 A highly enantioselective Cu-catalyzed borylation of 2-substituted 1,3-dienes is reported.
A highly enantioselective Cu-catalyzed borylation of 2-substituted 1,3-dienes is reported.
Abstract
A highly enantioselective Cu-catalyzed borylation of 2-substituted 1,3-dienes is reported. The use of a chiral phosphanamine ligand is essential in achieving high chemo-, regio-, diastereo- and enantioselectivity. It provides access to a variety of homoallylic boronates in consistently high yield and enantiomeric excess with 2-aryl and 2-heteroaryl 1,3-dienes as well as sterically demanding 2-alkyl 1,3-dienes. Preliminary investigations based on a non-linear effect study point to a mechanism involving more than one metal center.
Introduction
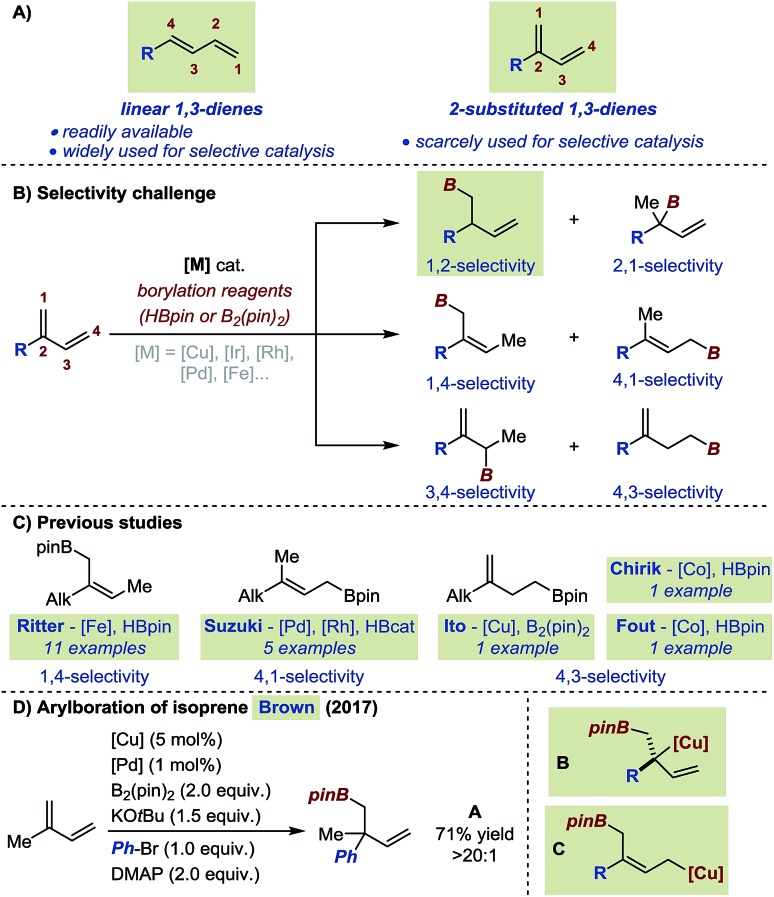
Conjugated 1,3-dienes represent a particularly attractive platform for selective functionalizations. They do occur in some natural products and have often been used as bifunctional building blocks for the synthesis of biologically active molecules as well as in several polymerization processes.1,2 From a selectivity standpoint, functionalization of 1,3-dienes represents a significant challenge due to the numerous coordination and insertion modes conceivable for a transition metal catalyst.3 To date, efforts to develop selective catalytic transformations have been essentially focused on linear 1,3-dienes (i.e. 4-substituted 1,3-dienes).4 Until recently, the limited synthetic accessibility of 2-substituted 1,3-dienes has hampered their use in the development of selective transformations.5 Moreover, most examples were focused on isoprene and myrcene, two readily available substrates of this subclass of conjugated dienes. Consequently, the effect of electronic and steric modifications has not been systematically investigated. For instance, the introduction of an aryl instead of an alkyl substituent at position 2- of a conjugated diene is likely to impart substantial changes in substrate polarity and/or in steric demand (Fig. 1A). Our laboratory recently reported a general procedure which streamlines access to 2-substituted 1,3-dienes and we decided to initiate a program on the selective functionalization of this underexplored scaffold.6
Fig. 1. (A) Linear vs. 2-substituted conjugated 1,3-dienes. (B) Selectivity challenges in hydroboration and borylation of 2-substituted 1,3-dienes. (C) Previous hydroborations/borylations of 2-substituted 1,3-dienes. (D) Arylboration of isoprene along with putative primary and tertiary Cu σ-allyl intermediates.
