Abstract
Objectives
5-HT storing enterochromaffin (EC) cells are believed to respond to nutrient and gut microbial components, and 5-HT receptor-expressing afferent vagal neurons have been described to be the major sensors of nutrients in the GI-tract. However, the molecular mechanism through which EC cells sense nutrients and gut microbiota is still unclear.
Methods and results
TPH1, the 5-HT generating enzyme, and chromogranin A, an acidic protein responsible for secretory granule storage of 5-HT, were highly enriched in FACS-purified EC cells from both small intestine and colon using a 5-HT antibody-based method. Surprisingly, EC cells from the small intestine did not express GPCR sensors for lipid and protein metabolites, such as FFAR1, GPR119, GPBAR1 (TGR5), CaSR, and GPR142, in contrast to the neighboring GLP-1 storing enteroendocrine cell. However, the GLP-1 receptor was particularly highly expressed and enriched in EC cells as judged both by qPCR and by immunohistochemistry using a receptor antibody. GLP-1 receptor agonists robustly stimulated 5-HT secretion from intestinal preparations using both HPLC and a specific amperometric method. Colonic EC cells expressed many different types of known and potential GPCR sensors of microbial metabolites including three receptors for SCFAs, i.e. FFAR2, OLF78, and OLF558 and receptors for aromatic acids, GPR35; secondary bile acids GPBAR1; and acyl-amides and lactate, GPR132.
Conclusion
Nutrient metabolites apparently do not stimulate EC cells of the small intestine directly but through a paracrine mechanism involving GLP-1 secreted from neighboring enteroendocrine cells. In contrast, colonic EC cells are able to sense a multitude of different metabolites generated by the gut microbiota as well as gut hormones, including GLP-1.
Keywords: Metabolite GPCR, Nutrient sensing, Gut microbiota, Gut hormone, Enteroendocrine, Afferent vagal nerves
Highlights
-
•
Pure intestinal 5-HT cells are obtained through antibody-based FACS sorting.
-
•
Small intestinal 5-HT cells do not express sensors for nutrient metabolites.
-
•
Colonic 5-HT cells express multiple types of receptors for gut microbial metabolites.
-
•
GLP-1 stimulates 5-HT release from ex vivo intestinal preparations.
-
•
GLP-1 and 5-HT act in series and synergy to control GI-tract and metabolism.
List of abbreviations
- CCK
Cholecystekinin
- ChgA
Chromogranin A
- EC
Enterochromaffin
- FACS
Fluorescence-activated cell sorting
- GIP
Gastric inhibitory polypeptide
- GI-tract
Gastrointestinal tract
- GLP-1
Glucagon-like peptide 1
- GPCR
G protein-coupled receptor
- HPLC
High-performance liquid chromatography
- hrGFP
Humanized Renilla reniformis-derived green fluorescent protein
- LDCV
Large dense core vesicle
- NKA
Neurokinin A
- NPY
Neuropeptide Y
- NTS
Neurotensin
- PYY
Peptide YY
- qPCR
Quantitative polymerase chain reaction
- SCFAs
Short chain fatty acids
- SP
Substance P
- SST
Somatostatin
- TPH1
Tryptophan hydroxylase 1
1. Introduction
The enterochromaffin (EC) cell is the most abundant cell type among the enteroendocrine cells throughout the entire gastrointestinal tract. The main secretory product of the EC cells is the biogenic amine serotonin or 5-hydroxy tryptamine (5-HT), which otherwise generally is known as a neurotransmitter in the CNS. However, the amount of ‘peripheral 5-HT’ produced by the EC cells in the GI tract is far larger than 5-HT in the CNS [1], [2]. As opposed to neurons, EC cells use the enzyme tryptophan hydroxylase 1 (TPH1) and not TPH2 to synthesize 5-HT [3], [4], and, instead of small neurosecretory vesicles, EC cells store 5-HT in large dense core vesicles (LDCV) in complex with acidic proteins such as chromogranin A (ChgA) and B [5]. Previously, we have generated a ChgA reporter mouse, which expressed hrGFP in monoamine producing enteroendocrine cells throughout the GI tract [6]. Although the major secretory product of EC cells is 5-HT, they can also store peptide hormones such as secretin in the villi of upper small intestine and tachykinins like substance P (SP) in the crypts throughout both the small and large intestine [7], [8].
In the intestinal capillaries, 5-HT from the EC cells is taken up, to a large degree, by platelets and stored in their granules together with ATP, ADP, and Ca2+ for eventual use in their blood clotting function [9]. However, approximately two percent of the 5-HT in the circulation is supposedly found in the free form, and peripheral 5-HT originating from the gut has recently been proposed to function as a major endocrine regulator of metabolism affecting adipose tissue, liver, muscles, pancreas and the immune system [1], [10], [11] although details of this endocrine function of 5-HT remains to be further characterized.
Locally, in the lamina propria, 5-HT acts as a major paracrine signal through G protein coupled 5-HT receptors on neighboring cells to affect epithelial growth [12], enterocyte secretion, and intestinal barrier function [13], for example, as well as to activate immune cells [14] and enteric nerves [9]. 5-HT is known to have major effects on GI tract motility, but it is still debated whether this is controlled by 5-HT released from EC cells or from enteric nerves [15], [16]. Stimulation of afferent vagal neurons by gut-derived 5-HT [17], [18] has been shown to play a role in the control of for example gastric emptying and pancreatic secretion [17], [19]. Importantly, vagal nerves are known to express the ionotropic 5-HT3a receptor, and it was recently demonstrated that the main afferent vagal neurons which sense dietary nutrients in the villi of the small intestine surprisingly were excited not by gut hormones such as GLP-1 but by 5-HT [20]. This would suggest that the EC cells are sensing dietary nutrient metabolites as previously shown for the peptide hormone secreting enteroendocrine cells [21], [22], [23].
Regulation of EC cells and release of 5-HT have been studied since its discovery in the 1950s, but, due to methodological problems in diligently measuring 5-HT release and the fact that EC cells like other enteroendocrine cell populations is a minor population of cells scattered and outnumbered by other cell types, the secretory mechanisms of the EC cells are still poorly understood. Nevertheless, various classical neurotransmitters and neuropeptides have been reported to affect 5-HT secretion and to modulate TPH1 expression [24], [25], and similar effects have been shown for dietary nutrients and, in particular, microbial metabolites such as short chain fatty acids (SCFAs) [26], [27]. Recently, by use of the ChgA reporter mouse [6] in combination with cultured intestinal organoids and single cell measurements, Bellono et al. could demonstrate that EC cells expressing chemosensory receptors such as receptors for short chain fatty acids, were electrically excitable and could modulate 5-HT-sensitive afferent vagal neurons via synaptic connections [28].
In the present study, we characterize the expression of receptors that function as sensors of luminal stimuli in FACS-purified EC cells from the small intestine and the colon focusing on nutrient and microbial metabolites and find that, surprisingly, in contrast to peptide-producing enteroendocrine cells of the small intestine [22], [29], [30], the EC cells do not express GPCRs for nutrient metabolites. Instead, we find that the receptor for the gut hormone GLP-1 is particularly highly expressed and enriched in EC cells and that GLP-1 efficiently stimulates 5-HT release. It thus appears that nutrients stimulate 5-HT secretion indirectly through GLP-1. In contrast, the colonic EC cells directly senses chemical stimuli of the gut lumen as they express a large repertoire of known and proposed sensors of microbial metabolites including several receptors for short chain fatty acids besides receptors for GLP-1 and other gut hormones.
2. Materials and methods
2.1. Intestinal cell preparation, intracellular antibody staining of 5-HT and FACS
Standard chow diet fed, non-fasted, wild type C57BL/6-J male mice, 8–12 weeks old, were euthanized by cervical dislocation. The first 10 cm of the small intestine including duodenum and most of jejunum were excised and will here be termed “small intestine” as was the entire large intestine without the rectum, which here will be termed “colon.” We obtained a single cell suspension following a previously developed method involving enzymatic digestion and physical shaking of inverted mucosal preparations [31]. To enable intracellular antibody labeling of 5-HT stored within the enterochromaffin cells, we fixed the pools of single cells with 1% paraformaldehyde/PBS solution (Sigma–Aldrich) for 20 min and subsequently permeabilized them with a solution consisting of 0.1% Saponin in 2% bovine serum albumin and 0.2% RNaseOUT (Invitrogen) in PBS for 15 min. The primary antibody against 5-HT (ab66047, Goat Anti 5-HT antibody, 1:3200, Abcam) was added and incubated with the cells for 60 min. A fraction of cells was not exposed to the primary antibody and used controls. After a brief wash with PBS the Alexa488 labeled secondary antibody (Donkey Anti-Goat 488, 1:400, Invitrogen) was added in cold PBS to the cell solution and incubated for 30 min. Another wash in ice-cold PBS was performed and the stained single cell suspension was re-suspended in cold FACS buffer (2% fetal bovine serum and 0.2% RNaseOUT in PBS). All the steps were performed at 4 °C while slowly rocking the tubes.
Cells were sorted into a 488 nm excitable fraction and a 488 nm non-excitable fraction using MoFLo Astrios (Beckman Coulter). The cells were sorted into tubes placed on dry ice.
Cells dissociated as described above from small intestine (n = 8) from GLU-Venus mice [32] were FACS isolated and used for the analysis of nutrient receptor expression in GLP-1 positive cells as previously described [33], [34]. After FACS-purification the proglucagon gene (GCG) was determined to be enriched approx. 500-fold in the Venus positive sorted cell fraction as compared with the Venus negative and the sample was used to determine the expression of nutrient metabolite sensors.
2.2. RNA extraction and quantitative RT-PCT analysis
Total RNA was isolated from FACS cells using a special kit for paraformaldehyde-fixated cells, RNeasy FFPE kit (Qiagen). Manufacturer's protocol was followed except for a few modifications; the first steps to remove xylene and subsequent ethanol precipitation were omitted, and the incubation steps at 55 °C and 80 °C were shortened to 12 min to minimize RNA degradation. RT-PCR was then performed using the SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA). Quantitative RT-PCR was performed to characterize isolated cells with SYBR PrecisionPLUS (Primerdesign) and primers (Tph1, ChgA, Ywhaz, GAPDH from Ref. [6]) in a LightCycler480 (Roche). Due to the small sample size, cDNA was amplified with QuantiTect Whole Transcriptome kit (Qiagen) and analyzed on custom-designed qPCR arrays targeting 88 peptides and granins of interest. The relative expression was calculated using the average of GAPDH, Ywhaz, and Tbp reference genes. Additional custom-designed qPCR array for various 7TM GPCRs (Qiagen) were used according to manufacturer's instructions. To calculate relative expression, we proceeded as in Ref. [34].
