Abstract
Background
Approximately 20% of patients with papillary thyroid carcinoma (PTC) will develop cancer recurrence, but no clinically available biomarker has been identified. Our study aimed to evaluate the prognostic value of G protein-coupled receptor kinase 6 (GRK6) in PTCs.
Material/Methods
We retrospectively enrolled 108 PTC patients in this study, and explored the expression of GRK6 in resected tumor samples by RT-qPCR and immunohistochemistry (IHC). The clinical data were interpreted by chi-square test, univariate analysis, and multivariate analysis. To investigate the functional mechanisms of GRK6 in regulating PTC progression, we also performed overexpression and silencing experiments in TPC-1 cells, a cell line generated from PTC tissues.
Results
RT-qPCR results showed a higher level of GRK6-mRNA in PTCs than in adjacent thyroid tissues. IHC revealed a distinct protein expression pattern of GRK6 among PTCs. Accordingly, we classified patients into low-GRK6 and high-GRK6 groups. The chi-square test showed that a higher GRK6 was associated with larger tumor size (P=0.045) and advanced TNM stage (P=0.001). Kaplan-Meier survival curve and log rank test demonstrated that higher GRK6 predicted poor disease-free survival (DFS) in PTC patients (P=0.002). Furthermore, Cox regression analysis confirmed that GRK6 was an independent prognostic factor for a higher recurrence risk of PTCs (P=0.047). MTT assay and Transwell assay demonstrated that GRK6 overexpression can significantly enhance tumor proliferation and invasion, which was consistent with clinical findings.
Conclusions
Our data show the oncogenic effects of GRK6 in promoting PTC progression.
MeSH Keywords: Cell Proliferation, Multiple Endocrine Neoplasia Type 2a, Prognosis
Background
Thyroid cancer is the most common endocrine malignancy, and more than 80% is papillary thyroid cancer (PTC) [1]. The overall survival for most PTC patients is satisfactory, but its general mortality is still higher than other endocrine neoplasms. Surgical resection is the most effective therapy for PTCs, ranging from thyroid lobectomy to a total thyroidectomy [2]. However, even after surgical treatment, local metastases and recurrence are observed in many patients [3,4]. Classification of these high-risk patients is helpful for clinical decision-making, such as resection extent, postoperative treatment, and prognosis prediction. At present, the most clinically applicable predictor for PTC recurrence is the status of regional lymph nodes [5], but increasing attention is being focussed on investigating novel biomarkers at the gene or protein level, which will not only help predict prognosis, but also direct therapy development.
G-protein-coupled receptor kinases (GRKs) are kinases that predominantly phosphorylate G protein-coupled receptors (GPCRs) [6]. Upon ligand stimulation, GRKs quickly phosphorylate GPCRs, leading to receptor desensitization and internalization [7]. From the aspect of physiology, GRKs exert critical regulatory functions in neurodevelopment [8], cardiovascular efficiency [9], and immunoreaction [10]. Accumulating evidence suggests that GRKs also participate in malignancy development [11,12]. Specifically, the function of GRK6 was shown to be suppressed in medulloblastoma by Src signaling, leading to enhanced tumor cell migration [13]. In addition, GRK6-knockout mice showed a higher risk of lung cancer metastasis [14], indicating the role of GRK6 in suppressing tumor progression. However, another study reported that GRK6 was highly expressed in hepatocellular carcinoma and was correlated with poor prognosis [15]. Therefore, GRK6 may play distinct roles in different tumor types.
The present study is the first to investigate the expression profile of GRK6 in PTCs. We found that GRK6 was up-regulated in PTCs, which was correlated with tumor size and lymph node metastasis. Also, high expression of GRK6 can independently indicate a high risk of PTC recurrence, as reflected by univariate and multivariate analyses. In addition, cellular results showed that GRK6 overexpression can directly enhance cell proliferation and invasion, whereby its siRNA silencing inhibits tumor progression. Our data reveal the role of GRK6 in predicting clinical outcomes and its potential as a novel drug target for PTCs.
Material and Methods
Patients and samples
A total of 108 PTC patients diagnosed and surgically treated in Weihai Hospital of Traditional Chinese Medicine from June 2009 to June 2016 were enrolled in this study. All the patients were confirmed as having PTC by pathologically examination [16]. None of the patients had received chemotherapy or radiotherapy before surgery. All the patients underwent therapeutic total or near-total thyroidectomy. This study was approved by the Ethics Committee of Weihai Hospital of Traditional Chinese Medicine, and written informed consents were obtained from all patients. Clinical information was retrieved for all patients (Table 1). Fresh-frozen PTC tissues and adjacent tissues were obtained from our hospital and stored in liquid nitrogen until use.
