Abstract
In the present study, we examined the effect of bexarotene (Targretin) and budesonide in chemoprevention of small cell lung carcinoma (mSCLC) using a lung specific knockout model of Rb1 and p53. Upon treatment with bexarotene, tumor incidence, number, and load were significantly reduced (p < 0.05). Budesonide treatment trended to inhibition, but the effect was not statistically significant (p > 0.05). Immunohistochemical staining indicated that bexarotene treatment decreased cell proliferation and increased apoptosis in tumors. The Rb1/p53 gene targeted mouse appears to be a valuable model for chemopreventive studies on human small cell lung cancer (hSCLC). Our results indicate that the retinoid X receptor (RXR) agonist bexarotene may be a potent chemopreventive agent in this cancer type.
INTRODUCTION
Small cell lung carcinoma (SCLC) is the most aggressive subtype of lung cancer with a mortality rate as high as 95% (2). The high frequency of relapse after initial chemotherapy accounts for the poor prognosis of this cancer type with a five-year survival rate of approximately 5% (3, 4). Cigarette smoking is associated with more than 90% of SCLC (2). SCLC is believed to originate from cells residing in the epithelial lining of the bronchi which have a neuroendocrine (NE) phenotype (5). Histopathologically, SCLC is formed by cancer cells small in size with highly pleomorphic involuted nuclei and a high nuclear/cytoplasmic ratio (5). They also express markers of NE differentiation, such as chromogranin A (CgA), neuron-specific enolase (NSE), synaptophysin (SYN), or neural cell adhesion molecule (NCAM; ref 1). Alterations in the tumor suppressor genes Rb1 and p53 are found in 90% of SCLCs (6). Besides retinoblastoma, SCLC is the only other human NE tumor that harbors RB1 mutations in almost all cases (7). Amplification of L-myc and N-myc oncogenes is exclusively present in SCLC compared to NSCLC (8). Increased BCL-2 and decreased BAX protein levels are often present coordinately with the loss of p53 (9).
Almost all mouse models of lung cancer produce adenomas and adenocarcinomas (10). Recently, Meuwissen et al. (1) generated mice with conditionally targeted alleles for both Rb1 and p53 that developed aggressive lung tumors with high incidence and with striking morphologic and immunophenotypic similarities to SCLC. Rb1 and P53 alleles were conditionally inactivated in the lung epithelium by using adenovirus-mediated somatic gene transfer of Cre recombinase (11). One potential strategy to prevent hSCLC in high-risk populations, such as smokers or ex-smokers, is to use chemopreventive agents to prevent the progression of pre-neoplastic lesions to late stage cancer and/or inhibit the development of new lesions. Bexarotene is a rexinoid and an RXR agonist. Budesonide is a synthetic glucocorticoid. Both of these agents had proven to be effective in blocking lung adenoma/adenocarcinoma formation in mouse models (16, 17). However, their effect on the development of SCLCs is not known. The present study employed this mouse model of hSCLC to test the chemopreventive activities of bexarotene and budesonide. In addition, we examined the effect of these agents on cell proliferation and apoptosis following short - term treatment with each agent.
MATERIALS AND METHODS
Reagents
Adeno-Cre virus (Ad5-CMV-Cre virus) was purchased from the University of Iowa Gene Transfer Vector Core (Iowa City, IA). Ketamine (NDC 0856-2013-01, Ketaset III, Ketamine HCl INJ USP) and Xylazine were obtained from Washington University School of Medicine Veterinarian Pharmacy. Bexarotene was obtained from the National Cancer Institute Chemical Repository (Bethesda, MD). Budesonide (>99% pure) was purchased from Sigma Chemical Co. (St. Louis, MO).
