Abstract
Objective
Reduction of brain glucose transporter GLUT1 results in severe neurological dysfunction. VEGF is required to restore and maintain brain glucose uptake across the blood brain barrier via GLUT1, which was shown to be acutely diminished in response to a high fat diet (HFD) in mice. The genetic and HFD-related regulation and association of VEGF and GLUT1 (SLC2A1) in humans was investigated in the NUtriGenomic Analysis in Twins (NUGAT) study.
Methods
92 healthy and non-obese twins were standardized to a high-carbohydrate low-fat diet for 6 weeks before switched to a 6-week HFD under isocaloric conditions. Three clinical investigation days were conducted: after 6 weeks of low-fat diet and after 1 and 6 weeks of HFD. Serum VEGF and other cytokine levels were measured using ELISA. Gene expression in subcutaneous adipose tissue was assessed by quantitative Real-Time PCR. Genotyping was performed using microarray. The Auditory Verbal Learning Task was conducted to measure cognitive performance.
Results
In this human study, we showed that the environmental regulation of SLC2A1 expression and serum VEGF by HFD was inversely correlated and both factors showed strong heritability (>90%). In response to the HFD containing 45% fat, serum VEGF levels increased (P = 0.002) while SLC2A1 mRNA expression in adipose tissue decreased (P = 0.001). Higher BMI was additionally associated with lower SLC2A1 expression. AA-genotypes of the rs9472159 polymorphism, which explained ∼39% of the variation in circulating VEGF concentrations, showed significantly reduced serum VEGF levels (P = 6.4 × 10−11) but higher SLC2A1 expression (P = 0.009) in adipose tissue compared to CC/CA-genotypes after 6 weeks of HFD. Memory performance in AA-genotypes declined in response to the HFD compared to CC- and CA-genotypes.
Conclusions
The results provide evidence to suggest the translatability of the dietary regulation of VEGF and GLUT1 from mouse models to humans. Our data demonstrate that HFD induces a genetically determined and correlated decrease of GLUT1 and increase of VEGF which may affect memory performance.
Clinical Trial Registration Number
Keywords: VEGF, GLUT1, High fat diet, Cognition
1. Introduction
Cognitive function is known to be affected by macronutrient intakes, with high fat diets and obesity being particularly deleterious [1], [2]. In mice, inflammatory responses in the central nervous system (CNS) to high fat diet (HFD) and subsequent brain insulin resistance appear to occur within days after initiating a high fat diet [3]. Although the pathways involved in central insulin resistance have been investigated in considerable detail, knowledge as to the precise mechanisms which transmit HFD associated signals are only partially understood [1], [2], [4].
The CNS depends on glucose as its energy substrate, and glucose needs to be transported across the blood brain barrier. Brain glucose uptake is mediated by facilitated transport via the glucose transporter GLUT1, and an important role of GLUT1 in cognitive function is becoming increasingly apparent [5], [6]. Disturbances in GLUT1 function due to genetic aberrations of the SLC2A1 gene lead to seizures, motor dysfunction, and encephalopathy in GLUT1-haploinsufficient mice and humans, while complete deletion of GLUT1 is lethal [5], [6]. Very recently, HFD was shown to reduce GLUT1 expression in brain endothelia, leading to cognitive impairment in mice [7]. This reaction was counterbalanced by an increased and delayed production of VEGF in myeloid cells, leading to elevated circulating VEGF levels, which increased GLUT1 expression and thereby compensated for the downregulation by HFD. Deletion of VEGF in myeloid cells, however, prevented this protective response and enhanced the development of dementia in mouse models, which underscores the importance of GLUT1 and VEGF expression for cognitive function. The increase in VEGF was proposed to represent an inflammatory response reducing peripheral but increasing central glucose uptake to maintain CNS glucose supply [7].
These data raise the question as to the HFD-related regulation and association of GLUT1 and VEGF in humans with regard to cognition. Serum levels of VEGF were shown to be determined by several genetic polymorphisms [8], [9]. It is unknown whether dietary intakes alter serum levels of VEGF or the expression of GLUT1 in humans. Moreover, it is unknown whether the levels or the reaction to nutritional intakes is determined genetically.
Therefore, we investigated the acute and delayed response of 92 human mono- and dizygous twins to an acute shift from low fat to high fat diet with respect to serum levels of VEGF and the expression of SLC2A1 in subcutaneous adipose tissue (AT). By comparing the degree of concordance of basal and diet induced responses within and between monozygous vs. dizygous twin pairs, we could determine the heritability of these responses. Moreover, we tested cognitive performance using a sensitive memory task previously shown to be modulated by dietary restriction [10], [11] before and at the end of the high fat diet and assessed its association with VEGF and GLUT1.
2. Material and methods
2.1. NUGAT study design
The NUGAT study protocol was approved by the independent ethics committee of the Charité-Universitätsmedizin Berlin and conducted in accordance with the principles of the Helsinki Declaration of 1975, as revised in 2000. All participants gave written informed consent prior to the study. The NUGAT study was registered with ClinicalTrials.gov: NCT01631123.
Details of recruitment and phenotyping of study participants as well as dietary interventions were published recently [12]. 92 healthy and non-obese mono- and dizygous twins (58 female and 34 male) with a mean (±SD) age of 31 (± 14) years and a mean (±SD) BMI of 22.8 (± 2.7) kg/m2 were included in the study. At screening, a standardized 3 h, 75 g OGTT (oral glucose tolerance test) with insulin measurements was performed.
The dietary intervention was carried out in a sequential design and under isocaloric conditions. Individual energy requirements were calculated based on participants resting energy expenditure (REE) determined by indirect calorimetry and physical activity level assessed by questionnaire. For 6 weeks, participants consumed a low fat (LF) diet with 30% of energy from dietary fat (55% carbohydrate, 15% protein), which served to achieve homogenous nutritional intakes. Subsequently, participants switched to a 6-week HFD (40% carbohydrate, 45% fat, 15% protein) with emphasis on foods high in saturated fat. An excellent compliance was indicated by increases in LDL- and HDL-cholesterol already after 1 week, which continued until week 6 of HFD [12]. Clinical investigation days (CIDs) were performed after 6 weeks of LF diet (LF6) and after 1 and 6 weeks of HFD (HF1 and HF6). At each CID, blood samples were drawn in the fasted state (>10 h since last food intake) in the morning from the forearm vein, centrifuged at 1.800 × g for 10 min at 4 °C, and stored at −80 °C until analysis. Furthermore, a biopsy of subcutaneous adipose tissue was performed by fine needle aspiration lateral to the umbilicus for gene expression analysis.