Table 1.
Characteristics of the PTC patients and associations with GRK6 expression level.
| Variable | Cases (n=108) | GRK6 expression | P value | |
|---|---|---|---|---|
| Low (n=61) | High (n=47) | |||
| Age (years) | 0.108 | |||
| ≤46 yrs | 48 | 23 | 25 | |
| >46 yrs | 60 | 38 | 22 | |
| Gender | 0.448 | |||
| Female | 86 | 47 | 39 | |
| Male | 22 | 14 | 8 | |
| Tumor size | 0.045* | |||
| ≤2.0 cm | 71 | 45 | 26 | |
| >2.0 cm | 37 | 16 | 21 | |
| ETE | 0.527 | |||
| Negative | 56 | 30 | 26 | |
| Positive | 52 | 31 | 21 | |
| TCI | 0.118 | |||
| Negative | 73 | 45 | 28 | |
| Positive | 35 | 16 | 19 | |
| LN metastasis | 0.233 | |||
| Negative | 84 | 50 | 34 | |
| Positive | 24 | 11 | 13 | |
| TNM stage | 0.001* | |||
| I–II | 75 | 50 | 25 | |
| III–IV | 33 | 11 | 22 | |
ETE – extrathyroidal extension; TCI – thyroid capsular invasion; LN – lymph node.
P<0.05.
RT-qPCR
Total RNA was isolated from 23 pairs of PTC tissues and adjacent thyroid tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). After quantified by Nanodrop, 1 μg of total RNA was reverse-transcribed into cDNA using the SuperScript cDNA system (Invitrogen) according to the manufacturer’s instructions. Quantitative PCR was conducted to test the mRNA level of GRK6 using SYBR Green PCR master mix (Applied Biosystems) with the following primers: sense, 5′-TTTGGGCTGGATGGGTCTGTTC-3′; antisense, 5′-CGCTGCAGTTCCCACAGCAATC-3′ [17]. Gene expression levels were evaluated by normalization against the housekeeping gene GAPDH (sense, 5′-TGCACCACCAACTGCTTAGC-3′; antisense. 5′-GGCATGGACTGTGGTCATGAG-3′) using the 2−ΔΔCT method [18]. Data are presented as mean ±SD from 3 independent experiments.
Immunohistochemistry (IHC)
Paraffin sections (4 μm) of the PTC tissues were deparaffinized and dehydrated with graded ethanol. After blocking the endogenous peroxidase with 3.0% H2O2, slides were incubated with citric acid buffer to retrieve antigen by heating in 120°C for 15 min. The slides were then blocked with 5% BSA for 1 h and incubated with primary rabbit polyclonal anti-GRK6 antibody (C20, Santa Cruz Biotechnology) overnight at 4°C. After that, slides were washed with PBS and further incubated with biotinylated second antibody for 15 min at room temperature. Finally, the slides were stained using the VECTASTAIN Elite ABC HRP kit and DAB reagents. For negative control, PBS was used instead of primary antibody.
IHC evaluation
We asked 2 independent pathologists blinded to this study to help us perform the IHC evaluation. The expression was calculated based on both the percent of positive cells and staining intensity, as described by others [19]. Briefly, the positivity percentage was scored as 0 (0–10%), 1 (11–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). The staining intensity was scored as 0 (no immunoreactivity), 1 (weakly stained), 2 (moderate stained), and 3 (strong stained). The final IHC score (IHCS) was weighted by multiplying the intensity score with percentage score (range, 0–12). Patients were classified into a GRK6 low expression group (IHCS ≥6) and a high expression group (IHCS <6) according to the IHCS.
Cell culture and transfection
The human PTC cell line, TPC-1, was cultured in DMEM supplemented with 10% FBS in a 5% CO2 atmosphere at 37°C. For the transfection, pRK5-GRK6 plasmid was purchased from Addgene (#32693) and GRK6-siRNA was synthesized by Santa Cruz Biotechnology (AACAGUAGGUUUGUAGUGAGC, position 724–744). Both overexpression and silencing were conducted using Fugene-6 (Roche) in OptiMEM (Invitrogen) for 48 h. The transfection efficiency was confirmed by Western blot.