Genotyping
Mice carrying conditional alleles for Rb1 (floxed at the exon 19) and p53 (floxed at the exons 2–10) were obtained from Dr. Anton Berns’ laboratory (1). The original mice were on a mixed background. These mice were backcrossed to A/J mice (Jackson Laboratory, Bar Harbor, ME) for five generations in our laboratory before use in the present study. For each generation, mouse tail-clipping was taken for genotyping of the Rb-Flox (RF) and p53-Flox (PF). Tail clips were homogenized and incubated overnight at 37°C in lysis buffer (0.4 mg/ml pronase, 10% (w/v) sodium dodecyl sulfate, 10 mM Tris, 400 mM NaCl, and 2 mM EDTA). DNA isolation was then carried out with saturated NaCl and precipitation with ice-cold alcohol. Genotyping was performed on DNA from each mouse for the presence of the transgenes by polymerase chain reaction (PCR). The PCR products were subjected to electrophoresis on a 2% agarose gel along with a DNA size marker and visualized by UV light after staining with ethidium bromide. For the p53-flox allele, PCR was carried out with primers p53-10F (5′AAGGGGTATGAGGGACAAGG 3′) and p53-10R (5′GAAGACAGAAAAGGGGAGGG 3′) to amplify a 460-bp product for the wild type (wt) allele and a 584-bp product for the p53-floxed allele. DNA with both wt p53 (p53wt/wt) displayed only single 460-bp fragment, DNA with wt p53 allele and Floxed (p53Flox/wt) allele showed both 460-bp and 584-bp fragments, whereas DNA with both Floxed (p53Flox/Flox) allele showed a single 584-bp fragment. For the Rb-flox, the PCR was carried out with primers Rb19E (5′CTCAAGAGCTCAGACTCATGG 3′) and Rb18 (5′GGCGTGTGCCATCAAT 3′) to amplify a 200-bp product for the wt allele and a 283-bp product for the Rb-floxed allele. DNA with both wt Rb (Rbwt/wt) displayed only single 200-bp fragment, DNA with wt Rb allele and Floxed (RbFlox/wt) allele showed both 200-bp and 283-bp fragments, whereas DNA with both Floxed (RbFlox/Flox) alleles showed a single 283-bp fragment. Only the B5 (AJ × Trp53F2-10/F2-10;Rb1F19/F19) mice were selected to use in this study.
Intratracheal Adeno-Cre virus administration
Adeno-Cre virus was suspended in 3% sucrose in PBS at concentration of 1 × 1012 particles/ml and stored at –80°C until use. Ad-Cre virus was administered via intratracheal injection to somatically inactivate p53 and Rb1 in pulmonary bronchial epithelial cells of B5 (AJ × Trp53F2-10/F2-10;Rb1F19/F19) mice. For each mouse, 5 × 1010 particles of virus were delivered through trachea. A cocktail was made with 1 ml Ketamine, 0.15 ml Xylazine, and 4 ml PBS. Mouse was anesthetized with 100 μl of the cocktail per 20-gram mouse through i.p. injection, placed in a supine position with a rubber “pillow” under its neck to ensure the airway was straight. A catheter (26G × 19mm (3/4″, Venisystems™, Abbocath-T, Abbott Ireland, Sigo, Republic of Ireland) was inserted slowly into the trachea, a 1 cc syringe attached and virus was delivered slowly. The mouse was hold by the performer with two hands at its forearms and soft rub was given to its chest gently for about 15 seconds in order for virus to move down into the lung and preventing death by bronchus blockage.
Histopathology Analysis
Lung tissue was fixed in Tellyesniczky’s solution (90% ethanol, 5% glacial acetic acid, 5% formalin) for 24 - 48 hours and stored in 70% ethanol. Tissue sections (5-μm each) were cut from each lung and stained with hematoxylin and eosin (H&E) for histological examination (Figure 1A) or unstained for future immunohistochemical analysis.
Figure 1. Development of small cell lung carcinomas in B5 (AJ × Trp53F2-10/F2-10;Rb1F19/F19) mice.
