Abstract
Objective
Laron syndrome (LS) is a rare, autosomal recessive disorder in humans caused by loss-of-function mutations of the growth hormone receptor (GHR) gene. To establish a large animal model for LS, pigs with GHR knockout (KO) mutations were generated and characterized.
Methods
CRISPR/Cas9 technology was applied to mutate exon 3 of the GHR gene in porcine zygotes. Two heterozygous founder sows with a 1-bp or 7-bp insertion in GHR exon 3 were obtained, and their heterozygous F1 offspring were intercrossed to produce GHR-KO, heterozygous GHR mutant, and wild-type pigs. Since the latter two groups were not significantly different in any parameter investigated, they were pooled as the GHR expressing control group. The characterization program included body and organ growth, body composition, endocrine and clinical-chemical parameters, as well as signaling studies in liver tissue.
Results
GHR-KO pigs lacked GHR and had markedly reduced serum insulin-like growth factor 1 (IGF1) levels and reduced IGF-binding protein 3 (IGFBP3) activity but increased IGFBP2 levels. Serum GH concentrations were significantly elevated compared with control pigs. GHR-KO pigs had a normal birth weight. Growth retardation became significant at the age of five weeks. At the age of six months, the body weight of GHR-KO pigs was reduced by 60% compared with controls. Most organ weights of GHR-KO pigs were reduced proportionally to body weight. However, the weights of liver, kidneys, and heart were disproportionately reduced, while the relative brain weight was almost doubled. GHR-KO pigs had a markedly increased percentage of total body fat relative to body weight and displayed transient juvenile hypoglycemia along with decreased serum triglyceride and cholesterol levels. Analysis of insulin receptor related signaling in the liver of adult fasted pigs revealed increased phosphorylation of IRS1 and PI3K. In agreement with the loss of GHR, phosphorylation of STAT5 was significantly reduced. In contrast, phosphorylation of JAK2 was significantly increased, possibly due to the increased serum leptin levels and increased hepatic leptin receptor expression and activation in GHR-KO pigs. In addition, increased mTOR phosphorylation was observed in GHR-KO liver samples, and phosphorylation studies of downstream substrates suggested the activation of mainly mTOR complex 2.
Conclusion
GHR-KO pigs resemble the pathophysiology of LS and are an interesting model for mechanistic studies and treatment trials.
Keywords: Growth hormone receptor, Laron syndrome, Pig model, Dwarfism, Hypoglycemia, Insulin-like growth factor 1, Signaling
Abbreviations: 4EBP1, eukaryotic initiation factor 4E binding protein 1; aa, amino acid; AKT, serine-threonine protein kinase; AMPK, AMP-activated protein kinase; CRISPR/Cas, clustered regularly interspaced short palindromic repeats/CRISPR-associated; DAB, 3,3′-diaminobenzidine; DXA, dual-energy X-ray absorptiometry; eIF4E, eukaryotic translation initiation factor 4E; ELISA, enzyme-linked immunosorbent assay; GH, growth hormone; GHR, growth hormone receptor; GSK3B, glycogen synthase 3 beta; HDL, high-density lipoprotein; HOMA, homeostatic model assessment; HSL, hormone-sensitive lipase; IGF1, insulin-like growth factor 1; IGFBP, IGF-binding protein; IgG, immunoglobulin G; INSR, insulin receptor; IRS1, insulin receptor substrate 1; JAK2, Janus kinase 2; LS, Laron syndrome; LSM, least squares mean; LDL, low-density lipoprotein; LEPR, leptin receptor; LPL, lipoprotein lipase; MAPK, mitogen-activated protein kinase; MRI, magnetic resonance imaging; mTOR, mechanistic target of rapamycin; mTORC, mTOR complex; PCR, polymerase chain reaction; PI3K, phosphoinositide 3 kinase; PPARG, peroxisome proliferator-activated receptor gamma; RIA, radioimmunoassay; S6K, protein S6 kinase 1; SE, standard error; sgRNA, single guide RNA; STAT, signal transducer and activator of transcription; TBS, Tris-buffered saline
Highlights
-
•
GHR-deficient pigs reveal postnatal growth retardation, disproportionate organ growth and an increased total body fat content.
-
•
GHR-deficient pigs show markedly reduced serum IGF1 and IGFBP3 levels, and transient juvenile hypoglycemia.
-
•
Increased expression and phosphorylation of IRS1 in liver of adult GHR-deficient pigs suggest increased insulin sensitivity.
-
•
Increased phosphorylation of JAK2 in liver of GHR-deficient pigs may be explained by higher serum leptin levels and activation of hepatic LEPR.
1. Introduction
Laron syndrome (LS) is a rare, autosomal recessive, hereditary disorder caused by loss-of-function mutations in the growth hormone receptor (GHR) gene (https://www.omim.org/entry/600946), initially described as a syndrome of primary growth hormone (GH) resistance or insensitivity ([1]; reviewed in [2], [3]). As a consequence, LS patients have low levels of insulin-like growth factor 1 (IGF1) and – due to the lack of feedback inhibition of GH secretion – high levels of GH [3]. A few hundred cases of LS have been reported world-wide, caused by a variety of GHR mutations (reviewed in [4]). Among them is an isolated, more homogeneous population of GHR deficient patients in Ecuador with only two distinct mutations of the GHR gene [5], [6], [7].
The main clinical feature is short stature. In addition, LS patients may exhibit reduced muscle strength and endurance, hypoglycemia in infancy, delayed puberty, obesity, and distinct facial features, including a protruding forehead, sunken bridge of the nose, and blue sclerae (reviewed in [3], [8]). The standard treatment of LS is long-term application of recombinant IGF1, which increases growth velocity and improves adult height, but it may lead to a spectrum of side effects, in particular hypoglycemia ([9], [10]; reviewed in [11]).
A particularly interesting observation in LS patients is their reduced incidence of malignancies ([12], [13]; reviewed in [8]). In addition, LS patients from the cohort in Ecuador have been shown to be protected against the development of type 2 diabetes despite severe obesity [7].
Although mechanistic studies have been performed in cell lines derived from LS patients and healthy controls [7], [14], animal models are of pivotal importance for understanding the pathophysiology of LS in vivo. In particular, GHR-deficient mice [15] have provided new insights into the consequences of GH insensitivity for body and organ growth, body composition, endocrine and metabolic functions, and reproduction, as well as aging and life expectancy (reviewed in [16]). More recently, inducible/tissue-specific Ghr knockout (KO) mouse models have helped to define the specific roles of GHR in liver, muscle, and adipose tissue and revealed interesting differences compared with constitutive Ghr KO mice [17], [18]. However, due to their small size, short life expectancy and physiological differences compared with humans, findings from mouse models may be difficult to extrapolate to the clinical situation of LS patients. In general, genetically tailored pig models are useful to bridge the gap between proof-of-concept studies in rodent models and clinical studies in patients (reviewed in [19], [20]). Thus, we have developed a GHR-deficient (GHR-KO) pig model and show that it resembles important aspects of LS pathophysiology and reveals altered activation of signaling cascades in the liver.
2. Materials and methods
2.1. Generation of GHR mutant pigs using CRISPR/Cas
All animal procedures in this study were approved by the responsible animal welfare authority (Regierung von Oberbayern; permission 55.2-1-54-2532-70-12) and performed according to the German Animal Welfare Act and Directive 2010/63/EU on the protection of animals used for scientific purposes.
For CRISPR/Cas-assisted GHR gene disruption using a single guide RNA (sgRNA) specific for exon 3 sequence 5′-TTCATGCCACTGGACAGATG-3′, a corresponding oligonucleotide was cloned into the pEX-A-U6-gRNA vector as described previously [21]. Cas9 mRNA and sgRNA were in vitro-transcribed using the Ambion Maxiscript SP6 kit (Thermo Fisher Scientific).
Porcine zygotes (German landrace background) were produced in vitro as described previously [22], and Cas9 mRNA (50 ng/μL) and sgRNA (100 ng/μL) was injected into their cytoplasm 8.5–9.5 h after in vitro fertilization. Recipient gilts were synchronized in the estrous cycle by oral administration of 4 mL Altrenogest (Regumate®; MSD Animal Health) for 15 days, followed by intramuscular injection of 750 IU ECG (Intergonan®; MSD Animal Health) and 750 IU HCG (Ovogest®; MSD Animal Health) after an additional 24 and 104 h, respectively. Embryo transfer was performed laparoscopically into one oviduct [23], [24]. Pregnancy was confirmed by ultrasonographic examination first on day 21 and again 4–6 weeks later.
Genomic DNA was isolated from tail tips of piglets using the Wizard DNA Extraction Kit (Promega). GHR mutations were detected by sequencing a GHR exon 3 PCR product obtained using primers GHR_Fw 5′-acc gct ctg aag ctg tga cc-3′ and GHR_Rv 5′-cac cct cag ata ctc tca tgc-3′. Based on the detected mutations, an XcmI restriction fragment length polymorphism assay was established, yielding fragments of 203 bp and 441 bp for wild-type GHR and a single fragment of 644 bp for the mutated GHR sequence.
Two female founder animals with different frameshift mutations were mated with wild-type boars to generate heterozygous F1 offspring. Heterozygous offspring of the same founder were intercrossed to obtain homozygous animals (GHR-KO) with the respective GHR mutations. The resulting pedigrees are shown in Suppl. Fig. 1.
2.2. Ligand immunostaining of porcine GHR
Liver and kidney tissue of GHR-KO and control pigs was fixed overnight in 4% formaldehyde and routinely embedded in paraffin. Paraffin sections were dewaxed, and endogenous peroxidase and biotin were blocked with 1% H2O2 in Tris-buffered saline (TBS) for 15 min and by using the avidin/biotin blocking kit (no. SP-2001; Vector Laboratories), respectively. After blocking with 0.5% fish gelatin for 30 min, the slides were incubated in 0.5 μg/mL recombinant rat GH (no. 16343667, ImmunoTools) in 0.2% fish gelatin solution overnight at 4 °C. They were then washed 3 times for 5 min in TBS and incubated in goat anti-rat GH polyclonal antibody solution (dilution 1:2,400, no. AF1566, R&D Systems) for 6 h at room temperature. After 3 washing steps in TBS (10 min each), the slides were incubated in biotinylated rabbit anti-goat IgG solution (dilution 1:100, no. BA-5000, Vector Laboratories) for 1 h at room temperature, washed 3 times for 10 min in TBS, and finally incubated with horseradish peroxidase-labelled avidin biotin complex for 30 min (no. PK-6100, VECTASTAIN Elite ABC-Peroxidase kit, Vector Laboratories). Immunoreactivity was visualized using 3,3′-diaminobenzidine tetrahydrochloride dihydrate (DAB) (brown color). Nuclear counterstaining was performed with Mayer's hemalum (blue color). As specificity controls for the ligand immunohistochemistry assay, rat GH as well as rat GH plus primary antibody were omitted.
2.3. Blood collection
Animals were fasted overnight (16 h) before blood collection from the jugular vein. After clotting for 30 min at room temperature, serum was separated by centrifugation (1200 × g) for 20 min at 6 °C and stored at −80 °C until analysis. For repeated blood sampling required to analyze GH secretion profiles, central venous catheters (Argon Careflow™; Merit Medical) were surgically inserted through the external ear vein. Blood samples were collected every 30 min for 9 h, starting at 11 a.m., and processed for serum collection as described above. During the test, the animals were fed regularly and had free access to water.