2.3. Immunohistochemistry
Intestinal tissue from three C57BL/6-J male mice was excised, rinsed and kept in 4% paraformaldehyde for 48 h at 4 °C afterwards stored in 70% ethanol and then paraffin embedded in a Shandon Excelsior (Thermo Fisher). Six μm sections were cut, put onto slides, and dried at 60 °C for 1 h. Before use, the sections were deparaffinized and rehydrated. To verify coexpression of 5-HT and various peptides, intestinal sections were stained with antibodies against Substance P (1:3200, “SSP 250-2” – generously provided by Prof. Steen Seier Poulsen) or against NKA (1:2000, an in-house antibody generated by E.T.) and counted as described in Ref. [6]. A minimum of 200 immunoreactive cells were counted per intestinal segment evaluated. When staining the sections for GLP-1 receptor, a Biotin-labeled Mouse anti-Mouse GLP-1r antibody was used (kind gift from Novo Nordisk); the tissue sections were treated with 0.1% pronase (Roche) in PBS for 10 min at 37 °C. The tissue sections were blocked with Avidin (Dako) and Biotin (Dako), respectively, for 10 min and additionally blocked using TBS with 3% skim milk (BioRad) and 7% Donkey serum (Jackson) for 30 min. Hereafter, the primary antibody was added 1:300 in pre-incubation solution overnight at 4 °C. The next day, the primary antibody was rinsed off with TBS, and Vectastain ABComplex (Vectorlabs) was added for 30 min. To amplify the primary antibody signal, Biotinyl Tyramide (Perkin Elmer) was applied for 5 min, washed off, and Streptavidin–FITC complex was added for 30 min to visualize binding sites of the primary antibody. To get an overview of coexpression of the receptor and 5-HT and relevant gut peptides, primary antibodies against 5-HT (same as above), somatostatin (sc-7819, 1:1600, Santa Cruz Biotechnology), and CCK (8007, 1:6000, SSP see [6]) were added, respectively, and left to incubate for 2 h at room temperature. After washing with TBS, a secondary antibody with a 568 nm fluorophore (1:200, Alexa568, Invitrogen) was added for 30 min. After the last washing, the slides were mounted with Prolong Gold Antifade with DAPI (Invitrogen) and analyzed using IX70 Olympus microscope with XM10 Olympus camera. Pseudocolor application and picture merging were done using Adobe Photoshop.
For coexpression studies, tissue from three mice was evaluated and a minimum of 200 cells counted. Structured Illumination Microscopy (SIM) was performed using the Zeiss Elyra PS.1 Super Resolution Microscope and the images were analyzed using Zeiss Zen Black 2012 software.
2.4. Measurements of 5-HT release
When verifying that activation of GLP-1R leads to 5-HT secretion, we utilized two methods: amperometric and HPLC measurements of secreted extracellular 5-HT from intestinal tissue segments as previously described [35], [36]. Briefly, for HPLC measurements, mucosal scrapings from small duodenum (sampled 1 cm from pyloric sphincter), jejunum, and colon (sampled 2 cm from rectum) were stimulated in oxygenated Krebs buffer with 10 nM Liraglutide (Novo Nordisk) or PBS as vehicle for 60 min. After incubation, the supernatant was removed, and 0.1 M HClO4 was added to preserve the 5-HT. The samples were centrifuged at 13,000 g for 10 min at 4 °C, and the supernatant was analyzed for 5-HT using HPLC with electrochemical detection (for details see Ref. [37]). The protein content of the mucosa was determined using a standard Bradford assay, and 5-HT concentrations were normalized to this.
To perform the continuous amperometric measurements, segments from small intestine and colon (n = 6) were pinned in a Sylgard® (Dow Corning) lined Teflon recording chamber and perfused with oxygenated Krebs buffer. The tissue was perfused for 10 min prior to the series of baseline measurements. For details on the amperometric recordings, see Ref. [37]. In short, a boron-doped diamond electrode with a potential of +650 mV oxidizes released 5-HT. Using a micromanipulator, this microelectrode was placed centimeters away from the tissue to measure background recordings. When measuring 5-HT overflow, the microelectrode was positioned 0.1 mm over the mucosa for 20s. This was repeated three times before 10 nM Liraglutide was added in the Krebs buffer and 10 min after three additional measurements were obtained. All results were analyzed using a repeated measure, 2-way ANOVA with post hoc Tukey test.
The tissue was harvested from 8 to 12 weeks-old C57BL/6-J mice and these procedures were in accordance to U.K. Home Office regulations and approved by the University of Brighton Ethics Committee.
3. Results
3.1. FACS purification of intestinal EC cells
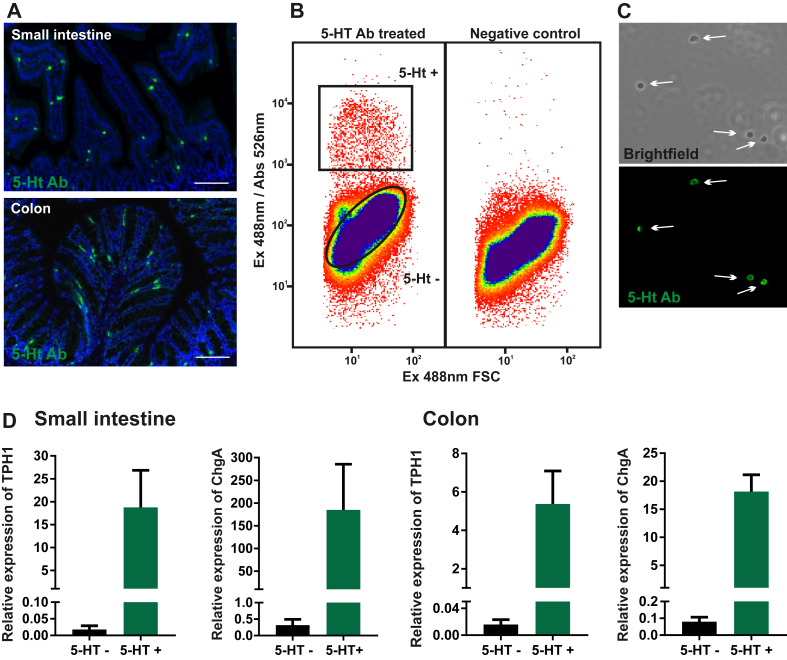
Although ChgA-GFP in many ways functions as a great reporter for monoamine producing cells of the GI tract, the tph1 transcript, coding for the key enzyme responsible for 5-HT synthesis in EC cells was only enriched 48- and 186-fold in positive FACS-purified ChgA-positive cells from the small intestine and colon, respectively [6]. By use of the same ChgA reporter, we observed a markedly higher enrichment, 3800-fold, for histidine decarboxylase (hdc), the enzyme responsible for histamine production in FACS-purified EC-like cells from the stomach. Hence, we decided mainly to employ a 5-HT antibody-based approach to purify EC cells from the intestine. Sorting cells using an intracellular epitope has been done previously [38], [39]. We used a 5-HT goat antibody as the primary antibody; in immunohistochemistry, this efficiently labels murine EC cells in both the small and large intestine (Figure 1A) [6]. For FACS-purification, this antibody was applied to preparations of saponin-permeabilized, fixed single cells, liberated by collagenase treatment of intestinal mucosa from the first 10 cm of the small intestine including duodenum and upper jejunum (henceforth termed ‘small intestine’) and from the colon. Approx. 0.3% and 0.5% of the total mucosal cells were sorted as 5-HT positive EC cells from the small intestine and colon, respectively (Figure 1B,C). Tph1 was on average enriched 1700-fold and ChgA 1400-fold in the small intestinal 5-HT positive cells and approx. 1000 times and 300 times in the purified colonic 5-HT cells. Thus, in both cases, the FACS-purified EC cells were very pure (Figure 1D).
Figure 1.
Antibody-mediated FACS purification of EC cells from the small intestine and colon of mice (A) Representative fluorescence microscopy showing 5-HT antibody staining (green and cell nuclei in blue) in wild type mouse small intestine and colon. Scale bars represent 50 μm. (B) FACS diagram presenting the sorted population (black boxes = gating) which were positive for 5-HT antibody staining marked with Alexa Fluor 488 and also the sorted negative population. The negative control also depicted in (B) is a control for unspecific binding by omitting the primary antibody. (C) Sorted 5-HT positive, single cells. (D) qPCR analysis of the expression of two EC marker genes; Tph1 and ChgA in positive vs. negative fraction of sorted cells from small intestine (n = 3) and colon (n = 4). The results are presented as relative expression to reference genes (average of GAPDH and Ywhaz).