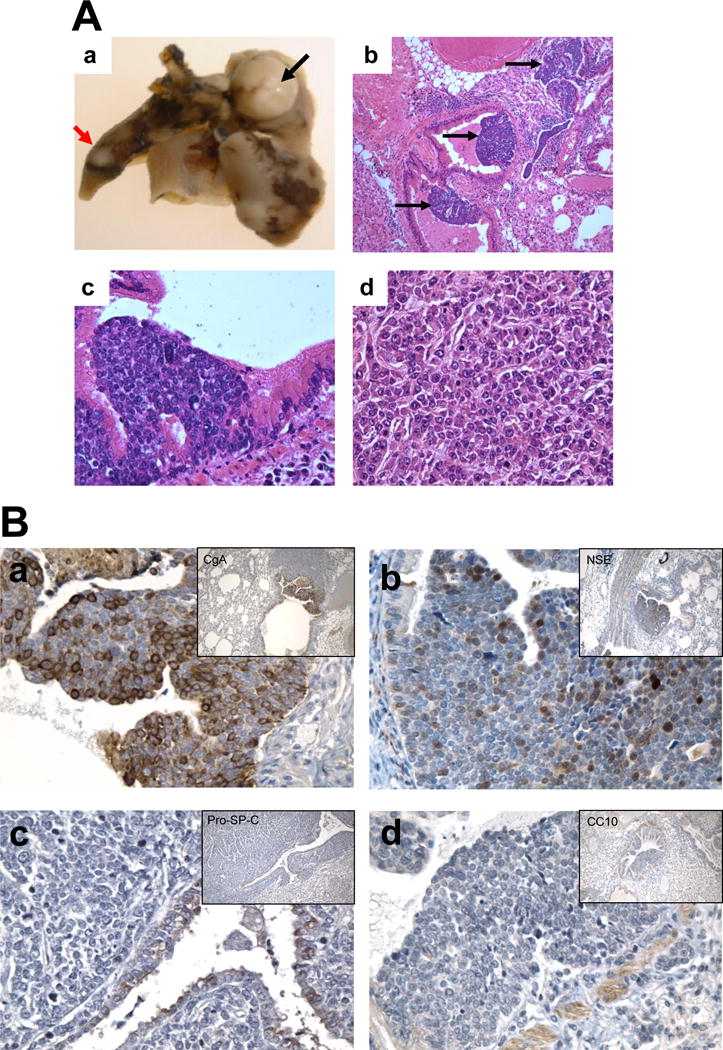
A. Gross and Histopathology of mSCLCs. a. Gross appearance of mSCLC (black arrow). b. Multiple dysplastic lesions (indicated by arrows) stained with H&E from a mouse 21 weeks after intratracheal Adeno-Cre (40×). c. Dysplastic lesion stained with H&E from a mouse 21 weeks after intratracheal Adeno-Cre (400×). d. H&E stain of mSCLC from mouse 36 weeks after intratracheal Adeno-Cre (400×). B. IHC staining on lung mSCLCs in B5 (AJ × Trp53F2-10/F2-10;Rb1F19/F19) mice with markers of neuroendocrine and epithelial differentiation. Anti-CgA antibody (a); Anti-NSE antibody (b); Anti-pro-SP-C antibody (c); Anti-CC 10 antibody (d).
Immunohistochemical (IHC) Study
Three lungs from each group of B5 (AJ × Trp53F2-10/F2-10;Rb1F19/F19) mice were analyzed. All slides were deparaffinized in xylene and rehydrated in gradient ethanol. Microwave antigen retrieval was carried out for 20 minutes in citrate buffer, pH 6.0. After blocking in 10% normal goat serum in PBS, primary antibody was diluted in 10% normal goat serum and incubated at 4°C overnight. NE markers, including CgA (12) and NSE (13) and epithelial markers, including pulmonary surfactant protein C (SP-/C) and Clara cell secretory protein (CC10; 10=10kD) were used to evaluate mSCLC tumors. Cell proliferation was assessed using primary monoclonal antibody against Ki-67 (1:200 dilution; Novo Castra, Burlington, Ontario, Canada). Cells undergoing apoptotic changes were detected using a terminal deoxynucleotidyl transferase biotin-dUTP nick end labelling (TUNEL) assay according to manufacturer’s instructions (ApopTagTM, in-situ Apoptosis Detection Kit; Intergen, New York, USA). Negative control slides were processed at the same time. Manual counting of labeled and total cells in high-powered (x400) fields of tumor tissue was conducted.
Chemoprevention study
Seven week old mice were randomized into three groups as shown in Figure 2. Chemopreventive agents (dotted line – bexarotene and budesonide) or control diet (solid line) were given to mice two weeks before intratracheal administration of Adeno-Cre virus (counted as week 0; indicated by arrow in Figure 2). Nine week-old B5 (AJ × Trp53F2-10/F2-10;Rb1F19/F19) mice were given AIN-76A Purified Diet # 100 000 (Dyets Inc, Bethlehem, PA, USA) with or without chemopreventive agent continuously until the end of experiment. Mice in group 1 were fed AIN-76A-purified diet as controls. Mice in group 2 were fed AIN-76A-purified diet containing budesonide (1.5 mg/kg diet), which was freshly made every week. Mice in group 3 were fed AIN-76A-purified diet and received bexarotene treatment. Bexarotene, 180 mg/kg body weight, was suspended in corn oil and delivered by oral gavage once a day and 5 days per week. Bexarotene suspension was made fresh daily. Food and water were available ad libitum. Lung tissue was fixed in Tellyesniczky’s solution overnight and stored in 70% ethanol. Lung tumor number was counted and the tumor diameter measured. For spherical tumors, the radius is used to calculate volume using the formula V = (4/3) π r3 (14). For irregular tumors, three measurements were taken at height (H), width (W), and length (L). The volume is then calculated using the formula V = (4/3) π × L/2 × W/2 × H/2 (15).