2.2. Serum concentrations of VEGF and inflammatory parameters
VEGF, IL6, IL8, and TNFα concentrations were measured in the serum of all participants at each CID using human ELISA Kits (R&D Systems Inc., Minneapolis, MN, USA). The human VEGF immunoassay ELISA had a lower detection limit of 9 pg/ml (VEGF intraassay coefficient of variation <6.7%, interassay coefficient of variation <8.8%; R&D Systems Inc, Minneapolis, MN, USA).
2.3. Analysis of gene expression in subcutaneous adipose tissue
A biopsy of subcutaneous adipose tissue was performed lateral to the umbilicus by fine needle aspiration. About 500 mg of adipose tissue were homogenized (Speed Mill, Analytik Jena, Jena, Germany), and total RNA was extracted by using the RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany). Gene expression was analyzed by quantitative Real-Time PCR. High-Capacity cDNA Reverse Transcription Kit™ (Applied Biosystems by Life Technologies, Carlsbad, CA, USA) was used to synthesize cDNA from 1 μg of RNA. Samples were labeled by Power SYBR Green Master Mix and measured in triplicates in optical 384-well plates with the ABI ViiA™7 Real-Time PCR System (Applied Biosystems by Life Technologies, Carlsbad, CA, USA). Samples were normalized to ribosomal protein large P0 (RPLP0) and for evaluation the standard curve method was used. The primer sequences were: EMR1 forward primer GGC TGT TCG GAC GGA ATA CTT, reverse primer TCT TAT CCC CTT GGC TAC CA; VEGFA forward primer CAT CTT CAA GCC ATC CTG TGT G, reverse primer CCG CAT AAT CTG CAT GGT GAT; KDR (VEGFR2) forward primer TGC GAA GTA CCT TGG TTA CCC A, reverse primer TAA TCG TCA GTA CAT GCC CCG; RPLP0 forward primer GCT TCC TGG AGG GTG TCC, reverse primer GGA CTC GTT TGT ACC CGT TG.
2.4. Genotyping
Genomic DNA was extracted from buffy coats (NucleoSpin, Macherey–Nagel, Düren, Germany). Genotyping was performed using HumanOmniExpressExome BeadChips (Illumina, Inc., San Diego, CA, USA) at the Interdisciplinary Center for Clinical Research (IZKF, Leipzig, Germany).
Genotype frequencies were analyzed for deviation from Hardy–Weinberg equilibrium by chi-square test using R 3.1.2 plus HardyWeinberg package 1.5.5.
2.5. Auditory Verbal Learning Task (AVLT)
The German version of the Auditory Verbal Learning Task (AVLT [13], [14]; was performed at the end of the LF dietary intervention and again at the end of the HFD period. A list containing 15 unrelated nouns was read to the participants, and they were asked to recall as many words as possible after the presentation irrespective of right order. This procedure was repeated 4 times (trial 1–5). After a delay of 30 min, the list had to be recalled by participants without further presentation (trial 7). Subsequently, the participants had to recognize these words from a 45 word comprised of the 15 known words plus 30 new words (trial 8).
Following measures were evaluated: learning score (sum of correctly repeated words after each presentation (trials 1–5)), delayed recall score (number of words correctly recalled after the 30 min delay), consolidation memory score (difference of recalled words between trial 5 and 7), and recognition score (number of correctly recognized words minus false assigned words).
2.6. Heritability
Heritability was estimated by applying the ACE structural equation model. This model analyzes covariance based on comparing the degree of concordance within and between monozygous vs. dizygous twin pairs. The proportion of variance is decomposed into (A) additive genetic influences, (C) common environmental, and (E) individual environmental influences. The ACE model was calculated using R 2.15.0 plus OpenMX package.
2.7. Statistical analysis
