Abstract
BACKGROUND
Reirradiation for locoregionally recurrent nasopharyngeal carcinoma (NPC) after a definitive dose of radiotherapy (RT) is challenging and usually associated with severe toxicities. Intensity‐modulated carbon ion RT (IMCT) offers physical/biologic advantages over photon‐based intensity‐modulated RT. Herein, the authors report their initial experience of IMCT in previously irradiated patients with locoregionally recurrent NPC.
METHODS
Patients with locoregionally recurrent, poorly differentiated or undifferentiated NPC who underwent salvage therapy with IMCT at the Shanghai Proton and Heavy Ion Center between May 2015 and August 2017 were included in the current study. The IMCT doses were 50 to 66 Gray equivalent (GyE) (2.0‐3.0 GyE/daily fraction), delivered via raster scanning technology. The 1‐year overall survival, disease‐specific survival, progression‐free survival (PFS), local recurrence‐free survival, regional recurrence‐free survival, and distant metastasis‐free survival were calculated. Univariate and multivariate analyses of PFS were performed to identify possible predictive factors.
RESULTS
Among the 75 patients included, 4 patients, 14 patients, 29 patients, and 28 patients, respectively, had recurrent American Joint Committee on Cancer stage I, stage II, stage III, and stage IVA/B disease. With a median follow‐up of 15.4 months (range, 2.6‐29.7 months), the 1‐year overall survival, disease‐specific survival, PFS, local recurrence‐free survival, regional recurrence‐free survival, and distant metastasis‐free survival rates were 98.1%, 98.1%, 82.2%, 86.6%, 97.9%, and 96.2%, respectively. A higher fraction size of 3 GyE (vs <3 GyE) or a higher biological equivalent dose significantly improved the PFS rate on univariate analysis, but not on multivariate analysis. No patient developed acute toxicity of grade ≥2 during IMCT. Late treatment‐induced severe (grade 3 or 4) toxicities were infrequent, but included mucosal necrosis (9.3%), xerostomia (1.3%), and temporal lobe necrosis (1.3%).
CONCLUSIONS
This initial experience in the first 75 patients with locoregionally recurrent NPC was encouraging. Carbon ion RT could provide promising survival rates with infrequent severe toxicities for patients with locoregionally recurrent NPC. Cancer 2018;124:2427‐37. © 2018 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Keywords: intensity‐modulated carbon ion radiotherapy, recurrent nasopharyngeal carcinoma, reirradiation, survival, toxicity
Short abstract
Reirradiation for locoregionally recurrent nasopharyngeal carcinoma after a definitive dose of radiotherapy is challenging and usually associated with severe toxicities. Carbon ion radiotherapy provides promising short‐term survival rates for patients with locoregionally recurrent nasopharyngeal carcinoma, with few treatment‐induced severe adverse effects noted.
INTRODUCTION
To the best of our knowledge, radiotherapy (RT) is the only curative modality for patients with nonmetastatic nasopharyngeal cancer (NPC). The prevailing use of photon‐based intensity‐modulated RT (IMRT) and the concurrent chemoradiotherapy strategy have significantly improved treatment outcomes.1, 2, 3 Nevertheless, locoregional disease recurrence after IMRT remains a major mode of treatment failure. Approximately 10% to 15% of patients with NPC will fail locoregionally after receiving definitive treatment.4
Reirradiation remains the most important modality for salvaging patients with locoregionally recurrent NPC (LR‐NPC). To our knowledge, surgery, stereotactic radiosurgery, and brachytherapy are effective only in patients with disease with limited recurrent tumor volume,5, 6 and IMRT currently is the most effective method for salvaging patients with LR‐NPC of any stage, including more extensive disease. However, reirradiation with IMRT to a definitive dose is associated with a considerable risk of severe complications, including mucosal necrosis, massive bleeding, cranial neuropathy, and temporal lobe necrosis.7, 8, 9, 10 Limited efficacy and a high probability of fatal complications limit the 2‐year overall survival (OS) rate for patients with recurrent (r) T3/T4 LR‐NPC to only approximately 40%.10
Accelerated beams of heavy, charged particles and protons have a finite range and a distant Bragg peak.11 Dosimetry studies have shown that carbon ion RT (CIRT) enables the delivery of high‐dose RT to the target volume(s) while sparing organs at risk (OAR), thereby enhancing the therapeutic ratio over IMRT in patients with head and neck cancer.12 Early data also have suggested that the use of proton or heavy ion (noncarbon) RT for patients with LR‐NPC is feasible and potentially effective.13, 14
Carbon ions have a relative biologic effectiveness ratio of 2 to 5:1 compared with photons and protons.15, 16 It is reasonable to postulate that the more precise intensity‐modulated CIRT (IMCT) used to salvage patients with LR‐NPC who failed prior courses of high‐dose RT may provide improved disease control and an improved toxicity profile compared with photon‐based IMRT due to the physical and biological characteristics of the carbon ion beam. However, to the best of our knowledge, the effectiveness and potential benefits in the toxicity profile of IMCT in the treatment of patients with LR‐NPC have never been addressed clinically. The objective of the current study was to present the initial results in terms of early efficacy and toxicity profiles among a group of patients with LR‐NPC who were treated prospectively according to our ongoing institutional protocols with IMCT.
