.7 | 0.064 | 0.623 |
| Medium HDL particle (μM) | 8.5 ± 0.6 | 10.6 ± 1.1 | 11.5 ± 1.5 | 0.121 | 0.850 |
| Large HDL particle (μM) | 3.1 ± 0.3 | 5.8 ± 0.9 | 5.5 ± 0.7 | 0.064 | 0.969 |
| Total Cholesterol Efflux Capacity (CEC %) | 11.6 ± 0.4 | 13.8 ± 1.2 | 13.2 ± 1.2 | 0.241 | 0.623 |
| Non-ABCA1 specific CEC (%) | 7.3 ± 0.3 | 8.7 ± 0.6 | 8.7 ± 0.5 | 0.076 | 0.969 |
| ABCA1 specific CEC (%) | 4.4 ± 0.3 | 5.1 ± 0.7 | 4.5 ± 0.6 | 0.623 | 0.570 |
Values are expressed as mean ± SEM. p1: probability value obtained by Mann–Whitney U test of baseline values between NR and R. p2: probability value obtained by Wilcoxon test between standard statin therapy (baseline; SST) and half dose statin with supplements (HDS), for responders only.
Bold font indicates statistically significant p values.
A positive linear correlation was observed between total CEC and HDL-C (r = 0.825; P = 0.003) and total HDL particle (r = 0.847; P = 0.002) for “R” (Figure S5). As to “NR,” a less strong correlation was observed between total CEC and total HDL particle (r = 0.620; P = 0.056), while it was positively correlated to VLDL-C (r = 0.769; P = 0.009) and negatively correlated with hs-CRP (r = −0.781; P = 0.008). ABCA1-specific CEC was also negatively correlated with hs-CRP for “NR” (r = −0.821; P = 0.004). TG and VLDL-C were positively correlated with ABCA1-specific CEC for both “R” (r = 0.662; P = 0.037 and r = 0.791; P = 0.006) and “NR” (r = 0.882; P = 0.001 and r = 0.861; P = 0.001) (Figure S5).
Regarding to the pilot study, standard statin therapy (SST) was compared with half dose statin therapy complemented with nutraceuticals (HDS). No differences were observed between both treatments for all biomarkers evaluated in this study (Table 3), and β risk was lower than 5% just for parameters associated to body weight (data not shown), suggesting that for some patients, nutraceutical supplementation can effectively combat any change in biomarkers despite a reduction in statin dose.
4. Discussion
Several epidemiological and clinical studies have investigated the effect of isolated bioactive compounds on biomarkers of atherosclerosis and end-points involved with cardiovascular diseases [14], [16], [17]. This is the first clinical study that investigated the effect of a concomitant combination of plant sterols, n-3 FA, and polyphenols on dyslipidemia, inflammation, and oxidative stress markers in patients treated with statins.
Our results showed that the supplementation was easily incorporated into daily routine, as observed by plasma concentrations of EPA + DHA and β-sitosterol after 6 weeks of NTR. Despite high adherence, the biochemical response to the combined bioactive supplementation varied according to the biomarkers. It was observed that TG reduction was relative to TG baseline values, being significant only in the subgroup of patients with baseline values above the median. The hypotriglyceridemic effect of n-3 FA is well established; increased β-oxidation, via activation of PPARα, and decreased lipogenesis by suppression of sterol regulatory element-binding protein-1c (SREBP-1c) gene transcription are the described mechanisms [16]. Previous data show that the magnitude of n-3 FA's hypotriglyceridemic effect is influenced by baseline TG concentration and n-3 FA dose, suggesting that clinically significant TG reduction occurs only if EPA + DHA dose exceeds 2 g/day [12]. In our study, despite an estimated daily intake of 2.1 g/d of EPA + DHA, quantification of fatty acids in softgels showed that the provided EPA + DHA dose was 1.7 g/d. In a recent study, Kleiner and colleagues showed that over 70% of commercial n-3 FA supplements sold in the United States did not contain the stated label amount of EPA and DHA [25], partially due to oxidative instability [26]. Interestingly, human clinical trials usually do not report the oxidative status of supplements, which may be in part responsible for conflicting results [27]. More rigorous quality control should be applied to commercial n-3 FA capsules, as consumption of oxidized lipids can be deleterious [28]. Nevertheless, TG reduction achieved by fish oil supplements seems to be beneficial in patients at risk of CVD, since hypertriglyceridemia is strongly associated with atherosclerosis pathogeneses and may also contribute to residual cardiovascular risk [29].