Figure 2. Chemopreventive effect of bexarotene and budesonide on mouse SCLC.
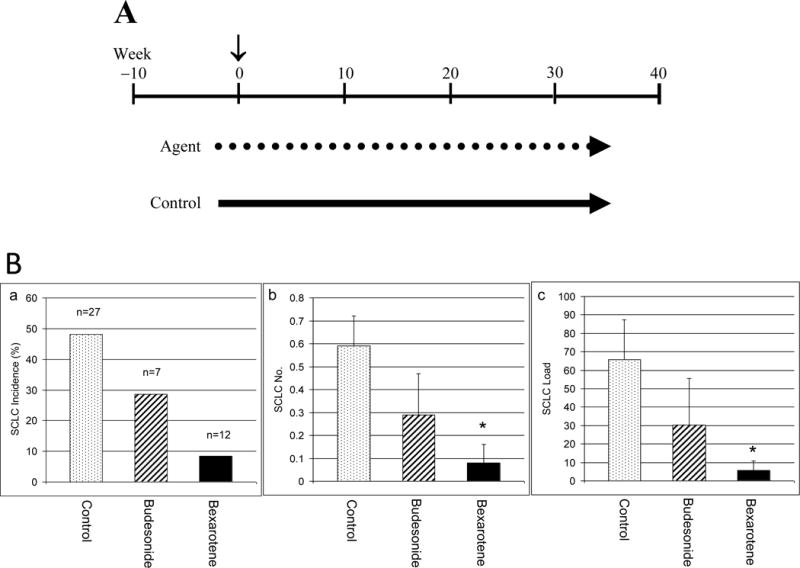
A. Experimental design of chemoprevention. The time (vertical arrow) of Adeno-Cre virus administration was counted as week 0 (~9-week old). Chemopreventive agent (dotted line) or control (solid line) groups were administered 2 weeks before Adeno-Cre (~7 week-old). Mice were given the agent continuously till the end of the experiment (36 weeks after Adeno-Cre virus; ~45 week-old). B. Chemopreventive effect of bexarotene and budesonide on mouse SCLC. Effect of bexarotene and budesonide treatments on mSCLC tumor incidence (a), SCLC tumor multiplicity (b), and SCLC tumor load (c) is shown. *P < 0.05. Bar, Standard Error (SE).
RESULTS
In B5 (AJ × Trp53F2-10/F2-10;Rb1F19/F19) mice that were received intratracheal Adeno-Cre virus, multiple foci of dysplastic cells were observed inside the bronchial and bronchiolar lumen 10 weeks after intratracheal Adeno-Cre administration (Figure 1A-b). Dysplastic cells were small and tightly clustered (Figure 1A-c). Visible tumors were apparent 36 weeks post intratracheal Adeno-Cre administration (Figure 1A-a). These tumors lacked the typical glandular and papillary features (Figure 1A-d) seen in mouse lung adenocarcinomas. In general, the cancer cells were small with high nuclear/cytoplasmic ratio. As shown in Figure 1A-d, additional features of the cancer cells included the hyperchromatic nuclei and a diffuse chromatin pattern that obscured the nucleolus. Cytoplasm was poorly developed and nuclear molding was commonly present. The cancer cells grew mostly in sheets and spread diffusely through the pulmonary tissues and air spaces, eliciting little stromal response. The mSCLC features were also determined by IHC staining. Neuriendocrine markers NSE and CgA were were detectable in mSCLC tumors (Figure 2B-ab), while the epithelial markers SPC and CC10 stained only in epithelial cells (Figure 1B-c & d). Average tumor multiplicity was 0.59 ± 0.13 tumors per mouse and average tumor load was 65.6 ± 21.9 mm3 (Figure 2B). No significant difference was observed between female and male mice in occurrence or phenotype of SCLC.