METHODS AND MATERIALS
Patients and Pretreatment Evaluation
All patients diagnosed with LR‐NPC who were previously treated with and reirradiated with IMCT >6 months after the completion of definitive photon‐based RT were treated prospectively with IMCT (raster scanning technology) using either a standardized treatment protocol or 1 of the 3 dose‐escalating phase 1/2 clinical trial protocols at the Shanghai Proton and Heavy Ion Center (SPHIC).17, 18
All protocols were registered to the institutional review board (IRB) of the SPHIC. IRB approvals were obtained for the 3 projects as well as the treatment protocol involved herein. All mentioned clinical trials were performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from all patients who were treated on the research protocols.
Required pretreatment baseline evaluations consisted of a complete history and physical examination, nasopharyngoscopy, complete blood counts, serum electrolyte tests, liver and renal function tests, electrocardiogram, and urinalysis. Magnetic resonance imaging (MRI) of the head and neck and whole‐body positron emission tomography (PET)‐computed tomography (CT) were mandatory for all patients to evaluate the extent of disease, unless clinically contraindicated. Patients who presented with distant metastasis were eligible if reirradiation was deemed beneficial for the patient's outcome, and those who had regional lymphadenopathy were required to be evaluated for potential neck dissection. The protocols also required that all patients were restaged according to the seventh edition of the American Joint Committee on Cancer staging classification for their recurrent disease.
Intensity‐Modulated CIRT
All patients were immobilized in the supine position with thermoplastic masks. CT simulation without intravenous contrast from the vertex to the inferior margin of the clavicular heads was performed at a 1.5‐mm cut. MRI‐CT fusion was performed for all patients planned.
The gross (macroscopic) tumor volume (GTV) of the primary site and neck included all disease noted on CT, PET‐CT, and MRI. The clinical target volumes (CTVs) of both the GTV of the primary site and neck were designed to include 5 mm beyond the GTV for microscopic extension (limited to as little as 1 mm near OAR), and a variable margin for occult tumor spread. An additional 3‐mm to 6‐mm margin was added to the CTV to create the planning target volume to allow for setup variability and uncertainty with regard to dose distribution. For patients whose tumors shrunk after chemotherapy, the prechemotherapy tumor volumes were defined universally as subclinical disease and were included in the CTV. Prophylactic irradiation to any uninvolved regions regardless of the probability of disease involvement was not administered. OAR required for all patients were defined according to the following priorities: brainstem, spinal cord, optic nerves/chiasm, temporal lobes, pituitary gland, eyes (including lens), temporomandibular joints, and parotid glands. Recovery from a previous IMRT dose was set at 70%, based on the radiobiological conclusions of Nieder et al,19 regardless of the latent time between the 2 courses of RT. The dose constraints of the OAR were based on TD5/5 (5% chance of injury showing up over the next 5 years) described by Emami,20 except for the optic nerve (dose to 20% of the volume (D20)<30 Gray equivalent [GyE]), brainstem (maximum dose <45 GyE), spinal cord (maximum dose <30 GyE), and temporal lobes (V40 <7.66 cm2; V50 <4.66 cm2), which were based on previous experience from the National Institute of Radiological Sciences in Japan.21
Treatment planning for IMCT was performed using the Syngo treatment planning software system (versions VC11 and VC13; Siemens Healthcare, Erlangen, Germany).