The Kolmogorov–Smirnov test was used to assess variables for normal distribution. Non-normally distributed variables were natural logarithm (ln)-transformed. Mean values for continuous data were compared using repeated measures or one-way ANOVA followed by Bonferroni adjusted posthoc test. The Kruskal–Wallis test as non-parametric equivalent of the ANOVA was used to verify significant results for non-normally distributed data. To compare two, not normally distributed, independent or dependent variables, the nonparametric Mann-Whitney-U and Wilcoxon test was used respectively.
Statistical analyses were processed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA) and p < 0.05 were considered significant. Values are expressed as mean ± SEM, unless otherwise stated.
3. Results
3.1. Heritability of serum VEGF concentrations and SLC2A1 expression in AT
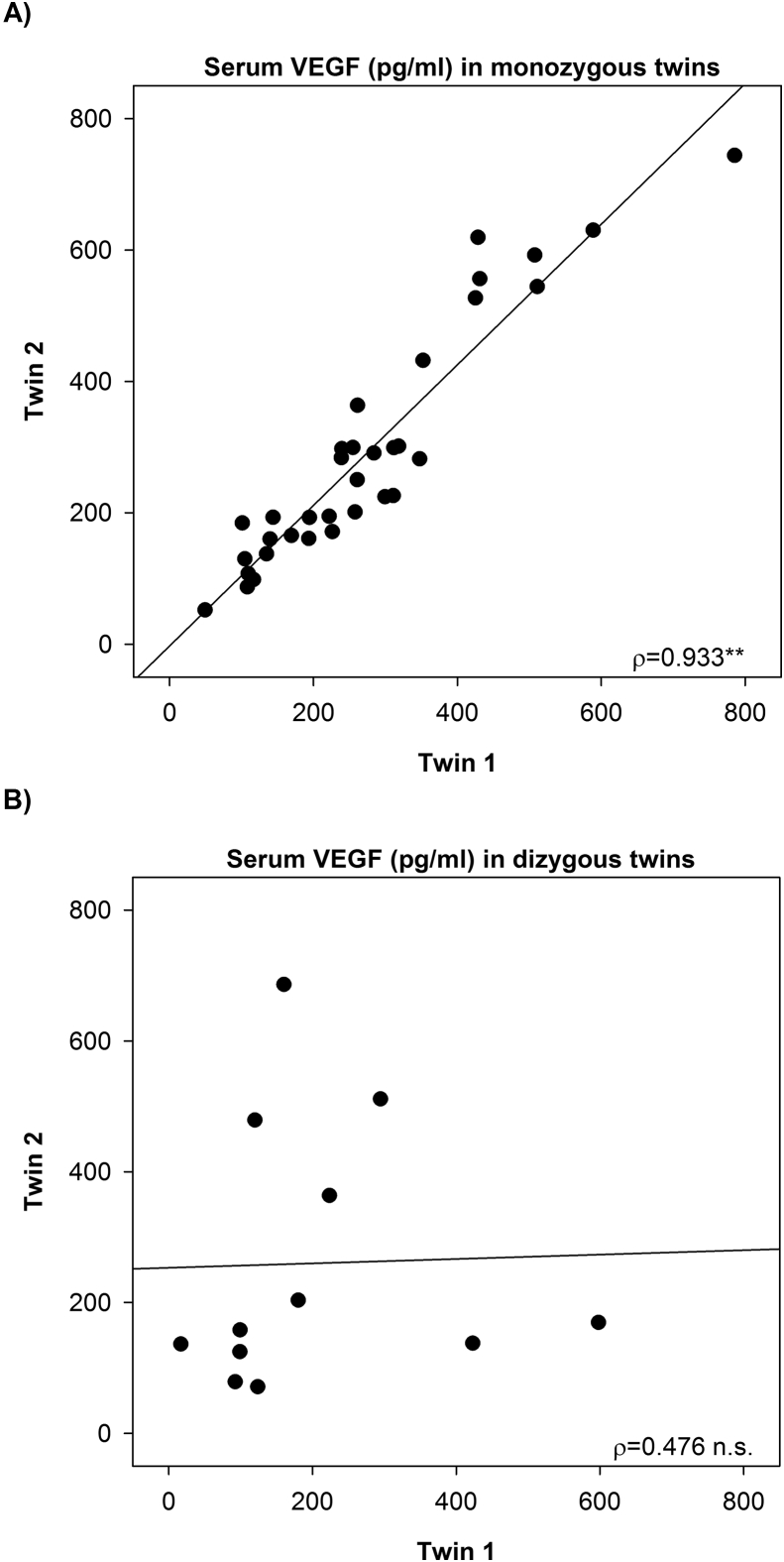
According to minimum and maximum values the fasting levels of VEGF varied 46-fold at LF6 (clinical investigation day (CID) after 6 weeks of low-fat diet), 32-fold at HF1 (CID after 1 week of HFD), and 19-fold at HF6 (CID after 6 weeks of HFD) and were highly correlated among the monozygous (LF6: ρ = 0.933, p = 9.9 × 10−16, Figure 1A) but not among the dizygous twin pairs (LF6: ρ = 0.476, p = 0.118, Figure 1B), indicating a strong heritability of 94%.
Figure 1.
VEGF levels are highly correlated in monozygous twins. Strong intrapair correlation of VEGF serum concentrations in (A) monozygous twins and no correlation in (B) dizygous twins. **p < 0.01.
The mRNA expression of SLC2A1 as assessed by quantitative Real-Time PCR (qPCR) in subcutaneous AT biopsies also showed a very high correlation among the monozygous (LF6: ρ = 0.771, p = 6.1 × 10−7) but not the dizygous twin pairs (LF6: ρ = 0.373, p = 0.259) with an estimated heritability of A = 0.938 at LF6 (A, proportion of variance due to additive genetic effects). For comparison, the gene expression of GLUT4 and GLUT5 was not heritable (A = 0 at LF6), confirming that GLUT1 shows a unique, strong heritability among the glucose transporters.
3.2. Increase in circulating VEGF concentrations in response to HFD
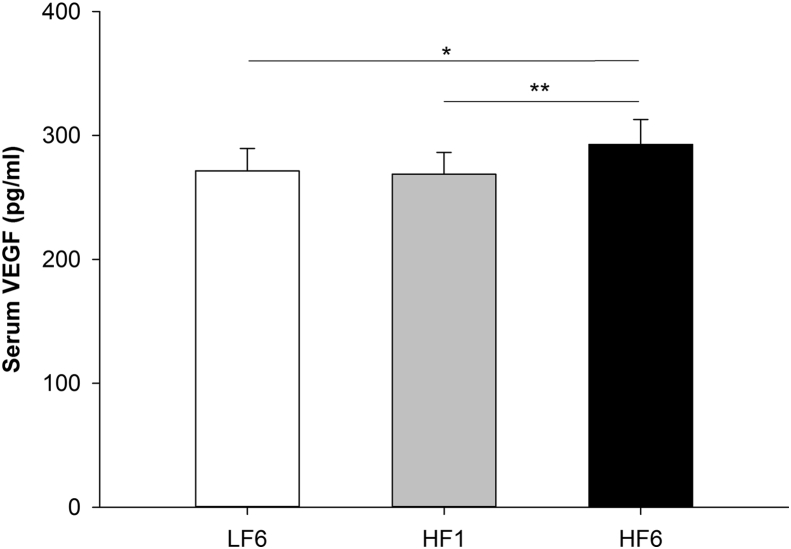
VEGF serum concentrations significantly increased in response to the HFD (repeated measures ANOVA, p = 0.002) with significantly higher levels after 6 weeks of the high fat dietary phase (mean ± SD: LF6 271.5 ± 173.1 pg/ml, HF1 268.8 ± 167.9 pg/ml, HF6 292.8 ± 193.1 pg/ml; Bonferroni adjusted posthoc test: HF6 vs. LF6: p = 0.023, HF6 vs. HF1: p = 0.008; Figure 2).
Figure 2.
VEGF levels increase in response to the 6-week HFD. VEGF serum concentrations at LF6, HF1 and HF6 (mean ± SEM; *p < 0.05, **p < 0.01). LF6, investigation day after 6 weeks of the low fat diet; HF1, investigation day after 1 week of the high fat diet; HF6, investigation day after 6 weeks of the high fat diet.
3.3. VEGF, VEGFR2, and GLUT1 gene expression in AT in response to HFD
VEGFA gene expression was significantly reduced in subcutaneous adipose tissue after 6 weeks of HFD (repeated measures ANOVA, p = 4.3 × 10−5; Bonferroni adjusted posthoc test LF6 vs. HF6: p = 9.5 × 10−5, HF1 vs. HF6: p = 0.030; Figure 3A). Gene expression levels did not correlate with circulating levels of VEGF, which suggests that circulating levels are not determined by adipose tissue derived VEGF (LF6: ρ = 0.112, p = 0.315; HF1: ρ = 0.017, p = 0.877; HF6: ρ = 0.056, p = 0.617). Gene expression of VEGF receptor 2 (VEGFR2, KDR), the primary receptor for VEGF, significantly decreased in response to the HFD (repeated measures ANOVA, p = 0.003; Bonferroni adjusted posthoc test LF6 vs. HF6: p = 0.011, LF6 vs. HF1: p = 0.031; Figure 3B).