2.4. Clinical chemistry, hormone assays, IGFBP ligand blot analysis
Clinical-chemical parameters in serum were determined using a Cobas 311 system (Hitachi) or an AU480 autoanalyzer (Beckman-Coulter) and adapted reagents from Roche Diagnostics or Beckman-Coulter, respectively. Serum GH concentrations were measured by an ELISA for rat/mouse GH (EZRMGH-45K; Merck) that cross-reacts with porcine GH. To calculate the area under the GH curve, values below the quantification limit of the assay (<0.07 ng/mL) were arbitrarily set to 0.07 ng/mL. IGF1 levels in serum were determined by RIA after dissociation of IGF1 from IGFBPs by acidification and blocking the IGF1 binding sites with an excess of IGF2 [25]. IGFBP ligand blot analysis of serum samples was performed as described previously [26] using serial dilutions of recombinant human IGFBP3 (41/38 kDa), IGFBP2 (32 kDa), IGFBP5 (29 kDa) and IGFBP4 (24 kDa) for quantification. Plasma insulin was determined using a species-specific RIA (Merck Millipore) as previously described [27]. Blood glucose levels were determined immediately using a Precision Xceed® glucometer and Precision XtraPlus® test strips (Abbott) [28]. Serum leptin levels were measured using a multi-species leptin radioimmunoassay (Cat. # XL-85K; EMD Millipore Corporation) that has been validated for porcine samples [29].
2.5. Growth parameters and body composition
Body weight and body length (distance between tip of the snout and tail root in straightened animals) of GHR-KO and control pigs were determined at weekly intervals. Relative body length was calculated by dividing the body length by the cube root of the body weight to retain the same dimensions. Since not all animals could be weighed/measured at exactly the same ages, raw data were adjusted by linear interpolation to defined ages/time points.
The percentage of total body fat was determined in 6-month-old GHR-KO and control pigs using dual-energy X-ray absorptiometry (DXA; Lunar iDXA, GE Healthcare) as previously described [30]. In addition, magnetic resonance imaging (MRI; Magnetom Open, Siemens) [31] was performed to visualize and determine the muscle to fat ratio as the area of longissimus dorsi muscle divided by the area of its overlying back fat at the last rib.
2.6. Necropsy
GHR-KO and control pigs were euthanized at 6 months of age under anesthesia by intravenous injection of T61® (Intervet) and immediately subjected to necropsy. Organs were dissected and weighed to the nearest mg. Tissue samples were collected as described previously [32] and routinely fixed in neutral buffered formalin solution (4%) for 24 h or frozen immediately on dry ice and stored at −80 °C for molecular profiling. Formalin-fixed tissue specimens were embedded in paraffin. Muscle sections were stained with hematoxylin and eosin (H&E).
2.7. Immunoblot analysis of signaling cascades
The concentrations and phosphorylation status of GHR-related signaling molecules in the liver were evaluated by Western blot analyses as described previously [33]. Briefly, liver tissue samples were homogenized in Laemmli extraction buffer, and the protein content was determined by the bicinchoninic acid protein assay. Forty micrograms of total protein was separated by SDS-PAGE and transferred to PVDF membranes (Millipore) by electro-blotting. Membranes were washed in TBS with 0.1% Tween-20 and blocked in 5% w/v fat-free milk powder (Roth) for 1 h. The membranes were then washed again and incubated in 5% w/v BSA (Roth) solution with the appropriate primary antibodies overnight at 4 °C. The antibodies and concentrations used are listed in Suppl. Table 1. After washing, the membranes were incubated in 5% w/v fat-free milk powder solution with the secondary antibody (donkey anti-rabbit; 1:2000; GE Healthcare) for 1 h. Bound antibodies were detected using the ECL Advance Western Blotting Detection Kit (GE Healthcare) and appropriate films from the same supplier. Band intensities were quantified using the ImageQuant software package (GE Healthcare).
2.8. Statistical analyses
Longitudinal data for body weight and body length or relative body length respectively were analyzed using PROC MIXED (SAS 8.2), taking the effects of pig line (#2529; #2533), group (GHR-KO; control), sex, age, and interaction group*age into account. Least squares means (LSMs) and standards errors (SEs) of LSMs were calculated for group*age and compared using Student's t-test. Data for glucose homeostasis and serum lipid concentrations were analyzed using PROC GLM (SAS 8.2), taking the effects of group, sex, age and the interaction group*age into account. LSMs and SEs were calculated for group*age and compared using Student's t-test. Body composition, organ weight and clinical-chemical data were analyzed using PROC GLM taking the effects of group and sex into account. LSMs and SEs were calculated for groups and compared using Student's t-test. IGFBP ligand blot and Western immunoblot data were evaluated for significant differences between GHR-KO and control pigs using the Mann-Whitney U test.
3. Results
3.1. Generation of a growth hormone receptor-deficient pig model
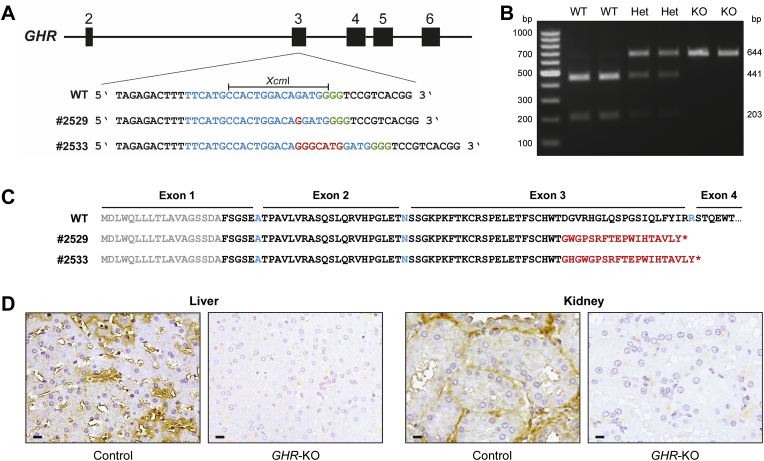
We employed CRISPR/Cas9 technology to generate GHR knockout (GHR-KO) pigs as a large animal model for Laron syndrome (LS). In vitro-transcribed RNA encoding Cas9 and sgRNA specific for GHR exon 3 was injected into in vitro fertilized porcine oocytes, which were transferred to recipient gilts. In total, 8 piglets were born, of which 3 showed monoallelic mutations in the GHR gene. Two female founder animals carried monoallelic insertions of 1 bp (#2529) or 7 bp (#2533) (Figure 1A). The two founder animals were mated with wild-type boars to establish pedigrees for phenotypic analyses of GHR-deficient (GHR-KO) vs. GHR-expressing F2 animals (Suppl. Fig. 1). The two lines were kept separate except in one experiment to test the fertility of GHR-KO pigs. Wild-type, heterozygous, and GHR-KO littermates were identified by PCR and restriction fragment length polymorphisms of the mutated and wild-type GHR alleles (Figure 1B). Since we did not observe significant differences between heterozygous GHR mutant and wild-type animals (Suppl. Fig. 2), they were pooled and used as the GHR-expressing control group.
Figure 1.
Generation of a GHR-deficient pig model using CRISPR/Cas technology. (A) Partial DNA sequence of GHR exon 3. The sgRNA binding site is indicated in blue and the protospacer adjacent motif (PAM) in green. Insertions (red) of 1 bp (founder #2529) or 7 bp (founder #2533) lead to a shift of the reading frame. WT = wild type. (B) Restriction fragment length polymorphism analysis to detect the WT GHR sequence as well as monoallelic (Het) and biallelic (KO) mutations. (C) Partial amino acid sequences encoded by the WT and mutant GHR alleles. The signal peptide is shown in gray, WT GHR aa sequence in black (aa encoded by adjacent non-symmetrical exons in blue), missense aa sequence in red, and the premature termination codon as an asterisk. (D) Ligand immunohistochemistry demonstrating the absence of functional GHR (brown staining in control) in GHR-KO pigs. Chromogen: DAB; counterstain: Mayer's hemalum; bar = 10 μm.
3.2. Homozygous frameshift mutations in GHR exon 3 result in GHR deficiency
The insertion of 1 bp (#2529) or 7 bp (#2533) leads to a shift in the reading frame in GHR exon 3. The mutant GHR transcripts encode the 18-aa signal peptide and 51 aa of the extracellular GHR domain, followed by an 18-aa or a 20-aa missense sequence and premature termination codon after 87 aa (#2529) or 89 aa (#2533) (Figure 1C).
The presence of GHR was investigated in liver and kidney sections since these tissues naturally express high levels of GHR [34]. To evaluate GH binding, the sections were incubated with recombinant GH, and bound GH was detected using specific antibodies. This ligand immunohistochemistry approach showed strong GH binding in control tissues, while GH binding was absent in tissue sections from GHR-KO animals (Figure 1D).
3.3. Decreased serum IGF1 and IGFBP3, and increased IGFBP2 in GHR-KO pigs
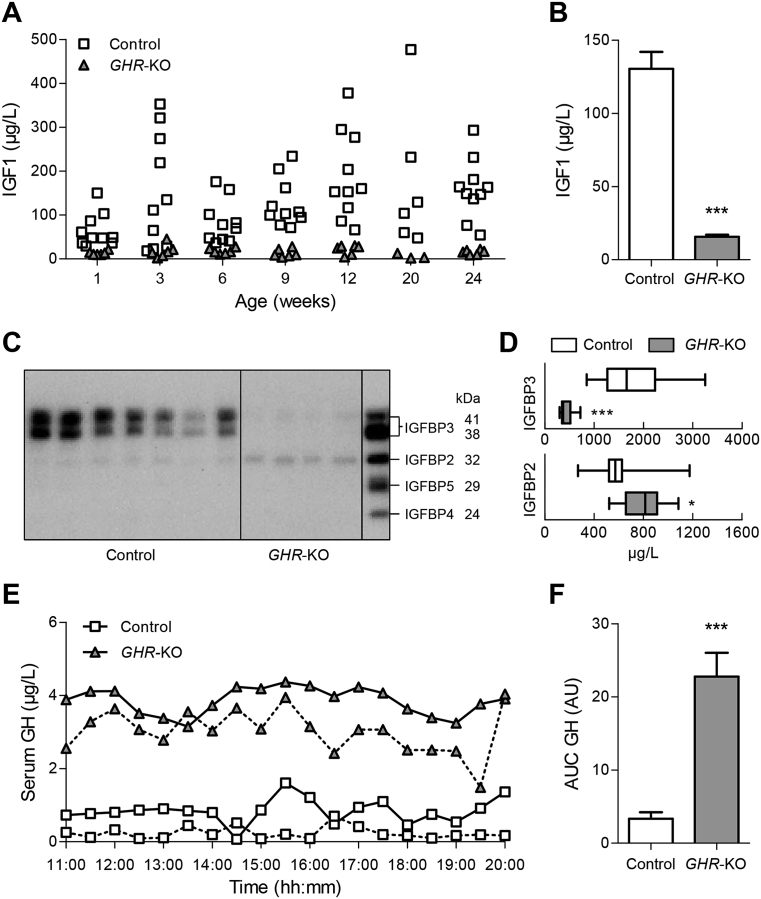
GHR-KO pigs of all ages showed a marked reduction in serum insulin-like growth factor 1 (Figure 2A,B). IGF binding proteins (IGFBPs) were evaluated by ligand blot analysis in 6-month-old animals using a dilution series of recombinant human IGFBPs for quantification (Figure 2C). IGFBP3 was significantly decreased in GHR-KO pigs (426 ± 41 μg/L vs. 1775 ± 205 μg/L in control animals; p < 0.0001), while IGFBP2 was significantly increased (799 ± 53 μg/L vs. 607 ± 66 μg/L in control animals; p = 0.0272) (Figure 2D).
Figure 2.
Serum IGF1, IGFBP and GH concentrations of GHR-KO compared with control pigs. (A) Scatter plot of serum IGF1 levels of GHR-KO and control pigs over time. (B) Means and standard deviations of all serum IGF1 values displayed in panel A (GHR-KO: n = 42; control: n = 69). (C) Representative IGFBP ligand blot. Right lane displays recombinant human IGFBP3 (41/38 kDa), IGFBP2 (32 kDa), IGFBP5 (29 kDa) and IGFBP4 (24 kDa). (D) Quantification of IGFBP3 and IGFBP2 in serum from GHR-KO (n = 10) and control pigs (n = 12). The figure shows medians, 25th and 75th percentiles (box), and extremes (whiskers). (E) Representative GH secretion profiles of two female GHR-KO and two female control pigs. (F) Area under the GH curve (AUC; means and standard deviations for 6 female GHR-KO and 5 female/1 male control pigs). AU = arbitrary units. *p < 0.05; ***p < 0.001.