The protocols for patients with LR‐NPC differ with regard to the total dose and fractionation used. The dose/fractionation of the pilot study used 50 to 66 GyE (2.0 GyE/daily fraction, 2.5 GyE/daily fraction, or 3.0 GyE/daily fraction, 5 days/week). The doses of the two phase 1/2 dose escalation trials (with or without concurrent chemotherapy) started at 55 GyE and 57.5 GyE (2.5 GyE/day, 5 days/week), respectively. The third dose escalation trial started at a dose of 54 GyE (3 GyE/day, 5 days/week) prescribed to the macroscopic disease. Treatments typically consisted of 2 to 3 beams. Individual factors such as reproducibility of patient positioning and/or beam angles were chosen for optimal dosimetry. A treatment plan for a patient with local disease recurrence in the postnasal space only is illustrated in Figure 1.
Figure 1.

A typical intensity‐modulated carbon ion radiotherapy treatment plan for a patient with rT3N0M0 locally recurrent nasopharyngeal carcinoma.
All patients were examined on a weekly basis for potential adverse events. Weekly CT scans of the head and neck were performed as per protocol requirement after week 2 of IMCT to ensure the consistency of the body contour and the dose distribution because any changes in the air/fluid composition in the sinuses or body anatomy may significantly alter the dose distribution of IMCT.
Chemotherapy
Induction chemotherapy was encouraged for all patients with locoregionally advanced recurrent disease, but no particular regimen was required by any of the protocols. Concurrent chemotherapy during IMCT was not encouraged except for patients enrolled into the phase 1/2 dose escalating study receiving concurrent IMCT plus chemotherapy. Adjuvant chemotherapy was not encouraged.
Follow‐Up
All patients were required to be followed by their primary radiation oncologist within 4 to 6 weeks after the completion of IMCT, every 3 months within the first 2 years, every 6 months in the following 3 years, and then annually thereafter. Complete history and physical examinations, nasopharyngoscopy examinations, and MRI scans of the head and neck were required at each follow‐up session, with the first scan performed during the last week of IMCT. PET‐CT was optional and was ordered at the discretion of the radiation oncologist.
Data Analysis
The duration of OS was calculated from the time of diagnosis of locoregional recurrence until death or the date of the last follow‐up visit for patients still alive at the time of last follow‐up. The duration of time to local, regional, or distant failure was measured from the time of diagnosis of disease recurrence until documented treatment failure.
The responses to treatment were based on the results of imaging studies such as MRI performed during the last week of IMCT and at each follow‐up session after the completion of salvage IMCT using the Response Evaluation Criteria In Solid Tumors (RECIST) (version 1.1).22Adverse events that occurred within 90 days after the initiation of IMCT were described as “acute,” and were measured using the Common Terminology Criteria for Adverse Events (version 4.03). Long‐term treatment‐induced side effects, which were defined as symptoms first occurring 90 days after or lasting >90 days after the initiation of IMCT, were graded according to the Radiation Therapy Oncology Group criteria.23 Univariate analysis regarding progression‐free survival (PFS) was performed using both the Kaplan‐Meier method (with the log‐rank test) and the univariate Cox proportional hazards model, whereas the Cox proportional hazards model was used for multivariate analysis of PFS. Potential prognostic factors, including sex, age, time to disease recurrence, stage of the primary tumor and cervical lymph nodes, receipt of either induction or concurrent chemotherapy, GTV dose, fractionation, and biological equivalent dose (BED), were included in the multivariate analysis. The total dose (to the GTV) and fractionation as well as the BED were analyzed separately in multivariate analysis. P values <.05 were considered to be statistically significant. All statistical analyses were performed using R statistical software (version 3.4.1; R Foundation, Vienna, Austria).