B5 (AJ × Trp53F2-10/F2-10;Rb1F19/F19) mice were used to determine the chemopreventive efficacy of bexarotene and budesonide. As shown in Figure 2B, 48% (13/27) of control mice developed SCLC compared to only 8% (1/12) of bexarotene-treated mice; an 83% decrease in tumor incidence. The number of SCLC per mouse was 0.59 ± 0.13 and 0.08 ± 0.08 in control and bexarotene-treated mice, respectively, representing an 86% decrease in mSCLC multiplicity (Figure 2B). Average tumor load was 65.6 ± 21.9 mm3 and 5.5 ± 5.4 mm3 in control and bexarotene-treated mice, respectively (Figure 2B), which is a decrease of 92%. Budesonide had a moderate inhibitory effect with a decrease in tumor incidence by 41% (48% in control mice vs. 28% in budesonide-treated mice; Figure 2B). The average number of mSCLC tumors per mouse was 0.29 ± 0.18 in budesonide-treated mice, representing a decrease in tumor multiplicity by 51% (p = 0.141; Figure 2B). The average tumor load was 30.4 ± 25.3 mm3, representing a decrease in tumor load by 54% (p = 0.221; Figure 2B).
The striking decrease in mSCLC growth is likely to be reflected in decreased proliferation and/or increased apoptosis. To investigate these two possible mechanisms, IHC assays with anti-Ki67 antibody for proliferative index and TUNEL assay for apoptotic index were performed (Figure 3A). Staining for Ki67 was present in 40.0%, 41.7%, and 1.8% of SCLC cells in control, budesonide, and bexarotene-treated tumors, respectively (Figure 3A-b). The Ki67 labeling index was decreased by 96% after bexarotene treatment (Figure 3A-b). TUNEL positive cells were present in 2.2%, 4.0%, and 12.9% of mSCLC in control, budesonide-treated and bexarotene-treated mSCLC samples, respectively (Figure 3A-d). TUNEL labeling was increased less than two fold by budesonide and almost four fold by bexarotene. These results indicate that treatment with bexarotene decreased the proliferative index and increased the apoptotic rate of the mSCLC cells. Finally, the presence of glucocorticoid receptor (GR) was determined in mouse SCLC using IHC staining with anti-GR antibody. GR staining was present bronchial epithelial cells (Figure 3Ba-d) and muscle cells (Figure 3B-d) but was not detected in mSCLC cells (Figure 3B-ac).
Figure 3. IHC staining.
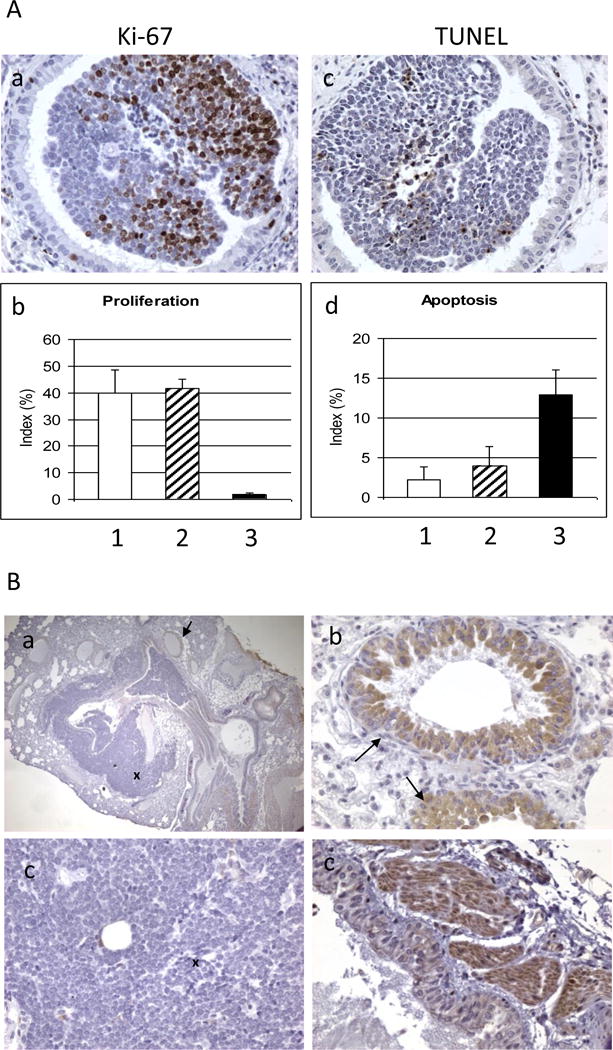
A. Effect of bexarotene on cell proliferation and apoptosis. Representative photo images of Ki-67 stains (a) and TUNEL stains (c) are shown. Bexarotene decreased cell proliferation (b; 1, control; 2, budesonide; 3, bexarotene) and increased apoptosis in mSCLC (d; 1, control; 2, budesonide; 3, bexarotene). B. IHC staining on mSCLC in B5 (AJ × Trp53F2-10/F2-10;Rb1F19/F19) mice with anti-glucocorticoid receptor (GR) antibody. Panel a shows positive staining of GR in bronchial epithelial cells (indicated by arrow) and negative staining in SCLC cells (indicated by cross); Panel b shows enlarged bronchial epithelial cells; Panel c shows enlarged SCLC cells; and Panel d shows the positive staining in muscle cells). Original magnification 100× in panel a; 400× in panels b-d.