Plant sterol consumption decreased LDL-C by 13%, independent of LDL-C baseline values, which corroborates previous studies [30], [31] and may correspond to a reduction of 20% in CVD risk over a lifetime [17]. The intensity of LDL-C reduction was shown to be influenced by subject's cholesterol absorption rate (as judged by basal campesterol concentration). Controversially, previous reports showed that basal lathosterol measures (cholesterol synthesis marker) could predict an individual's LDL-C lowering response to plant sterol supplementation, as lathosterol concentration negatively correlated with intensity of LDL-C reduction after intake of plant sterols [32], [33]. However, these observations were conducted with untreated hypercholesterolemic individuals, while subjects in our study were treated with statins and therefore had limited lathosterol synthesis.
A limitation in our study was that statin treatment was not restricted to a single type (atorvastatin or simvastatin) and dose. However, we suggest that treatment with different statins did not affect the LDL-C response to plant sterols, since baseline cholesterol synthesis was similar between subgroups with lowest and highest LDL-C reduction. The same was reported by Hallikainen and colleagues [34], when individuals using three different statins with varying doses were provided with plant stanol esters. These authors observed that neither the type nor the dose of statin influenced the results.
As stated above, the degree of LDL-C reduction was associated with the baseline cholesterol absorption rate. Whether statin treatment was responsible for upregulating cholesterol absorption rate remains to be determined [35]. This could be better elucidated if lathosterol and campesterol measurements were taken in all patients prior to statin therapy assignment. In fact, identifying an individual's sterol synthesizer/absorber profile could be a powerful tool for personalized medicine in primary prevention. The information about sterol synthesizer/absorber profile would allow clinicians to introduce cholesterol absorption inhibitors, like ezetimibe or plant sterols, at the beginning of treatment, preventing adverse effects of high statin doses.
In our study, NTR also reduced hs-CRP, possibly through an additive anti-inflammatory effect of n-3 FA and polyphenols. N-3 FA, especially EPA and DHA, give rise to inflammation-resolving mediators like resolvins and protectins [36]. Increased availability of n-3 FA also reduces synthesis of pro-inflammatory eicosanoids (through substrate competition with omega-6 fatty acids) and decreases activation of NF-kB [36], [37]. However, n-3 FA effects on CVD risk remains inconclusive [38]. The addition of n-3 FA to statin therapy was previously shown to reduce hs-CRP by 22% after 4 g EPA/day [39]. In our study, 1.7 g/day EPA + DHA prompted a 35% hs-CRP reduction. Likewise, the consumption of a polyphenol extract by statin-treated patients was previously shown to reduce hs-CRP by 23%, accompanied by a 29% reduction of oxidized LDL [40]. In the present study, a reduction of MDA (lipid oxidation secondary product) was observed only in a subgroup of patients with higher baseline values, possibly promoted by the antioxidant activity of green tea polyphenols, since both groups received the same amount of chocolate. Although green tea intake has been widely associated with antioxidant and anti-atherosclerotic effects [41], results have been inconsistent, and there is a lack of high quality studies linking green tea intake to CVD risk reduction [42].
A critical principle of personalized medicine is to provide the right therapy to the right patient at the right time [43]. Our study shows for the first time that, for some patients, statin dosage can be reduced by half, without change in biomarkers of CVD risk, provided that specific dietary compounds are consumed daily at pre-specified amounts. This is consistent with previous observations that doubling statin dose causes only about 6% additional decrease of LDL-C [44], which can easily be attained by plant sterol supplementation. The effect of statin therapy on HDL metabolism is less clear. Previous reports have shown that statins may both decrease [45], [46] or increase [47] CEC – the only metric of HDL functionality that has been shown to be predictive of prospective CVD events. A recent study showed that on-statin CEC was inversely associated with incident cardiovascular events and that, in fact, HDL particle number was the strongest HDL-related biomarker of residual risk in statin-treated patients [48]. In our pilot study, a 50% reduction on statin dosage did not alter CEC or HDL particle concentration.