RESULTS
Study Population
A total of 75 consecutive and nonselected patients with LR‐NPC (poorly differentiated or undifferentiated squamous cell carcinoma [SCC]) were treated at SPHIC between May 2015 and August 2017. Locoregional disease recurrences were diagnosed histologically or clinically using repeated MRI and PET‐CT scans. No patient presented with distant metastasis. Among these patients, 9 presenting with both local and cervical lymph node disease recurrence underwent neck dissection before IMCT, and 3 patients underwent neck dissection after IMCT. The dose of the initial RT to the GTV of the primary site and lymphadenopathy ranged from 66 to 76 Gy in 28 to 38 fractions among all analyzed patients.
The characteristics of the patients and their disease are detailed in Table 1.
Table 1.
Characteristics of All Patients and Their Disease
| Characteristics | No. (%) |
|---|---|
| Total | 75 (100%) |
| Sex | |
| Male | 54 (72%) |
| Female | 21 (28%) |
| Age at time of locoregional disease recurrence, y | |
| Median (range) | 48 (17‐70) |
| ≤50 | 40 (53.33%) |
| >50 | 35 (46.67%) |
| Initial radiation dose, Gy | |
| Median (range) | 70 (66‐75.75) |
| AJCC stage of disease at time of initial diagnosis | |
| I and II | 17 (22.67%) |
| III and IVA/B | 58 (77.33%) |
| Initial radiotherapy technique (IMRT) | |
| IMRT | 72 (96%) |
| Non‐IMRT | 3 (4%) |
| Time to disease recurrence | |
| Median (range), mo | 28.70 (7.36‐214.95) |
| ≤2 y | 31 (41.33%) |
| >2 y | 44 (58.67%) |
| AJCC stage of disease at time of disease recurrence | |
| I and II | 18 (24%) |
| III and IVA/B | 57 (76%) |
| Recurrent T/N classification | |
| rT0 (retropharyngeal lymph node positive) | 4 (5.33%) |
| rT1 | 5 (6.67%) |
| rT2 | 11 (14.67%) |
| rT3 | 28 (37.33%) |
| rT4 | 27 (36%) |
| rN+ | 22 (29.33%) |
| Interval between diagnosis of NPC recurrence and re‐IMCT, mo | |
| Median (range) | 2 (0‐8) |
| Interval between first RT and re‐IMCT, mo | |
| Median (range) | 29 (11‐216) |
| Total dose of re‐IMCT, GyE | |
| Median (range) | 57.5 (50‐66) |
| <60 | 39 (52%) |
| ≥60 | 36 (48%) |
| Fractionation of re‐IMCT, GyE | |
| Median (range) | 3 |
| 2 | 3 (4%) |
| 2.5 | 29 (38.67) |
| 3 | 43 (57.33%) |
| Induction chemotherapy | |
| TP | 19 (25.33%) |
| GP | 17 (22.67%) |
| Others | 10 (13.33%) |
| No induction chemotherapy | 29 (38.67%) |
| Concurrent chemotherapy | |
| Cisplatin (wkly dose at 40 mg/m2) | 8 (10.67%) |
| Cisplatin (3‐wk dose at 80 mg/m2) | 4 (5.33%) |
| No concurrent chemotherapy | 63 (84%) |
Abbreviations: AJCC, American Joint Committee on Cancer; GP, gemcitabine plus cisplatin; Gy, gray; GyE, Gray equivalent; IMCT, intensity‐modulated carbon ion radiotherapy; IMRT, intensity‐modulated radiotherapy; NPC, nasopharyngeal carcinoma; RT, radiotherapy; TP, docetaxel plus cisplatin.
With the exception of 1 patient, all patients completed their planned treatments without a treatment break for any reason. One patient declined planned further IMCT after a total dose of 50 GyE (the planned total dose was 54 GyE at 2.0 GyE/daily fraction) due to concerns regarding side effects, although no side effects were observed during IMCT.
A total of 46 patients received 1 to 6 cycles of induction chemotherapy consisting of docetaxel plus cisplatin, gemcitabine plus cisplatin, or 5‐fluorouracil plus cisplatin before IMCT. Twelve patients received concurrent cisplatin (8 with a weekly dose of 40 mg/m2 and 4 patients with a 3‐weekly dose of 80 mg/m2); of these, 6 patients were in the IMCT plus concurrent chemotherapy protocol. One patient who did not receive induction or concurrent chemotherapy because he was recovering from a cerebrovascular accident received adjuvant chemotherapy.