3.4. GHR-KO pigs have high levels of circulating GH
To evaluate effects of GHR deficiency on the pulsatile secretion of GH, we collected serial blood samples at 30-min intervals over a period of 9 h (starting at 11 a.m.) from five female and one male 9-month-old GHR-KO pigs and from six age-matched female controls. Serum GH levels of GHR-KO pigs were high with partially preserved pulsatility (area under the GH curve was increased 6.8-fold in GHR-KO compared with control pigs), indicating a disturbance in the negative feedback control of GH secretion (Figure 2E,F; Suppl. Fig. 3).
3.5. GHR-KO pigs show severe growth retardation
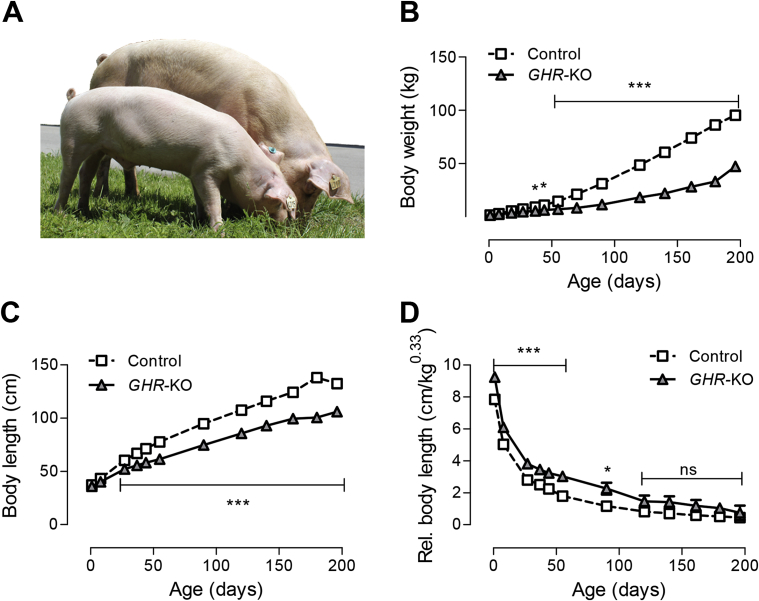
The birth weight of GHR-KO piglets did not differ from control littermates. First significant growth retardation became obvious at five weeks of age (p = 0.045), leading to a 62% reduction in body weight of 6-month-old GHR-KO pigs (33.0 ± 1.5 kg) compared with age-matched control animals (86.2 ± 1.1 kg; p < 0.0001) (Figure 3A,B). Body length at birth did not differ between GHR-KO and control piglets (33.9 ± 1.4 cm vs. 37.3 ± 1 cm; p = 0.4068). Significant differences in body length appeared at four weeks of age (52.2 ± 1.4 cm in GHR-KO vs. 60.3 ± 1 cm in control animals; p < 0.0001). At six months of age, the body length of GHR-KO pigs was reduced by 27% compared with control pigs (100.6 ± 1.6 cm vs. 138.1 ± 1.4 cm; p < 0.0001) (Figure 3C). Up to an age of four months, weight gain was more affected than linear growth by GHR deficiency, as indicated by an increased relative body length (body length divided by the cube root of body weight) of GHR-KO pigs (Figure 3D). No growth parameters exhibited significant sex-related differences.
Figure 3.
Body weight gain and growth of GHR-KO compared with control pigs. (A) GHR-KO pig (front) and control littermate aged 6 months. (B) Body weight gain. (C) Body length. (D) Relative body length (body length divided by the cube root of body weight). These parameters were determined in 12 GHR-KO and 25 control pigs. Panels A–D show least squares means (LSMs) and standard errors of LSMs estimated for group*age (see 2.8 for the statistical model). *p < 0.05; **p < 0.01; ***p < 0.001; ns = not significant.
3.6. GHR-KO pigs show an increased proportion of body fat and a reduced ratio of muscle to fat tissue
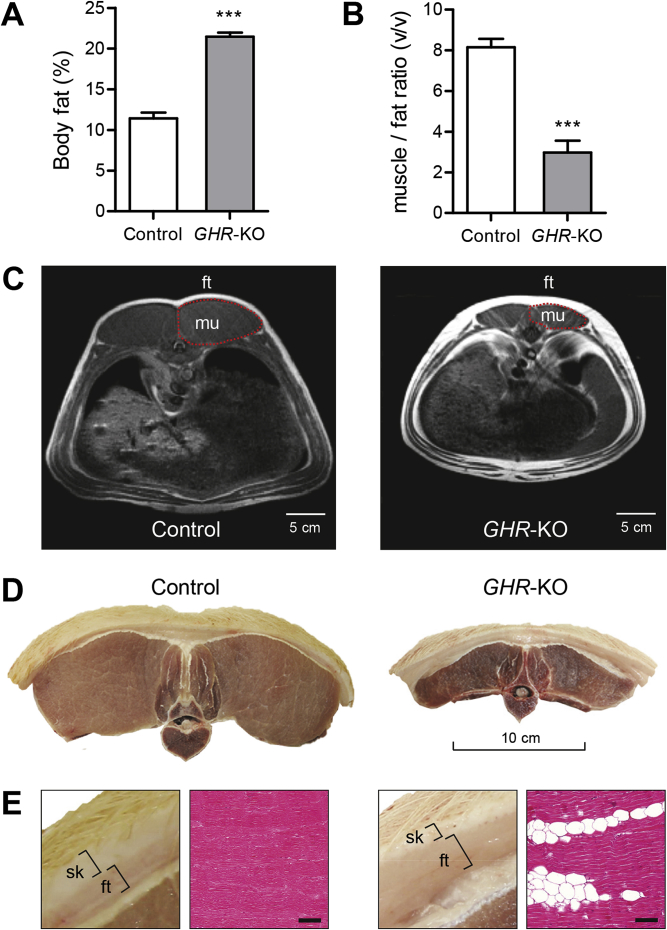
DXA analysis revealed a markedly increased percentage of total body fat in 6-month-old GHR-KO pigs (21.5 ± 0.7% vs. 11.4 ± 0.5% in age-matched control animals; p < 0.0001) (Figure 4A). While female pigs in the control group displayed significantly higher body fat content than male pigs (13.2 ± 0.7% vs. 9.6 ± 0.7%; p = 0.0009), no sex-related differences were observed in GHR-KO pigs (22.9 ± 1% in males vs. 22 ± 1% in females; p = 0.4530). To determine the ratio of muscle to fat tissue, MRI scans were performed at the location of the last rib, and the volume ratio of the longissimus dorsi muscle and its overlying back fat was calculated (Figure 4B,C). GHR-KO pigs showed a significantly reduced muscle to fat tissue ratio compared with control pigs (3.0 ± 0.6 vs. 8.2 ± 0.4; p < 0.0001) (Figure 4B). No sex-related differences were observed in this parameter (Suppl. Table 2). The MRI findings were confirmed upon necropsy, showing a marked increase in the thickness of the subcutaneous fat tissue and a reduction in the size of the longissimus dorsi muscle in GHR-KO pigs (Figure 4D,E). Histological sections of skeletal muscle samples from GHR-KO pigs revealed markedly increased numbers of adipocyte section profiles between muscle fibers (Figure 4E).
Figure 4.
Body composition of 6-month-old GHR-KO compared with control pigs. (A) DXA analysis revealed a significantly higher amount of total body fat in GHR-KO pigs. (B) The calculated ratio of muscle to fat tissue from MRI images at the last rib revealed a significant shift towards fat tissue in GHR-KO pigs (GHR-KO: n = 12; control: n = 25; ***p < 0.001). Panels A and B show least squares means (LSMs) and standard errors of LSMs estimated for the 2 groups (see 2.8 for the statistical model). (C) Representative magnetic resonance images used to evaluate the volume of the longissimus dorsi muscle (mu) and its overlying back fat (ft) at the last rib in GHR-KO and control pigs. Note the larger subcutaneous and visceral fat depots in GHR-KO pigs. (D) Representative macroscopic cross-sections of the first lumbar vertebra, the two longissimus dorsi muscles and the overlying back fat and skin. (E) Higher magnification of D showing an increased ratio of subcutaneous fat (ft) to skin (sk) thickness in a GHR-KO compared with a control pig. Histological section (H&E stain) showing an increased amount of intramuscular fat in GHR-KO pigs (bar = 100 μm).
3.7. Disproportionate organ growth of GHR-KO pigs
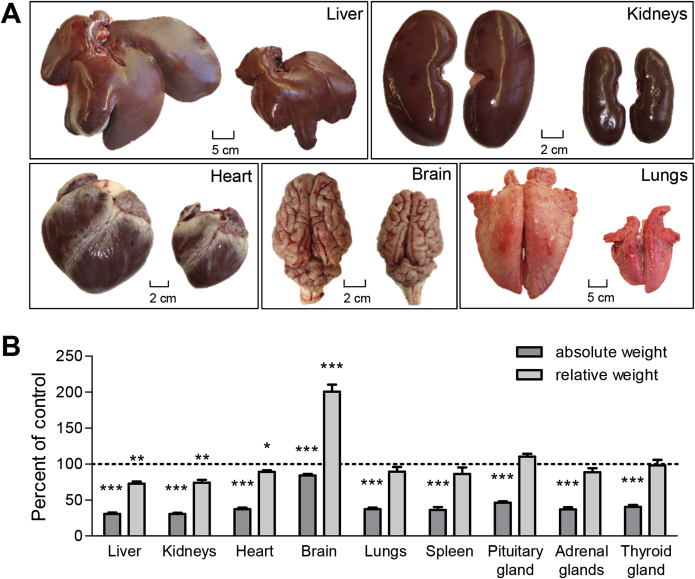
In 6-month-old GHR-KO pigs, absolute weights of all organs were significantly smaller than in age-matched control animals (Figure 5, Suppl. Table 3). Most organ weights of GHR-KO pigs were reduced proportionally to body weight. However, the relative weights of liver (73% of control animal relative liver weight; p = 0.0005), kidneys (73% of control animal relative kidney weight; p = 0.0002) and heart (87% of control animal relative heart weight; p = 0.0119) were significantly reduced, while relative brain weight was doubled (200% of control animal relative brain weight; p < 0.0001) (Figure 5, Suppl. Table 3).
Figure 5.
Disproportionate organ growth in GHR-KO compared with control pigs. GHR-deficiency led to a proportionate and disproportionate reduction in organ sizes. (A) Representative organs from control (left) and GHR-KO pigs (right). (B) Relative differences between GHR-KO and control pigs in absolute organ weights and in organ weight-to-body weight ratios (relative organ weights). These parameters were determined in 9 GHR-KO and 25 control pigs, and least squares means (LSMs) and standard errors of LSMs were estimated for the 2 groups (see 2.8 for the statistical model). *p < 0.05; **p < 0.01; ***p < 0.001.
3.8. Male and female GHR-KO pigs are fertile
The ovaries of 6-month-old GHR-KO gilts did not show obvious morphological differences from control ovaries. Mating of an 8-month-old GHR-KO boar (line #2529) with a GHR-KO sow (line #2533) of the same age resulted in a litter of 6 healthy GHR-KO piglets (Suppl. Fig. 4A). Their birth weight tended to be reduced in comparison to GHR-KO piglets derived from heterozygote × heterozygote matings (on average 0.75 kg compared with 1.3 kg). However, the animals showed catch-up growth and achieved a higher body weight at 6 months of age than GHR-KO offspring from heterozygous GHR-KO parents (43.8 ± 1.3 kg vs. 33.0 ± 2.2 kg; p < 0.0001; Suppl. Fig. 4B).