Western blot
For protein expression analysis, transfected cells were homogenized in radioimmune precipitation assay (RIPA) buffer at 4°C for 30 min. After centrifugation at 13 000 rpm for 20 min, the protein concentration in supernatant was tested using a Pierce BCA kit (Thermo Fisher Scientific). Approximately 20 μg of total proteins were resolved on 12% SDS-PAGE, blotted to PVDF membrane, and probed with primary antibodies, followed by incubating with HRP-conjugated secondary antibodies, and visualized by adding ECL reagent.
Proliferation assay
The cell viability assay was achieved using the Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan). Briefly, transfected TPC-1 cells were seeded at 4×103 cells/well in 96-well plates in triplicate and cultured at 37°C. At designated time points (1, 2, 3, and 4 days), 10 μl of Cell Counting Kit-8 reagent was added into the well and incubated for another 3 h. The absorbance of each well was measured at 450 nm under a Bio-Rad microplate reader. Each experiment was performed 3 times.
Invasion assay
After being coated with Matrigel (BD Biosciences, San Jose, CA), transfected TPC-1 cells were seeded into the upper chamber of the Transwell apparatus (8.0 μm, Corning, NY, USA) at a density of 5×104 cells/chamber (200 μl serum-free medium). Another 800 μl of medium supplemented with 20% serum was added to the lower chamber to induce cell invasion. After we had cultured the cells at 37°C for 36 h, the cells on the upper side of the membrane was gently removed with cotton swabs. Invaded cells on the lower side of the membrane were fixed and stained with 0.5% crystal violet. Cells were counted under an inverted light microscope (Olympus) in 5 randomly selected visual fields. The experiment was performed 3 times independently.
Statistical analyses
Data are presented as mean ±SD, and all statistical analyses were performed using SPSS software (version 16.0; Chicago, IL, USA). Correlations between GRK6 expression and patients’ clinical parameters were calculated by the Pearson chi-square test. Univariate survival analysis was performed by Kaplan-Meier method and tested with the log-rank test. Cox regression modelling was used to investigate the independent effect of prognostic factors. For the cellular results, the difference was compared by Student’s t-test. P<0.05 was considered statistically significant.
Results
Patients information
This study included 108 PTC patients with a mean age of 49.7 years (range, 20–76 years). As shown in Table 1, most of the patients were female (86/108, 79.63%). Seventy-one patients (71/108, 65.74%) had a tumor diameter smaller than 2.0 cm, while the other 37 patients (37/108, 34.26%) had a tumor diameter larger than 2.0 cm. Fifty-six patients (56/108, 51.85%) were diagnosed without extrathyroidal extension (ETE), and the other 52 patients (52/121, 48.15%) had positive ETE. The status of thyroid capsular invasion (ETI) was also evaluated; about one-third of patients (35/108, 32.41%) had positive TCI. Most of the patients (84/108, 77.78%) had negative regional lymph nodes. Seventy-five (75/108, 69.44%) patients were classified as TNM stage I/II, and the other 33 (33/108, 30.56%) had TNM stage III/IV. The 5-year disease-free survival rate of the entire cohort was 84.22%.
GRK6 expression pattern and its associations with clinicopathological characteristics
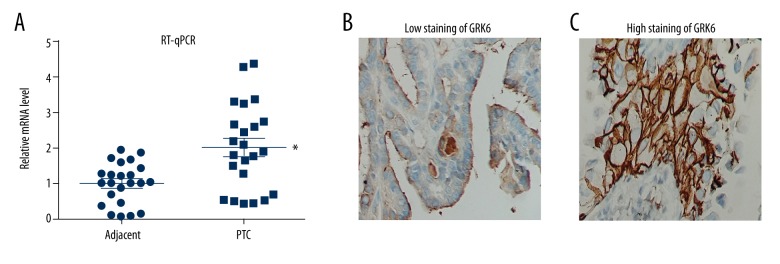
We first assessed the transcription level of GRK6 by RT-qPCR in 23 pairs of PTC and adjacent thyroid tissues. In 17 patients (17/23, 73.91%), GRK6-mRNA showed a higher level in PTC tissues than in adjacent normal tissues. The mean GRK6-mRNA level was 2.7 times higher in PTC tissues (Figure 1A), and the protein expression of GRK6 was predominantly identified in the cytoplasm of tumor cells (Figure 1B, 1C).