OS, Local, Regional and Distant Failure, and PFS
All patients were followed according to the planned schedule. At a median follow‐up of 15.4 months (range, 2.6‐29.7 months), 2 patients had died. One patient developed mucosal necrosis 3 months after the completion of salvage IMCT and subsequently died of massive hemorrhage 4 months thereafter. Another patient had experienced no moderate or severe acute or late adverse events and died of a cardiovascular accident unrelated to LR‐NPC or retreatment at 16 months after her diagnosis (or 12 months after the completion of salvage IMCT). The 1‐year OS rate and disease‐specific survival (DSS) rate both were 98.1% (95% confidence interval [95% CI], 94.4%‐100%) (Fig. 2).
Figure 2.
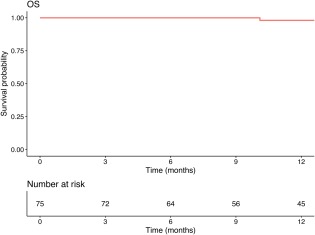
The 1‐year overall survival (OS) rate for patients with locoregionally recurrent nasopharyngeal carcinoma treated with intensity‐modulated carbon ion radiotherapy.
At the time of the current analysis, 21 patients, 1 patient, and 2 patients, respectively, developed local, regional, and distant failure. The 1‐year PFS, local recurrence‐free survival (LRFS), regional recurrence‐free survival (RRFS), and distant metastasis‐free survival (DMFS) rates were 82.2% (95% CI, 72.8%‐92.9%), 86.6% (95% CI, 77.8%‐96.4%), 97.9% (95% CI, 94.0%‐100%), and 96.2% (95% CI, 91.2%‐100%), respectively (Fig. 3).
Figure 3.

The 1‐year (A) progression‐free survival (PFS), (B) local recurrence‐free survival (LRFS), (C) regional recurrence‐free survival (RRFS), and (D) distant metastasis‐free survival (DMFS) rates of patients with locoregionally recurrent nasopharyngeal carcinoma treated with intensity‐modulated carbon ion radiotherapy.
Among the patients who developed local recurrence or disease progression after IMCT reirradiation, 3 patients were recruited into a phase 2/3 trial (chemotherapy with radiosensitization) at SPHIC. The only patient who developed neck disease recurrence alone (out of the IMCT field) was salvaged successfully with surgery. All other patients who developed local or distant disease recurrence were treated with salvage chemotherapy, including 7 patients who also underwent nasopharyngectomy.
Acute and Late Adverse Events
Reirradiation with IMCT after a definitive dose of IMRT for LR‐NPC was found to be well tolerated. None of the 75 patients developed grade ≥2 reirradiation‐induced toxicity during or within 90 days after the initiation of IMCT. The only observed acute adverse events associated with IMCT were grade 1 erythema of the nasopharyngeal/oropharyngeal mucosa in 9 patients. No patient experienced persistent alopecia or skin erythema.
Seven patients developed necrosis at the tumor bed after 3 months of follow‐up. However, at the time of the onset of tumor bed necrosis, no necrosis or erythema was observed in the adjacent mucosa. One of the 7 patients who was not salvaged with debridement subsequently died of massive hemorrhage (grade 5) at 10 months after the diagnosis of disease recurrence (or 7 months after the completion of IMCT). Late toxicity profiles are detailed in Table 2.
Table 2.
Type and Frequency of Late Toxicitiesa
| Grade 1 or 2 | ≥Grade 3 | |
|---|---|---|
| Toxicity | No. of Patients (%) | No. of Patients (%) |
| Nasopharyngeal mucositisb | 0 | 7 (9.3%)b |
| Temporal lobe necrosis | 7 (9.3%) | 1 (1.3%) |
| Xerostomia | 1 (1.3%) | 1 (1.3%) |
| Hearing loss | 1 (1.3%) | 0 |
| Cranial nerve neuropathy | 1 (1.3%) | 0 |
Toxicities were graded according to the Radiation Therapy Oncology Group criteria.