DISCUSSION
In this study, we employed a recently developed model of SCLC (1) to test for the potential chemopreventive activity of two agents. We found that bexarotene is highly effective in inhibiting the development of mSCLC. Bexarotene is a RXR-selective agonist that minimally binds RAR receptors (18), and it is the first synthetic RXR-selective agonist to enter clinical trials for cancer therapy indications (19). The RXR receptors form heterodimers with a wide variety of nuclear receptors, including the peroxisome proliferator-activated receptors (PPARα, PPARγ, and PPARδ), the farnesoid X receptor (FXR), the constitutive androstane receptor (CAR) receptor, the RAR receptors (α, β, γ), the vitamin D receptors, and the liver X receptors (LXRα and LXRβ) (20, 21, 22). The resulting heterodimers serve as transcription activators of a wide variety of genes. These receptors play major roles in glucose (PPARγ), triglyceride (PPARα), cholesterol (PPARδ, LXR), bile acid (FXR), and xenobiotic (CAR receptor) metabolism. Bexarotene has been shown to have antiproliferative activity in preclinical in vitro and in vivo models of many cancers. In the N-nitroso-N-methylurea-induced estrogen receptor (ER) - positive rat mammary tumor model, bexarotene caused a 90% reduction in tumor burden and tumor incidence compared with control rats (23). Similarly, bexarotene and other RXR agonists have proven highly effective in preventing ER negative mammary tumors in transgenic mice (24, 31). We and others have shown that the RXR agonist bexarotene is an effective chemopreventive agent in an adenocarcinoma model of lung cancer (17, 32). Other investigators have similarly shown that other RXR agonists are similarly effective in preventing lung non-SCLC in human (33). Rosati et al. (25) have shown that a RXR-selective synthetic retinoid, LG100153, was a potent agent in SCLC cell lines (25). The present finding that bexarotene profoundly reduced mSCLC tumor incidence and multiplicity as well as decrease tumor size is consistent with previous studies and suggests that bexarotene is a potent chemopreventive agent against mSCLC.
Budesonide treatment did not have a statistical significant effect on chemoprevention of mSCLC in this model as measured by tumor incidence, tumor number, or tumor load (Figure 2). Budesonide has been shown to be one of the most potent chemopreventive agents in mouse adenoma/adenocarcinoma models. It prevented lung adenoma formation in benzo[a]pyrene (B[a]P) - induced A/J mice (wild type) when delivered via diet (26) or by aerosol inhalation (27). It was also effective against lung adenoma/adenocarcinoma development in p53 and/or Ink4A/Arf mutant mice (16). One possible reason for budesonide’s lack of efficacy in this model is the absence of detectable GR in mouse lung SCLC (Figure 3B). A correlation between decreased glucocorticoid receptor expression and resistance to the antiproliferative effects of GR in human SCLC has previously been observed (28, 29). A recent study found that human hSCLC cells (DMS 79, DMS 53 and COR L24) are profoundly resistant to glucocorticoid primarily due to deficient GR expression (30).
In summary, we found that mouse SCLC development can be effectively prevented by the RXR agonist bexarotene. The glucocorticoid budesonide which was highly effective in a mouse lung adenocarcinoma model was ineffective in mouse SCLC likely due to a lack of GR expression in SCLC cells. The preventive effect of bexarotene is accompanied by decreased proliferation and increased apoptosis. To the best of our knowledge, this is the first chemopreventive investigation in a mouse model of SCLC. B5 (AJ × Trp53F2-10/F2-10;Rb1F19/F19) mice will be a valuable model for pre-clinical chemopreventive and potentially chemotherapeutic studies to identify agents active against human SCLC.
Footnotes
Supported by grants (N01CN43308, R01CA113793, R01AT003203, P50CA089019) from the National Institutes of Health.