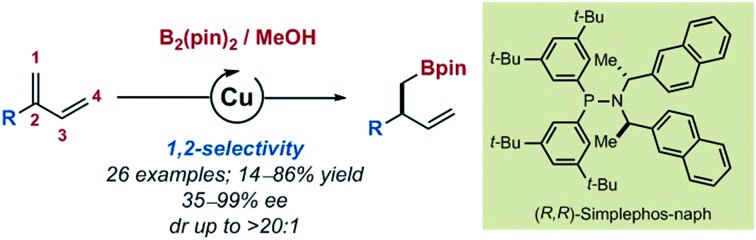
Among the approaches to functionalize 1,3-dienes, transition metal-catalyzed hydroborations and borylations have emerged as attractive strategies providing access to polyfunctionalized – potentially enantioenriched – structural motifs. For 2-substituted substrates, the selectivity challenge is highlighted by the fact that up to 6 different isomers can be generated upon exclusive mono-functionalization (Fig. 1B). Allylic boronates resulting from formal 1,4- or 4,1-hydroborations of 2-substituted 1,3-dienes have been reported by the groups of Suzuki and Ritter using highly chemo-, regio- and stereoselective Pd, Rh and Fe catalysts.7 Homoallylic boronates obtained by a 4,3-selective hydroboration are less common. Ito, Fout and Chirik only described such products in studies where the focus was initially set on the hydroboration of cyclic 1,3-dienes and terminal alkenes.8 To our knowledge, methods to access products of 1,2-, 2,1- and 3,4-selective hydroboration are absent from the current literature (Fig. 1B). Cu/Pd-catalyzed cooperative carboboration of alkenes represents another emerging class of transformation which involves the transient generation of copper-alkyl intermediates prior to transmetalation to a palladium catalyst.9 In this context, only few reports have placed emphasis on 1,3-dienes.10 The Brown group recently disclosed the highly selective arylboration of isoprene and myrcene unexpectedly leading to the exclusive formation of A when 4-dimethylaminopyridine (DMAP) was employed as additive (Fig. 1D).10a Interestingly, to account for this unusual selectivity preliminary mechanistic investigations do not support the formation of a congested Cu-σ-allyl intermediate such as B (Fig. 1D) but rather transmetalation to Pd from a putative 1,4-borocupration species such as C. Within this context, at the outset of our investigations, we aimed at establishing a perfectly 1,2-selective borylation of 2-substituted 1,3-dienes to access valuable enantioenriched homoallylic boronates.11 Herein, we describe the successful realization of our objective with the development of a highly chemo-, regio-, and enantioselective Cu-catalyzed borylation of a variety of 2-substituted 1,3-dienes.
Results and discussion
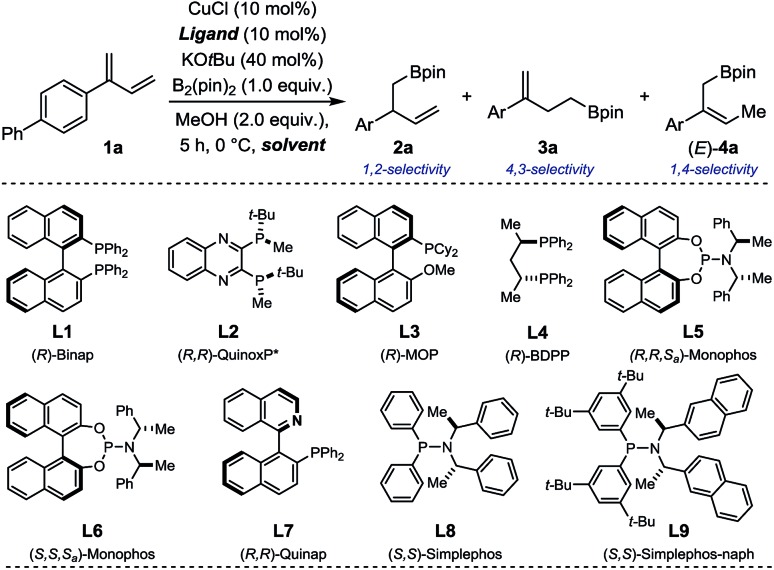
Our study commenced by evaluating several chiral ligands for the Cu-catalyzed borylation of 1a using prototypical reaction conditions favoring borylcupration (B2(pin)2/MeOH; Table 1).12,13 With ligands L1–3, homoallylic boronate 3a was obtained predominantly along with trace amount of the desired 1,2-borylated product 2a (conv. = 67–86%; Entry 1–3). The commercially available bisphosphine (R)-BDPP afforded 2a and 3a in a nearly 1 : 1 ratio with a low but measurable enantioselectivity for the former (2a: 24% ee, Entry 4). Using either diastereoisomers of the well-established phosphoramidite ligands L5 and L6, 2a was obtained as major regioisomer albeit in mixture with both 3a and (E)-4a (a product of formal 1,4-borylation) and in low ee (Entry 5–6). The chiral heterotopic ligand (R)-Quinap L7, enabled to increase both the relative ratio in favor of 2a as well as its enantiomeric excess (2a: 43% ee; Entry 7). A slightly improved result was achieved with Simplephos L8 – a structure introduced by the Alexakis group which rapidly established itself as a ubiquitous ligand in numerous Cu-catalyzed enantioselective transformations.14 Given the higher modularity of L8 compared to L7, several members of the Simplephos family were evaluated next (See ESI† for details) and allowed for identification of L9 as the best congener for the borylation of 1a (Entry 9). Further optimization of the solvent, temperature, concentration, time and copper source led to a system which afforded homoallylic boronate 2a as a single regioisomer (2a : 3a > 20 : 1) in 82% yield and 90% ee (Entry 10–15).