All necrosis initiated at the tumor bed without evidence of mucosal necrosis or erythema. One patient died of hemorrhage (grade 5).
Predictive Factors for Local and Regional Disease Control
Using both univariate and multivariate analyses, we assessed the value of all significant predictive factors for local and regional disease control previously reported in the literature for LR‐NPC after reirradiation using IMRT excluding tumor volume. These potential prognosticators included age, sex, stage of disease at the time of disease recurrence, time to disease recurrence, use of chemotherapy (induction and/or concurrent chemotherapy), dose of salvage IMCT to the GTV, fractionation (dose/fraction), and BED. Because only 2 patients in the current study cohort had died and only 1 patient had died of disease/treatment‐associated factors, analysis for predictive indicators of OS or DSS was not performed.
On univariate analyses using the log‐rank test (Table 3), the fraction size (<3 GyE vs 3 GyE) and BED (<74.1 GyE vs ≥74.1 GyE) were found to be significant for the prediction of PFS (P =.027 and .035, respectively) (Fig. 4). Cox regression analysis was used to examine the factors on univariate and multivariate analyses of PFS (Tables 4 and 5). Both fraction size (hazard ratio, 0.27; 95% CI, 0.08‐0.94 [P =.039]) and BED (hazard ratio, 0.91; 95% CI, 0.94‐0.99 [P =.031]) were found to be significantly associated with PFS on univariate analyses. No significant association was observed on multivariate analyses.
Table 3.
Univariate Analysis by the Kaplan‐Meier Methoda
| Characteristic | PFS | LRFS | RRFS | DMFS |
|---|---|---|---|---|
| Sex | 0.15 | 0.059 | 0.56 | 0.42 |
| Age (≤50 y vs >50 y) | 0.37 | 0.70 | 0.38 | 0.12 |
| Time to disease recurrence (≤2 y vs >2 y) | 0.23 | 0.37 | 0.30 | 0.18 |
| T classification (T1/2 vs T3/4) | 0.38 | 0.59 | 0.50 | 0.49 |
| N classification (N+ vs N‐) | 0.14 | 0.12 | 0.56 | 0.42 |
| Chemotherapyb | 0.13 | 0.43 | 0.50 | 0.51 |
| GTV dose (<60 GyE vs ≥60 GyE) | 0.19 | 0.30 | 0.61 | 0.37 |
| Fractionation (<3 GyE vs 3 GyE) | 0.027 | 0.058 | 0.48 | 0.24 |
| BED (<74.1 GyE vs ≥74.1 GyE)c | 0.035 | 0.10 | 0.50 | 0.26 |
Abbreviations: BED, biological equivalent dose; DMFS, distant metastasis‐free survival, GTV, gross (macroscopic) tumor volume; GyE, Gray equivalent; LRFS, local recurrence‐free survival; PFS, progression‐free survival; RRFS, regional recurrence‐free survival.
Bold type indicates statistical significance.
Induction and/or concurrent chemotherapy.
The median BED was 74.1 GyE.
Figure 4.
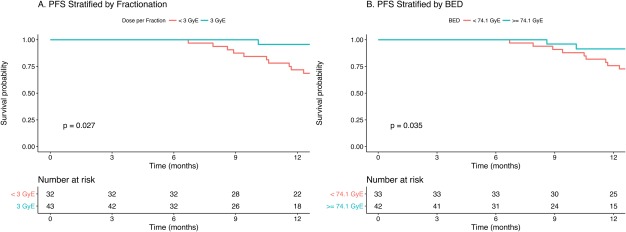
Progression‐free survival (PFS) of patients with locoregionally recurrent nasopharyngeal carcinoma treated with intensity‐modulated carbon ion radiotherapy stratified by (A) fractionation or (B) biological equivalent dose (BED). GyE, Gray equivalent.
Table 4.