References
- 1.Meuwissen R, Linn SC, Linnoila RI, Zevenhoven J, Mooi WJ, Berns A. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4:181–9. doi: 10.1016/s1535-6108(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 2.Brownson RC, Chang JC, Davis JR. Gender and histologic type variations in smoking-related risk of lung cancer. Epidemiology. 1992;3:61–4. doi: 10.1097/00001648-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Junker K, Wiethege T, Muller KM. Pathology of small-cell lung cancer. J Cancer Res Clin Oncol. 2000;126:361–8. doi: 10.1007/PL00008483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worden FP, Kalemherian GP. Therapeutic advances in small cell lung cancer. Expert Opin Investig Drugs. 2000;9:565. doi: 10.1517/13543784.9.3.565. [DOI] [PubMed] [Google Scholar]
- 5.Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol. 2001;28(2 Suppl 4):3–13. Review. [PubMed] [Google Scholar]
- 6.Gouyer V, Gazzeri S, Bolon I, Drevet C, Brambilla C, Brambilla E. Mechanism of retinoblastoma gene inactivation in the spectrum of neuroendocrine lung tumors. Am J Respir Cell Mol Biol. 1998;18:188–96. doi: 10.1165/ajrcmb.18.2.3008. [DOI] [PubMed] [Google Scholar]
- 7.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–12. doi: 10.1016/s1535-6108(02)00102-2. Review. [DOI] [PubMed] [Google Scholar]
- 8.Meuwissen R, Berns A. Mouse models for human lung cancer. Genes Dev. 2005;19:643. doi: 10.1101/gad.1284505. [DOI] [PubMed] [Google Scholar]
- 9.Brambilla E, Negoescu A, Gazzeri S, et al. Apoptosis-related factors p53, Bcl2, and Bax in neuroendocrine lung tumors. Am J Pathol. 1996;149:1941–52. [PMC free article] [PubMed] [Google Scholar]
- 10.Tuveson DA, Jacks T. Modeling human lung cancer in mice: similarities and shortcomings. Oncogene. 1999;18:5318–24. doi: 10.1038/sj.onc.1203107. Review. [DOI] [PubMed] [Google Scholar]
- 11.Meuwissen R, Linn SC, van der Valk M, Mooi WJ, Berns A. Mouse model for lung tumorigenesis through Cre/lox controlled sporadic activation of the K-Ras oncogene. Oncogene. 2001;20:6551–8. doi: 10.1038/sj.onc.1204837. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari L, Seregni E, Bajetta E, Martinetti A, Bombardieri E. The biological characteristics of chromogranin A and its role as a circulating marker in neuroendocrine tumours. Anticancer Res. 1999;19:3415–27. [PubMed] [Google Scholar]
- 13.Kobayashi S, Okada S, Hasumi T, Sato N, Fujimura S. The significance of NSE and CEA as a diffrentiation marker for the cellular heterogeneity of small cell lung cancer. Tohoku J Exp Med. 1999;189:37–49. doi: 10.1620/tjem.189.37. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Liu Q, Lantry LE, et al. A germ-line p53 mutation accelerates pulmonary tumorigenesis: p53-independent efficacy of chemopreventive agents green tea or dexamethasone/myo-inositol and chemotherapeutic agents taxol or adriamycin. Cancer Res. 2000;60:901–7. [PubMed] [Google Scholar]
- 15.Eshleman JS, Carlson BL, Mladek AC, Kastner BD, Shide KL, Sarkaria JN. Inhibition of the Mammalian Target of Rapamycin Sensitizes U87 Xenografts to Fractionated Radiation Therapy. Cancer Res. 2002;62:7291–7. [PubMed] [Google Scholar]
- 16.Wang Y, Zhang Z, Kastens E, Lubet RA, You M. Mice with alterations in both p53 and Ink4a/Arf display a striking increase in lung tumor multiplicity and progression: differential chemopreventive effect of budesonide in wild-type and mutant A/J mice. Cancer Res. 2003;63:4389–95. [PubMed] [Google Scholar]
- 17.Wang Y, Zhang Z, Yao R, Jia D, Wang D, Lubet RA, You M. Prevention of lung cancer progression by bexarotene in mouse models. Oncogene. 2006;25:1320–9. doi: 10.1038/sj.onc.1209180. [DOI] [PubMed] [Google Scholar]