Figure 3.
Reduced gene expression of VEGFA, KDR, and SLC2A1 in subcutaneous adipose tissue in response to HFD. Relative mRNA expression assessed by quantitative Real-Time PCR of (A)VEGFA, (B)KDR and (C)SLC2A1. Values are shown as mean ± SEM. *p < 0.05, ***p < 0.001. To compare the main effects a Repeated Measures ANOVA and Bonferroni adjusted posthoc test was used. LF6, investigation day after 6 weeks of the low fat diet; HF1, investigation day after 1 week of the high fat diet; HF6, investigation day after 6 weeks of the high fat diet.
The mRNA expression of SLC2A1 in AT was significantly downregulated after 6 weeks of HFD (repeated measures ANOVA p = 0.001; Bonferroni adjusted posthoc test LF6 vs. HF6: p = 4 × 10−6) and negatively correlated with serum levels of VEGF at LF6 (LF6: ρ = −0.296, p = 0.007). The correlation between SLC2A1 and VEGF became more stringent upon switching to the HFD (HF1: ρ = −0.428, p = 5.4 × 10−5; HF6: ρ = −0.311, p = 0.005).
This raised the question whether GLUT1 expression is linked to inflammatory stimuli from macrophages as suggested by the study of Jais et al [7]. Indeed, an extraordinary correlation was observed between SLC2A1 expression in adipose tissue and the macrophage marker EMR1 (EGF-like module-containing mucin-like hormone receptor-like 1, human homolog of F4/80; LF6: ρ = 0.656, p = 3.0 × 10−11, HF1: ρ = 0.629, p = 1.5 × 10−10, HF6: ρ = 0.583, p = 1.2 × 10−8), supporting the idea that stimuli emanating from macrophages upregulate GLUT1. Notably, SLC2A1 did not correlate with circulating levels of IL6, IL8, or TNFα, demonstrating that the correlation was rather exceptional.
Further, it was brought into question whether there may be a similar regulation of GLUT1 in adipose tissue and blood brain barrier (BBB) cells. Therefore, this was tested in the mouse model in which a positive correlation of VEGF and HFD had been shown [7]. We could not observe a statistically significant reduced Slc2a1 mRNA expression in white adipose tissue of wildtype mice fed a high fat diet for 4 weeks (p = 0.251, Figure S1).
Myeloid cells represent a significant source of VEGF. Therefore, we isolated peripheral blood mononuclear cells and circulating monocytes from the participants (n = 30) and determined VEGFA gene expression. Remarkably, there was no change in response to HFD (Figure S2), and the gene expression was not correlated with circulating levels of VEGF (LF6: p = 0.806), suggesting that circulating VEGF does not significantly originate from circulating monocytes.
Stimulation of primary human macrophages, adipocytes, or co-cultures, respectively, with VEGF for 24 h did not influence gene expression of GLUT1 (Figure S3).
3.4. Altered SLC2A1 gene expression in normal weight vs. overweight subjects
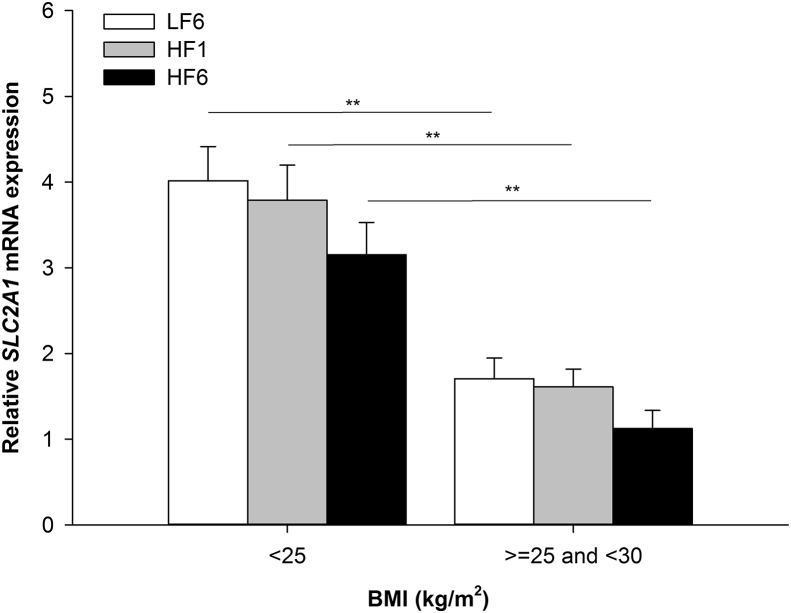
SLC2A1 gene expression significantly correlated with BMI (LF6: ρ = −0.381, p = 3.8 × 10−4; HF1: ρ = −0.302, p = 0.005; HF6: ρ = −0.322, p = 0.003). SLC2A1 gene expression in AT was significantly lower in overweight subjects (BMI ≥ 25 kg/m2 and <30 kg/m2) compared to normal weight subjects (BMI < 25 kg/m2; LF6: p = 0.001, HF1: p = 0.003, HF6: p = 0.003; Figure 4). SLC2A1 gene expression also correlated with age (LF6: ρ = −0.289, p = 0.008; HF1: ρ = −0.263, p = 0.016; HF6: ρ = −0.250, p = 0.024).
Figure 4.
SLC2A1 gene expression in AT differed between BMI categories.SLC2A1 gene expression in AT at LF6, HF1, and HF6 after stratification for BMI class (**p < 0.01). LF6, investigation day after 6 weeks of the low fat diet; HF1, investigation day after 1 week of the HFD; HF6, investigation day after 6 weeks of the HFD; AT, adipose tissue.
In contrast, VEGF serum levels only significantly correlated with age (LF6: ρ = 0.288, p = 0.005; HF1: ρ = 0.341, p = 0.001; HF6: ρ = 0.248, p = 0.017).
3.5. Polymorphism rs9472159 is associated with serum VEGF concentrations and SLC2A1 gene expression in AT
In view of the extraordinarily high heritability of VEGF, we analyzed polymorphisms in the NUGAT study previously reported in the literature to be associated with VEGF serum levels [8], [9]. Of 10 markers covered by the array, 2 variants, rs9472159 and rs9369434, were significantly associated with circulating VEGF levels (rs9472159 (C > A), LF6: corrected p = 1.2 × 10−4, HF1: corrected p = 1.0 × 10−4, HF6: corrected p = 8.8 × 10−6; rs9369434 (C > T), LF6: corrected p = 0.009, HF1: corrected p = 0.010, HF6: corrected p = 0.004).