The individual characteristics that distinguish “R” from “NR” subjects remain to be elucidated. Despite having 40% more HDL-C, responders did not show marked alteration in CEC, compared to non-responders. Although both groups also had similar HDL particle concentrations, we observed a shift in size distribution, with “R” having fewer small particles and higher concentrations of large particles, which carry most of the HDL cholesterol. “R” and “NR” subjects may have a different inflammatory context since smaller HDL particles have been associated with anti-inflammatory properties [49] and hs-CRP was negatively correlated with CEC for non-responders. Further studies are necessary to better understand and predict response to both drugs and nutraceuticals.
Apart from our small sample size, another limitation of our study is that we did not compare the effects of reduced statin dose by itself, without any supplementation. Therefore, it is not clear if the 6 weeks of reduced-dose statin therapy was adequate to induce statistical changes in biomarkers. However, it would not be prudent or advisable to lower statin dose of high risk patients without providing alternative treatment. Long-term studies are necessary to confirm that biomarkers are kept unchanged during HDS therapy.
5. Conclusion
The combined intake of n-3 FA, plant sterols, and polyphenols promoted changes thought to be cardioprotective when added to statin therapy, observed by reductions of lipids, and of markers of inflammation and oxidative stress. Furthermore, it became clear that the hypocholesterolemic effect depends on the individual's sterol synthesis/absorption capacity. Although limited by the small sample size, our study has also shown the potential for adding nutraceuticals to statin therapy for primary prevention, instead of for example, doubling drug dosage whenever LDL-C levels are suboptimal. This may be particularly helpful in the many patients with and at risk for CVD who cannot tolerate high dose statin therapy. Such an approach, however, should be particular to subjects identified as good responders to the proposed treatment. Further studies are needed to establish new markers as a means to predict individual response to nutraceuticals.
Financial support
This work was financially supported by the Coordination for the Improvement of Higher Education Personnel (CAPES), São Paulo Research Foundation (FAPESP grants 14/04247-3, 14/18576-9, 15/16243-5, 15/18859-3), and National Institutes of Health grant P01HL092969. Funding sources had no role in the design, execution, analyses, or interpretation of study data nor the decision to submit the results of this study.
Authors' contributions
BS, IC, and AB designed the study. BS, IC, AP, and AB conducted clinical aspects of the study. BS, MN, AP, and TV conducted laboratory aspects of the study. BS, IC, LPB, TV, SH, and EF analyzed and interpreted the data. Critical revision of the manuscript for important intellectual content: IC, SH, and EF. BS wrote first draft of the manuscript and has primary responsibility for final content. All authors contributed to subsequent drafts, and approved the final manuscript.
Acknowledgements
We are grateful to the staff of the Hospital, specially Dr. Andre Faludi, Chief of the Dyslipidemia Medical Section of Dante Pazzanese Institute of Cardiology. We also thank Dr. Marcelo Bertolami who provided insight and expertise that greatly assisted the research.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.02.005.