Figure 1.
GRK6 was up-regulated in PTCs. (A) RT-qPCR results from 23 matched PTCs and adjacent thyroid tissues showed an elevated RNA level of GRK6 in PTCs (P=0.004). IHC images showed representative low expression (B) and high expression (C) of GRK6 protein in PTC cells, especially in the cytoplasm.
According to the staining percentage and staining intensity, we classified patients into low-GRK6 and high-GRK6 groups (Table 1). By chi-square testing, we found that GRK6 was positively correlated with the tumor size (P=0.045) and TNM stage (P=0.001). No significant association was observed between GRK6 expression and other parameters.
GRK6 is a novel biomarker indicating high recurrence risk
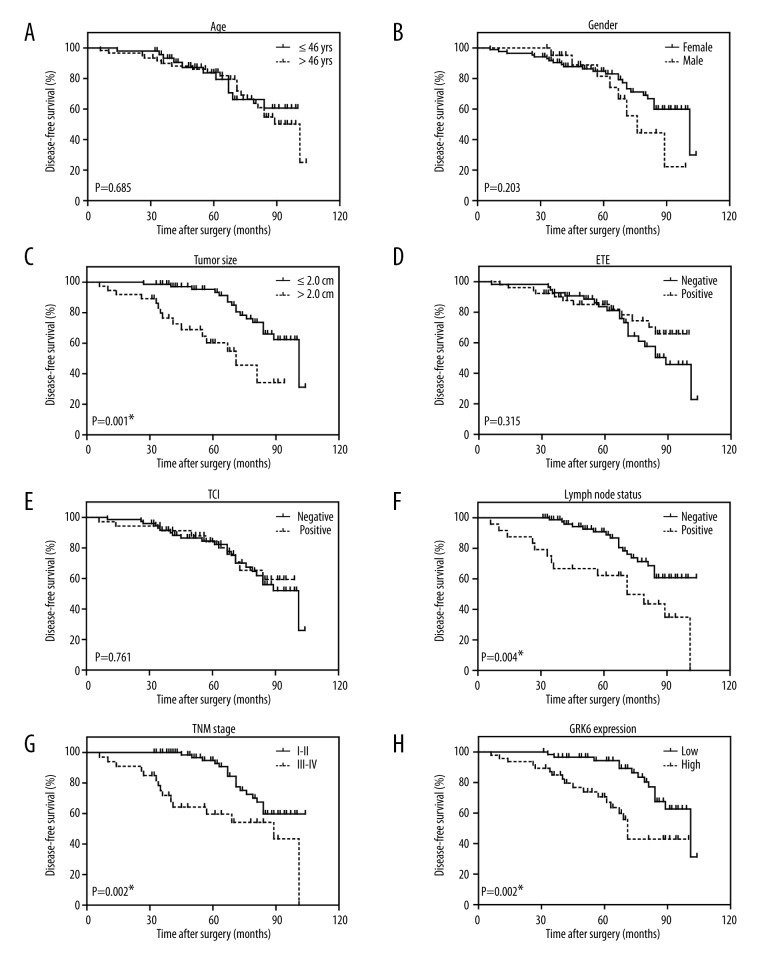
Since higher GRK6 was observed more frequently in PTCs with larger tumor size and advanced TNM stages, we suspected it may help predict patients’ clinical outcomes. We therefore analyzed the prognostic factors for disease-free survival (DFS) in PTCs using Kaplan-Meier method (Figure 2, Table 2). A larger tumor size (P<0.001), positive lymph node metastasis (P=0.004), and advanced TNM stage (P=0.002) were shown as unfavorable factors affecting DFS. Importantly, patients with low GRK6 expression showed a better prognosis than those with high GRK6 expression (DFS 91.8±2.7 months vs. 71.7±4.8 months, P=0.002).
Figure 2.
Kaplan-Meier survival analyses of the disease-free survival of PTC patients. All statistical significance was tested by log-rank test. * P<0.05.
Table 2.