3.2. Co-expression and storage of peptides and proteins with 5-HT in EC cells
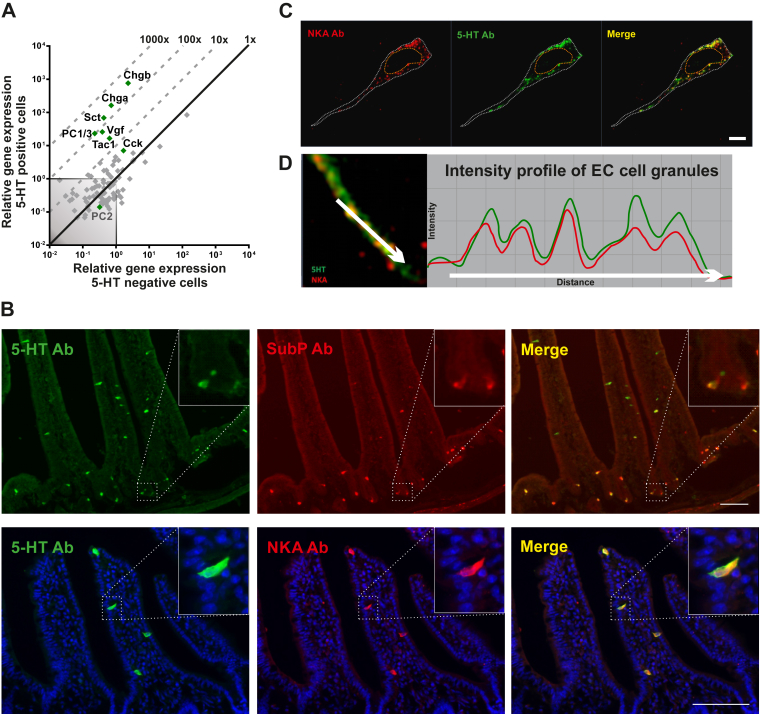
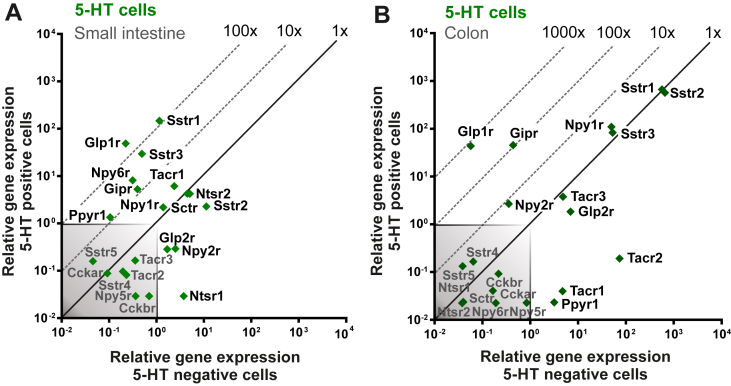
Based on immunohistochemical analysis, different peptide hormones and granin proteins have been reported to be co-expressed with 5-HT in EC cells [40]. We therefore probed the FACS-purified EC cells for expression of an array of 88 different ‘enteroendocrine proteins’: granins, peptide precursors, and precursor convertases. Concerning granins, i.e. acidic proteins, which help store monoamines in secretory granules, not only chromogranin A but also chromogranin B and the VGF granin were all highly expressed and enriched in both small intestinal and colonic EC cells (Figure 2A and Suppl. Figure 1A). With respect to prohormone convertases, only prohormone convertase 1/3 (PC1/3), and not prohormone convertase 2, was expressed in the EC cells from both small and large intestine (Figure 2A and Suppl. Figure 1A). Concerning peptide precursors, the transcripts for the secretin precursor Sct, the tachykinin precursor Tac1, and, to some degree, the CCK precursor Cc, were all expressed and enriched in EC cells from the small intestine (Figure 2A). The colonic EC cells did not express Sct and Cck but still expressed Tac1 (Suppl. Figure 1A). Immunohistochemistry demonstrated that not only Substance P but also Neurokinin A (NKA), i.e. the two major tachykinin peptides, are expressed almost exclusively in EC cells in the intestinal mucosa, as close to 100% of the SP and NKA positive cells also were positive for 5-HT (Figure 2B, Suppl. Figure 1B). However, not all 5-HT storing cells stained positive for SP and NKA possibly due to variable expression level and detection limit of the peptide antibodies (Suppl. Figure 2C).
Figure 2.
qPCR and histological analysis of co-expressed peptides and granins in EC cells. (A) qPCR analysis targeting the expression of 88 common peptide hormones, neuropeptides and granins in 5-HT positive cells (y-axis) versus 5-HT negative cells (x-axis) in small intestine. The enriched peptide transcripts are depicted as green dots whereas the rest are gray. The 45°-angled gray dotted lines depict the fold change enrichment in 5-HT positive cells versus 5-HT negative cells and the gray-shaded square is marking what is considered noise. (B) Fluorescent microscope pictures of small intestine labeled with 5-HT (green) and Substance P or Neurokinin A (Red) depicts co-expression in EC cells. Scale bars correspond to 50 μm. (C) Fluorescent structural illumination microscopy visualizes granules in single EC cells when stained with 5-HT (green) and NKA (red) antibodies. (D) The intensity profiles of the different fluorochromes in granules show that they are positive for both 5-HT and NKA.
In EC cells as opposed to neurons, 5-HT is stored in large dense core vesicles conceivably in complex with the large acidic chromogranin proteins [5]. In enteroendocrine cells expressing more than one peptide hormone, these peptides are in some cases stored in the same secretory granules in other cases not [41]. In order to study to what degree 5-HT was co-stored with the tachykinin peptides, we focused on the long basolateral extensions which enteroendocrine cells use for paracrine, targeted secretion [42]. These extensions are loaded with secretory granules which are aligned like pearls on a string (Figure 2C). By employing super-resolution fluorescence microscopy to such basolateral EC cell extensions, we could demonstrate that the NKA peptide and 5-HT in fact are co-stored in the large dense core vesicles (Figure 2D).
3.3. Expression of nutrient metabolite GPCRs in EC cells
EC cells are classical enteroendocrine cells, which are located in the lumen and morphologically equipped to respond to luminal stimuli. Table S1 lists the main receptors known to sense nutrient metabolites in the small intestine when they are absorbed [23] and those which. according to the present literature. conceivably sense metabolites generated by the gut microbiota (Table SI). Receptors such as GPR119 and GPBAR1 (TGR5) can probably serve both functions.
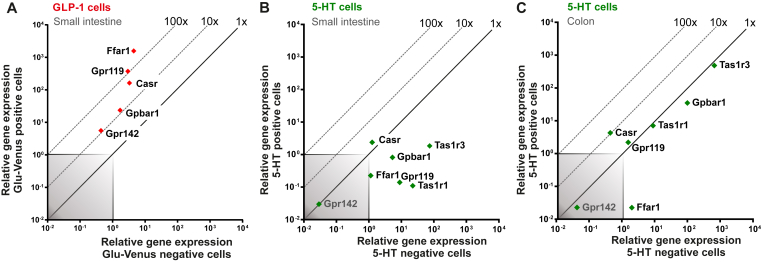
As shown in Figure 3A, the main receptors for lipid and protein metabolites, i.e. FFAR1 sensing free fatty acids, GPR119 sensing 2-acyl glycerol, GPBAR1 sensing bile acids from the lipid micelles, CaSR sensing amino acids and oligopeptides, and GPR142 sensing aromatic amino acids all are highly expressed and enriched in FACS-purified GLP-1 cells from the small intestine as also previously reported [22], [30]. Surprisingly, however, none of these nutrient sensors including the sweet taste receptors TAS1R1 and TAS1R3 was enriched in 5-HT storing EC cells from the small intestine (Figure 3B). In fact, most of the nutrient metabolite receptors were negatively enriched and expressed below noise level in small intestinal EC cells. This was particularly puzzling since 5-HT has been shown to be a major stimulus for afferent vagal fibers sensing nutrients in the small intestine [20].
Figure 3.
qPCR analysis of nutrient metabolite receptors on GLP-1 positive cells and 5-HT positive cells. (A) qPCR analysis expression data for main nutrient metabolite 7TM GPCRs in Glu-Venus positive cells (y-axis) compared with surrounding negative cells (x-axis) isolated from small intestine. (B and C) 5-HT positive (y-axis) and 5-HT negative (x-axis) cell gene mRNA expression from small intestine (B) and colon (C). The 45°-angled gray dotted lines display the fold change enrichment in 5-HT positive cells versus 5-HT negative cells and the gray-shaded square is marking what is considered noise.
In the colonic EC cells, GPBAR1 was highly expressed but not enriched, and the expression of GPR119 and CaSR was just above noise level (Figure 3C). However, it is likely that in the colonic EC cells, these three receptors are not sensing nutrient metabolites but instead microbial metabolites such as N-acyl amides and secondary bile acids, respectively (Table SI). Bile acids have previously been reported to stimulate 5-HT release from EC cells in [43]. The colonic EC cells also expressed TAS1R1 at a high level, but, like the other receptors in this group, they are expressed at the same level in the non-5-HT neighboring colonic mucosa cells (Figure 3C).
3.4. Expression of microbial metabolite receptors in EC cells
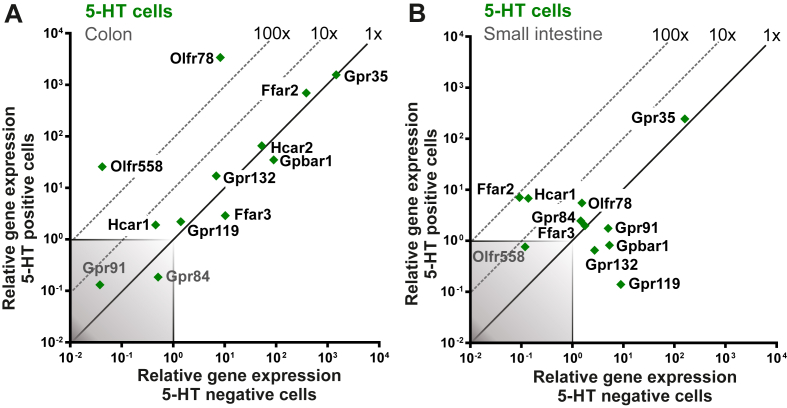
As shown in Figure 4, most of the receptors for microbial metabolites were highly expressed, several of which were also highly enriched, particularly in colonic EC cells, which is in agreement with the notion that these cells should be sensing the gut microbiota (Figure 4A). In particular, the transcripts for the SCFA receptors FFAR2 and OLFR78 and the transcript for GPR35, which is a sensor of aromatic amino acids, were all very highly expressed in colonic EC cells, which concerning SCFAs is in agreement with their reported stimulatory effects on Tph1 expression and 5-HT synthesis [26], [27], [37]. Of these microbial metabolite sensors, Olfr78 stood out in being approx. 400-fold enriched in the colonic EC cells in contrast to Ffar2 and Gpr35, which were equally expressed in EC cells and in the neighboring mucosal cells (Figure 4A). Another member of the olfactory receptor superfamily, OLFR558 was recently shown to be a receptor for branched SCFAs such as isovalerate and was found to be expressed in EC cells [28]. We could confirm that Olfr558 is expressed and highly enriched in colonic EC cells (640-fold), although at a considerably lower actual expression level than, for example Olfr78 (Figure 4A)
Figure 4.
qPCR analysis of microbial metabolite receptors on 5-HT positive cells. (A and B) qPCR analysis expression data for known microbiota metabolite 7TM GPCRs in 5-HT positive cells (y-axis) compared with surrounding negative cells (x-axis) isolated from colon (A) and small intestine (B). The 45°-angled gray dotted lines display the fold change enrichment in 5-HT positive cells versus 5-HT negative cells and the gray-shaded square is marking what is considered noise.
A number of other receptors for microbial metabolites were like FFAR2 relatively highly expressed but not enriched in the colonic EC cells as described for GPBAR1 above, meaning that they likely are expressed by the colonic mucosal cells in general. In order of decreasing relative expression these are: Hca2 (Niacr1), sensing SCFAs and nicotinic acid; GPBAR1, sensing secondary bile acids; GPR132, sensing acyl amides and lactate; GPR119, sensing acyl amides, and HCAR1, sensing lactate, were both expressed just above noise level (Figure 4A).
In EC cells from the small intestine, the microbial metabolite sensors were expressed at a lower level than in the colonic EC cells, although GPBAR1 was relatively highly expressed, but still not enriched (Figure 4B). FFAR2 and HCAR1 were both relatively enriched, i.e. 78- and 50-fold, respectively, but only expressed at a relatively low level (Figure 4B). GPR119, a potential sensor of microbial produced acyl amides, was in fact negatively enriched and below noise level in the small intestinal EC cells, as discussed above in relation to the nutrient metabolite 2-acyl-glycerol.