Table 1. Reaction optimization a .
| |||||
| Entry | L | Solvent | Conv. b (%) | 2a : 3a : 4a b | ee 2a c (%) |
| 1 | L1 | THF | 86 | 1 : 15 : — | nd |
| 2 | L2 | THF | 74 | 1 : 3 : — | 5 (S) |
| 3 d | L3 | THF | 67 | 1 : 7 : — | 21 (R) |
| 4 | L4 | THF | 81 | 1 : 1.5 : — | 24 (R) |
| 5 d | L5 | THF | 63 | 4.6 : 1.3 : 1 | 4 (R) |
| 6 d | L6 | THF | 80 | 4 : 1 : 1 | 20 (R) |
| 7 | L7 | THF | 74 | 6 : 1 : 2 | 43 (R) |
| 8 d | L8 | THF | 87 | 8 : 2 : 1 | 48 (R) |
| 9 d | L9 | THF | 91 | 27 : 2.7 : 1 | 67 (R) |
| 10 d , e | L9 | Et2O | 51 | 12 : 1 : — | 72 (R) |
| 11 d , e | L9 | Tol. | 83 | 13 : 1 : — | 70 (R) |
| 11 d , e | L9 | C6H5Cl | 85 | 12 : 1 : — | 74 (R) |
| 13 d , e | L9 | C5H12 | 88 | 17 : 1 : — | 70 (R) |
| 14 e , g | L9 | C5H12 | 77 | 20 : 1 : — | 85 (R) |
| 15 f , g , h | L9 | C5H12 | 89 (82) i | 20 : 1 : — | 90 (R) |
aReaction conditions: 1a (0.12 mmol), B2(pin)2 (0.10 mmol), 0.3 M.
bDetermined by 1H NMR using an internal standard.
cDetermined by HPLC using a chiral stationary phase after oxidation to the corresponding alcohol 2′a.
d20 mol% of ligand.
e0.1 M.
f0.15 M.
g–40 °C, 40 h.
hCuOtBu (10 mol%), without base.
iYield over 2 steps after oxidation to 2′a and purification by column chromatography.
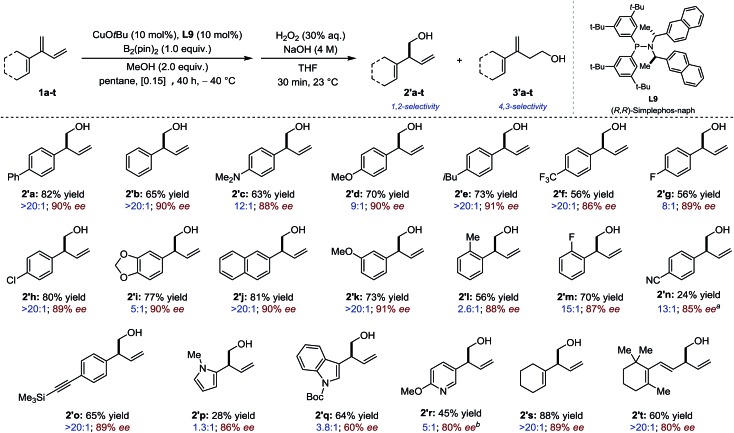
The scope of the process was first evaluated for 2-(hetero) aryl substituted 1,3-dienes. In most cases only products resulting from 1,2- and 4,3-borylation were detected. The selectivity was assessed by analysis of the crude reaction mixture after borylation and the yields and enantiomeric excess were measured after oxidation into the corresponding homoallylic alcohols (20 examples, Fig. 2). Overall, most conjugated 1,3-dienes containing electron-rich, electron-neutral and electron-deficient aromatic substituents participated well in the Cu-catalyzed borylation reaction, delivering the product in moderate to good yield, good to excellent regioselectivity and high enantioselectivity (2′a–2′l: 56–82% yield; chemoselectivity: 2.6 : 1 to > 20 : 1; 86–91% ee). Several functional groups (methoxy, fluoro, amino, trifluoromethyl, chloro) are tolerated. Whereas a homoallylic alcohol having a p-CN substituent (2′n) was isolated in much reduced yield albeit in high ee, 2′o which displayed an alkyne substituent was generated in high yield, chemo- and enantioselectivity. Reduced performances were observed in the borylation of heteroaromatic-containing 1,3-dienes although satisfactory enantioinduction were still measured for 2′p and 2′r. Finally, skipped dienes 2's-t – which derived from the parent dendralenic 1,3-dienes – were isolated as single regioisomers in good yield and high enantioselectivity.