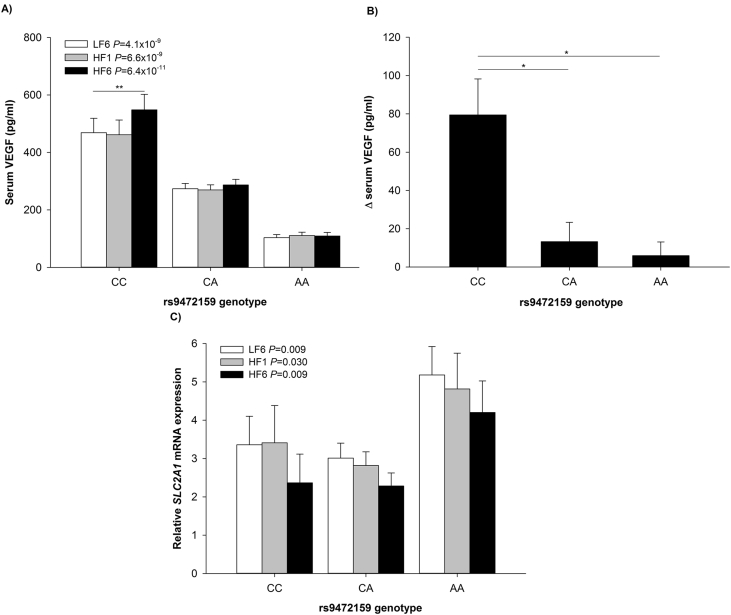
SNP rs9472159 was previously reported to be located in an enhancer- and promoter-associated histone mark region and therefore proposed to be able to regulate expression of VEGF [9], [15]. Genotype frequencies for the VEGF rs9472159 polymorphism were CC = 13, CA = 63 and AA = 16 and did deviate from values predicted by the Hardy–Weinberg equilibrium (χ2 = 12.66, p = 3.7 × 10−4). The frequency of the A allele was 0.52. VEGF serum levels were significantly lower in carriers of the A-allele compared to carriers of the major allele C (LF6: CC vs. CA vs. AA, 468.9 ± 50.1 pg/ml vs. 273.5 ± 18.6 pg/ml vs. 103.2 ± 10.9 pg/ml, p = 4.1 × 10−9, HF1: p = 6.6 × 10−9, HF6: p = 6.4 × 10−11; Figure 5A). The polymorphism explained up to 39% of the variation in VEGF serum concentrations (LF6: corrected R2 = 0.344, p = 5.0 × 10−10; HF1: corrected R2 = 0.339, p = 8.6 × 10−10; HF6: corrected R2 = 0.393, p = 1.4 × 10−11). An increase in VEGF serum concentrations in response to the high saturated fat diet was confirmed only for wildtype CC-genotypes (repeated measures ANOVA; CC p = 0.043, CA p = 0.067 and AA p = 0.490; ΔVEGF ANOVA p = 0.009, CC vs. CA and AA genotype, p = 0.011 and p = 0.024, Figure 5B). Most notably, rs9472159 was also linked to the adipose tissue gene expression of SLC2A1 (LF6: p = 0.009, HF1: p = 0.030, HF6: p = 0.009; Figure 5C) suggesting that the correlation of the two proteins has some genetic determinants.
Figure 5.
VEGF serum levels and SLC2A1 gene expression levels differed between rs9472159 genotypes. (A) VEGF serum concentrations at LF6, HF1, and HF6 after stratification for rs9472159 genotype and (B) change of VEGF serum concentrations (HF6-LF6) stratified for rs9472159 genotype. (C)SLC2A1 gene expression in AT after stratification rs9472159 genotype. *p < 0.05, **p < 0.01; LF6, investigation day after 6 weeks of the low fat diet; HF1, investigation day after 1 week of the HFD; HF6, investigation day after 6 weeks of the HFD.
Genotype frequencies for SNP rs9369434, also located intergenically and in proximity to rs9472159 on chromosome 6, were CC = 26, CT = 55 and TT = 11 and were in Hardy–Weinberg disequilibrium (χ2 = 4.80, p = 0.029). The frequency of the T allele was 0.42. Carriers of the T-allele had significantly lower VEGF serum levels compared to carriers of the major allele C (LF6: CC vs. CT vs. TT; 375.1 ± 42.2 pg/ml vs. 254.9 ± 18.0 pg/ml 109.1 ± 13.5 pg/ml, p = 2.2 × 10-5, HF1: p = 3.7 × 10-5, HF6: p = 6.0 × 10-6). Up to 23% of the variation in circulating VEGF levels could be explained by the rs9369434 polymorphism (LF6: corrected R2 = 0.204, p = 4.0 × 10-6; HF1: corrected R2 = 0.196, p = 7.0 × 10-6; HF6: corrected R2 = 0.229, p = 8.5 × 10-7). An increase in VEGF serum concentrations in response to the high saturated fat diet was again only observed for the wildtype CC-genotypes (repeated measures ANOVA; CC p = 0.005, CT p = 0.157 and TT p = 0.901). Polymorphism rs9369434 was also associated with adipose tissue gene expression of SLC2A1 (LF6: p = 0.134, HF1: p = 0.030, HF6: p = 0.039).
3.6. Auditory Verbal Learning Task (AVLT)
In an exploratory approach, the German version of the Auditory Verbal Learning Task (AVLT [10], [11], [14]) was conducted to evaluate whether the HFD affected learning and memory performance. We did not observe a significant decline of learning and memory performance in response to the 6 weeks of HFD but a significantly improved learning score, delayed recall score and consolidation memory score (Wilcoxon test, p = 1.3 × 10−4, p = 3.4 × 10−4, p = 0.036 respectively; Table S1 and Figure 6A) most likely as effect of repetitive testing. Recognition did not change in response to the HFD (Wilcoxon test, p = 0.817, Table S1).