3.9. GHR-KO pigs show transient hypoglycemia, while insulin levels remain unaffected
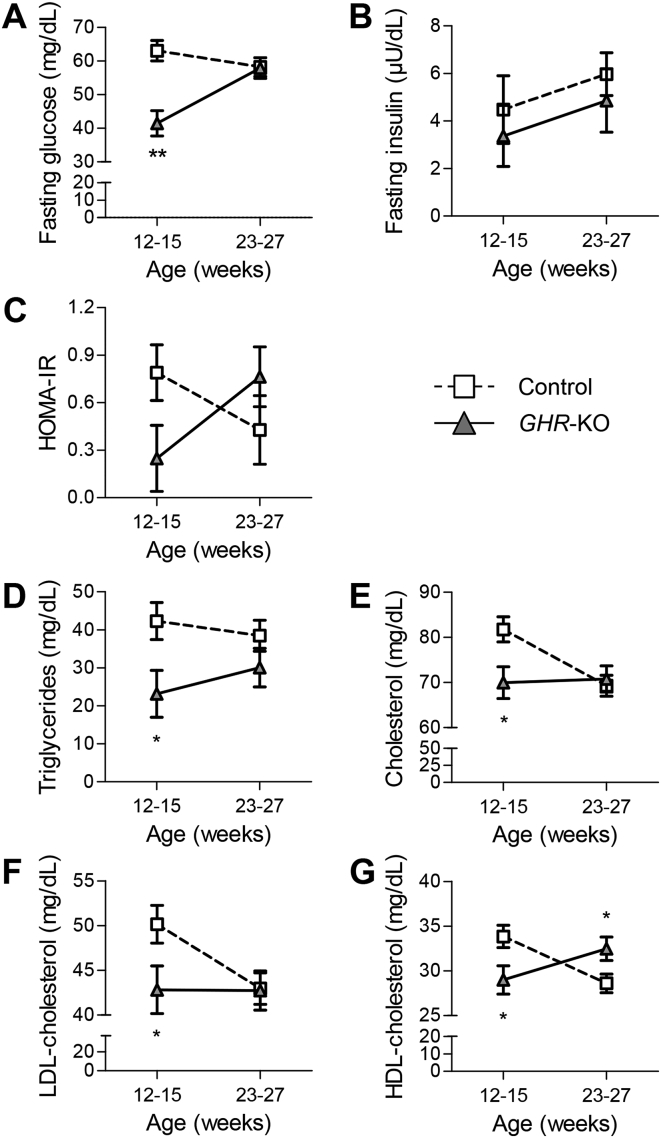
Young GHR-KO pigs (12–15 weeks old) showed significantly reduced fasting blood glucose levels (41.5 ± 3.8 mg/dL vs. 63.1 ± 3.1 mg/dL in age-matched control animals; p = 0.0001), while this difference disappeared in older animals (23–27 weeks) (Figure 6A). Fasting serum insulin levels of GHR-KO pigs did not differ from control pigs at any age (Figure 6B). In addition, the homeostatic model assessment (HOMA) for evaluating insulin resistance was calculated from fasting glucose and insulin concentrations. In agreement with their low fasting blood glucose concentrations, HOMA-IR values of young GHR-KO pigs tended to be reduced (0.25 ± 0.20 vs. 0.76 ± 0.19 in age-matched control pigs; p = 0.0635), but increased to 0.76 ± 0.18 in the older age group (p = 0.0839), when controls had a HOMA-IR of 0.43 ± 0.20 (p = 0.2584; Figure 6C). Analysis of variance revealed a significant (p < 0.05) interaction of group*age.
Figure 6.
Age-dependent changes in glucose and lipid homeostasis parameters in GHR-KO and control pigs. (A) Transient juvenile hypoglycemia in GHR-KO pigs. (B) Unchanged serum insulin concentrations. (C) Initially lower, then higher HOMA-IR score (interaction group*age: p < 0.05). Serum concentrations of (D) triglycerides, (E) cholesterol, (F) low-density lipoprotein (LDL)-cholesterol, and (G) high-density lipoprotein (HDL)-cholesterol levels were significantly lower in young GHR-KO pigs than in age-matched controls, but normalized with age. HDL-cholesterol levels of 23- to 27-week-old GHR-KO pigs were even higher than in their control littermates. At least 6 animals per group and age-class were investigated. Panels A–G show least squares means (LSMs) and standard errors of LSMs estimated for group*age (see 2.8 for the statistical model). *p < 0.05; **p < 0.01.
3.10. Serum lipid levels are reduced in young GHR-KO pigs
Young GHR-KO pigs (aged 12–15 weeks) revealed significantly decreased serum concentrations of triglycerides (23.2 ± 6.8 mg/dL vs. 42.3 ± 4.9 mg/dL in controls; p = 0.021; Figure 6D), cholesterol (70 ± 3.5 mg/dL vs. 81.8 ± 2.8 mg/dL in controls; p = 0.0129; Figure 6E), low-density lipoprotein (LDL)-cholesterol (42.8 ± 2.7 mg/dL vs. 50.1 ± 2.1 mg/dL in controls; p = 0.0392; Figure 6F) and high-density lipoprotein (HDL)-cholesterol (29.0 ± 1.6 mg/dL vs. 33.8 ± 1.3 mg/dL in controls; p = 0.0224; Figure 6G). In older GHR-KO pigs (23–27 weeks), serum triglyceride, cholesterol and LDL-cholesterol concentrations were similar to those of control pigs, while serum HDL-cholesterol levels were increased (32.5 ± 1.3 mg/dL vs. 28.6 ± 1.0 mg/dL in controls; p = 0.0272) (Figure 6D–G). Excluding triglycerides, all investigated lipid parameters were significantly (p < 0.0002) affected by sex, with higher levels in female than in male animals (Suppl. Table 4).
3.11. Additional alterations of clinical-chemical parameters in GHR-KO pigs
To screen for changes in organ functions and metabolic pathways, a broad spectrum of clinical-chemical parameters in serum of 6-month-old GHR-KO and control pigs were analyzed. While all parameters remained within physiological ranges, GHR-KO pigs displayed lower levels of creatinine (91.2 ± 6.5 μmol/L vs. 126.4 ± 5.0 μmol/L in controls; p = 0.0004) but higher levels of urea (7.4 ± 0.5 mmol/L vs. 4.3 ± 0.3 mmol/L in controls; p < 0.0001) (Suppl. Table 5).
3.12. GHR-KO pigs display significant changes in the activation of hepatic signaling cascades
Since the liver is a major target tissue of insulin and GH, we evaluated changes in associated signaling cascades by Western blot analyses of liver samples from fasted 6-month-old GHR-KO and control animals.
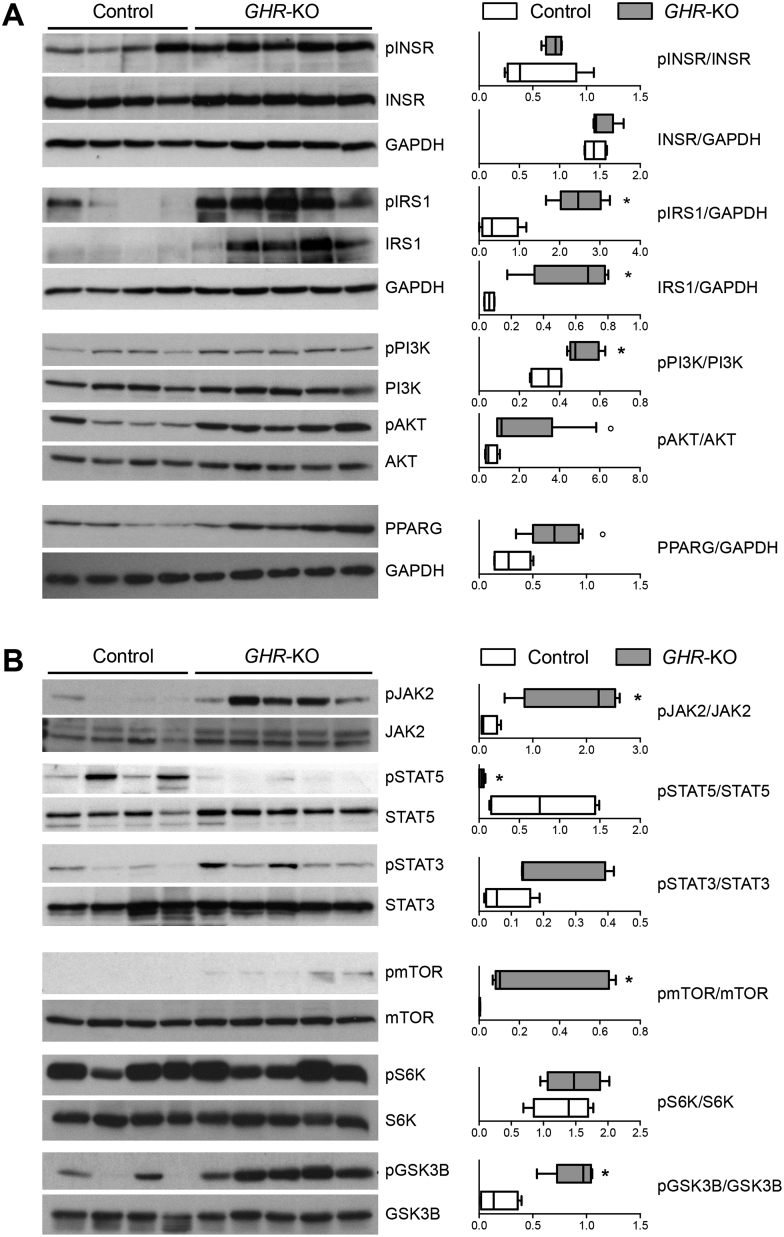
The total amounts of insulin receptor (INSR) and of phosphorylated INSR were unchanged in GHR-KO liver samples. In contrast, the concentrations of total and phosphorylated insulin receptor substrate 1 (IRS1) were significantly (p = 0.0159) increased in liver samples from GHR-KO vs. control pigs. The investigation of signal transducers downstream of the INSR revealed significantly increased phosphorylation of phosphoinositide 3 kinase (PI3K) and a trend (p = 0.0635) toward an increase in serine/threonine protein kinase AKT phosphorylation in GHR-KO liver samples. Furthermore, a trend (p = 0.0635) toward increased levels of total peroxisome proliferator-activated receptor gamma (PPARG) was observed (Figure 7A).
Figure 7.
Western blot analysis of signaling cascades in liver samples of 6-month-old fasted GHR-KO (n = 5) and control pigs (n = 4). (A) Insulin receptor-related signaling pathway and PPARG. (B) GHR- and mTOR-related signaling pathways. The box plots show medians, 25th and 75th percentiles (box), and extremes (whiskers). *p < 0.05; °p = 0.0635; evaluated using the Mann–Whitney U test.
GHR-KO liver samples showed significantly increased phosphorylation levels of Janus kinase 2 (JAK2). The phosphorylation levels of signal transducer and activator of transcription 5 (STAT5) were significantly reduced, while STAT3 phosphorylation showed a tendency to increase (p = 0.1111). Phosphorylation of STAT1 was not significantly different between GHR-KO and control pigs, whereas significantly increased phosphorylation levels of mitogen-activated protein kinase (MAPK) were detected (Suppl. Fig. 5).
In addition, liver extracts from GHR-KO pigs showed a significant increase in phosphorylated mechanistic target of rapamycin (mTOR), which was not detected in control liver samples. To distinguish between the activation of mTOR complex 1 (mTORC1) and 2 (mTORC2), we analyzed several specific up- and downstream signal transducers for each complex [35], [36] (Figure 7B; Suppl. Fig. 5).