Fig. 2. Sequential borylation/oxidation of 2-(hetero)aryl substituted 1,3-dienes. Reaction conditions: 1a (0.36 mmol), B2(pin)2 (0.30 mmol). Chemoselectivity assessed by 1H NMR after borylation. Yields of isolated alcohols 2′. Enantioselectivity determined after oxidation by HPLC, GC or SFC using a chiral stationary phase. aThe minor isomer is 4′n. bIsolated as a 5 : 1 mixture.
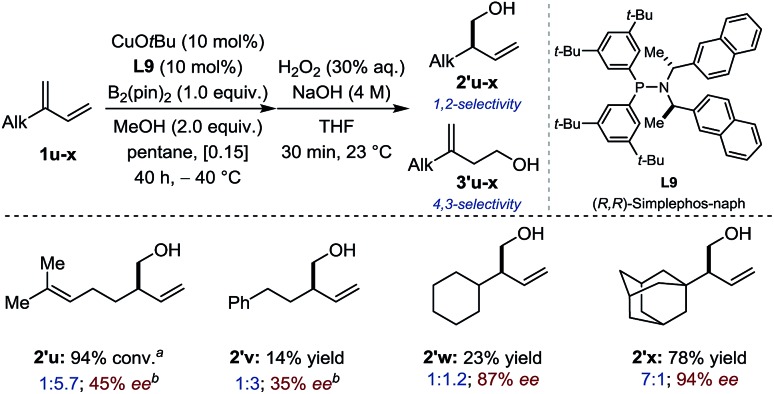
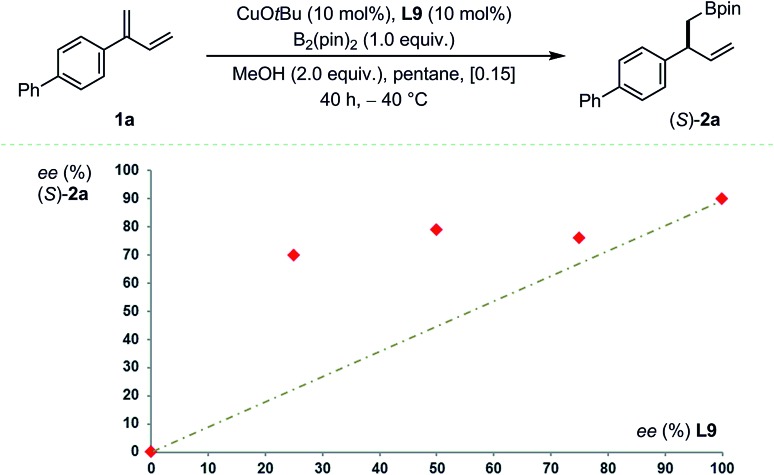
Subsequently, conjugated 2-alkyl-1,3-dienes were investigated (Fig. 3). Counter-intuitively – perhaps – the reactivity, the chemoselectivity and the enantioselectivity were all found to increase drastically when going from primary (1u-v), to secondary (1w) and finally tertiary alkyl substituents (1x). While primary and secondary alkyl favored 4,3-borylation, a sterically demanding 1-adamantyl group restored preferential 1,2-borylation (7 : 1) generating 2′x in good yield and excellent enantioselectivity. At first sight, these data suggest that enantio-differentiation may preferentially occur during 1,4-protodecupration from intermediate C rather than during 1,2-borocupration as a particularly congested quaternary Cu-σ-allyl species B would be formed in the case of 2′x. The situation is likely to be more subtle and complex. Indeed, non-linear-effect studies revealed a substantial deviation from linearity – a signature for catalyst aggregation as is apparent from Fig. 4.15
Fig. 3. Sequential borylation/oxidation of 2-alkyl substituted 1,3-dienes. Reaction conditions: 1u-x (0.18–0.36 mmol), B2(pin)2 (0.15–0.30 mmol). Chemoselectivity assessed by 1H NMR after borylation. Isolated yields for alcohols 2′v-x. Enantioselectivity determined after oxidation by HPLC, GC, SFC using a chiral stationary phase. aConversion of non-separable isomers determined by 1H NMR (2′u + 3′u). bAt 0 °C for 24 h.
Fig. 4. Non-linear effect study. Reaction conditions: 1a (0.12 mmol), B2(pin)2 (0.10 mmol). Chemoselectivity > 20 : 1 in all cases as assessed by 1H NMR. Enantioselectivity determined after oxidation by HPLC using a chiral stationary phase. Average of two experiments.