Univariate analysis for the disease-free survival of PTC patients.
| Variable | Cases (n=158) | Disease-free survival | P value | |
|---|---|---|---|---|
| Mean ±SD (months) | 5-year (%) | |||
| Age (years) | 0.685 | |||
| ≤46 yrs | 48 | 83.3±4.2 | 83.6% | |
| >46 yrs | 60 | 82.7±3.6 | 84.0% | |
| Gender | 0.203 | |||
| Female | 86 | 84.9±3.2 | 84.7% | |
| Male | 22 | 76.5±5.1 | 81.5% | |
| Tumor size | <0.001* | |||
| ≤2.0 cm | 71 | 90.6±2.6 | 95.3% | |
| >2.0 cm | 37 | 65.6±5.3 | 60.2% | |
| ETE | 0.315 | |||
| Negative | 56 | 82.1±3.6 | 83.7% | |
| Positive | 52 | 84.6±3.9 | 85.1% | |
| TCI | 0.761 | |||
| Negative | 73 | 83.6±3.3 | 84.5% | |
| Positive | 35 | 82.2±4.6 | 84.0% | |
| LN metastasis | 0.004* | |||
| Negative | 84 | 89.4±2.8 | 90.8% | |
| Positive | 24 | 67.0±7.3 | 62.2% | |
| TNM stage | 0.002* | |||
| I–II | 75 | 90.6±2.6 | 94.8% | |
| III–IV | 33 | 69.9±6.5 | 59.6% | |
| GRK6 expression | 0.002* | |||
| Low | 61 | 91.8±2.7 | 94.4% | |
| High | 47 | 71.7±4.8 | 70.6% | |
ETE – extrathyroidal extension; TCI – thyroid capsular invasion; LN – lymph node.
P<0.05.
Furthermore, we analyzed tumor size, lymph node metastasis, TNM stage, and GRK6 expression in a Cox regression multivariate analysis model to explore the independent hazard effect of each factor (Table 3). Although lymph node metastasis showed no statistical significance, tumor size (HR 3.146, 95% CI 1.362–7.267, P=0.007), TNM stage (HR 2.423, 95% CI 1.147–5.117, P=0.020), and GRK6 expression (HR 2.132, 95% CI 1.009–4.507, P=0.047) were all independently correlated with disease recurrence.
Table 3.
Multivariate analysis for the disease-free survival of PTC patients.
| Variable | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Tumor size (vs. ≤2.0 cm) | 3.146 | 1.362–7.267 | 0.007* |
| LN metastasis (vs. negative) | 1.455 | 0.668–3.172 | 0.345 |
| TNM stage (vs. I–II) | 2.423 | 1.147–5.117 | 0.020* |
| GRK6 expression (vs. low) | 2.132 | 1.009–4.507 | 0.047* |
LN – lymph node; CI – confidence interval.
P<0.05.
GRK6 directly promotes PTC cell proliferation and invasion
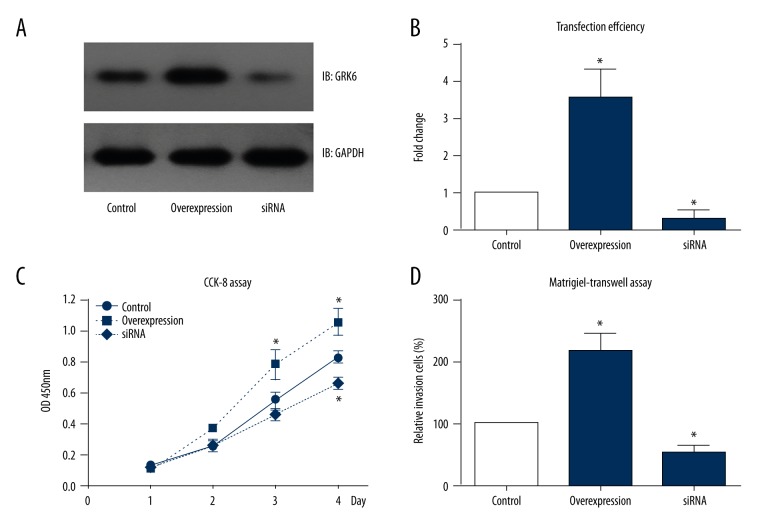
Weperformed cellular studies to investigate the underlying mechanisms of GRK6 in prompting PTC progression. We transfected TPC-1 cells with GRK6 plasmid or GRK6 siRNA (Figure 3A, 3B). After we confirmed the transfection efficiency by Western blot, the cells were tested by MTT assay to evaluate cell viability, which showed a significantly enhanced proliferation pattern in GRK6-overexpressing cells. In contrast, cells transfected with GRK6 siRNA exhibited decreased cell viability (Figure 3C). We also assessed the invasion of TPC-1 cells by use of Matrigel-Transwell strategy. As expected, GRK6 can positively regulate the invasion process of PTC tumor cells (Figure 3D). Cells with overexpressed GRK6 showed approximately 2.3 times more invasion cells than in the control group, while cells silencing GRK6 showed a 40% decrease in invasion capacity.