A key element of mTORC1 action is protein S6 kinase 1 (S6K), which phosphorylates S6. GHR-KO liver samples did not show increased phosphorylation of S6K (Figure 7B). Phosphorylation of other effectors downstream of mTORC1 — eukaryotic initiation factor 4E binding protein 1 (4EBP1) and eukaryotic translation initiation factor 4E (eIF4E) — was unchanged in GHR-KO liver samples (Suppl. Fig. 5). Phosphorylation of AMP-activated protein kinase (AMPK), an inhibitor of mTORC1, was significantly increased in GHR-KO liver tissue (Suppl. Fig. 5). Collectively these data suggest that mTORC1 is not activated in liver of GHR-KO pigs.
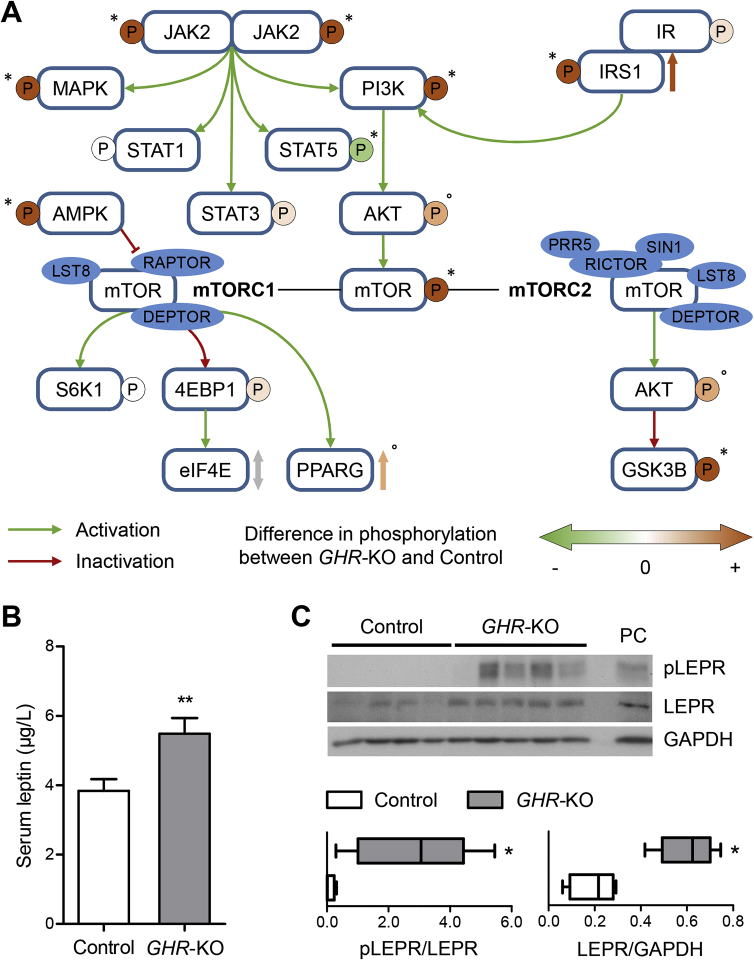
In contrast, one of the most important downstream substrates of mTORC2 — glycogen synthase 3 beta (GSK3B) – showed significantly increased phosphorylation in GHR-KO liver tissue (Figure 7B). Furthermore, the phosphorylation levels of potent regulators of mTORC2 action, PI3K and AKT, were also increased (Figure 7A). These findings suggest that mTORC2 is activated in the liver of GHR-deficient pigs. A summary of these findings is provided in Figure 8A.
Figure 8.
(A) Schematic summary of the changes in phosphorylation in INSR- and GHR-related signaling molecules in liver samples of GHR-KO compared with control pigs. *p < 0.05; °p = 0.0635; evaluated using the Mann–Whitney U test. (B) Significantly increased fasting serum leptin concentrations in 6-month-old GHR-KO vs. control pigs. The figure shows the estimated least squares means (LSMs) and standard errors of the LSMs for the two groups, taking into account the effect of sex (9 male/13 female control pigs; 6 male/6 female GHR-KO pigs). **p < 0.01 for the effect of group (PROC GLM). (C) Significantly increased expression and phosphorylation of LEPR in liver samples from GHR-KO compared with control pigs. PC = protein lysate from choroid plexus of a wild-type pig used as positive control. *p < 0.05; evaluated using the Mann–Whitney U test.
3.13. Serum leptin levels and hepatic leptin receptor activation are increased in GHR-KO pigs
Considering the expected reduced STAT5 phosphorylation but unexpected increased JAK2 phosphorylation in the liver of GHR-KO pigs, we measured serum leptin levels and hepatic leptin receptor (LEPR) activation. LEPR signaling involves activation of JAK2 and STAT3, but not STAT5 (reviewed in [37]). Overall, serum leptin concentrations of 6-month-old fasted GHR-KO pigs were significantly elevated compared with the controls (Figure 8B), with higher levels in females than in males (Suppl. Table 2). In addition, LEPR phosphorylation was significantly increased in GHR-KO vs. control liver samples (Figure 8C).
4. Discussion
This study reports a new large animal model for GHR deficiency (Laron syndrome, LS). Previously described pig models with impaired GH function, which are either GHR-deficient [38] or express a dominant negative GHR [39], were established on a minipig background that is already growth impaired and, thus, may not resemble all pathophysiological consequences of human LS. Therefore, we established our GHR-deficient model on a German landrace background with the physiological growth potential of domestic pigs and performed a comprehensive characterization of body and organ growth, body composition, endocrine and metabolic changes, and signaling cascades in liver.
Our GHR-deficient pig model exhibits important hallmarks of human LS, such as insensitivity to GH, low circulating IGF1 concentrations, and reduced postnatal body and organ growth. Similar findings have been reported for minipig models with impaired GH function [38], [39]. Our study provides a more comprehensive phenotypic analysis, including serial GH measurements, effects on serum IGFBPs, age-dependent changes in glucose homeostasis and lipid profiles, and analyses of hepatic signaling cascades.
Circulating IGF1 originates in large part from the liver, as shown by liver specific Igf1 KO mice [40], and is complexed by high-affinity IGFBPs, with IGFBP3 and an acid labile subunit (ALS) forming a 150-kDa complex, which is the main reservoir of IGF1 in the bloodstream (reviewed in [41], [42]). ALS is directly regulated by GH, and reduced ALS levels are observed in GHR-deficient patients [43]. The production of IGFBP3 is also stimulated by GH [44], and GHR deficiency in mice and humans leads to reduced levels of IGFBP3 in the circulation [15], [45]. In agreement with these reports, serum IGFBP3 concentrations of GHR-KO pigs were significantly reduced, presumably leading to a decrease in the IGF1 reservoir in the circulation and thus to a shortened half-life. In addition, the concentration of IGFBP2 was increased in serum of GHR-KO pigs. Studies in transgenic IGFBP2-overexpressing mice have shown that IGFBP2 inhibits the growth of normal [46] and even of GH-overexpressing mice [47]. Therefore, the reduced growth of GHR-KO pigs most likely results from a combination of a decline in the production and half-life of circulating IGF1 and, possibly, IGF1 sequestration by inhibitory IGFBP2.
Circulating IGF1 is also responsible for the feedback inhibition of pituitary GH secretion (reviewed in [48]). In accordance with this concept, GHR-KO pigs with low serum IGF1 levels showed high serum GH concentrations with partially preserved pulsatility. Serial blood samples for the analysis of hormone profiles can be obtained easily from pigs equipped with permanent central venous catheters [49], whereas serial blood sampling in mice is more difficult.
Interestingly, a growth deficiency phenotype of GHR-KO pigs was observed no earlier than postnatal week 5, which indicated that GH was not required as promoter of intrauterine and early postnatal growth. This finding is consistent with observations in infants with LS [50] and in neonatal Ghr KO mice [15]. Instead, intrauterine growth depends on IGF2 and IGF1, with the latter acting independently of GH during this period of development [51]. An essential role of GH as a postnatal growth promoter is established during the maturation of the endocrine growth axis, which is associated with an increase in expression of hepatic GHR [52]. In general, intrauterine maturation of endocrine functions in pigs is similar to humans, whereas rodents are born in a more immature state (reviewed in [53]).
In agreement with observations in human LS patients [54] and Ghr KO mice [55], GHR-KO pigs displayed an increase in total body fat and a decrease in the muscle to fat ratio. Adipose tissue mass is determined by the storage and removal of triglycerides in adipocytes. A recent study in which human adipocyte lipid age was determined by measuring 14C from nuclear bomb tests revealed that triglycerides are renewed up to 6 times during the average adipocyte life-span of 10 years [56]. In GHR-KO pigs, physiological adipocyte lipid turnover is apparently disturbed at different levels. First, the lipolytic action of GH via an increase in adipose tissue hormone-sensitive lipase (HSL) activity (reviewed in [16], [57]) is lost in the absence of GHR. Second, the synthesis of storage lipids in adipocytes is likely to increase in GHR-KO pigs. This phenomenon requires hydrolysis by lipoprotein lipase (LPL) of the triglyceride component of circulating chylomicrons and very low-density lipoproteins (VLDL) into free fatty acids and 2-monoacylglycerol, which can be taken up by adipocytes. Adipose tissue LPL activity is increased by insulin but inhibited by GH and sex steroids (reviewed in [58]). Reduced serum triglyceride levels in young GHR-KO pigs (12–15 weeks) with normal insulin levels, but lacking the counteracting effects of GH and sex steroids, may reflect an increased use of triglycerides for lipid synthesis in adipocytes, leading to an increase in total body fat. In older GHR-KO pigs (23–27 weeks), the increased total body fat content may lead to decreased triglyceride turnover of adipocytes [56], limiting the use of circulating triglycerides for storage lipid synthesis in adipocytes. Furthermore, the animals became sexually mature, with sex steroids potentially inhibiting adipose tissue LPL and the hydrolysis of serum triglycerides, thus resulting in normal serum triglyceride levels. Decreased serum cholesterol levels, as observed in young GHR-KO pigs, have also been reported in human LS patients [3] and Ghr KO mice (reviewed in [16]).
Measurements of organ weights in GHR-KO pigs revealed that growth of liver, kidneys, and heart is particularly dependent on GHR/GH action, as their relative weights were significantly decreased in comparison to control pigs. Reduced relative liver and kidney weights have also been observed in Ghr KO mice [55], and interestingly LS patients have disproportionately reduced cardiac dimensions [59]. The important role of the GH/IGF1 system in the growth of these organs is supported by their disproportionate overgrowth in conditions of GH/IGF1 excess, as in GH-overexpressing transgenic mice [60], [61] and in patients with acromegaly [62]. In contrast, brain growth is less dependent on intact GHR signaling, as shown by a relatively moderate reduction in absolute brain weight and a marked increase in the relative brain weight of GHR-KO pigs. Based on similar observations in Ghr KO mice, Sjogren et al. [63] speculated that a large proportion of brain growth occurs relatively early in development in a GH-independent stage.
Patients with Laron syndrome exhibit delayed puberty, but they are able to achieve full sexual development and reproduce [2]. In Ghr KO mice, delayed puberty and reduced litter sizes have been observed. The latter has been attributed to reduced ovarian function due to the lack of IGF1 (reviewed in [16]). To assess the fertility of GHR-KO pigs, a GHR-KO boar and a GHR-KO sow were mated, resulting in a litter of 6 healthy piglets. Although not significant, the mean birth weight of these piglets was 40% lower than that of GHR-KO piglets from heterozygote x heterozygote mating, most likely because of the limited fetal growth capacity in a smaller mother. Interestingly, GHR-KO piglets from homozygous parents showed catch-up growth and had a 33% higher body weight at six months than GHR-KO piglets from heterozygous parents. An explanation for this increased growth performance may be hybrid vigor, potentially resulting from the mating of GHR-KO pigs from two different lines (Suppl. Fig. 1).