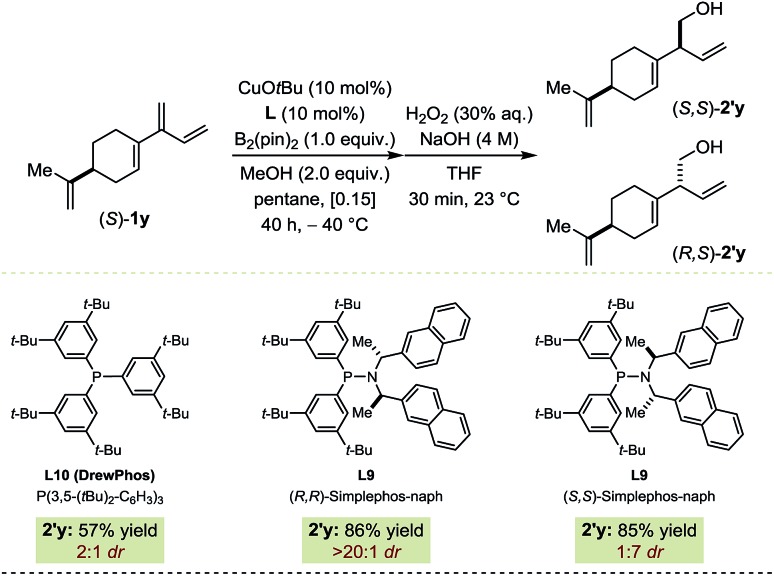
Catalyst-controlled diastereoselective transformations where a chiral catalyst can install one (or several) new stereocenter(s) independently of the stereochemical complexity of the substrate have gained a certain momentum recently.16,17 Hence, the innate bias imposed by (S)-1y on the Cu-catalyzed borylation was assessed using DrewPhos (L10) leading to a 2 : 1 diastereomeric ratio in favor of (S,S)-2′y (Fig. 5).18 Diastereoselective borylation reactions were performed with both enantiomers of the chiral ligand. In the match situation (S,S)-2′y was isolated in 86% yield and > 20 : 1 dr with (R,R)-L9. In the mismatch situation, the enantiomeric ligand (S,S)-L9 proved able to substantially override the substrate bias, generating (R,S)-2′y preferentially (85% yield; 1 : 7 dr). In addition to serving as a prototype for further developments, this example highlights the high chemoselectivity of our protocol as only one of the four olefinic functionalities present in (S)-1y underwent borylation.
Fig. 5. Catalyst-controlled diastereoselective borylation of (S)-1y. Reaction conditions: (S)-1y (0.24 mmol), B2(pin)2 (0.20 mmol). Chemoselectivity > 20 : 1 in all cases as assessed by 1H after borylation. Diastereoselectivity assessed by 1H and 13C{1H} NMR after borylation and oxidation. Isolated yields after oxidation.
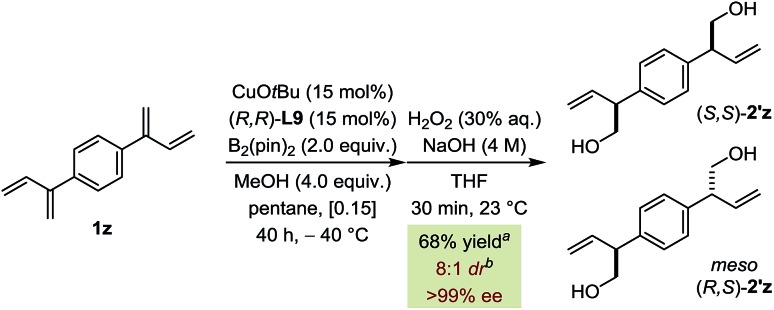
Finally, double borylation of bis-diene 1z led preferentially to the formation of (S,S)-2′z (>99% ee) over the meso isomer (R,S)-2′z (8 : 1 dr) (Fig. 6). This approach is based on the Horeau duplication principle and demonstrates that the catalyst can induce high levels of selectivity of each diene moiety independently of the other leading to an overall amplification.19,20
Fig. 6. Borylation/oxidation sequence of bis-diene 1z (0.36 mmol scale). a1,2-/4,3-selectivity = 10 : 1. bInseparable mixture.
Conclusions
In conclusion, we have developed a highly chemo-, regio- and enantioselective Cu-catalyzed borylation of 2-aryl and 2-alkyl substituted 1,3-dienes using a chiral phosphanamine ligand. The method is compatible with a broad range of functional groups and provides rapid access to synthetically relevant homoallylic boronates/alcohols and is amenable to a catalyst-controlled diastereoselective process. Further mechanistic investigations into the origin of the selectivity of this catalytic reaction are underway.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by the University of Geneva. S. Rosset is acknowledged for technical assistance.