Univariate Analysis of PFS by Cox Regression
| Variable | Univariate Analysis | |
|---|---|---|
| HR (95% CI) | P a | |
| Sex (female as referent) | ||
| Male | 0.53 (0.22‐1.28) | .15 |
| Age, y | 0.99 (0.95‐1.03) | .55 |
| Time to disease recurrence, y | 1.23 (1.03‐1.46) | .023 |
| T classification (T1/2 as referent) | ||
| T3/4 | 1.62 (0.55‐4.83) | .38 |
| N classification (N‐ as referent) | ||
| N+ | 0.41 (0.12‐1.39) | .15 |
| Chemotherapy (no chemotherapy as referent) | ||
| With chemotherapy | 2.25 (0.76‐6.68) | .14 |
| GTV dose, GyE | 0.90 (0.80‐1.02) | .095 |
| Fractionation (<3 GyE as referent) | ||
| 3 GyE | 0.27 (0.08‐0.94) | .039 |
| BED, GyEb | 0.91 (0.84‐0.99) | .031 |
Abbreviations: 95% CI, 95% confidence interval; BED, biological equivalent dose; GTV, gross (macroscopic) tumor volume; GyE, Gray equivalent; HR, hazard ratio; PFS, progression‐free survival.
Bold type indicates statistical significance.
The BED was analyzed as a continuous variable.
Table 5.
Multivariate Analyses of PFS
| Variable | Fractionation and Total Dose Included | BED Included | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Sex (female as referent) | ||||
| Male | 0.59 (0.20‐1.71) | .33 | 0.67 (0.24‐1.85) | .44 |
| Age, y | 0.96 (0.92‐1.01) | .13 | 0.97 (0.93‐1.02) | .20 |
| Time to recurrence, y | 1.14 (0.90‐1.44) | .29 | 1.16 (0.93‐1.44) | .19 |
| T classification (T1/2 as referent) | ||||
| T3/4 | 0.86 (0.26‐2.86) | .80 | 0.84 (0.25‐2.80) | .78 |
| N classification (N‐ as referent) | ||||
| N+ | 0.37 (0.09‐1.48) | .16 | 0.34 (0.09‐1.36) | .13 |
| Chemotherapy (no chemotherapy as referent) | ||||
| With chemotherapy | 1.42 (0.39‐5.24) | .60 | 1.80 (0.51‐6.29) | .36 |
| GTV dose, GyE | 0.96 (0.85‐1.09) | .55 | NA | NA |
| Fractionation (<3 GyE as referent) | ||||
| 3 GyE | 0.32 (0.08‐1.23) | .097 | NA | NA |
| BED, GyE | NA | NA | 0.94 (0.86‐1.03) | .17 |
Abbreviations: 95% CI, 95% confidence interval; BED, biological equivalent dose; GTV, gross (macroscopic) tumor volume; GyE, Gray equivalent; HR, hazard ratio; NA, not applicable; PFS, progression‐free survival.
DISCUSSION
In the current study, we analyzed the 1‐year treatment outcomes for the first 75 consecutive patients with LR‐NPC (poorly or undifferentiated SCC) who failed locoregionally after definitive RT and were salvaged with IMCT at SPHIC. To the best of our knowledge, the current study is the first to examine the treatment of patients with LR‐NPC using CIRT. At the time of this analysis, all patients with the exception of 2 were alive. One patient died of massive hemorrhage 7 months after the completion of IMCT and another died of an interconcurrent cardiovascular incident unrelated to recurrent NPC. With a median follow‐up of 15.4 months, the OS, DSS, PFS, LRFS, RRFS, and DMFS rates at 1 year were 98.1%, 98.1%, 82.2%, 86.6%, 97.9%, and 96.2%, respectively. No patient developed acute toxicity of grade ≥2 during IMCT. Late treatment‐induced severe (grade 3 or 4) toxicities were infrequent, and included xerostomia (1 patient) and temporal lobe necrosis (1 patient). Seven patients developed necrosis at the tumor bed but had no evidence of a severe mucosal reaction at the onset of the analysis. Only 1 patient who did not receive sufficient debridement subsequently died of massive hemorrhage (grade 5 toxicity). Although the median follow‐up of 15.4 months is relatively short, the outcomes of patients in the current study, including the short‐term survival data and the absence of acute serious adverse events (SAEs) and infrequently observed late SAEs, are encouraging. This is especially apparent given that in previous reports regarding salvage treatments for patients with LR‐NPC in which IMRT or conventional RT was used, the median time to the development of SAEs was reported to be approximately 6 months, and the majority of the reported rates of developing SAEs were >50%, while significantly contributing to patient mortality.7, 8, 9, 10, 24 Therefore, the current study data provide early evidence that IMCT may be a potentially superior modality compared with current and traditional treatment approaches for patients with locoregionally recurrent head and neck malignancies who have failed prior high‐dose RT. Not surprisingly, the results of the current study also indicated that fractionation and/or BED could be important predictive factors for PFS after IMCT. On univariate analysis, a dose of 3 GyE per fraction (compared with smaller fraction sizes) and a higher BED were found to significantly improve PFS. However, no significant covariates were detected on multivariate analysis.