GHR-KO pigs showed juvenile hypoglycemia that normalized when the animals reached sexual maturity. Hypoglycemia is a hallmark of juvenile age LS [3], [64] and has been mainly explained by the lack of stimulatory GH effects on hepatic gluconeogenesis (reviewed in [57]). Contrasting observations have been obtained concerning the role of altered insulin sensitivity in the development of juvenile hypoglycemia (reviewed in [65]). While no evidence for increased insulin sensitivity, and even some cases of insulin-resistant diabetes mellitus, have been reported in the Israeli cohort of LS patients [64], [66], individuals from the Ecuadorian LS cohort are more insulin-sensitive than their GHR intact relatives [67], and no cases of diabetes mellitus were reported [7]. The reasons for this discrepancy remain elusive [65]. Although HOMA-IR is not a routinely established parameter to assess insulin (in)sensitivity in pigs (reviewed in [68]), the trend (p = 0.0635) toward lower HOMA-IR scores in young GHR-KO vs. control pigs suggests that increased insulin sensitivity is a contributing factor to juvenile hypoglycemia, as also observed in some Ghr KO mouse models ([69]; reviewed in [16]). While the HOMA-IR scores of GHR-KO pigs increased with age, and tended to be higher in sexually mature GHR-KO than in age-matched control pigs (interaction group*age: p < 0.05), there was no evidence for insulin resistance. Interestingly, we observed increased levels of total and phosphorylated IRS1 in liver samples from fasted adult GHR-KO pigs, which could represent a mechanism for increased insulin sensitivity. Similar observations and conclusions were obtained in GH-deficient Ames dwarf mice with increased insulin sensitivity [70]. Future studies involving state-of-the-art in vivo measurements of gluconeogenesis [71] and investigation of insulin sensitivity using hyperinsulinemic, euglycemic clamp studies in juvenile and sexually mature GHR-KO and control pigs will help to clarify the relative contributions of these mechanisms to juvenile hypoglycemia in LS and its normalization in adult LS patients. Such studies can be performed more accurately in pigs than in rodent models due to their larger size [49].
While all clinical-chemical parameters measured in the serum of GHR-KO pigs remained within the normal reference ranges for pigs, creatinine levels were reduced and serum urea concentrations were increased compared with the control pigs. The serum creatinine concentration correlates with muscle mass [72], which explains the reduced levels in GHR-KO pigs and in LS patients [3]. Increased serum concentrations of urea, the end product of amino acid catabolism in mammals, have also been detected in Ghr KO mice [69] and are most likely due to IGF1 deficiency and the lack of its protein anabolic action (reviewed in [73]).
Since the liver is a major target organ for GH, we investigated alterations in the activity of selected hepatic signaling pathways in adult fasted GHR-KO and control pigs. Upon binding of GH, the GHR homodimer undergoes a conformational change that brings the originally parallel receptor transmembrane domains into a rotated crossover orientation, thus separating the lower parts of the transmembrane helices. Thereby, the two JAK2 molecules that are associated with the membrane proximal Box1 motif of the GHR chains are separated, and the inhibitory pseudokinase domain of one JAK2 is removed from the kinase domain of the other JAK2 and vice versa [74]. The kinase domains of the two JAK2 molecules can then be transactivated and initiate tyrosine phosphorylation of the GHR cytoplasmic domains and STAT5, the key transcription factor mediating most genomic actions of GH (reviewed in [75]), including stimulation of IGF1 gene expression [76]. In agreement with this concept, the phosphorylation of STAT5 in liver of GHR-KO pigs was significantly reduced and the circulating IGF1 levels were markedly decreased compared with control pigs. However, unexpectedly, phosphorylation of JAK2 was significantly increased in liver of GHR-KO pigs, together with a significant increase in the phosphorylation of MAPK, PI3K, and mTOR, which are known to be – directly or indirectly – activated by JAK2 [77].
In addition to GHR, many other class I cytokine receptors also use the non-receptor tyrosine kinase JAK2 for signaling, including the receptors for erythropoietin, prolactin, interleukins 3, 5 and 6, granulocyte-macrophage colony-stimulating factor, interferon-gamma, thrombopoietin, and leptin (reviewed in [78]). GHR is abundantly expressed in liver and can dimerize and associate with JAK2 in the absence of GH (reviewed in [75]). Since GHR-bound JAK2 can only be activated in the presence of GH, elimination of GHR may increase the pool of JAK2 that can be phosphorylated by other class I cytokine receptors. In addition, the loss of GHR may alter the abundance of these receptors or their ligands and thus lead to increased JAK2 phosphorylation.
A candidate is leptin, since increased serum leptin levels have been detected in LS patients [79] and in the Ghr KO mouse model (reviewed in [16]). Leptin is known to induce the expression of its receptor in liver [80], and leptin receptor activation involves the phosphorylation of JAK2 and STAT3, but not STAT5 (reviewed in [37]). Consistent with this hypothesis, we observed significantly increased serum leptin levels and increased expression and phosphorylation of leptin receptors in liver of GHR-KO pigs, providing a potential explanation for the significantly increased phosphorylation of JAK2, while phosphorylation of STAT5 was significantly reduced.
Phosphorylation of the serine/threonine kinase mTOR that forms the catalytic core of mTOR complex-1 (mTORC1) and mTORC2 was significantly increased in GHR-KO pigs. While mTOR phosphorylation was not directly investigated in Ghr KO mice, Dominick et al. [35] have evaluated the activities of mTORC1 and mTORC2 in fed and fasted animals based on the phosphorylation status of downstream substrates of these complexes. In fasted Ghr KO mice, the authors observed reduced phosphorylation of the ribosomal protein S6, while the phosphorylation status of S6K and 4EBP1 was unaltered compared with control mice. In fed Ghr KO mice, the phosphorylation status of all three indicators of mTORC1 activity was significantly reduced. In contrast, the phosphorylation level of several target substrates of mTORC2, including AKT, was significantly increased in fasted Ghr KO vs. control mice, while this difference disappeared after feeding [35]. The authors concluded that mTORC2 activity was increased in Ghr KO mice – at least in fasted animals — whereas mTORC1 activity was unaltered or reduced in fasted and fed Ghr KO mice, respectively. Our study of liver samples from fasted GHR-KO pigs did not reveal an increase in the phosphorylation of S6K or of 4EBP1 that would trigger its dissociation from eIF4E and allow cap-dependent mRNA translation (reviewed in [36]). Moreover, we observed a significant increase in phosphorylation (= activation) of the mTORC1 inhibitor AMPK [81]. Collectively, these findings argue against a major activation of mTORC1 in liver of GHR-KO pigs. In contrast, a significant increase in GSK3B phosphorylation and a trend (p = 0.0635) toward an increase in AKT phosphorylation suggested a rise in the activity of mTORC2. While mTORC1 has profound effects on mRNA translation, metabolism, and protein turnover, mTORC2 signaling is implicated in the regulation of ion transport, apoptosis, glucose metabolism, cell migration, and cytoskeleton rearrangement (reviewed in [36]). Future molecular profiling studies of liver and other tissues from GHR-KO and control pigs will clarify whether these biological processes are altered in the absence of GHR signaling.
Another interesting observation in liver samples from GHR-KO pigs was the trend (p = 0.0635) toward an increased abundance of PPARG. Discrepant findings have been reported regarding the consequences of increased PPARG activity in liver, ranging from the promotion of hepatic steatosis through the upregulation of genes involved in lipid uptake and storage, to the prevention of hepatic steatosis and fibrosis, possibly by sequestering fatty acids in adipose tissue and preventing hepatic stellate cell activation (reviewed in [82]). Studies of GHR-KO and control pigs, e.g., after being fed a high-fat diet, may provide additional insight into these mechanisms.
One of the most striking effects of GHR deficiency is the increased life-span that has been observed in Ghr KO mice [83], [84]. Moreover, patients with LS show reduced incidences of cancer and diabetes, associated with reduced pro-aging signaling in cells incubated with serum derived from these patients [7]. While life expectancy studies in humans are difficult due to their long duration and to multiple confounding factors, they are possible in pigs, which can be maintained under standardized conditions and have a normal life expectancy of 15–17 years (reviewed in [85]). Moreover, protective effects of GHR deficiency against tumors and diabetes can be evaluated by crossing the GHR KO mutation in existing pig models that are genetically predisposed to tumor development (e.g., [86]) or (pre-)diabetes [27], [87], thus bridging the gap between rodents and humans in longevity research with tailored large animal models. In addition to these long-lasting in vivo experiments, the effects of GHR deficiency on resistance to stress – such as UV light and heat – may be evaluated in primary cell cultures from GHR-KO pigs, similarly to studies that have been performed using cultured cells from dwarf mice [88].
Investigation of the neurological consequences of GHR deficiency is another interesting field, since mental retardation has been reported in a proportion of LS patients [2], while subjects from the Ecuadorian cohort display normal intelligence [89] or even enhanced cognitive performance [90]. As sophisticated methods for testing cognitive functions of pigs are available [91], the GHR-KO pig may also serve as a model to address these questions.
Finally, GHR-KO pigs are interesting animal models for developing and evaluating the efficacy and safety of new treatment options for LS, such as PASylated IGF1 with a prolonged plasma half-life [92]. In addition, it would be interesting to determine whether the phenotype of GHR-KO pigs can be rescued by correction of the GHR mutation in a proportion of liver cells via gene editing. In a mouse model, hydrodynamic injection into the tail vein has been used to deliver components of a CRISPR/Cas9 system to correct a mutated fumarylacetoacetate hydrolase (Fah) gene in hepatocytes in vivo [93]. In pigs, refined techniques are available for direct application into the liver [94], [95].
In summary, GHR-KO pigs resemble important aspects of the pathophysiology of human Laron syndrome and are thus an interesting model for mechanistic studies and treatment trials.
Funding
This study was supported in part by the German Center for Diabetes Research (DZD) and by the German Research Council (TRR127).
Author contributions
A. Hi., B. K., M. D., and E. W. conceived the experiments. A. Hi. and E. W. wrote the manuscript. All authors contributed to the manuscript and read and approved the final version. M. D., S. B., and H. L. developed the CRISPR/Cas system for GHR KO. B. K., M. K., and H. N. performed the in vitro fertilization, microinjection and embryo transfer experiments; A. Hi. and B. K. managed the breeding and performed the phenotypic characterization of GHR-KO and control pigs. A. Hi., A. B., and R. W. performed the necropsies. E. K. conducted the GHR ligand immunohistochemistry. M. B. and A. M. S. performed the DXA and MRI studies. A. Ho. carried out the IGFBP ligand blots analyses. W. F. B. and M. B. performed the IGF1 and GH assays. S. R. performed the insulin and glucose measurements, B. R. and M. H. d. A. the clinical-chemical measurements. M. D. performed the Western blot analysis of signaling molecules. A. Hi. and E. W. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
The authors thank Christina Blechinger, Luong Chau, Tamara Holy, Eva-Maria Jemiller, Sebastian Kaidel, Franziska Kress, and Tatjana Schröter for excellent technical assistance, and Christian Erdle, Sylvia Hering, and Harald Paul for expert animal management.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.03.006.