Figure 3.
GRK6 enhanced PTC cell proliferation and invasion. (A) GRK6-plasmids and GRK6-siRNA were transfected into TPC-1 cells using Fugene6 transcription reagent, and immunoblotting was performed 48 h after transfection. (B) Semi-quantification of the transfection efficiency compared to the protein level in the control group. (C) Cell viability was tested using a CCK-8 kit, which revealed a positive regulatory role of GRK6 in the proliferation of TPC-1 cells. (D) Similarly, transfected cells were subjected to a Transwell assay pre-coated with Matrigel. Data analysis showed that GRK6-overexpression enhanced cell invasion, while GRK6-siRNA attenuated cell invasion. * P<0.05 compared to control group.
Discussion
Post-translational modification is critical in regulating protein stability and functions, which is catalyzed by various enzymes. Accumulating results show the nonnegligible roles of modification enzymes in tumor progression, such as the ubiquitinases [20], deubiquitinases [21], kinases [22], and phosphatases [23]. GRKs were first reported to catalyze the phosphorylation and desensitization of GPCRs. Nevertheless, recent studies identified its GPCR-independent roles in regulating cellular functions [24]. For example, GRK2, GRK5, and GRK6 can mediate TNFα-induced NF-κB signaling via direct phosphorylation of IκBα [25,26]. Moreover, GRKs were reported to be involved in tumor development and progression. GRK5 can functionally regulate well-known cancer-related proteins such as Wnt and tumor suppressor p53 [27]. Another example is the upregulation of GRK5 in glioblastoma stem cells, indicating its significance in promoting tumor proliferation [12].
Here, we identified the expression pattern of GRK6 in PTCs for the first time. According to our data, GRK6 was up-regulated in PTCs, and was significantly correlated with tumor size and tumor stage. In addition, univariate and multivariate analyses of disease-free survival suggested that high GRK6 level was an unfavorable factor indicating a higher disease recurrence risk. In other words, GRK6 showed tumor-promoting roles in PTCs. However, it seems that GRK6 showed completely different functions in medulloblastoma, in which it can suppress tumor proliferation [13]. In our opinion, the distinct roles of GRK6 can be explained by its subcellular localization and catalyzed substrates. In medulloblastoma, GRK6 may function through phosphorylating and desensitizing the CXCR4 receptor, which was previously reported to promote medulloblastoma [28].
To better confirm the role of GRK6 in PTCs, we used a PTC cell line, TPC-1 cells, as a cell model to assess the tumor biology. Our data revealed that heterogenous overexpression of GRK6 can enhance both cell proliferation and invasion processes, while silencing of GRK6 attenuated cell viability and invasion. These data provide a novel direction for drug development and tumor therapy. However, the detailed molecular mechanisms of its tumor-promoting role, such as its substrates and signaling pathways, in PTCs need further investigation.