3.5. Expression of peptide hormone receptors in EC cells
Surprisingly and in contrast to other enteroendocrine cells of the small intestine, 5- HT storing EC cells did not express receptors for nutrient metabolites (Figure 3A). We reasoned that perhaps nutrient metabolites could affect EC cells indirectly through one or more of the gut hormones. Therefore, we tested whether EC cells express receptors for these peptide hormones.
As shown in Figure 5A, the somatostatin receptors 1 and 3 and the GLP-1 receptor were the most highly expressed peptide hormone receptors in EC cells from the small intestine followed by the PYY/NPY receptor Y6, the tachykinin receptor NK1, and the GIP receptor. Importantly the GLP-1 receptor was the most highly enriched receptor (220-fold), which was surprising as no effect of GLP-1 on EC cells has previously been reported. GLP-1 is usually expressed, released and functions together with PYY and neurotensin [41]. PYY could affect EC cells through the Y6 receptor, which however is a pseudogene in man [44]. The NTSR1 receptor, which neurotensin normally acts through in the periphery, is not expressed at all in the EC cells and the normally centrally expressed NTSR2 receptor is only expressed at a low level in the EC cells. Somatostatin has long been known to markedly inhibit 5-HT release [24], [25] but exactly which receptors are present on EC cells has not been described before.
Figure 5.
qPCR analysis of peptide hormone receptors on 5-HT positive EC cells. (A and B) qPCR analysis expression data for relevant peptide hormone 7TM GPCRs in 5-HT positive (y-axis) and 5-HT negative (x-axis) cells from small intestine (A) and colon (B). The genes are depicted as green squares. The 45°-angled gray dotted lines display the fold change enrichment in 5-HT positive cells versus 5-HT negative cells and the gray-shaded square is marking what is considered noise.
In colonic EC cells, the somatostatin receptors (SSTR1, 2 and 3), the PYY Y1 receptor, the GIP receptor, and the GLP-1 receptor were all relatively highly expressed, again with the GLP-1 receptor being the most highly enriched, i.e. 760-fold (Figure 5B). The GIP receptor was also highly enriched in the colonic EC cells, 105-fold. The more classical PYY receptors Y1 and Y2 were clearly expressed, with the Y1 receptor being highly expressed but not enriched, which fits with its function as an important regulator of enterocyte function [45], [46], while the Y2 receptor was expressed at a lower level but enriched approx. 10-fold (Figure 5B). A similar pattern of expression levels of gut hormone peptide receptors was seen in FACS isolated ChgA-hrGFP positive cells (Suppl. Figure 2B).
3.6. Expression of the GLP-1 receptor in EC cells
All analysis of expression presented above has been performed at the RNA level by qPCR. In principle the transcriptional expression does not necessarily correspond linearly to expression at the protein level. Previously, we have noticed that receptors that are not truly highly expressed or in particular highly enriched at the RNA level in enteroendocrine cells may not be functionally important at least in respect of control of hormone secretion [33], probably because they are not expressed at the protein level.
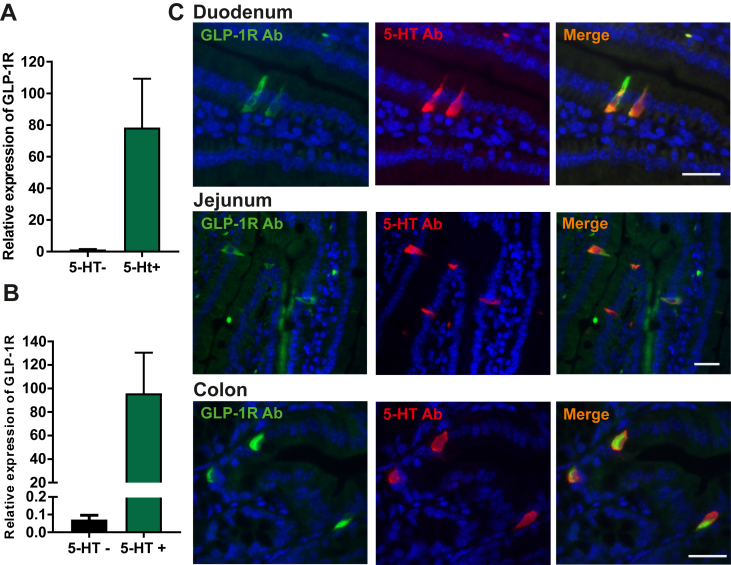
To verify the presence of the GLP-1 receptor in EC cells at the protein level, we performed immunohistochemistry using a novel monoclonal GLP-1 receptor antibody with a specificity which has been verified in GLP-1R deficient mice [47].1 Two distinct populations of GLP-1R positive cells were observed throughout the intestine. A population of round cells exhibiting rather strong immunoreactivity was observed in the lamina propria (Suppl. Figure 3). These are likely intestinal intraepithelial lymphocytes that known to express the GLP-1 receptor [48] and will not be further characterized here. The other population of GLP-1 receptor immunoreactive cells was flask shaped, enteroendocrine like cells located in the intestinal epithelium, the vast majority of which co-stained with the 5-HT antibody (Figure 6C). GLP-1 receptor immunoreactive 5-HT positive enteroendocrine cells were observed throughout the intestine, except in the ileum. Not all 5-HT positive cells stained positively for GLP-1 receptor, i.e. 42% of EC cells in the small intestine and 54% in the colon, which as discussed above for NKA and SP could be due to differences in expression level and due to the detection limits for in this case the GLP-1 receptor antibody; but, it could also be due to true differences in populations of EC cells. Few GLP-1R positive enteroendocrine cells were not positive for 5-HT, but instead staining positively for the peptide hormone somatostatin (Suppl. Figure 3). Somatostatin cells of the stomach have previously been shown to express the GLP-1 receptor [34].
Figure 6.
Expression of the GLP-1 receptor in EC cells. (A and B) qPCR expression data of GLP-1R in 5-HT positive cells (green bar) versus 5-HT negative cells (black bar) from small intestine (n = 3) (A) and colon (n = 4) (B). (C) Representative fluorescent microscopy picture with monoclonal GLP-1R antibody (green) in combination with 5-HT antibody (red) in duodenum, jejunum, and colon. Cell nuclei are depicted in blue and scale bars represent 20 μm.
3.7. Proximity of GLP-1 and 5-HT enteroendocrine cells
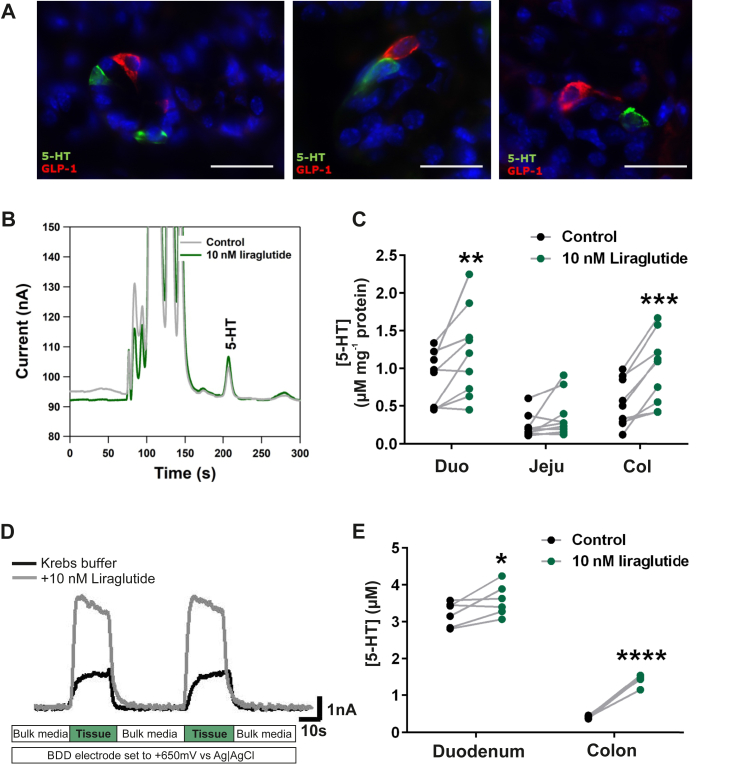
Although in many cases we could demonstrate GLP-1 and 5-HT cells in close proximity to each other (Figure 7A), we did not attempt to quantify this, which would require a major stereological effort and would not resolve how close the cells need to be in the intestinal villus to function in a paracrine manner. Importantly, enteroendocrine cells including GLP-1 cells are often equipped with relatively long basolateral extensions, enabling targeted paracrine signaling even at a distance [42], [49] (Figure 7A, right panel).
Figure 7.
Proximity of GLP-1 and EC cells and functional studies of GLP-1 receptor on the EC cell. Panel A – Representative fluorescent microscopy pictures of GLP-1 (red) and 5-HT (green) positive cells localized closely in the duodenum. Cell nuclei are depicted in blue and scale bars represent 20 μm. Note in the panel to the right, the long basolateral extension of the GLP-1 cell approaching the EC cell. Panels B–E: Ex vivo 5-HT release data from murine intestine stimulated with liraglutide (10 nm) (A) Example of chromatographic responses from HPLC-ECD analysis of buffer from control (gray) and stimulated (green) excised intestinal pieces. (B) 5-HT secretion from excised duodenum (Duo), jejunum (Jeju) or colon pieces (Col). Samples from the same animal were treated with liraglutide (green dots) or vehicle as control (black dots) and are connected with a gray line (n = 9). (C) Example of real-time amperometric measurements of ex vivo murine colon stimulated with liraglutide (10 nm). (D) 5-HT release data from amperometric measurements (n = 6). The connected dots represent samples coming from the same animal stimulated with vehicle (black) or liraglutide (green).
3.8. Functional studies of the GLP-1 receptor in EC cells
Determination of 5-HT secretion is far from simple and accordingly we used two different ex vivo experimental approaches to test whether activation of the GLP-1 receptors on EC cells stimulates 5-HT secretion.
Firstly, pieces of murine intestine, specifically duodenum, jejunum, and colon, were excised and stimulated with liraglutide, a selective and potent GLP-1 receptor agonist, or control vehicle. Released 5-HT was quantified in the buffer by HPLC and electrochemical detection [36] (Figure 7B). Overall, stimulation with a GLP-1 receptor agonist resulted in an increase of released 5-HT compared with vehicle (Figure 7C). More specifically, in duodenum, we observed a statistically significant increase in 5-HT concentrations, i.e. an increase in seven out of nine samples, with a two-fold response in two of the tissue samples. In the samples from jejunum, the 5-HT responses were not statistically significant although a numerical increase was observed in three mice (Figure 7C). A very robust and statistically significant increase of 5-HT release was detected in the colon, where samples from all mice were releasing 5-HT in response to the 10 nM liraglutide (n = 9) (Figure 7C).