Conflict of interest
No potential conflicts of interest relevant to this article are reported.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Laron Z., Pertzelan A., Mannheimer S. Genetic pituitary dwarfism with high serum concentation of growth hormone–a new inborn error of metabolism? Israel Journal of Medical Sciences. 1966;2:152–155. [PubMed] [Google Scholar]
- 2.Laron Z. Clinical evidence of growth hormone resistance in patients with Laron syndrome. In: Laron Z., Kopchick J.J., editors. Laron Syndrome - from Man to Mouse. Springer; 2011. pp. 21–25. [Google Scholar]
- 3.Laron Z. Laron syndrome (primary growth hormone resistance or insensitivity): the personal experience 1958–2003. The Journal of Clinical Endocrinology and Metabolism. 2004;89:1031–1044. doi: 10.1210/jc.2003-031033. [DOI] [PubMed] [Google Scholar]
- 4.Laron Z. Epilogue: the future of Laron syndrome — the need for changes. Growth Hormone & IGF Research. 2016;28:79–80. doi: 10.1016/j.ghir.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Guevara-Aguirre J., Rosenbloom A.L., Fielder P.J., Diamond F.B., Jr., Rosenfeld R.G. Growth hormone receptor deficiency in Ecuador: clinical and biochemical phenotype in two populations. The Journal of Clinical Endocrinology and Metabolism. 1993;76:417–423. doi: 10.1210/jcem.76.2.7679400. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld R.G., Rosenbloom A.L., Guevara-Aguirre J. Growth hormone (GH) insensitivity due to primary GH receptor deficiency. Endocrine Reviews. 1994;15:369–390. doi: 10.1210/edrv-15-3-369. [DOI] [PubMed] [Google Scholar]
- 7.Guevara-Aguirre J., Balasubramanian P., Guevara-Aguirre M., Wei M., Madia F., Cheng C.W. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Science Translational Medicine. 2011;3 doi: 10.1126/scitranslmed.3001845. 70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laron Z., Kauli R., Lapkina L., Werner H. IGF-I deficiency, longevity and cancer protection of patients with Laron syndrome. Mutation Research Reviews in Mutation Research. 2017;772:123–133. doi: 10.1016/j.mrrev.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Chernausek S.D., Backeljauw P.F., Frane J., Kuntze J., Underwood L.E. Long-term treatment with recombinant insulin-like growth factor (IGF)-I in children with severe IGF-I deficiency due to growth hormone insensitivity. The Journal of Clinical Endocrinology and Metabolism. 2007;92:902–910. doi: 10.1210/jc.2006-1610. [DOI] [PubMed] [Google Scholar]
- 10.Backeljauw P.F., Kuntze J., Frane J., Calikoglu A.S., Chernausek S.D. Adult and near-adult height in patients with severe insulin-like growth factor-I deficiency after long-term therapy with recombinant human insulin-like growth factor-I. Hormone Research in Paediatrics. 2013;80:47–56. doi: 10.1159/000351958. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J., Blethen S., Kuntze J., Smith S.L., Lomax K.G., Mathew P.M. Managing the child with severe primary insulin-like growth factor-1 deficiency (IGFD): IGFD diagnosis and management. Drugs in R & D. 2014;14:25–29. doi: 10.1007/s40268-014-0039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shevah O., Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Hormone & IGF Research. 2007;17:54–57. doi: 10.1016/j.ghir.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Steuerman R., Shevah O., Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. European Journal of Endocrinology. 2011;164:485–489. doi: 10.1530/EJE-10-0859. [DOI] [PubMed] [Google Scholar]
- 14.Lapkina-Gendler L., Rotem I., Pasmanik-Chor M., Gurwitz D., Sarfstein R., Laron Z. Identification of signaling pathways associated with cancer protection in Laron syndrome. Endocrine-Related Cancer. 2016;23:399–410. doi: 10.1530/ERC-16-0054. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y., Xu B.C., Maheshwari H.G., He L., Reed M., Lozykowski M. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.List E.O., Sackmann-Sala L., Berryman D.E., Funk K., Kelder B., Gosney E.S. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocrine Reviews. 2011;32:356–386. doi: 10.1210/er.2010-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duran-Ortiz S., Noboa V., Kopchick J.J. Disruption of the GH receptor gene in adult mice and in insulin sensitive tissues. Growth Hormone & IGF Research. 2018;38:3–7. doi: 10.1016/j.ghir.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Young J.A., List E.O., Kopchick J.J. Deconstructing the growth hormone receptor (GHR): physical and metabolic phenotypes of tissue-specific GHR gene-disrupted mice. Progress in Molecular Biology and Translational Science. 2016;138:27–39. doi: 10.1016/bs.pmbts.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Rogers C.S. Genetically engineered livestock for biomedical models. Transgenic Research. 2016;25:345–359. doi: 10.1007/s11248-016-9928-6. [DOI] [PubMed] [Google Scholar]
- 20.Aigner B., Kessler B., Klymiuk N., Kurome M., Renner S., Wünsch A. Genetically tailored pig models for translational biomedical research. In: Conn P.M., editor. Animal Models for the Study of Human Disease. Academic Press; London, Oxford, San Diego, Cambridge: 2017. pp. 671–701. [Google Scholar]
- 21.Dahlhoff M., Gaborit N., Bultmann S., Leonhardt H., Yarden Y., Schneider M.R. CRISPR-assisted receptor deletion reveals distinct roles for ERBB2 and ERBB3 in skin keratinocytes. FEBS Journal. 2017;284:3339–3349. doi: 10.1111/febs.14196. [DOI] [PubMed] [Google Scholar]
- 22.Umeyama K., Honda K., Matsunari H., Nakano K., Hidaka T., Sekiguchi K. Production of diabetic offspring using cryopreserved epididymal sperm by in vitro fertilization and intrafallopian insemination techniques in transgenic pigs. Journal of Reproduction and Development. 2013;59:599–603. doi: 10.1262/jrd.2013-069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurome M., Kessler B., Wuensch A., Nagashima H., Wolf E. Nuclear transfer and transgenesis in the pig. Methods in Molecular Biology (Clifton, NJ) 2015;1222:37–59. doi: 10.1007/978-1-4939-1594-1_4. [DOI] [PubMed] [Google Scholar]
- 24.Besenfelder U., Modl J., Muller M., Brem G. Endoscopic embryo collection and embryo transfer into the oviduct and the uterus of pigs. Theriogenology. 1997;47:1051–1060. doi: 10.1016/s0093-691x(97)00062-9. [DOI] [PubMed] [Google Scholar]
- 25.Blum W.F., Breier B.H. Radioimmunoassays for IGFs and IGFBPs. Growth Regulation. 1994;4(Suppl 1):11–19. [PubMed] [Google Scholar]
- 26.Wirthgen E., Hoflich C., Spitschak M., Helmer C., Brand B., Langbein J. Quantitative Western ligand blotting reveals common patterns and differential features of IGFBP-fingerprints in domestic ruminant breeds and species. Growth Hormone & IGF Research. 2016;26:42–49. doi: 10.1016/j.ghir.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Renner S., Fehlings C., Herbach N., Hofmann A., von Waldthausen D.C., Kessler B. Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function. Diabetes. 2010;59:1228–1238. doi: 10.2337/db09-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blutke A., Renner S., Flenkenthaler F., Backman M., Haesner S., Kemter E. The Munich MIDY Pig Biobank - a unique resource for studying organ crosstalk in diabetes. Molecular Metabolism. 2017;6:931–940. doi: 10.1016/j.molmet.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papadopoulos G.A., Erkens T., Maes D.G., Peelman L.J., van Kempen T.A., Buyse J. Peripartal feeding strategy with different n-6:n-3 ratios in sows: effect on gene expression in backfat white adipose tissue postpartum. British Journal of Nutrition. 2009;101:197–205. doi: 10.1017/S0007114508994782. [DOI] [PubMed] [Google Scholar]
- 30.Kremer P.V., Fernandez-Figares I., Forster M., Scholz A.M. In vivo body composition in autochthonous and conventional pig breeding groups by dual-energy X-ray absorptiometry and magnetic resonance imaging under special consideration of Cerdo Iberico. Animal : An International Journal of Animal Bioscience. 2012;6:2041–2047. doi: 10.1017/S1751731112001267. [DOI] [PubMed] [Google Scholar]
- 31.Kremer P.V., Forster M., Scholz A.M. Use of magnetic resonance imaging to predict the body composition of pigs in vivo. Animal : An International Journal of Animal Bioscience. 2013;7:879–884. doi: 10.1017/S1751731112002340. [DOI] [PubMed] [Google Scholar]
- 32.Albl B., Haesner S., Braun-Reichhart C., Streckel E., Renner S., Seeliger F. Tissue sampling guides for porcine biomedical models. Toxicologic Pathology. 2016;44:414–420. doi: 10.1177/0192623316631023. [DOI] [PubMed] [Google Scholar]
- 33.Streckel E., Braun-Reichhart C., Herbach N., Dahlhoff M., Kessler B., Blutke A. Effects of the glucagon-like peptide-1 receptor agonist liraglutide in juvenile transgenic pigs modeling a pre-diabetic condition. Journal of Translational Medicine. 2015;13:73. doi: 10.1186/s12967-015-0431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fagerberg L., Hallström B.M., Oksvold P., Kampf C., Djureinovic D., Odeberg J. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Molecular & Cellular Proteomics : MCP. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dominick G., Berryman D.E., List E.O., Kopchick J.J., Li X., Miller R.A. Regulation of mTOR activity in Snell dwarf and GH receptor gene-disrupted mice. Endocrinology. 2015;156:565–575. doi: 10.1210/en.2014-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rawlings J.S., Rosler K.M., Harrison D.A. The JAK/STAT signaling pathway. Journal of Cell Science. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 38.Cui D., Li F., Li Q., Li J., Zhao Y., Hu X. Generation of a miniature pig disease model for human Laron syndrome. Scientific Reports. 2015;5:15603. doi: 10.1038/srep15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li F., Li Y., Liu H., Zhang X., Liu C., Tian K. Transgenic Wuzhishan minipigs designed to express a dominant-negative porcine growth hormone receptor display small stature and a perturbed insulin/IGF-1 pathway. Transgenic Research. 2015;24:1029–1042. doi: 10.1007/s11248-015-9912-6. [DOI] [PubMed] [Google Scholar]
- 40.Yakar S., Liu J.L., Stannard B., Butler A., Accili D., Sauer B. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf E., Schneider M.R., Zhou R., Fisch T.M., Herbach N., Dahlhoff M. Functional consequences of IGFBP excess - lessons from transgenic mice. Pediatric Nephrology (Berlin, Germany) 2005;20:269–278. doi: 10.1007/s00467-004-1657-z. [DOI] [PubMed] [Google Scholar]
- 42.Hwa V., Oh Y., Rosenfeld R.G. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocrine Reviews. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 43.Labarta J.I., Gargosky S.E., Simpson D.M., Lee P.D., Argente J., Guevara-Aguirre J. Immunoblot studies of the acid-labile subunit (ALS) in biological fluids, normal human serum and in children with GH deficiency and GH receptor deficiency before and after long-term therapy with GH or IGF-I respectively. Clinical Endocrinology. 1997;47:657–666. doi: 10.1046/j.1365-2265.1997.2581078.x. [DOI] [PubMed] [Google Scholar]
- 44.Laron Z., Klinger B., Blum W.F., Silbergeld A., Ranke M.B. IGF binding protein 3 in patients with Laron type dwarfism: effect of exogenous rIGF-I. Clinical Endocrinology. 1992;36:301–304. doi: 10.1111/j.1365-2265.1992.tb01449.x. [DOI] [PubMed] [Google Scholar]
- 45.Savage M.O., Blum W.F., Ranke M.B., Postel-Vinay M.C., Cotterill A.M., Hall K. Clinical features and endocrine status in patients with growth hormone insensitivity (Laron syndrome) The Journal of Clinical Endocrinology and Metabolism. 1993;77:1465–1471. doi: 10.1210/jcem.77.6.7505286. [DOI] [PubMed] [Google Scholar]
- 46.Hoeflich A., Wu M., Mohan S., Foll J., Wanke R., Froehlich T. Overexpression of insulin-like growth factor-binding protein-2 in transgenic mice reduces postnatal body weight gain. Endocrinology. 1999;140:5488–5496. doi: 10.1210/endo.140.12.7169. [DOI] [PubMed] [Google Scholar]