Conflict of interest
The authors declare that they have no competing interests.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Gotto A.M., Moon J.E. Pharmacotherapies for lipid modification: beyond the statins. Nature Reviews Cardiology. 2013;10:560–570. doi: 10.1038/nrcardio.2013.117. [DOI] [PubMed] [Google Scholar]
- 2.Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 3.Sabatine M.S., Giugliano R.P., Keech A.C., Honarpour N., Wiviott S.D., Murphy S.A. Evolocumab and clinical outcomes in patients with cardiovascular disease. New England Journal of Medicine. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 4.Nicholls S.J., Puri R., Anderson T., Ballantyne C.M., Cho L., Kastelein J.J.P. Effect of evolocumab on progression of coronary disease in statin-treated patients. JAMA. 2016;316(22):2373. doi: 10.1001/jama.2016.16951. [DOI] [PubMed] [Google Scholar]
- 5.Parathath S., Grauer L., Huang L.-S., Sanson M., Distel E., Goldberg I.J. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes. 2011;60:1759–1769. doi: 10.2337/db10-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kingwell B.A., Chapman M.J., Kontush A., Miller N.E. HDL-targeted therapies: progress, failures and future. Nature Reviews Drug Discovery. 2014;13(6):445–464. doi: 10.1038/nrd4279. [DOI] [PubMed] [Google Scholar]
- 7.Davidson M.H. Reducing residual risk for patients on statin therapy: the potential role of combination therapy. The American Journal of Cardiology. 2005;96(Suppl):3K–13K. doi: 10.1016/j.amjcard.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Stoekenbroek R.M., Kastelein J.J.P. Dyslipidaemia: statin-associated muscle symptoms — really all in the mind? Nature Reviews Cardiology. 2017;14(8):445–446. doi: 10.1038/nrcardio.2017.92. [DOI] [PubMed] [Google Scholar]
- 9.Serban M., Colantonio L.D., Manthripragada A.D., Monda K.L., Bittner V.A., Banach M. Statin intolerance and risk of coronary heart events and all-cause mortality following myocardial infarction. Journal of the American College of Cardiology. 2017;69(11):1386–1395. doi: 10.1016/j.jacc.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 10.Rideout T.C., Harding S.V., Marinangeli C.P.F., Jones P.J.H. Combination drug-diet therapies for dyslipidemia. Translational Research. 2010;155(5):220–227. doi: 10.1016/j.trsl.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Moss J.W.E., Ramji D.P. Nutraceutical therapies for atherosclerosis. Nature Reviews Cardiology. 2016;13(9):513–532. doi: 10.1038/nrcardio.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson T.A. Role of n-3 fatty acids in the treatment of hypertriglyceridemia and cardiovascular disease. American Journal of Clinical Nutrition. 2008;87:1981S–1990S. doi: 10.1093/ajcn/87.6.1981S. [DOI] [PubMed] [Google Scholar]
- 13.De Smet E., Mensink R.P., Plat J. Effects of plant sterols and stanols on intestinal cholesterol metabolism: suggested mechanisms from past to present. Molecular Nutrition & Food Research. 2012;56:1058–1072. doi: 10.1002/mnfr.201100722. [DOI] [PubMed] [Google Scholar]
- 14.Manach C., Mazur A., Scalbert A. Polyphenols and prevention of cardiovascular diseases. Current Opinion in Lipidology. 2005;16(1):77–84. doi: 10.1097/00041433-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Scolaro B., Soo Jin Kim H., de Castro I.A. Bioactive compounds as an alternative for drug co-therapy: overcoming challenges in cardiovascular disease prevention. Critical Reviews in Food Science and Nutrition. 2016 doi: 10.1080/10408398.2016.1235546. (Article in Press) [DOI] [PubMed] [Google Scholar]
- 16.Mozaffarian D., Wu J.H.Y. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. Journal of the American College of Cardiology. 2011;58(20):2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 17.Katan M.B., Grundy S.M., Jones P., Law M., Miettinen T., Paoletti R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clinic Proceedings. 2003;78:965–978. doi: 10.4065/78.8.965. [DOI] [PubMed] [Google Scholar]
- 18.Moss J.W.E., Davies T.S., Garaiova I., Plummer S.F., Michael D.R., Ramji D.P. A unique combination of nutritionally active ingredients can prevent several key processes associated with atherosclerosis in vitro. PLoS One. 2016;11(3):e0151057. doi: 10.1371/journal.pone.0151057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botelho P.B., Galasso M., Dias V., Mandrioli M., Lobato L.P., Rodriguez-Estrada M.T. Oxidative stability of functional phytosterol-enriched dark chocolate. LWT – Food Science and Technology. 2014;55(2):444–451. [Google Scholar]