- 18.Boehm MF, Zhang L, Badea BA, et al. Synthesis and structure-activity relationships of novel retinoid X receptor-selective retinoids. J Med Chem. 1994;37:2930–41. doi: 10.1021/jm00044a014. [DOI] [PubMed] [Google Scholar]
- 19.Miller VA, Benedetti FM, Rigas JR, et al. Initial clinical trial of a selective retinoid X receptor ligand, LGD1069. J Clin Oncol. 1997;15:790–5. doi: 10.1200/JCO.1997.15.2.790. [DOI] [PubMed] [Google Scholar]
- 20.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–50. doi: 10.1016/0092-8674(95)90200-7. Review. [DOI] [PubMed] [Google Scholar]
- 21.Chawla A, Boisvert WA, Lee CH, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–71. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 22.Zhang-Gandhi CX, Drew PD. Liver X receptor and retinoid X receptor agonists inhibit inflammatory responses of microglia and astrocytes. J Neuroimmunol. 2007;183:50–9. doi: 10.1016/j.jneuroim.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bischoff ED, Gottardis MM, Moon TE, Heyman RA, Lamph WW. Beyond tamoxifen: the retinoid X receptor-selective ligand LGD1069 (TARGRETIN) causes complete regression of mammary carcinoma. Cancer Res. 1998;58:479–84. [PubMed] [Google Scholar]
- 24.Wu K, Zhang Y, Xu XC, et al. The retinoid X receptor-selective retinoid, LGD1069, prevents the development of estrogen receptor-negative mammary tumors in transgenic mice. Cancer Res. 2002;62:6376–80. [PubMed] [Google Scholar]
- 25.Rosati R, Ramnath N, Adil MR, Ou X, Ali MA, Heyman RA, Kalemkerian GP. Activity of 9-cis-retinoic acid and receptor-selective retinoids in small cell lung cancer cell lines. Anticancer Res. 1998;18:4071–5. [PubMed] [Google Scholar]
- 26.Wattenberg LW, Estensen RD. Studies of chemopreventive effects of budenoside on benzo[a]pyrene- induced neoplasia of the lung of female A/J mice. Carcinogenesis. 1997a;18:2015–7. doi: 10.1093/carcin/18.10.2015. [DOI] [PubMed] [Google Scholar]
- 27.Wattenberg LW, Wiedmann TS, Estensen RD, Zimmerman CL, Steele VE, Kelloff GJ. Chemoprevention of pulmonary carcinogenesis by aerosolized budesonide in female A/J mice. Cancer Res. 1997b;57:5489–92. [PubMed] [Google Scholar]
- 28.Hofmann J, Kaiser U, Maasberg M, Havemann K. Glucocorticoid receptors and growth inhibitory effects of dexamethasone in human lung cancer cell lines. Eur J Cancer. 1995;31A:2053–8. doi: 10.1016/0959-8049(95)00431-9. [DOI] [PubMed] [Google Scholar]
- 29.Lu YS, Lien HC, Yeh PY, Yeh KH, Kuo ML, Kuo SH, et al. Effects of glucocorticoids on the growth and chemosensitivity of carcinoma cells are heterogeneous and require high concentration of functional glucocorticoid receptors. World J Gastroenterol. 2005;11:6373–80. doi: 10.3748/wjg.v11.i40.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sommer P, Le Rouzic P, Gillingham H, Berry A, Kayahara M, Huynh T, White A, Ray DW. Glucocorticoid receptor overexpression exerts an antisurvival effect on human small cell lung cancer cells. Oncogene. 2007;26:7111–21. doi: 10.1038/sj.onc.1210524. [DOI] [PubMed] [Google Scholar]
- 31.Liby K, Risingsong R, Royce DB, et al. Prevention and treatment of experimental estrogen receptor-negative mammary carcinogenesis by the synthetic triterpenoid CDDO-methyl Ester and the rexinoid LG100268. Clin Cancer Res. 2008;14:4556–63. doi: 10.1158/1078-0432.CCR-08-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira MA, Kramer PM, Nines R, et al. Prevention of mouse lung tumors by targretin. Int J Cancer. 2006;118:2359–62. doi: 10.1002/ijc.21618. [DOI] [PubMed] [Google Scholar]
- 33.Dragnev KH, Petty WJ, Ma Y, Rigas JR, Dmitrovsky E. Nonclassical retinoids and lung carcinogenesis. Clin Lung Cancer. 2005;6:237–44. doi: 10.3816/CLC.2005.n.003. Review. [DOI] [PubMed] [Google Scholar]