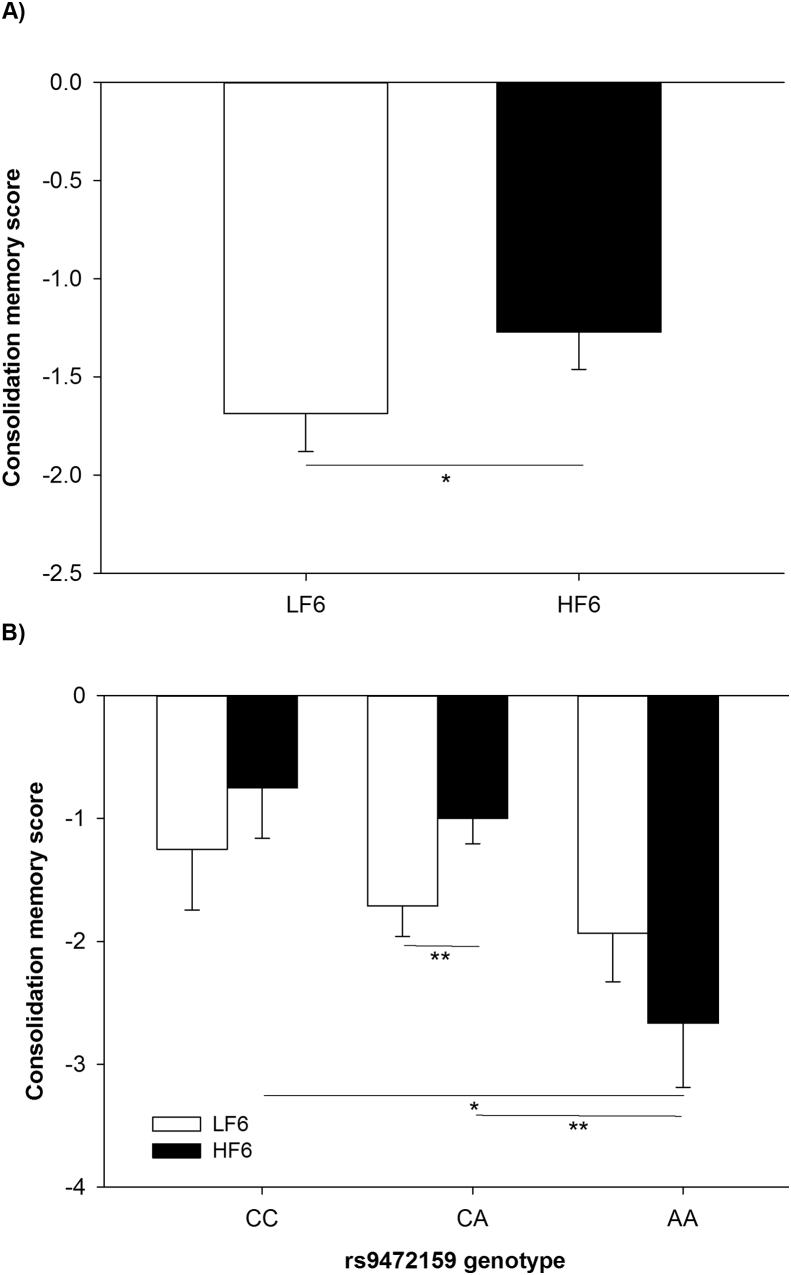
Figure 6.
Consolidation memory scores according to the Auditory Verbal Learning Task (A) before and after 6 weeks of HFD and (B) before and after HFD stratified by rs9472159 genotype. Consolidation memory score was defined as the number of correct words recalled after the fifth trial subtracted from the number of correct words recalled after 30 min delay, multiplied by −1 to create positive relations. Data are shown as mean ± SEM, *p < 0.05, **p < 0.01. LF6, investigation day after 6 weeks of the low fat diet; HF6, investigation day after 6 weeks of the HFD.
However, after stratification by the rs9472159 genotype, we observed that the consolidation memory score, which is per definition adjusted for the initial learning rate and therefore less prone to test-retest/ceiling effects, declined in homozygous carriers of the polymorphism in response to the HFD compared to wildtype CC and heterozygous carriers CA (Kruskal–Wallis test p = 0.009; Mann-Whitney-U test CC vs. AA p = 0.021, CA vs. AA p = 0.003; Figure 6B and Table S2). Comparing measures of delayed recall and recognition elucidated a trend for better memory performance in CC/CA-genotypes compared to AA-genotypes, respectively (recessive model; Mann-Whitney-U test p = 0.078 and p = 0.102 respectively; Table S2). We did not observe any genotype-based difference with regard to learning score in response to the HFD (Kruskal–Wallis test p = 0.273; Table S2).
Polymorphism rs9369434 was also associated with cognitive impairment after 6 weeks of HFD (Table S3).
4. Discussion
Stimulated by studies linking cognitive function to the dietary regulation of GLUT1 and its regulation by VEGF, we investigated the translatability of these findings into humans. Our data confirm an inverse link between the expression of GLUT1 and serum levels of VEGF through their regulation by food intake. In addition, we report extensive heritability of the expression of both factors and identified a genetic variant partially explaining the heritability. Moreover, this variant was linked to cognitive functions, which again concurs with the data from mice and provides possible links for the well-established role of high fat diet and in particular high saturated fat for the risk of developing dementia [16], [17], [18], [19].
GLUT1 shows the highest expression in the blood brain barrier and the brain requires GLUT1 for adequate function [6], [20], [21], [22]. Earlier studies demonstrated an upregulation of GLUT1 expression and glucose transport upon treatment with VEGF in rat brain cortical endothelia and in retinal endothelial cells [23], [24] as well as in bovine aortic endothelial cells in which VEGF strongly upregulated glucose transport via GLUT1 while GLUT4 was unaffected [25]. The upregulation of GLUT1 by VEGF therefore was shown in several vascular tissues across different species. Adipocytes primarily express GLUT4 while GLUT1 shows a low level of expression and is not regulated by insulin or glucose [21]. Adipose tissue biopsies contain endothelial cells, macrophages, and preadipocytes, which account for about 50% of the cells present. We therefore tested whether GLUT1 is regulated by VEGF in primary human adipocytes and did not observe any regulation, suggesting that the changes might be related to other cell types present in the biopsies.
The inverse correlation between VEGF and GLUT1 was rather exceptional and was not observed for other glucose transporters. The correlation was reproduced at all investigation days and increased upon switching to the HFD, confirming the solid relation of the two factors in humans. The increase in VEGF was not associated with increases in cytokines IL-6 and TNFα and thus was not part of a generalized inflammatory reaction suggesting a specific role of VEGF in regulating GLUT1 in humans.
An additional aspect was introduced by the extensive heritability of VEGF, which was the highest among several cytokines analyzed in our twin study including IL6 and TNFα, which showed no or a very modest heritability. A certain heritability of VEGF had been shown in family studies [26]. Our heritability estimates for VEGF are considerably higher since we chose twins without significant differences in body weight and standardized dietary intakes and thereby corrected for the significant environmental alterations of VEGF levels. This raises the possibility that the impact of VEGF may differ substantially in humans depending on the inherited level of expression.