- 20.García-Llatas G., Vidal C., Cilla A., Barberá R., Lagarda M.J. Simultaneous quantification of serum phytosterols and cholesterol precursors using a simple gas chromatographic method. European Journal of Lipid Science and Technology. 2012;114(5):520–526. [Google Scholar]
- 21.Shirai N., Suzuki H., Wada S. Direct methylation from mouse plasma and from liver and brain homogenates. Analytical Biochemistry. 2005;343(1):48–53. doi: 10.1016/j.ab.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 22.Hong Y., Yeh S., Chang C., Hu M. Total plasma malondialdehyde levels in 16 Taiwanese college students determined by various thiobarbituric acid tests and an improved high-performance liquid chromatography-based method. Clinical Biochemistry. 2000;33(8):619–625. doi: 10.1016/s0009-9120(00)00177-6. [DOI] [PubMed] [Google Scholar]
- 23.De La Llera-Moya M., Drazul-Schrader D., Asztalos B.F., Cuchel M., Rader D.J., Rothblat G.H. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutchins P.M., Ronsein G.E., Monette J.S., Pamir N., Wimberger J., He Y. Quantification of HDL particle concentration by calibrated ion mobility analysis. Clinical Chemistry. 2014;60(11):1393–1401. doi: 10.1373/clinchem.2014.228114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleiner A.C., Cladis D.P., Santerre C.R. A comparison of actual versus stated label amounts of EPA and DHA in commercial omega-3 dietary supplements in the United States. Journal of the Science of Food and Agriculture. 2015;95:1260–1267. doi: 10.1002/jsfa.6816. [DOI] [PubMed] [Google Scholar]
- 26.Albert B.B., Derraik J.G.B., Cameron-Smith D., Hofman P.L., Tumanov S., Villas-Boas S.G. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Scientific Reports. 2015;5:7928. doi: 10.1038/srep07928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert B.B., Cameron-Smith D., Hofman P.L., Cutfield W.S. Oxidation of marine omega-3 supplements and human health. BioMed Research International. 2013;2013:464921. doi: 10.1155/2013/464921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nogueira M.S., Kessuane M.C., Lobo Ladd A.A.B., Lobo Ladd F.V., Cogliati B. Effect of long-term ingestion of weakly oxidised flaxseed oil on biomarkers of oxidative stress in LDL-receptor knockout mice. British Journal of Nutrition. 2016;116(2):258–269. doi: 10.1017/S0007114516001513. [DOI] [PubMed] [Google Scholar]
- 29.Ng T.W.K., Ooi E.M.M., Watts G.F., Chan D.C., Barrett P.H.R. Atorvastatin plus omega-3 fatty acid ethyl ester decreases very-low-density lipoprotein triglyceride production in insulin resistant obese men. Diabetes, Obesity and Metabolism. 2014;16:519–526. doi: 10.1111/dom.12243. [DOI] [PubMed] [Google Scholar]
- 30.Scholle J.M., Baker W.L., Talati R., Coleman C.I. The effect of adding plant sterols or stanols to statin therapy in hypercholesterolemic patients: systematic review and meta-analysis. Journal of the American College of Nutrition. 2009;28:517–524. doi: 10.1080/07315724.2009.10719784. [DOI] [PubMed] [Google Scholar]
- 31.Bertolami A., Botelho P.B., Macedo L.F.L., Abdalla D.S.P., Faludi A.A., Galasso M. Effect of plant sterols compared with ezetimibe on oxidative stress in patients treated with statins. Journal of Functional Foods. 2014;10:178–186. [Google Scholar]
- 32.Rideout T.C., Harding S.V., Mackay D., Abumweis S.S., Jones P.J.H. High basal fractional cholesterol synthesis is associated with nonresponse of plasma LDL cholesterol to plant sterol therapy. American Journal of Clinical Nutrition. 2010;92:41–46. doi: 10.3945/ajcn.2009.29073. [DOI] [PubMed] [Google Scholar]
- 33.Mackay D.S., Gebauer S.K., Eck P.K., Baer D.J., Jones P.J.H. Lathosterol-to-cholesterol ratio in serum predicts cholesterol-lowering response to plant sterol consumption in a dual-center, randomized, single-blind placebo-controlled trial. The American Journal of Clinical Nutrition. 2015;101:432–439. doi: 10.3945/ajcn.114.095356. [DOI] [PubMed] [Google Scholar]