5-HT release can be measured by continuous amperometric detection in samples of excised intestinal mucosa [35]. 5-HT is detected using a boron doped diamond electrode, which was set to a specific voltage known to specifically detect and quantify 5-HT. When the tissue sample is approached with the electrode, overflow of 5-HT can be quantified in high resolution every second. The excised mucosa (duodenum or colon) is submerged in a perfusion dish, and control measurements were performed with vehicle, Krebs buffer overflowing the tissue (Figure 7D). Hereafter, the agonist was added into the buffer, and new measurements were performed. A clear increase in amperometric signal, corresponding to 5-HT release, was observed in particular when the mucosa from the colon was exposed to 10 nM liraglutide (Figure 7D,E). As shown in Figure 7E, the spontaneous release of 5-HT was higher in the duodenum than in the colonic preparations; nevertheless, a significant increase in 5-HT release was observed in response to the GLP-1 receptor agonist both in the colon and in the duodenum, although the relative response was smaller in the duodenum due to the higher level of spontaneous release of 5-HT (Figure 7E).
4. Discussion
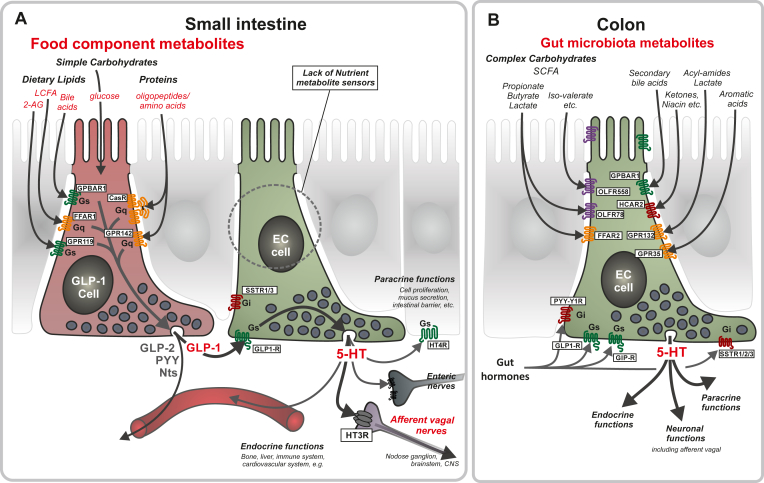
5-HT storing EC cells constitute the largest population of enteroendocrine cells, which, with respect to anatomical localization and cell morphology, are clearly specialized to sense the content of the gut lumen and believed to respond to nutrients in the small intestine and to gut microbiota metabolites in the colon. Surprisingly, we find in the present study that the EC cells themselves are devoid of nutrient metabolite sensing receptors in the small intestine; instead, they are highly enriched in GLP-1 receptors. Given that neighboring GLP-1-storing enteroendocrine cells massively express nutrient metabolite receptors, which stimulate GLP-1 secretion, and that we here demonstrate that GLP-1 stimulates 5-HT secretion, we propose that the EC cells sense nutrient metabolites indirectly through the GLP-1 cell and thereby convey the signal of food components to 5HT3 receptor-expressing afferent vagal neurons, which constitute the major populations of food-sensing neurons in the GI-tract [20] (Figure 8A). In contrast, the EC cells of the colon apparently do sense the chemical content of the lumen directly, as we find multiple different GPCR sensors of microbial metabolites to be highly expressed and enriched in colonic EC cells, which also express receptors for GLP-1 and other gut hormones (Figure 8B).
Figure 8.
Schematic overview of the proposed mechanism through which nutrient metabolites stimulate 5-HT secretion from small intestinal EC cells indirectly through GLP-1 cells and how a series of different gut microbial metabolites can stimulate colonic EC cells directly. Panel A: In the small intestine food components are digested to different metabolites which are sensed by GPCRs expressed on peptide hormones storing enteroendocrine cells: FFAR1/GPR40 sensing LCFAs; GPR119 sensing 2-acyl glycerol (2-AG); GPBAR1/TGR5 sensing bile acids; CasR and GPR142 sensing amino acids and oligopeptides (Figure 3A). Remarkably, these nutrient sensing GPCRs were absent in EC cells from the small intestine (Figure 3B). However, receptors for gut hormones, in particular the GLP-1 receptor, were highly expressed and enriched on the EC cells and since GLP-1 stimulates 5HT secretion from intestinal preparations (Figure 5, Figure 7) it is proposed that nutrient metabolites stimulates 5-HT secretion from EC cells indirectly through GLP-1 cells and thereby stimulate the many indicated paracrine and endocrine functions of 5-HT. Importantly, the main afferent vagal neurons, which innervate the intestinal mucosa and respond to nutrients express the 5HT3 receptor, i.e. a ligand gated ion-channel [20]. Panel B: colonic EC cells express many different types of receptors for microbial metabolites including: FFAR2/GPR43, OLF78 and OLF558 sensing different types of short chain fatty acids; GPBARR1/TGR5 sensing secondary bile acids; GPR35 sensing small aromatic acids and GPR132 sensing lactate and acyl amides. In particular the SCFAs have been shown to control maturation, gene expression and secretion from the EC cells. The colonic EC cells also express several receptors for gut hormones including GLP-1, GIP, and PYY.
4.1. EC cells sense nutrients indirectly through GLP-1 cells
EC cells are classical open-type flask-shaped enteroendocrine cells with apical microvilli-decorated extensions reaching the gut lumen. Accordingly, they would be expected to sense the luminal content both in respect of mechano- and chemical sensing, although it is becoming more and more clear that a major part of at least the sensing of nutrient and microbial metabolites occurs after or during their absorption through receptors expressed on the basolateral membrane of the enteroendocrine cells [23], [50], [51]. Recently, it was shown through the application of a number of elegant techniques including in vivo recordings of afferent vagal neurons in the nodose ganglion and genetically guided anatomical mapping that the population of neurons which densely innervates the intestinal villi and which detects nutrients express the 5-HT HTR3 ionotropic receptor and not as otherwise expected, for example receptors for GLP-1 or CCK. It was proposed by Williams et al. that these neurons, named ‘GPR65 neurons’ due to their high expression of an orphan GPCR, would communicate directly with EC cells and that the EC cells would function as the main primary sensors of nutrients [20]. Therefore it was highly surprising to find that the GPCRs, which sense dietary lipid metabolites and bile acids associated with the lipid micelles, were basically absent from EC cells s, i.e. FFAR1 (LCFAs), GPR119 (2-acyl glycerol) and GPBAR1 (TGR5) (bile acids) as well as the GPCRs sensing amino acid and oligopeptide protein metabolites; i.e. CaSR and GPR142 [23]. Several of these receptors were even ‘negatively enriched’ in the FACS-purified EC cells, meaning that they are more highly expressed and enriched in the neighboring cells (Figure 3B). Recently it was reported that FFAR1 and GPR119 were indeed expressed in a preparation of EC cells; however, purification was by a density gradient centrifugation method [52], [53] and not a selective, antibody-guided FACS purification method as in the present study. Thus, perhaps the contradictory receptor expression patterns can be due to differences and purity of the enteroendocrine cell type populations obtained through these two very different methods.
In contrast to EC cells, peptide producing enteroendocrine cells such as GLP-1 cells express all of the nutrient metabolite receptors and in large amounts (Figure 3A), and agonists for the nutrient metabolite receptors are known to be efficient secretagogues for hormones including GLP-1 [21], [22], [29], [30], which potentially could target neighboring EC cells. Accordingly, we specifically looked for expression of receptors for gut hormone peptides. Among the potentially stimulating receptors, the receptor for GLP-1, Glp1r stood out as the most highly enriched and also highly expressed receptor in EC cells from both the small intestine and the colon, which, to our knowledge, has not previously been reported. Importantly, expression of the GLP-1 receptor could be demonstrated also at the protein level through staining of 5-HT cells with a selective monoclonal receptor antibody (Figure 6C) [47].
Although a few pure GLP-1 cells do exist, GLP-1 in most enteroendocrine cells is expressed, stored, and released together with PYY and neurotensin [41]. However, with respect to receptors for these other gut hormones, only the PYY/NPY Y6 receptor was expressed at a reasonable mRNA level in the small intestinal EC cells; however, in humans this is a pseudogene. The expected receptor targets for PYY and neurotensin, i.e. the Y2 and NTS-R1 receptors, respectively, were both expressed below noise level in EC cells from the small intestine. Thus, with respect to potential mediators of the signal of incoming nutrients to EC cells, which themselves lack nutrient metabolite receptors, GLP-1 was the prime candidate.
5-HT release from intestinal EC cells is, in contrast to peptide hormone secretion, far from trivial to quantify; this is due both to issues related to rapid uptake of the monoamine by various cells including the EC cells themselves and to assay technological problems associated with, for example 5-HT ELISAs [54]. Nevertheless, both by employing HPLC analysis with electrochemical detection and by amperometric technology, we were able to demonstrate that a GLP-1 receptor agonist robustly releases 5-HT in both the small intestine and in the colon (Figure 7).
Thus, we propose that GLP-1 released in response to nutrient metabolites functions as a paracrine regulator of 5-HT secretion from the neighboring EC cells, which conceivably convey the nutrient signals from the gut to the CNS by activating 5HT3 receptors expressed on the afferent vagal GPR65-neurones, which strongly innervates the small intestinal villi [20] and which have been shown to directly interact with EC cells [28]. As indicated schematically in Figure 8A and as shown by immunohistochemistry in Figure 7A, the GLP-1 cells are often equipped with long basolateral extensions, which were described originally for somatostatin cells as means of obtaining targeted, paracrine regulation of neighboring cells in the stomach [42]. Thus, a paracrine effect of GLP-1 on EC cells may occur either through bulk diffusion or through more specific targeting of EC cells by the basolateral cellular extensions (Figure 7A). It should be noted that similar basolateral extensions have been proposed to function as connectors to nerves [49].
Although the EC cells of the small intestine apparently are not sensing food through GPCR sensors of nutrient metabolites, they are likely still sensing the presence of food through mechano-sensing. Since 1959, it has been known that small intestinal EC cells are able to sense mechanical stimuli and react with 5-HT secretion [55], [56]. Mechanical forces in the form of stretching and poking or stroking of the mucosa have been reported to stimulate 5-HT release and recently a mechanosensitive ion channel (Piezo2) was proposed to be involved in this process [57]. Consequently, during food intake, the sensing of the physical passage of food through the duodenum will stimulate EC cells, possibly through their apical extensions, which reach the lumen. Very likely, the mechanical stimulus will act in synergy with the chemically induced GLP-1 stimulus of the EC cells.