- 47.Hoeflich A., Nedbal S., Blum W.F., Erhard M., Lahm H., Brem G. Growth inhibition in giant growth hormone transgenic mice by overexpression of insulin-like growth factor-binding protein-2. Endocrinology. 2001;142:1889–1898. doi: 10.1210/endo.142.5.8149. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan S.A., Cohen P. The somatomedin hypothesis 2007: 50 years later. The Journal of Clinical Endocrinology and Metabolism. 2007;92:4529–4535. doi: 10.1210/jc.2007-0526. [DOI] [PubMed] [Google Scholar]
- 49.Kleinert M., Clemmensen C., Hofmann S., Moore M., Renner S., Woods S. Animal models of obesity and diabetes. Nature Reviews Endocrinology. 2018;14:140–162. doi: 10.1038/nrendo.2017.161. [DOI] [PubMed] [Google Scholar]
- 50.Laron Z. The diagnostic and prognostic importance of neonatal length measurements. The Israel Medical Association Journal : IMAJ. 2000;2:84–85. [PubMed] [Google Scholar]
- 51.Baker J., Liu J.P., Robertson E.J., Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 52.Hetz J.A., Menzies B.R., Shaw G., Rao A., Clarke I.J., Renfree M.B. Growth axis maturation is linked to nutrition, growth and developmental rate. Molecular and Cellular Endocrinology. 2015;411:38–48. doi: 10.1016/j.mce.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Symonds M.E., Sebert S.P., Hyatt M.A., Budge H. Nutritional programming of the metabolic syndrome. Nature Reviews Endocrinology. 2009;5:604–610. doi: 10.1038/nrendo.2009.195. [DOI] [PubMed] [Google Scholar]
- 54.Laron Z., Ginsberg S., Lilos P., Arbiv M., Vaisman N. Body composition in untreated adult patients with Laron syndrome (primary GH insensitivity) Clinical Endocrinology. 2006;65:114–117. doi: 10.1111/j.1365-2265.2006.02558.x. [DOI] [PubMed] [Google Scholar]
- 55.Berryman D.E., List E.O., Palmer A.J., Chung M.-Y., Wright-Piekarski J., Lubbers E. Two-year body composition analyses of long-lived GHR null mice. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2010;65:31–40. doi: 10.1093/gerona/glp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arner P., Bernard S., Salehpour M., Possnert G., Liebl J., Steier P. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. 2011;478:110–113. doi: 10.1038/nature10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vijayakumar A., Novosyadlyy R., Wu Y., Yakar S., LeRoith D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Hormone & IGF Research. 2010;20:1–7. doi: 10.1016/j.ghir.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H., Eckel R.H. Lipoprotein lipase: from gene to obesity. American Journal of Physiology Endocrinology and Metabolism. 2009;297:E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 59.Feinberg M.S., Scheinowitz M., Laron Z. Echocardiographic dimensions and function in adults with primary growth hormone resistance (Laron Syndrome) The American Journal of Cardiology. 2000;85:209–213. doi: 10.1016/s0002-9149(99)00642-6. [DOI] [PubMed] [Google Scholar]
- 60.Shea B.T., Hammer R.E., Brinster R.L. Growth allometry of the organs in giant transgenic mice. Endocrinology. 1987;121:1924–1930. doi: 10.1210/endo-121-6-1924. [DOI] [PubMed] [Google Scholar]
- 61.Wanke R., Hermanns W., Folger S., Wolf E., Brem G. Accelerated growth and visceral lesions in transgenic mice expressing foreign genes of the growth hormone family: an overview. Pediatric Nephrology (Berlin, Germany) 1991;5:513–521. doi: 10.1007/BF01453693. [DOI] [PubMed] [Google Scholar]
- 62.Lombardi G., Colao A., Ferone D., Marzullo P., Orio F., Longobardi S. Effect of growth hormone on cardiac function. Hormone Research. 1997;48(Suppl 4):38–42. doi: 10.1159/000191311. [DOI] [PubMed] [Google Scholar]
- 63.Sjogren K., Bohlooly Y.M., Olsson B., Coschigano K., Tornell J., Mohan S. Disproportional skeletal growth and markedly decreased bone mineral content in growth hormone receptor -/- mice. Biochemical and Biophysical Research Communications. 2000;267:603–608. doi: 10.1006/bbrc.1999.1986. [DOI] [PubMed] [Google Scholar]
- 64.Laron Z., Avitzur Y., Klinger B. Carbohydrate metabolism in primary growth hormone resistance (Laron syndrome) before and during insulin-like growth factor-I treatment. Metabolism: Clinical and Experimental. 1995;44:113–118. doi: 10.1016/0026-0495(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 65.Guevara-Aguirre J., Rosenbloom A.L. Obesity, diabetes and cancer: insight into the relationship from a cohort with growth hormone receptor deficiency. Diabetologia. 2015;58:37–42. doi: 10.1007/s00125-014-3397-3. [DOI] [PubMed] [Google Scholar]
- 66.Laron Z. Growth hormone insensitivity (Laron syndrome) Reviews in Endocrine & Metabolic Disorders. 2002;3:347–355. doi: 10.1023/a:1020905725012. [DOI] [PubMed] [Google Scholar]
- 67.Guevara-Aguirre J., Rosenbloom A.L., Balasubramanian P., Teran E., Guevara-Aguirre M., Guevara C. GH receptor deficiency in ecuadorian adults is associated with obesity and enhanced insulin sensitivity. The Journal of Clinical Endocrinology and Metabolism. 2015;100:2589–2596. doi: 10.1210/jc.2015-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Renner S., Dobenecker B., Blutke A., Zols S., Wanke R., Ritzmann M. Comparative aspects of rodent and nonrodent animal models for mechanistic and translational diabetes research. Theriogenology. 2016;86:406–421. doi: 10.1016/j.theriogenology.2016.04.055. [DOI] [PubMed] [Google Scholar]
- 69.Liu J.-L., Coschigano K.T., Robertson K., Lipsett M., Guo Y., Kopchick J.J. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. American Journal of Physiology Endocrinology and Metabolism. 2004;287:E405–E413. doi: 10.1152/ajpendo.00423.2003. [DOI] [PubMed] [Google Scholar]
- 70.Dominici F.P., Hauck S., Argentino D.P., Bartke A., Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-1 and IRS-2 in liver of Ames dwarf mice. Journal of Endocrinology. 2002;173:81–94. doi: 10.1677/joe.0.1730081. [DOI] [PubMed] [Google Scholar]
- 71.Chung S.T., Chacko S.K., Sunehag A.L., Haymond M.W. Measurements of gluconeogenesis and glycogenolysis: a methodological review. Diabetes. 2015;64:3996–4010. doi: 10.2337/db15-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baxmann A.C., Ahmed M.S., Marques N.C., Menon V.B., Pereira A.B., Kirsztajn G.M. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clinical Journal of the American Society of Nephrology : CJASN. 2008;3:348–354. doi: 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.LeRoith D., Yakar S. Mechanisms of disease: metabolic effects of growth hormone and insulin-like growth factor 1. Nature Clinical Practice Endocrinology & Metabolism. 2007;3:302–310. doi: 10.1038/ncpendmet0427. [DOI] [PubMed] [Google Scholar]
- 74.Brooks A.J., Dai W., O'Mara M.L., Abankwa D., Chhabra Y., Pelekanos R.A. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science. 2014;344 doi: 10.1126/science.1249783. 1249783. [DOI] [PubMed] [Google Scholar]
- 75.Waters M.J. The growth hormone receptor. Growth Hormone & IGF Research. 2016;28:6–10. doi: 10.1016/j.ghir.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 76.Mukherjee A., Alzhanov D., Rotwein P. Defining human insulin-like growth factor I gene regulation. American Journal of Physiology Endocrinology and Metabolism. 2016;311:E519–E529. doi: 10.1152/ajpendo.00212.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rawlings J.S., Rosler K.M., Harrison D.A. The JAK/STAT signaling pathway. Journal of Cell Science. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 78.Waters M.J., Brooks A.J. JAK2 activation by growth hormone and other cytokines. The Biochemical Journal. 2015;466:1–11. doi: 10.1042/BJ20141293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laron Z., Silbergeld A., Lilos P., Blum F.W. Serum leptin in obese patients with Laron syndrome before and during IGF-I treatment. Journal of Pediatric Endocrinology & Metabolism : JPEM. 1998;11:653–656. doi: 10.1515/JPEM.1998.11.5.653. [DOI] [PubMed] [Google Scholar]
- 80.Cohen P., Yang G., Yu X., Soukas A.A., Wolfish C.S., Friedman J.M. Induction of leptin receptor expression in the liver by leptin and food deprivation. Journal of Biological Chemistry. 2005;280:10034–10039. doi: 10.1074/jbc.M413684200. [DOI] [PubMed] [Google Scholar]
- 81.Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmadian M., Suh J.M., Hah N., Liddle C., Atkins A.R., Downes M. PPARgamma signaling and metabolism: the good, the bad and the future. Nature Medicine. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coschigano K.T., Clemmons D., Bellush L.L., Kopchick J.J. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 84.Coschigano K.T., Holland A.N., Riders M.E., List E.O., Flyvbjerg A., Kopchick J.J. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 85.Burgstaller J.P., Brem G. Aging of cloned animals: a mini-review. Gerontology. 2017;63:417–425. doi: 10.1159/000452444. [DOI] [PubMed] [Google Scholar]
- 86.Flisikowska T., Merkl C., Landmann M., Eser S., Rezaei N., Cui X. A porcine model of familial adenomatous polyposis. Gastroenterology. 2012;143:1173–1175. doi: 10.1053/j.gastro.2012.07.110. [DOI] [PubMed] [Google Scholar]
- 87.Renner S., Braun-Reichhart C., Blutke A., Herbach N., Emrich D., Streckel E. Permanent neonatal diabetes in INS(C94Y) transgenic pigs. Diabetes. 2013;62:1505–1511. doi: 10.2337/db12-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murakami S., Salmon A., Miller R.A. Multiplex stress resistance in cells from long-lived dwarf mice. The FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- 89.Kranzler J.H., Rosenbloom A.L., Martinez V., Guevara-Aguirre J. Normal intelligence with severe insulin-like growth factor I deficiency due to growth hormone receptor deficiency: a controlled study in a genetically homogeneous population. The Journal of Clinical Endocrinology and Metabolism. 1998;83:1953–1958. doi: 10.1210/jcem.83.6.4863. [DOI] [PubMed] [Google Scholar]
- 90.Nashiro K., Guevara-Aguirre J., Braskie M.N., Hafzalla G.W., Velasco R., Balasubramanian P. Brain structure and function associated with younger adults in growth hormone receptor-deficient humans. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2017;37:1696–1707. doi: 10.1523/JNEUROSCI.1929-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schuldenzucker V., Schubert R., Muratori L.M., Freisfeld F., Rieke L., Matheis T. Behavioral testing of minipigs transgenic for the Huntington gene - A three-year observational study. PLoS One. 2017;12:e0185970. doi: 10.1371/journal.pone.0185970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schlapschy M., Binder U., Borger C., Theobald I., Wachinger K., Kisling S. PASylation: a biological alternative to PEGylation for extending the plasma half-life of pharmaceutically active proteins. Protein Engineering Design and Selection : PEDS. 2013;26:489–501. doi: 10.1093/protein/gzt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yin H., Xue W., Chen S., Bogorad R.L., Benedetti E., Grompe M. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nature Biotechnology. 2014;32:551–553. doi: 10.1038/nbt.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kamimura K., Suda T., Xu W., Zhang G., Liu D. Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Molecular Therapy : The Journal of the American Society of Gene Therapy. 2009;17:491–499. doi: 10.1038/mt.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stoller F., Schlegel A., Viecelli H.M., Rufenacht V., Cesarovic N., Viecelli C. Hepatocyte transfection in small pigs after weaning by hydrodynamic intraportal injection of naked DNA/minicircle vectors. Human Gene Therapy Methods. 2015;26:181–192. doi: 10.1089/hgtb.2014.140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.