Conclusions
In summary, our data provide the first evidence that GRK6 expression is up-regulated in PTCs. We also explored the clinical value of GRK6 in PTC patients, such as assessing disease progression and predicting recurrence risk. We showed that GRK6 can directly promote PTC cell proliferation and invasion, revealing its potential in therapeutic development.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–67. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Roh J-L, Park J-Y, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: Pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg. 2007;245(4):604–10. doi: 10.1097/01.sla.0000250451.59685.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Taskforce. Thyroid. 2006;16(2):109–42. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 4.Grant CS. Recurrence of papillary thyroid cancer after optimized surgery. Gland Surg. 2015;4(1):52–62. doi: 10.3978/j.issn.2227-684X.2014.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow SM, Law SC, Chan JK, et al. Papillary microcarcinoma of the thyroid – prognostic significance of lymph node metastasis and multifocality. Cancer. 2003;98(1):31–40. doi: 10.1002/cncr.11442. [DOI] [PubMed] [Google Scholar]
- 6.Dohlman HG, Thorner J, Caron MG, Lefkowitz RJ. Model systems for the study of seven-transmembrane-segment receptors. Ann Rev Biochem. 1991;60(1):653–88. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- 7.Xiao K, Liu H. “Barcode” and differential effects of GPCR phosphorylation by different GRKs. G Protein-Coupled Receptor Kinases. 2016:75–120. [Google Scholar]
- 8.Gainetdinov RR, Premont RT, Bohn LM, et al. Desensitization of G protein – coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–44. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 9.Dorn GW. GRK mythology: G-protein receptor kinases in cardiovascular disease. J Mol Med. 2009;87(5):455–63. doi: 10.1007/s00109-009-0450-7. [DOI] [PubMed] [Google Scholar]
- 10.Lombardi MS, Kavelaars A, Cobelens PM, et al. Adjuvant arthritis induces down-regulation of G protein-coupled receptor kinases in the immune system. J Immunol. 2001;166(3):1635–40. doi: 10.4049/jimmunol.166.3.1635. [DOI] [PubMed] [Google Scholar]
- 11.Miyagawa Y, Ohguro H, Odagiri H, et al. Aberrantly expressed recoverin is functionally associated with G-protein-coupled receptor kinases in cancer cell lines. Biochem Biophys Res Commun. 2003;300(3):669–73. doi: 10.1016/s0006-291x(02)02888-7. [DOI] [PubMed] [Google Scholar]
- 12.Kaur G, Kim J, Kaur R, et al. G-protein coupled receptor kinase (GRK)-5 regulates proliferation of glioblastoma-derived stem cells. J Clin Neurosci. 2013;20(7):1014–18. doi: 10.1016/j.jocn.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Yuan L, Zhang H, Liu J, et al. Growth factor receptor-Src-mediated suppression of GRK6 dysregulates CXCR4 signaling and promotes medulloblastoma migration. Mol Cancer. 2013;12(1):18. doi: 10.1186/1476-4598-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutton N, Smith N, Thomas AJ, et al. GRK6 deficiency promotes tumor aggressiveness and metastasis in a murine model of human lung cancer. AACR. 2013 [Google Scholar]
- 15.Li Y-P. GRK6 expression in patients with hepatocellular carcinoma. Asian Pac J Trop Med. 2013;6(3):220–23. doi: 10.1016/S1995-7645(13)60027-9. [DOI] [PubMed] [Google Scholar]
- 16.DeLellis RA. Pathology and genetics of tumours of endocrine organs. IARC; 2004. [Google Scholar]
- 17.Ren X-R, Reiter E, Ahn S, et al. Different G protein-coupled receptor kinases govern G protein and β-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 2005;102(5):1448–53. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8(3):4888–900. doi: 10.18632/oncotarget.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakayama KI, Nakayama K. Ubiquitin ligases: Cell-cycle control and cancer. Nat Rev Cancer. 2006;6(5):369–81. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Zhang Q, Li K, et al. Prognostic significance of USP33 in advanced colorectal cancer patients: new insights into β-arrestin-dependent ERK signaling. Oncotarget. 2016;7(49):81223–40. doi: 10.18632/oncotarget.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gollob JA, Wilhelm S, Carter C, Kelley SL, editors. Role of Raf kinase in cancer: Therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway Seminars in oncology. Elsevier; 2006. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–47. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 24.Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: More than just kinases and not only for GPCRs. Pharm Ther. 2012;133(1):40–69. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter J, Benovic JL, Parameswaran N. G-protein coupled receptor kinases mediate TNFa-induced NFKB signaling via direct interaction with and phosphorylation of IKBCX. inflammatory signaling in macrophages: regulation by G-protein coupled receptor kinase-2 and 5. 2010:80. [Google Scholar]
- 26.Ohba Y, Nakaya M, Watari K, et al. GRK6 phosphorylates IκBα at Ser 32/Ser 36 and enhances TNF-α-induced inflammation. Biochem biophys Res Commun. 2015;461(2):307–13. doi: 10.1016/j.bbrc.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 27.Kim JI, Chakraborty P, Wang Z, Daaka Y. G-protein coupled receptor kinase 5 regulates prostate tumor growth. J Urol. 2012;187(1):322–29. doi: 10.1016/j.juro.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 28.Ozawa PMM, Ariza CB, Ishibashi CM, et al. Role of CXCL12 and CXCR4 in normal cerebellar development and medulloblastoma. Int J Cancer. 2016;138(1):10–13. doi: 10.1002/ijc.29333. [DOI] [PubMed] [Google Scholar]