It should be noted that the nutrient sensing mechanisms and interactions between GLP-1 and EC cells described above may be particularly important in the duodenum and proximal small intestine, which is particularly rich in EC cells and which we have focused on in the present study. In the ileum, which is rich in GLP-1 cells, we could not detect immunohistochemical staining of GLP-1 receptor in the EC cells. In this respect, it is also important to emphasize that Williams et al. in their study of the afferent vagal mechanisms in the small intestine also focused on the duodenum [20]. In fact endogenous intestinal GLP-1 may exert some of its effects such as effects on gastric emptying directly through GLP-1 receptor-expressing afferent vagal neurons as demonstrated by viral mediated knock-down of the GLP-1 receptor in nodose ganglia in rats [58]. Thus, there appear to be major regional differences even within the small intestine, and it could be hypothesized that GLP-1 in the proximal small intestine mainly acts through paracrine activation of neighboring EC cells and in the distal small intestine mainly acts through paracrine direct activation of afferent vagal nerves and through endocrine mechanisms.
4.2. Physiological versus pharmacological effects of GLP-1 potentially mediated through EC cells and 5-HT
Over the years, the notion that endogenous, intestinal GLP-1, which is rapidly inactivated by DPP-IV, could mediate part of its physiological effects through paracrine activation of GLP-1 receptor expressing afferent vagal nerves has gained general acceptance despite not being firmly proven [59]. As mentioned above, one recent argument against this notion is that the afferent vagal neurons expressing GLP-1 receptors apparently do not directly innervate the intestinal mucosa; surprisingly, they originate in the muscle layers of the stomach and small intestine [20]. Importantly, the present study demonstrates that intestinal GLP-1 probably activates another population of afferent vagal neurons indirectly by stimulating 5-HT release from neighboring EC cells (Figure 8A). As reviewed by Krieger et al., it is very difficult to ascertain to what degree endogenous intestinal GLP-1 in fact exerts physiological functions through afferent vagal nerves. One problem is that the key experiments are performed with exogenously administered GLP-1 or GLP-1 mimetics, which will stimulate GLP-1 receptors expressed throughout the body [59]. A possible activation of a physiological afferent vagal mechanism may be overshadowed by other pharmacological effects. In this context, it should be emphasized that several such studies indicate that the beneficial pharmacological effects of GLP-1 mimetics on appetite, food intake, and, consequently, body weight appear to be mediated through GLP-1 receptors expressed in the hypothalamus, including the arcuate nucleus [60], [61]. The GLP-1 receptor system, however, is very complex, as selective deletion of the GLP-1 receptor in specific populations of hypothalamic neurons, which previously had been shown to be targeted by GLP-1 mimetics [61], demonstrated that although these GLP-1 receptors are sufficient, they are in fact surprisingly not necessary for the pharmacological regulation of energy balance and glucose homeostasis by GLP-1 mimetics in mice [62].
In relation to our observation that GLP-1 stimulates 5-HT secretion from EC cells, it is interesting that antagonism of 5HT3 receptors has been shown to abolish glucose- and flavor-induced suppression of gastric emptying [17], [63], indicating that 5-HT secretion from the EC cells and subsequent stimulation of 5HT3 receptors on vagal afferents possibly could be involved in this physiological mechanism. Thus, 5-HT secretion from EC cells could either add to or act in synergy with the suppression of gastric emptying by endogenous GLP-1 or it could be the mechanism through which endogenous, intestinal GLP-1 and perhaps also some GLP-1 mimetics affect gastric emptying. It should be emphasized that effects of exogenously administered GLP-1 mimetics on gastric emptying varies dependent upon the compound and the duration of treatment [64].
In addition, both 5-HT and GLP-1 have been implicated as regulators of the intestinal immune system. Pharmacological inhibition of TPH1 or serotonin receptor antagonism reduces inflammation and necrosis in several intestinal inflammation models [14], [65] suggesting a pro-inflammatory role for 5-HT. However, under normal conditions 5-HT appears to stabilize the intestinal barrier and promote secretion of protective mucus [1]. Intestinal immune cells express GLP-1 receptors, which we also observed in the present study (Figure S3B). In particular, focus has been on GLP-1 expressing intraepithelial lymphocytes and GLP-1 has been shown to suppress the production of inflammatory cytokines thus decreasing severity of intestinal injury [48]. The fact that GLP-1 receptor knockout mice display reduced expression of genes responsible for endothelial protection and repair compared with wild type mice [48] makes it potentially interesting to investigate the interplay between gut GLP-1 and 5-HT in relation to the paracrine modulation of intestinal immunity.
In principle, it should be possible to test whether a given physiological effect of GLP-1 is mediated through 5-HT release from EC cells by use of TPH1 deficient animals, which should not be able to synthesize 5-HT selectively in EC cells [11]. However, at least in the global TPH1 knock-out animals, we obtained a large number of EC cells in both the small intestine and the colon that are still 5-HT immunoreactive, and, although the amounts of 5-HT in both the small intestine and the colon are decreased, these tissues still contain considerable amounts of extractable 5-HT (Suppl. Figure 4). This is likely, due to the fact that the catalytic domain of TPH1 is not removed in the knock-out construct [11], [66]. Thus, although this model apparently works well for studying peripheral endocrine effects of 5-HT, due to the fact that the circulating plasma levels of 5-HT are strongly reduced, the model is not suitable to study intestinal paracrine mechanisms, as the EC cells still contain 5-HT. Currently available pharmacological tools are also inadequate due to the fact that 5HT3 receptor antagonists readily cross the blood brain barrier making it impossible to investigate the selective peripheral contribution to the physiological effects.
4.3. Colonic EC cells express multiple different known and potential receptors for microbial metabolites
The gut microbiota affects host metabolism to a large degree through generation or modifications of metabolites which are being sensed by host metabolite GPCRs [23]. Gut microbiota derived products have previously been shown to directly modulate EC cell function and regulate production of 5-HT [26], [27], [37]. Thus, 5-HT is produced in larger amounts in the colon from conventionally raised/microbiota re-colonized mice than in germ free mice, and SCFAs such as acetate and butyrate have been reported to increase TPH1 expression in the colon [26].
In the present study, we find a large number of GPCR sensors of microbial metabolites to be highly expressed and enriched in colonic EC cells. In relation to sensing of SCFAs, it is interesting that the EC cells not only express FFAR2, which is well-known to function as a SCFA receptor in adipose tissue and leukocytes, but also OLF78, which recently has been described to be a SCFA receptor [67] and which we find is very highly expressed and in fact the most highly enriched GPCR in the colonic EC cells, in contrast to FFAR2, which is not enriched (Figure 5). We also find that another ‘olfactory’ GPCR, Olf558, which is a sensor of isovalerate and similar branched SCFAs, is highly expressed and enriched in the colonic EC cells in agreement with a recent report by Bellono et al. [28]. Moreover, GPR35 sensing aromatic acidic metabolites, GPBAR1 (TGR5) sensing secondary bile acids, HCAR2 sensing nicotinic acid and butyrate, and GPR132 sensing lactate and aryl-amides are all highly expressed but not enriched in EC cells of the colon, meaning that they are equally highly expressed in the neighboring cells of the colonic mucosa (Figure 5). Thus, colonic EC cells are able to directly sense a large variety of microbial metabolites and express several different types of receptors for SCFAs. In contrast, in the small intestine, the expression of receptors for microbial metabolites in the EC cells is at a much lower level. Although the small intestinal EC cells do express a number of microbial metabolite sensors above detection, the expression of, for example FFAR2 and Olf78 is 100-fold lower than in colonic EC cells (Figure 4B).
Concluding remarks – The first key finding of the present study, that EC cells of the small intestine do not sense nutrient metabolites directly through GPCRs but instead via a paracrine GLP-1 mediated mechanism, could very likely have major implications on our concept of how endogenous intestinal GLP-1 exerts its physiological functions in general and potentially how GLP-1 mimetic drugs exerts some of their many effects, although central GLP-1 receptors currently are dominating the picture. These issues will have to be characterized through application of various novel genetic and pharmacological tools, conceivably in combination. Nevertheless, the identification of expression of a large repertoire of receptors for gut microbial metabolites, including novel ones, in colonic EC cells opens the door for studies of how the gut microbiota controls our physiology and our metabolism through specific metabolites and intestinal 5-HT. An important issue which we have not addressed in the present study is the presumed sensing of toxins by the EC cells. These cells are believed to act as detectors of emetogenic agents and the released 5-HT is believed to function as the key mediator of nausea and vomiting, which is reflected in the fact that antagonists of the 5HT3 receptor such as ondansetron are effective antiemetic agents. It will be interesting to determine the expression pattern of the many different Tas2R bitter taste receptors in the EC cells.
Acknowledgments
We thank Lotte Bjerre Knudsen and Charles Pyke from Novo Nordisk A/S for providing the GLP-1 receptor antibody and for valuable guidance and discussions. Furthermore, we thank Fiona Gribble and Frank Reimann for giving us the possibility to use the GLU-Venus mice and Gerard Karsenty for providing the global TPH1KO mice. Additionally, we thank Barbara Thaysen and Stine Lindberg Vedersø for expert technical assistance. The Novo Nordisk Foundation Center for Basic Metabolic Research is supported by an unconditional grant (NNF10CC1016515) from the Novo Nordisk Foundation to University of Copenhagen. The work on metabolite receptors is further supported by an Immunometabolism grant NNF15CC0018346 and Challenge Grants NNF15OC0016798 and NNF14OC0016798 from the Novo Nordisk Foundation. The study related to Sensory Neurons of the Gut–Brain Axis study is supported by grant 7016-00389A from the Danish Council for Independent Research.
Footnotes
It is generally known in the field that most commercially available antibodies against GPCRs do not work despite reports in the literature. Accordingly, we are not able to check the expression of the other receptors dealt with in the present paper by immunohistochemistry.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.03.004.