- 34.Hallikainen M., Kurl S., Laakso M., Miettinen T.A., Gylling H. Plant stanol esters lower LDL cholesterol level in statin-treated subjects with type 1 diabetes by interfering the absorption and synthesis of cholesterol. Atherosclerosis. 2011;217:473–478. doi: 10.1016/j.atherosclerosis.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 35.Van Himbergen T.M., Matthan N.R., Resteghini N.A., Otokozawa S., Ai M., Stein E.A. Comparison of the effects of maximal dose atorvastatin and rosuvastatin therapy on cholesterol synthesis and absorption markers. Journal of Lipid Research. 2009;50:730–739. doi: 10.1194/jlr.P800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calder P.C. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Molecular Nutrition & Food Research. 2012;56:1073–1080. doi: 10.1002/mnfr.201100710. [DOI] [PubMed] [Google Scholar]
- 37.Li K., Huang T., Zheng J., Wu K., Li D. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor α: a meta-analysis. PLoS One. 2014;9(2):e88103. doi: 10.1371/journal.pone.0088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rangel-Huerta O.D., Aguilera C.M., Mesa M.D., Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. The British Journal of Nutrition. 2012;107:S159–S170. doi: 10.1017/S0007114512001559. [DOI] [PubMed] [Google Scholar]
- 39.Ballantyne C.M., Bays H.E., Kastelein J.J., Stein E., Isaacsohn J.L., Braeckman R.A. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study) American Journal of Cardiology. 2012;110(7):984–992. doi: 10.1016/j.amjcard.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 40.Naruszewicz M., Łaniewska I., Millo B., Dłuzniewski M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI) Atherosclerosis. 2007;194:179–184. doi: 10.1016/j.atherosclerosis.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 41.Pang J., Zhang Z., Zheng T., Bassig B.A., Ge J., Yang Y. Green tea consumption and the risk of the related factors of cardiovascular diseases and ischemic related diseases: a meta-analysis. International Journal of Cardiology. 2016;202:967–974. doi: 10.1016/j.ijcard.2014.12.176. [DOI] [PubMed] [Google Scholar]
- 42.Murray M., Walchuk C., Suh M., Jones P.J. Green tea catechins and cardiovascular disease risk factors: should a health claim be made by the United States Food and Drug Administration? Trends in Food Science & Technology. 2015;41(2):188–197. [Google Scholar]
- 43.Ridker P.M. How common is residual inflammatory risk? Circulation Research. 2017;120(4):617–619. doi: 10.1161/CIRCRESAHA.116.310527. [DOI] [PubMed] [Google Scholar]
- 44.Reiner Z. Combined therapy in the treatment of dyslipidemia. Fundamental & Clinical Pharmacology. 2010;24(1):19–28. doi: 10.1111/j.1472-8206.2009.00764.x. [DOI] [PubMed] [Google Scholar]
- 45.Ronsein G.E., Hutchins P.M., Isquith D., Vaisar T., Zhao X.Q., Heinecke J.W. Niacin therapy increases high-density lipoprotein particles and total cholesterol efflux capacity but not ABCA1-specific cholesterol efflux in statin-treated subjects. Arteriosclerosis, Thrombosis, and Vascular Biology. 2016;36(2):404–411. doi: 10.1161/ATVBAHA.115.306268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicholls S.J., Ruotolo G., Brewer H.B., Kane J.P., Wang M.D., Krueger K.A. Cholesterol efflux capacity and pre-beta-1 HDL concentrations are increased in dyslipidemic patients treated with evacetrapib. Journal of the American College of Cardiology. 2015;66(20):2201–2210. doi: 10.1016/j.jacc.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Kini A.S., Vengrenyuk Y., Shameer K., Maehara A., Purushothaman M., Yoshimura T. Intracoronary imaging, cholesterol efflux, and transcriptomes after intensive statin treatment. Journal of the American College of Cardiology. 2017;69(6):628–640. doi: 10.1016/j.jacc.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 48.Khera A.V., Demler O.V., Adelman S.J., Collins H.L., Glynn R.J., Ridker P.M. Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: an analysis from the JUPITER trial (Justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin) Circulation. 2017;135(25):2494–2504. doi: 10.1161/CIRCULATIONAHA.116.025678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duprez D.A., Otvos J., Tracy R.P., Feingold K.R., Greenland P., Gross M.D. High-Density lipoprotein subclasses and noncardiovascular, noncancer chronic inflammatory-related events versus cardiovascular events: the Multi-ethnic study of atherosclerosis. Journal of the American Heart Association. 2015;4(9):e002295. doi: 10.1161/JAHA.115.002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.