Footnotes
†Electronic supplementary information (ESI) available: Experimental procedures, characterization of all new compounds and spectral data. See DOI: 10.1039/c8sc01538d
References
- (a) Nicolaou K. C., Snyder S. A., Montagnon T., Vassilikogiannakis G. Angew. Chem., Int. Ed. 2002;41:1668. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; (b) Negishi E.-I., Huang Z., Wang G., Mohan S., Wang C., Hattori H. Acc. Chem. Res. 2008;41:1474. doi: 10.1021/ar800038e. [DOI] [PubMed] [Google Scholar]
- (a) Leicht H., Göttker-Schnetmann I., Mecking S. ACS Macro Lett. 2016;5:777. doi: 10.1021/acsmacrolett.6b00321. [DOI] [PubMed] [Google Scholar]; (b) Yao C., Liu N., Long S., Wu C., Cui D. Polym. Chem. 2016;7:1264. [Google Scholar]; (c) Leicht H., Göttker-Schnetmann I., Mecking S. J. Am. Chem. Soc. 2017;139:6823. doi: 10.1021/jacs.7b03087. [DOI] [PubMed] [Google Scholar]
- (a) Mahatthananchai J., Dumas A. M., Bode J. W. Angew. Chem., Int. Ed. 2012;51:10954. doi: 10.1002/anie.201201787. [DOI] [PubMed] [Google Scholar]; (b) Xiong Y., Sun Y., Zhang G. Tetrahedron Lett. 2018;59:347. [Google Scholar]
- (a) Ely R. J., Morken J. P. J. Am. Chem. Soc. 2010;132:2534. doi: 10.1021/ja910750b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sharma R. K., RajanBabu T. V. J. Am. Chem. Soc. 2010;132:3295. doi: 10.1021/ja1004703. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Watkins A. L., Landis C. R. Org. Lett. 2011;13:164. doi: 10.1021/ol102797t. [DOI] [PubMed] [Google Scholar]; (d) Zbieg J. R., Yamaguchi E., McInturff E. L., Krische M. J. Science. 2012;336:324. doi: 10.1126/science.1219274. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Kliman L. T., Mlynarski S. N., Ferris G. E., Morken J. P. Angew. Chem., Int. Ed. 2012;51:521. doi: 10.1002/anie.201105716. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Goldfogel M. J., Roberts C. C., Meek S. J. J. Am. Chem. Soc. 2014;136:6227. doi: 10.1021/ja502275w. [DOI] [PubMed] [Google Scholar]; (g) Chen T. Y., Tsutsumi R., Montgomery T. P., Volchkov I., Krische M. J. J. Am. Chem. Soc. 2015;137:1798. doi: 10.1021/ja5130258. [DOI] [PubMed] [Google Scholar]; (h) Saini V., O'Dair M., Sigman M. S. J. Am. Chem. Soc. 2015;137:608. doi: 10.1021/ja511640g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Wu X., Lin H.-C., Li M.-L., Li L.-L., Han Z.-Y., Gong L.-Z. J. Am. Chem. Soc. 2015;137:13476. doi: 10.1021/jacs.5b08734. [DOI] [PubMed] [Google Scholar]; (j) Liu Y., Xie Y., Wang H., Huang H. J. Am. Chem. Soc. 2016;138:4314. doi: 10.1021/jacs.6b00976. [DOI] [PubMed] [Google Scholar]; (k) Korkis S. E., Burns D. J., Lam H. W. J. Am. Chem. Soc. 2016;138:12252. doi: 10.1021/jacs.6b06884. [DOI] [PubMed] [Google Scholar]; (l) Yang X.-H., Dong V. M. J. Am. Chem. Soc. 2017;139:1774. doi: 10.1021/jacs.6b12307. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Adamson N. J., Hull E., Malcolmson S. J. Am. Chem. Soc. 2017;139:7180. doi: 10.1021/jacs.7b03480. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Gui Y.-Y., Hu N., Chen X.-W., Liao L.-L., Ju T., Ye J.-H., Zhang Z., Li J., Yu D.-G. J. Am. Chem. Soc. 2017;139:17011. doi: 10.1021/jacs.7b10149. [DOI] [PubMed] [Google Scholar]
- (a) Smejkal T., Han H., Breit B., Krische M. J. J. Am. Chem. Soc. 2009;131:10366. doi: 10.1021/ja904124b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ebe Y., Nishimura T. J. Am. Chem. Soc. 2014;136:9284. doi: 10.1021/ja504990a. [DOI] [PubMed] [Google Scholar]; (c) Chen Q.-A., Kim D. K., Dong V. M. J. Am. Chem. Soc. 2014;136:3772. doi: 10.1021/ja500268w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) McCammant M. S., Sigman M. S. Chem. Sci. 2015;6:1355. doi: 10.1039/c4sc03074e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Nguyen K. D., Herkommer D., Krische M. J. J. Am. Chem. Soc. 2016;138:14210. doi: 10.1021/jacs.6b09333. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Oda S., Franke J., Krische M. J. Chem. Sci. 2016;7:136. doi: 10.1039/c5sc03854e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Jiang L., Cao P., Wang M., Chen B., Wang B., Liao J. Angew. Chem., Int. Ed. 2016;55:1385. doi: 10.1002/anie.201607493. [DOI] [PubMed] [Google Scholar]; (h) Li X., Meng F., Torker S., Shi Y., Hoveyda A. H. Angew. Chem., Int. Ed. 2016;55:9997. doi: 10.1002/anie.201605001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Yang X.-H., Lu A., Dong V. M. J. Am. Chem. Soc. 2017;139:14049. doi: 10.1021/jacs.7b09188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Li H., Fiorito D., Mazet C. ACS Catal. 2017;7:1554. [Google Scholar]; (b) Fiorito D., Folliet S., Liu Y., Mazet C. ACS Catal. 2018;8:1392. [Google Scholar]
- (a) Satoh M., Nomoto Y., Miyaura N., Suzuki A. Tetrahedron Lett. 1989;30:3789. [Google Scholar]; (b) Wu J. Y., Moreau B., Ritter T. J. Am. Chem. Soc. 2009;131:12915. doi: 10.1021/ja9048493. [DOI] [PubMed] [Google Scholar]
- (a) Sasaki Y., Zhong C., Sawamura M., Ito H. J. Am. Chem. Soc. 2010;132:1226. doi: 10.1021/ja909640b. [DOI] [PubMed] [Google Scholar]; (b) Obligacion J. V., Chirik P. J. J. Am. Chem. Soc. 2013;135:19107. doi: 10.1021/ja4108148. [DOI] [PubMed] [Google Scholar]; (c) Ibrahim A. D., Entsminger S. W., Fout A. R. ACS Catal. 2017;7:3730. [Google Scholar]
- (a) Semba K., Nakao Y. J. Am. Chem. Soc. 2014;136:7567. doi: 10.1021/ja5029556. [DOI] [PubMed] [Google Scholar]; (b) Smith K. B., Logan K. M., You W., Brown M. K. Chem.–Eur. J. 2014;20:12032. doi: 10.1002/chem.201404310. [DOI] [PubMed] [Google Scholar]; (c) Logan K. M., Smith K. B., Brown M. K. Angew. Chem., Int. Ed. 2015;54:5228. doi: 10.1002/anie.201500396. [DOI] [PubMed] [Google Scholar]; (d) Jia T., Cao P., Wang B., Lou Y., Yin X., Wang M., Liao J. J. Am. Chem. Soc. 2015;137:13760. doi: 10.1021/jacs.5b09146. [DOI] [PubMed] [Google Scholar]; (e) Logan K. M., Brown M. K. Angew. Chem., Int. Ed. 2017;56:851. doi: 10.1002/anie.201609844. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chen B., Cao P., Yin X., Liao Y., Jiang L., Ye J., Wang M., Liao J. ACS Catal. 2017;7:2425. [Google Scholar]
- (a) Smith K. B., Brown M. K. J. Am. Chem. Soc. 2017;139:7721. doi: 10.1021/jacs.7b04024. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sardini S. R., Brown M. K. J. Am. Chem. Soc. 2017;139:9823. doi: 10.1021/jacs.7b05477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Miura T., Takahashi Y., Murakami M. Chem. Commun. 2007:595. doi: 10.1039/b612710j. [DOI] [PubMed] [Google Scholar]; (b) Yu B., Menard F., Isono N., Lautens M. Synthesis. 2009:853. [Google Scholar]; (c) Kim J., Park S., Park J., Cho S. H. Angew. Chem., Int. Ed. 2016;55:1498. doi: 10.1002/anie.201509840. [DOI] [PubMed] [Google Scholar]; (d) Garza V. J., Krische M. J. J. Am. Chem. Soc. 2016;138:3655. doi: 10.1021/jacs.6b01078. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Shi Y., Hoveyda A. H. Angew. Chem., Int. Ed. 2016;55:3455. doi: 10.1002/anie.201600309. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhan M., Li R.-Z., Mou Z.-D., Cao C.-G., Liu J., Chen Y.-W., Niu D. ACS Catal. 2016;6:3381. [Google Scholar]
- (a) Semba K., Fujihara T., Terao J., Tsuji Y. Tetrahedron. 2015;71:2183. [Google Scholar]; (b) Neeve E. C., Geier S. J., Mkhalid I. A. I., Westcott S. A., Marder T. B. Chem. Rev. 2016;116:9091. doi: 10.1021/acs.chemrev.6b00193. [DOI] [PubMed] [Google Scholar]; (c) Diner C., Szabó K. J. J. Am. Chem. Soc. 2017;139:2. doi: 10.1021/jacs.6b10017. [DOI] [PubMed] [Google Scholar]