Conflict of interest
The authors declare no competing financial interests.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Main Nutrient and Microbial metabolites which are being recognized by GPCR sensors and which are probed for expression in EC cells in the present study. Sensing of triglycerides is mainly performed by FFAR1 and GPR119, which recognize LCFAs and 2-acyl glycerol, respectively, and TGR5 recognizing primary bile acids required for triglyceride micelle formation [29]. FFAR4 is not listed in this part of the table as it mainly is a Gi couple receptor inhibition hormone secretion and apparently not involved in EEC cell sensing of triglycerides. CaSR and GPR142 are currently considered to be the main sensors of dietary protein metabolites (ref). The sweet taste receptor is part of the analysis despite the fact that glucose in enteroendocrine cells in general is sensed via uptake through SGLT or GLUT2 and not through GPCRs. The signaling metabolites and their receptors are not yet well characterized; however, the most well-established sensors of microbial metabolites are listed.
qPCR and histological analysis of co-expressed peptides and granins in colonic EC cells. (A) qPCR analysis targeting the expression of 88 common peptide hormones, neuropeptides and granins in 5-HT positive cells (y-axis) versus 5-HT negative cells (x-axis) in colon. The enriched peptide transcripts are depicted as green dots whereas the rest are gray. The 45°-angled gray dotted lines depict the fold change enrichment in 5-HT positive cells versus 5-HT negative cells and the gray-shaded square is marking what is considered noise. (B) Fluorescent microscope pictures of small intestine labeled with 5-HT (green) and Substance P or Neurokinin A (Red) depicts co-expression in EC cells. Scale bars correspond to 50 μm. (C) Overview in percentage of how many 5-HT positive cells also stained positive for Substance P (n = 4).
qPCR analysis of various receptors on ChgA-GFP positive colon cells (A) qPCR analysis expression data for main nutrient metabolite 7TM GPCRs and microbiota metabolite in ChgA-GFP positive cells (y-axis) compared with surrounding negative cells (x-axis) isolated from colon. (B) qPCR analysis expression data for main gut hormone 7TM GPCRs in ChgA-GFP positive cells (y-axis) compared with surrounding negative cells (x-axis) isolated from colon. The 45°-angled gray dotted lines display the fold change enrichment and the gray-shaded square is marking what is considered noise.
Immunohistochemistry (A and B) Representative fluorescent microscopy picture with monoclonal GLP-1R antibody (green) in combination with Somatostatin antibody (red) in duodenum.
Global TPH1KO mice still synthesize 5-HT in the intestine. (A) Example of genotype analysis results of TPH1KO +/+ (Wt), TPH1KO +/− (Hz) and TPH1KO −/− (KO). (B) HPLC-ECD measurements of 5-HT concentration in homogenized tissue from duodenum and colon (n = 7). (C) Representative images from immunohistochemistry on intestinal segments marked with 5-HT antibody to show EC cells. Scale bars represent 50 μm.
References
- 1.Spohn S.N., Mawe G.M. Non-conventional features of peripheral serotonin signalling – the gut and beyond. Nature Reviews Gastroenterology & Hepatology. 2017;14(7):412–420. doi: 10.1038/nrgastro.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erspamer V., Asero B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature. 1952;169(4306):800–801. doi: 10.1038/169800b0. [DOI] [PubMed] [Google Scholar]
- 3.Walther D.J., Bader M. A unique central tryptophan hydroxylase isoform. Biochemical Pharmacology. 2003;66(9):1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 4.Cote F., Thevenot E., Fligny C., Fromes Y., Darmon M., Ripoche M.A. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(23):13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machado J.D., Diaz-Vera J., Dominguez N., Alvarez C.M., Pardo M.R., Borges R. Chromogranins A and B as regulators of vesicle cargo and exocytosis. Cellular and Molecular Neurobiology. 2010;30(8):1181–1187. doi: 10.1007/s10571-010-9584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelstoft M.S., Lund M.L., Grunddal K.V., Egerod K.L., Osborne-Lawrence S., Poulsen S.S. Research resource: a chromogranin a reporter for serotonin and histamine secreting enteroendocrine cells. Molecular Endocrinology. 2015;29(11):1658–1671. doi: 10.1210/me.2015-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aiken K.D., Roth K.A. Temporal differentiation and migration of substance P, serotonin, and secretin immunoreactive enteroendocrine cells in the mouse proximal small intestine. Developmental Dynamics. 1992;194(4):303–310. doi: 10.1002/aja.1001940406. [DOI] [PubMed] [Google Scholar]
- 8.Grun D., Lyubimova A., Kester L., Wiebrands K., Basak O., Sasaki N. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature. 2015;525(7568):251–255. doi: 10.1038/nature14966. [DOI] [PubMed] [Google Scholar]
- 9.Mawe G.M., Hoffman J.M. Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nature Reviews Gastroenterology & Hepatology. 2013;10(8):473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Merahbi R., Loffler M., Mayer A., Sumara G. The roles of peripheral serotonin in metabolic homeostasis. FEBS Letters. 2015;589(15):1728–1734. doi: 10.1016/j.febslet.2015.05.054. [DOI] [PubMed] [Google Scholar]
- 11.Yadav V.K., Ryu J.H., Suda N., Tanaka K.F., Gingrich J.A., Schutz G. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135(5):825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tackett J.J., Gandotra N., Bamdad M.C., Muise E.D., Cowles R.A. Enhanced serotonin signaling stimulates ordered intestinal mucosal growth. Journal of Surgical Research. 2017;208(Supplement C):198–203. doi: 10.1016/j.jss.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Akiba Y., Maruta K., Narimatsu K., Said H., Kaji I., Kuri A. FFA2 activation combined with ulcerogenic COX inhibition induces duodenal mucosal injury via the 5-HT pathway in rats. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2017;313(2):G117–G128. doi: 10.1152/ajpgi.00041.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolis K.G., Stevanovic K., Li Z., Yang Q.M., Oravecz T., Zambrowicz B. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut. 2014;63(6):928–937. doi: 10.1136/gutjnl-2013-304901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heredia D.J., Gershon M.D., Koh S.D., Corrigan R.D., Okamoto T., Smith T.K. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. Journal of Physiology. 2013;591(Pt 23):5939–5957. doi: 10.1113/jphysiol.2013.256230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer N.J., Sia T.C., Brookes S.J., Costa M., Keating D.J. CrossTalk opposing view: 5-HT is not necessary for peristalsis. Journal of Physiology. 2015;593(15):3229–3231. doi: 10.1113/JP270183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raybould H.E., Glatzle J., Robin C., Meyer J.H., Phan T., Wong H. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2003;284(3):G367–G372. doi: 10.1152/ajpgi.00292.2001. [DOI] [PubMed] [Google Scholar]
- 18.Browning K.N. Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Frontiers in Neuroscience. 2015;9:413. doi: 10.3389/fnins.2015.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Hao Y., Zhu J., Owyang C. Serotonin released from intestinal enterochromaffin cells mediates luminal non-cholecystokinin-stimulated pancreatic secretion in rats. Gastroenterology. 2000;118(6):1197–1207. doi: 10.1016/s0016-5085(00)70373-8. [DOI] [PubMed] [Google Scholar]
- 20.Williams E.K., Chang R.B., Strochlic D.E., Umans B.D., Lowell B.B., Liberles S.D. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166(1):209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelstoft M.S., Egerod K.L., Holst B., Schwartz T.W. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metabolism. 2008;8(6):447–449. doi: 10.1016/j.cmet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Tolhurst G., Reimann F., Gribble F.M. Nutritional regulation of glucagon-like peptide-1 secretion. Journal of Physiology. 2009;587(1):27–32. doi: 10.1113/jphysiol.2008.164012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Husted A.S., Trauelsen M., Rudenko O., Hjorth S.A., Schwartz T.W. GPCR-Mediated signaling of metabolites. Cell Metabolism. 2017;25(4):777–796. doi: 10.1016/j.cmet.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Kidd M., Modlin I.M., Eick G.N., Champaneria M.C. Isolation, functional characterization, and transcriptome of Mastomys ileal enterochromaffin cells. American Journal of Physiology–Gastrointestinal and Liver Physiology. 2006;291(5):G778–G791. doi: 10.1152/ajpgi.00552.2005. [DOI] [PubMed] [Google Scholar]
- 25.Racke K., Schworer H. Regulation of serotonin release from the intestinal mucosa. Pharmacological Research. 1991;23(1):13–25. doi: 10.1016/s1043-6618(05)80101-x. [DOI] [PubMed] [Google Scholar]
- 26.Reigstad C.S., Salmonson C.E., Rainey J.F., 3rd, Szurszewski J.H., Linden D.R., Sonnenburg J.L. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. The FASEB Journal. 2015;29(4):1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellono N.W., Bayrer J.R., Leitch D.B., Castro J., Zhang C., O'Donnell T.A. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170(1):185–198. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekberg J.H., Hauge M., Kristensen L.V., Madsen A.N., Engelstoft M.S., Husted A.S. GPR119, a major enteroendocrine sensor of dietary triglyceride metabolites coacting in synergy with FFA1 (GPR40) Endocrinology. 2016;157(12):4561–4569. doi: 10.1210/en.2016-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engelstoft M.S., Schwartz T.W. Opposite regulation of ghrelin and glucagon-like Peptide-1 by metabolite g-protein-coupled receptors. Trends in Endocrinology and Metabolism. 2016;27(9):665–675. doi: 10.1016/j.tem.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Egerod K.L., Engelstoft M.S., Grunddal K.V., Nohr M.K., Secher A., Sakata I. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153(12):5782–5795. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reimann F., Habib A.M., Tolhurst G., Parker H.E., Rogers G.J., Gribble F.M. Glucose sensing in L cells: a primary cell study. Cell Metabolism. 2008;8(6):532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engelstoft M.S., Park W.M., Sakata I., Kristensen L.V., Husted A.S., Osborne-Lawrence S. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Molecular Metabolism. 2013;2(4):376–392. doi: 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egerod K.L., Engelstoft M.S., Lund M.L., Grunddal K.V., Zhao M., Barir-Jensen D. Transcriptional and functional characterization of the G protein-coupled receptor repertoire of gastric somatostatin cells. Endocrinology. 2015;156(11):3909–3923. doi: 10.1210/EN.2015-1388. [DOI] [PubMed] [Google Scholar]
- 35.Patel B.A. Continuous amperometric detection of co-released serotonin and melatonin from the mucosa in the ileum. Analyst. 2008;133(4):516–524. doi: 10.1039/b717034c. [DOI] [PubMed] [Google Scholar]