The same applies to GLUT1, which is the only glucose transporter with a strongly inherited expression. GLUT1 was markedly downregulated by increased body weight, which is an important aspect closely linked to metabolism. The expression of both factors was additionally affected by HFD and correlated with age and in both cases again in opposite direction. Therefore, VEGF and GLUT1 are inversely regulated by unfavorable, frequently coinciding metabolic impacts on a strong genetic background of inheritance of basal levels.
GLUT1 mediates basal, insulin-independent glucose uptake, which might be reduced in adipose tissue given reduced GLUT1 mRNA expression in response to the HFD. In view of GLUT4, which is responsible for the majority of glucose uptake in adipose tissue, it is not known whether a reduced GLUT1 expression in adipose tissue exerts significant metabolic consequences.
A potentially helpful finding in our NUGAT study was that the rs9472159 polymorphism was associated with levels of VEGF and GLUT1. Being located in an enhancer- and promoter-associated histone mark region, it was assumed to potentially alter expression of surrounding genes, like VEGFA [15]. The additional association with GLUT1 concurs with the well-established regulation of GLUT1 by VEGF providing one possible explanation for the association. This genetic polymorphism should facilitate further studies on the role of VEGF and GLUT1 since this information is readily available in many human studies. Although Hardy–Weinberg equilibrium was not present in our sample, previous studies assessed its association with serum VEGF [9] suggesting that our sample was only slightly unbalanced. However, the role of this polymorphism certainly needs to be viewed with caution since earlier studies have shown the modest reproducibility of genetic associations derived from relatively small studies.
The discovery that GLUT1 in the microvasculature represents an important target of VEGF thereby affecting brain function is highly relevant. The work of Jais et al. identified VEGF as a determining player in the regulation of cognitive performance and provides an important link to the unfavorable effects of obesity and HFD for the risk of developing dementia [7]. Peripheral insulin resistance was suggested to represent an attempt to improve brain glucose supply by reducing peripheral glucose utilization [7], [27]. Indeed, in our study insulin sensitivity decreased in response to HFD as suggested by a significant increase in HOMA-IR values [28].
Cognitive function, operationalized by a sensitive verbal memory task, was not directly affected by the high fat diet and was unrelated to VEGF serum concentrations and SLC2A1 gene expression in AT. Improvements in measures of learning, delayed recall, and consolidation memory were most likely affected by the repetitive testing. The large variation of basal expression levels of SLC2A1 and VEGF apparently did not translate into differences in these tests in healthy and non-obese subjects. However, after stratification for rs9472159 genotypes, we observed significantly declined consolidation memory scores in carriers of the polymorphism (AA) compared to non- or heterozygous carriers (CC/CA). Consolidation memory score is generally considered to represent a quite reliable measure of delayed memory function [10], [11], [13], [14]. Homozygous carriers of the polymorphism (AA) showed the lowest concentrations of serum VEGF and highest levels of GLUT1 gene expression in AT. The observation therefore warrants further investigation and the availability of a single SNP facilitates testing in more adequate larger cohorts. Notably, it was shown that under insulin-induced hypoglycemia, increases in serum VEGF were significantly correlated with preserved cognitive performance in healthy man [29]. Furthermore, low VEGF has been identified as a biomarker for Alzheimer's disease in cerebrospinal fluid [30].
We attempted to identify a possible source of the elevated serum VEGF levels. The absence of any correlation of circulating VEGF with its expression in circulating PBMCs, monocytes or adipose tissue suggests that other sources might be involved. Jais and coworkers established a myeloid source in mice using genetic deletion and suggested that perivascular macrophages might provide the increased VEGF [7]. The same may apply in humans. A difference remains that we did not observe a rapid upregulation of VEGF upon the introduction of high fat intake, which may imply a slower regulation of the system in humans. Our intervention was relatively modest since we used an isocaloric high fat diet, which induces mild insulin resistance in contrast to paradigms of hypercaloric high fat diets which very rapidly elicit massive insulin resistance [31] and also corresponds to the dietary approach in the animal experiments. Moreover, responses may be more pronounced in obese subjects.
In summary, our data indicate VEGF as a determining factor linking dietary fat intake to cognitive function in humans.
Author contributions
R.S. researched data and substantially contributed to analysis and interpretation of the data. N.S., M.A.O., V.W., A.F., A.B., A.J., J.C.B., and T.F. contributed to data acquisition and evaluation. S.H. and M.K. contributed to study design, subject recruitment and data collection. A.F.H.P. conceived, designed and supervised the NUGAT study and significantly contributed to data interpretation and critical review of the manuscript. R.S. and A.F.H.P. drafted the manuscript, which was critically reviewed by all authors.
Funding
The NUGAT study was funded by the German Federal Ministry of Education and Research (BMBF; grant no. 0315424). The BMBF had no role neither in designing the study nor with respect to analysis or interpretation of the data.
Acknowledgements
The authors thank all study participants for their cooperation. The authors also wish to acknowledge Katrin Sprengel, Andrea Borchert, and Tanja Ahrens for their excellent technical assistance.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.02.004.