- (a) Lee J.-E., Yun J. Angew. Chem., Int. Ed. 2008;47:145. doi: 10.1002/anie.200703699. [DOI] [PubMed] [Google Scholar]; (b) Lee Y., Hoveyda A. H. J. Am. Chem. Soc. 2009;131:3160. doi: 10.1021/ja809382c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lee Y., Jang H., Hoveyda A. H. J. Am. Chem. Soc. 2009;131:18234. doi: 10.1021/ja9089928. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lee J. C. H., McDonald R., Hall D. G. Nat. Chem. 2011;3:894. doi: 10.1038/nchem.1150. [DOI] [PubMed] [Google Scholar]; (e) Corberán R., Mszar N. W., Hoveyda A. H. Angew. Chem., Int. Ed. 2011;50:7079. doi: 10.1002/anie.201102398. [DOI] [PubMed] [Google Scholar]; (f) Sasaki Y., Horita Y., Zhong C., Sawamura M., Ito H. Angew. Chem., Int. Ed. 2011;50:2778. doi: 10.1002/anie.201007182. [DOI] [PubMed] [Google Scholar]; (g) Semba K., Shinomiya M., Fujihara T., Terao J., Tsuji Y. Chem.–Eur. J. 2013;19:7125. doi: 10.1002/chem.201300443. [DOI] [PubMed] [Google Scholar]; (h) Kubota K., Hayama K., Iwamoto H., Ito H. Angew. Chem., Int. Ed. 2015;54:8809. doi: 10.1002/anie.201502964. [DOI] [PubMed] [Google Scholar]; (i) Jarava-Barrera C., Parra A., López A., Cruz-Acosta F., Collado-Sanz D., Cárdenas D. J., Tortosa M. ACS Catal. 2016;6:442. doi: 10.1021/acscatal.5b02742. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Kubota K., Watanabe Y., Hayama K., Ito H. J. Am. Chem. Soc. 2016;138:4338. doi: 10.1021/jacs.6b01375. [DOI] [PubMed] [Google Scholar]; (k) Wang Z., He X., Zhang R., Zhang G., Xu G., Zhang Q., Xiong T., Zhang Q. Org. Lett. 2017;19:3067. doi: 10.1021/acs.orglett.7b01135. [DOI] [PubMed] [Google Scholar]
- (a) Palais L., Mikhel I. S., Bournaud C., Micouin L., Falciola C. A., Augustin M. V., Rosset S., Bernardinelli G., Alexakis A. Angew. Chem., Int. Ed. 2007;46:7462. doi: 10.1002/anie.200702186. [DOI] [PubMed] [Google Scholar]; (b) Hawner C., Li K., Cirriez V., Alexakis A. Angew. Chem., Int. Ed. 2008;47:8211. doi: 10.1002/anie.200803436. [DOI] [PubMed] [Google Scholar]; (c) Palais L., Alexakis A. Chem.–Eur. J. 2009;15:10473. doi: 10.1002/chem.200901577. [DOI] [PubMed] [Google Scholar]; (d) Palais L., Bournaud C., Micouin L., Alexakis A. Chem.–Eur. J. 2010;16:2567. doi: 10.1002/chem.200902417. [DOI] [PubMed] [Google Scholar]; (e) Müller D., Alexakis A. Org. Lett. 2012;14:1842. doi: 10.1021/ol3004436. [DOI] [PubMed] [Google Scholar]; (f) Müller D., Guénée L., Alexakis A. Eur. J. Org. Chem. 2013:6335. [Google Scholar]; (g) Li H., Grassi D., Guénée L., Bürgi T., Alexakis A. Chem.–Eur. J. 2014;20:16694. doi: 10.1002/chem.201404668. [DOI] [PubMed] [Google Scholar]
- (a) Kagan H. B., in Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer, Berlin, 1999, ch. 4. [Google Scholar]; (b) Blackmond D. G. Acc. Chem. Res. 2000;33:402. doi: 10.1021/ar990083s. [DOI] [PubMed] [Google Scholar]; (c) Satyanarayana T., Abraham S., Kagan H. B. Angew. Chem., Int. Ed. 2009;48:456. doi: 10.1002/anie.200705241. [DOI] [PubMed] [Google Scholar]
- (a) Comprehensive AsymmetricComprehensive Asymmetric CatalysisCatalysis, ed. E. N. Jacobsen, A. Pfaltz, and H. Yamamoto, Springer, Berlin, 1999. [Google Scholar]; (b) Fundamentals of Asymmetric Catalysis, ed. P. J. Walsh and M. C. Kozlowski, University Science Books, Sausalito, CA, 2009. [Google Scholar]
- (a) Hassan A., Lu Y., Krische M. J. Org. Lett. 2009;11:3112. doi: 10.1021/ol901136w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shin I., Wang G., Krische M. J. Chem.–Eur. J. 2014;20:13382. doi: 10.1002/chem.201404065. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Li H., Mazet C. J. Am. Chem. Soc. 2015;137:10720. doi: 10.1021/jacs.5b06281. [DOI] [PubMed] [Google Scholar]
- (a) Cinderella A. P., Vulovic B., Watson D. A. J. Am. Chem. Soc. 2017;139:7741. doi: 10.1021/jacs.7b04364. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Vulovic B., Cinderella A. P., Watson D. A. ACS Catal. 2017;7:8113. doi: 10.1021/acscatal.7b03465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Vigneron J.-P., Dhaenes M., Horeau A. Tetrahedron. 1973;29:1055. [Google Scholar]; (b) Baba S., Sartor K., Kagan H. B. Bull. Soc. Chim. Fr. 1994;131:525. [Google Scholar]; (c) Lagasse F., Tsukamoto M., Kagan H. B. J. Am. Chem. Soc. 2003;125:7490. doi: 10.1021/ja0300315. [DOI] [PubMed] [Google Scholar]
- When 1,3-dienes with other substitutions patterns (2,3-disubstituted, linear, cyclic…) were investigated with our optimized conditions, unsatisfactory levels of selectivity were observed. See ESI.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.