- 36.Patel B.A., Arundell M., Parker K.H., Yeoman M.S., O'Hare D. Simple and rapid determination of serotonin and catecholamines in biological tissue using high-performance liquid chromatography with electrochemical detection. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 2005;818(2):269–276. doi: 10.1016/j.jchromb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Nzakizwanayo J., Dedi C., Standen G., Macfarlane W.M., Patel B.A., Jones B.V. Escherichia coli Nissle 1917 enhances bioavailability of serotonin in gut tissues through modulation of synthesis and clearance. Scientific Reports. 2015;5:17324. doi: 10.1038/srep17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hrvatin S., Deng F., O'Donnell C.W., Gifford D.K., Melton D.A. MARIS: method for analyzing RNA following intracellular sorting. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0089459. e89459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada H., Maruo R., Watanabe M., Hidaka Y., Iwatani Y., Takano T. Messenger RNA quantification after fluorescence activated cell sorting using intracellular antigens. Biochemical and Biophysical Research Communications. 2010;397(3):425–428. doi: 10.1016/j.bbrc.2010.05.112. [DOI] [PubMed] [Google Scholar]
- 40.Diwakarla S., Fothergill L.J., Fakhry J., Callaghan B., Furness J.B. Heterogeneity of enterochromaffin cells within the gastrointestinal tract. Neuro-Gastroenterology and Motility. 2017;29(6) doi: 10.1111/nmo.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grunddal K.V., Ratner C.F., Svendsen B., Sommer F., Engelstoft M.S., Madsen A.N. Neurotensin is coexpressed, coreleased, and acts together with GLP-1 and PYY in enteroendocrine control of metabolism. Endocrinology. 2016;157(1):176–194. doi: 10.1210/en.2015-1600. [DOI] [PubMed] [Google Scholar]
- 42.Larsson L.I., Goltermann N., de Magistris L., Rehfeld J.F., Schwartz T.W. Somatostatin cell processes as pathways for paracrine secretion. Science. 1979;205(4413):1393–1395. doi: 10.1126/science.382360. [DOI] [PubMed] [Google Scholar]
- 43.Kidd M., Modlin I.M., Gustafsson B.I., Drozdov I., Hauso O., Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol. 2008;295(2):G260–G272. doi: 10.1152/ajpgi.00056.2008. [DOI] [PubMed] [Google Scholar]
- 44.Harding S.D., Sharman J.L., Faccenda E., Southan C., Pawson A.J., Ireland S. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research. 2018;46(D1):D1091–D1106. doi: 10.1093/nar/gkx1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox H.M., Tough I.R., Woolston A.M., Zhang L., Nguyen A.D., Sainsbury A. Peptide YY is critical for acylethanolamine receptor Gpr119-induced activation of gastrointestinal mucosal responses. Cell Metabolism. 2010;11(6):532–542. doi: 10.1016/j.cmet.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz T.W., Holst B. An enteroendocrine full package solution. Cell Metabolism. 2010;11(6):445–447. doi: 10.1016/j.cmet.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Jensen C.B., Pyke C., Rasch M.G., Dahl A.B., Knudsen L.B., Secher A. Characterization of the glucagonlike Peptide-1 receptor in male mouse brain using a novel antibody and in situ hybridization. Endocrinology. 2018;159(2):665–675. doi: 10.1210/en.2017-00812. [DOI] [PubMed] [Google Scholar]
- 48.Yusta B., Baggio L.L., Koehler J., Holland D., Cao X., Pinnell L.J. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes. 2015;64(7):2537–2549. doi: 10.2337/db14-1577. [DOI] [PubMed] [Google Scholar]
- 49.Bohorquez D.V., Chandra R., Samsa L.A., Vigna S.R., Liddle R.A. Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. Journal of Molecular Histology. 2011;42(1):3–13. doi: 10.1007/s10735-010-9302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brighton C.A., Rievaj J., Kuhre R.E., Glass L.L., Schoonjans K., Holst J.J. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located g protein-coupled bile acid receptors. Endocrinology. 2015;156(11):3961–3970. doi: 10.1210/en.2015-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Christensen L.W., Kuhre R.E., Janus C., Svendsen B., Holst J.J. Vascular, but not luminal, activation of FFAR1 (GPR40) stimulates GLP-1 secretion from isolated perfused rat small intestine. Physiological Reports. 2015;3(9) doi: 10.14814/phy2.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin A.M., Lumsden A.L., Young R.L., Jessup C.F., Spencer N.J., Keating D.J. Regional differences in nutrient-induced secretion of gut serotonin. Physiological Reports. 2017;5(6) doi: 10.14814/phy2.13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin A.M., Lumsden A.L., Young R.L., Jessup C.F., Spencer N.J., Keating D.J. The nutrient-sensing repertoires of mouse enterochromaffin cells differ between duodenum and colon. Neuro-Gastroenterology and Motility. 2017;29(6) doi: 10.1111/nmo.13046. [DOI] [PubMed] [Google Scholar]
- 54.Brand T., Anderson G.M. The measurement of platelet-poor plasma serotonin: a systematic review of prior reports and recommendations for improved analysis. Clinical Chemistry. 2011;57(10):1376–1386. doi: 10.1373/clinchem.2011.163824. [DOI] [PubMed] [Google Scholar]
- 55.Bulbring E., Crema A. The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. Journal of Physiology. 1959;146(1):18–28. doi: 10.1113/jphysiol.1959.sp006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bertrand P.P. Real-time detection of serotonin release from enterochromaffin cells of the Guinea-pig ileum. Neuro-Gastroenterology and Motility. 2004;16(5):511–514. doi: 10.1111/j.1365-2982.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 57.Wang F., Knutson K., Alcaino C., Linden D.R., Gibbons S.J., Kashyap P. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. Journal of Physiology. 2017;595(1):79–91. doi: 10.1113/JP272718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krieger J.P., Arnold M., Pettersen K.G., Lossel P., Langhans W., Lee S.J. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2016;65(1):34–43. doi: 10.2337/db15-0973. [DOI] [PubMed] [Google Scholar]
- 59.Krieger J.P., Langhans W., Lee S.J. Vagal mediation of GLP-1's effects on food intake and glycemia. Physiology & Behavior. 2015;152(Pt B):372–380. doi: 10.1016/j.physbeh.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Sisley S., Gutierrez-Aguilar R., Scott M., D'Alessio D.A., Sandoval D.A., Seeley R.J. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. Journal of Clinical Investigation. 2014;124(6):2456–2463. doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Secher A., Jelsing J., Baquero A.F., Hecksher-Sorensen J., Cowley M.A., Dalboge L.S. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. Journal of Clinical Investigation. 2014;124(10):4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burmeister M.A., Ayala J.E., Smouse H., Landivar-Rocha A., Brown J.D., Drucker D.J. The hypothalamic glucagon-like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes. 2017;66(2):372–384. doi: 10.2337/db16-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hira T., Yahagi A., Nishimura S., Sakaino M., Yamashita T., Hara H. Diunsaturated aldehyde, trans,trans-2,4-decadienal in the intestinal lumen suppresses gastric emptying through serotonin signaling in rats. Journal of Agricultural and Food Chemistry. 2015;63(37):8177–8181. doi: 10.1021/acs.jafc.5b03126. [DOI] [PubMed] [Google Scholar]
- 64.Jelsing J., Vrang N., Hansen G., Raun K., Tang-Christensen M., Bjerre Knudsen L. Liraglutide: short-lived effect on gastric emptying—long lasting effects on body weight. Diabetes, Obesity and Metabolism. 2012;14(6):531–538. doi: 10.1111/j.1463-1326.2012.01557.x. [DOI] [PubMed] [Google Scholar]
- 65.Gross Margolis K., Vittorio J., Talavera M., Gluck K., Li Z., Iuga A. Enteric serotonin and oxytocin: endogenous regulation of severity in a murine model of necrotizing enterocolitis. American Journal of Physiology–Gastrointestinal and Liver Physiology. 2017;313(5):G386–G398. doi: 10.1152/ajpgi.00215.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinez A., Knappskog P.M., Haavik J. A structural approach into human tryptophan hydroxylase and its implications for the regulation of serotonin biosynthesis. Current Medicinal Chemistry. 2001;8(9):1077–1091. doi: 10.2174/0929867013372616. [DOI] [PubMed] [Google Scholar]
- 67.Pluznick J.L., Protzko R.J., Gevorgyan H., Peterlin Z., Sipos A., Han J. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(11):4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Main Nutrient and Microbial metabolites which are being recognized by GPCR sensors and which are probed for expression in EC cells in the present study. Sensing of triglycerides is mainly performed by FFAR1 and GPR119, which recognize LCFAs and 2-acyl glycerol, respectively, and TGR5 recognizing primary bile acids required for triglyceride micelle formation [29]. FFAR4 is not listed in this part of the table as it mainly is a Gi couple receptor inhibition hormone secretion and apparently not involved in EEC cell sensing of triglycerides. CaSR and GPR142 are currently considered to be the main sensors of dietary protein metabolites (ref). The sweet taste receptor is part of the analysis despite the fact that glucose in enteroendocrine cells in general is sensed via uptake through SGLT or GLUT2 and not through GPCRs. The signaling metabolites and their receptors are not yet well characterized; however, the most well-established sensors of microbial metabolites are listed.
qPCR and histological analysis of co-expressed peptides and granins in colonic EC cells. (A) qPCR analysis targeting the expression of 88 common peptide hormones, neuropeptides and granins in 5-HT positive cells (y-axis) versus 5-HT negative cells (x-axis) in colon. The enriched peptide transcripts are depicted as green dots whereas the rest are gray. The 45°-angled gray dotted lines depict the fold change enrichment in 5-HT positive cells versus 5-HT negative cells and the gray-shaded square is marking what is considered noise. (B) Fluorescent microscope pictures of small intestine labeled with 5-HT (green) and Substance P or Neurokinin A (Red) depicts co-expression in EC cells. Scale bars correspond to 50 μm. (C) Overview in percentage of how many 5-HT positive cells also stained positive for Substance P (n = 4).
qPCR analysis of various receptors on ChgA-GFP positive colon cells (A) qPCR analysis expression data for main nutrient metabolite 7TM GPCRs and microbiota metabolite in ChgA-GFP positive cells (y-axis) compared with surrounding negative cells (x-axis) isolated from colon. (B) qPCR analysis expression data for main gut hormone 7TM GPCRs in ChgA-GFP positive cells (y-axis) compared with surrounding negative cells (x-axis) isolated from colon. The 45°-angled gray dotted lines display the fold change enrichment and the gray-shaded square is marking what is considered noise.
Immunohistochemistry (A and B) Representative fluorescent microscopy picture with monoclonal GLP-1R antibody (green) in combination with Somatostatin antibody (red) in duodenum.
Global TPH1KO mice still synthesize 5-HT in the intestine. (A) Example of genotype analysis results of TPH1KO +/+ (Wt), TPH1KO +/− (Hz) and TPH1KO −/− (KO). (B) HPLC-ECD measurements of 5-HT concentration in homogenized tissue from duodenum and colon (n = 7). (C) Representative images from immunohistochemistry on intestinal segments marked with 5-HT antibody to show EC cells. Scale bars represent 50 μm.