Conflict of interest
None.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Vogt M.C., Bruning J.C. CNS insulin signaling in the control of energy homeostasis and glucose metabolism - from embryo to old age. Trends in Endocrinology and Metabolism. 2013;24:76–84. doi: 10.1016/j.tem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Williamson R., McNeilly A., Sutherland C. Insulin resistance in the brain: an old-age or new-age problem? Biochemical Pharmacology. 2012;84:737–745. doi: 10.1016/j.bcp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Thaler J.P., Yi C.X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O. Obesity is associated with hypothalamic injury in rodents and humans. Journal of Clinical Investigation. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benoit S.C., Kemp C.J., Elias C.F., Abplanalp W., Herman J.P., Migrenne S. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. Journal of Clinical Investigation. 2009;119:2577–2589. doi: 10.1172/JCI36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Pascual J.M., Yang H., Engelstad K., Mao X., Cheng J. A mouse model for Glut-1 haploinsufficiency. Human Molecular Genetics. 2006;15:1169–1179. doi: 10.1093/hmg/ddl032. [DOI] [PubMed] [Google Scholar]
- 6.Winkler E.A., Nishida Y., Sagare A.P., Rege S.V., Bell R.D., Perlmutter D. GLUT1 reductions exacerbate Alzheimer's disease vasculo-neuronal dysfunction and degeneration. Nature Neuroscience. 2015;18:521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jais A., Solas M., Backes H., Chaurasia B., Kleinridders A., Theurich S. Myeloid-cell-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell. 2016;166:1338–1340. doi: 10.1016/j.cell.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Choi S.H., Ruggiero D., Sorice R., Song C., Nutile T., Vernon Smith A. Six novel loci associated with circulating VEGF levels identified by a meta-analysis of genome-wide association studies. PLoS Genetics. 2016;12:e1005874. doi: 10.1371/journal.pgen.1005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debette S., Visvikis-Siest S., Chen M.H., Ndiaye N.C., Song C., Destefano A. Identification of cis- and trans-acting genetic variants explaining up to half the variation in circulating vascular endothelial growth factor levels. Circulation Research. 2011;109:554–563. doi: 10.1161/CIRCRESAHA.111.243790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witte A.V., Fobker M., Gellner R., Knecht S., Floel A. Caloric restriction improves memory in elderly humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witte A.V., Kerti L., Margulies D.S., Floel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. Journal of Neuroscience. 2014;34:7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuler R., Osterhoff M.A., Frahnow T., Seltmann A.C., Busjahn A., Kabisch S. High-saturated-fat diet increases circulating angiotensin-converting enzyme, which is enhanced by the rs4343 polymorphism defining persons at risk of nutrient-dependent increases of blood pressure. Journal of the American Heart Association. 2017;6 doi: 10.1161/JAHA.116.004465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helmstaedter C., Kurthen M. Memory and epilepsy: characteristics, course, and influence of drugs and surgery. Current Opinion in Neurology. 2001;14:211–216. doi: 10.1097/00019052-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Lezak M.D. 4th ed. Oxford University Press; New York: 2004. Neuropsychological assessment. [Google Scholar]
- 15.Stathopoulou M.G., Bonnefond A., Ndiaye N.C., Azimi-Nezhad M., El Shamieh S., Saleh A. A common variant highly associated with plasma VEGFA levels also contributes to the variation of both LDL-C and HDL-C. Journal of Lipid Research. 2013;54:535–541. doi: 10.1194/jlr.P030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalmijn S., Launer L.J., Ott A., Witteman J.C., Hofman A., Breteler M.M. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Annals of Neurology. 1997;42:776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 17.Laitinen M.H., Ngandu T., Rovio S., Helkala E.L., Uusitalo U., Viitanen M. Fat intake at midlife and risk of dementia and Alzheimer's disease: a population-based study. Dementia and Geriatric Cognitive Disorders. 2006;22:99–107. doi: 10.1159/000093478. [DOI] [PubMed] [Google Scholar]
- 18.Morris M.C., Evans D.A., Bienias J.L., Tangney C.C., Bennett D.A., Aggarwal N. Dietary fats and the risk of incident Alzheimer disease. Archives of Neurology. 2003;60:194–200. doi: 10.1001/archneur.60.2.194. [DOI] [PubMed] [Google Scholar]
- 19.Morris M.C., Tangney C.C. Dietary fat composition and dementia risk. Neurobiology of Aging. 2014;35(Suppl 2):S59–S64. doi: 10.1016/j.neurobiolaging.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelhardt S., Patkar S., Ogunshola O.O. Cell-specific blood-brain barrier regulation in health and disease: a focus on hypoxia. British Journal of Pharmacology. 2014;171:1210–1230. doi: 10.1111/bph.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson A.L., Pessin J.E. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annual Review of Nutrition. 1996;16:235–256. doi: 10.1146/annurev.nu.16.070196.001315. [DOI] [PubMed] [Google Scholar]
- 22.Pardridge W.M., Boado R.J., Farrell C.R. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative western blotting and in situ hybridization. Journal of Biological Chemistry. 1990;265:18035–18040. [PubMed] [Google Scholar]
- 23.Mani N., Khaibullina A., Krum J.M., Rosenstein J.M. Activation of receptor-mediated angiogenesis and signaling pathways after VEGF administration in fetal rat CNS explants. Journal of Cerebral Blood Flow and Metabolism. 2003;23:1420–1429. doi: 10.1097/01.WCB.0000090620.86921.9C. [DOI] [PubMed] [Google Scholar]
- 24.Sone H., Deo B.K., Kumagai A.K. Enhancement of glucose transport by vascular endothelial growth factor in retinal endothelial cells. Investigative Ophthalmology and Visual Science. 2000;41:1876–1884. [PubMed] [Google Scholar]
- 25.Pekala P., Marlow M., Heuvelman D., Connolly D. Regulation of hexose transport in aortic endothelial cells by vascular permeability factor and tumor necrosis factor-alpha, but not by insulin. Journal of Biological Chemistry. 1990;265:18051–18054. [PubMed] [Google Scholar]
- 26.Berrahmoune H., Herbeth B., Lamont J.V., Masson C., Fitzgerald P.S., Visvikis-Siest S. Heritability for plasma VEGF concentration in the Stanislas family study. Annals of Human Genetics. 2007;71:54–63. doi: 10.1111/j.1469-1809.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- 27.Fehm H.L., Kern W., Peters A. The selfish brain: competition for energy resources. Progress in Brain Research. 2006;153:129–140. doi: 10.1016/S0079-6123(06)53007-9. [DOI] [PubMed] [Google Scholar]
- 28.Schuler R., Osterhoff M.A., Frahnow T., Mohlig M., Spranger J., Stefanovski D. Dietary fat intake modulates effects of a frequent ACE gene variant on glucose tolerance with association to type 2 diabetes. Scientific Reports. 2017;7:9234. doi: 10.1038/s41598-017-08300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dantz D., Bewersdorf J., Fruehwald-Schultes B., Kern W., Jelkmann W., Born J. Vascular endothelial growth factor: a novel endocrine defensive response to hypoglycemia. Journal of Clinical Endocrinology and Metabolism. 2002;87:835–840. doi: 10.1210/jcem.87.2.8215. [DOI] [PubMed] [Google Scholar]
- 30.Leung Y.Y., Toledo J.B., Nefedov A., Polikar R., Raghavan N., Xie S.X. Identifying amyloid pathology-related cerebrospinal fluid biomarkers for Alzheimer's disease in a multicohort study. Alzheimer's & Dementia: diagnosis. Assessment & Disease Monitoring. 2015;1:339–348. doi: 10.1016/j.dadm.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brons C., Jensen C.B., Storgaard H., Hiscock N.J., White A., Appel J.S. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. The Journal of Physiology. 2009;587:2387–2397. doi: 10.1113/jphysiol.2009.169078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.