To the best of our knowledge, RT is the only curative treatment modality for patients with nonmetastatic NPC. Despite the improved outcome with the prevailing use of IMRT and combined chemoradiotherapy, approximately 10% of patients will fail locally after receiving definitive treatment.4 The treatment of patients with LR‐NPC is challenging, and precision radiation technologies such as stereotactic radiosurgery and brachytherapy can be used only to treat small recurrent foci in the postnasal space, but their application is highly limited by the tumor volume.5, 6 Therefore, IMRT remains the mainstay treatment technology for patients with LR‐NPC. To our knowledge, however, results from all published series to date have demonstrated that retreatment using IMRT is potentially curative for patients with LR‐NPC but at the cost of a high incidence and severity of radiation‐induced toxicities. IMRT doses ranging from 50 to 70 Gy in conventional fractionations have been used for patients with recurrent NPC in those reports; however, to our knowledge, the optimal dose is unknown. Long‐term side effects, especially mucosal necrosis and fatal hemorrhage, after reirradiation using high‐dose IMRT (>66 Gy) for patients with recurrent NPC were considered unacceptable in 2 larger series.8, 9 To our knowledge, according to the largest series published to date regarding IMRT reirradiation for patients with LR‐NPC, 120 of the 239 patients studied died. Approximately 70% of the dead patients died of SAEs such as mucosal necrosis, cranial neuropathy, and/or symptomatic brain necrosis, whereas <30% died of local or distant failure.9 These findings were echoed by a more recently published series of 77 patients (60% with early recurrent stage and 40% with locally advanced recurrent stage NPC) who were treated with reirradiation using IMRT after failing IMRT.10 In essence, although IMRT was the best treatment modality available for locally advanced NPC, it conversely also was the major cause of death in patients undergoing reirradiation.
The majority of the patients included in the above‐mentioned publications developed local recurrence after conventional RT. Tumor volume coverage is considered suboptimal with 2‐dimensional RT, and marginal miss of disease, especially subclinical disease, could occur more frequently compared with IMRT. At the same time, doses to normal nasopharyngeal mucosa surrounding the primary NPC lesion might be uneven. Locoregional recurrence of NPC after high‐dose IMRT, which to our knowledge has been the standard technology for the past decade, represents a new scenario and its management could be more challenging. Recurrence of a typically radiosensitive malignancy such as NPC after highly conformal, high‐dose irradiation appears to select for greater resistance to a second course of photon‐based RT. Normal tissues such as the mucosa, temporal lobes of the brain, cavernous sinus, and brainstem encompassed within the planning target volume for the reirradiation usually have been irradiated intensely in the previous IMRT, especially for patients with locoregionally advanced disease. In a recently analyzed series, Kong et al discovered that patients with NPC who failed initial IMRT and were reirradiated with a second course of IMRT demonstrated particularly poor outcomes. Approximately 40% of patients experienced mucosal necrosis but, more important, approximately 67% of cases of mucosal necrosis occurred within 6 months after the second course of IMRT.10 The majority of dead patients died of treatment toxicities, and the 3‐year OS rate in patients who developed SAEs was 26.1%. Therefore, it is reasonable to postulate that patients with LR‐NPC after definitive IMRT are more prone to develop severe adverse events and are more resistant to reirradiation after a second course of IMRT. Previous results regarding reirradiation with IMRT for patients with LR‐NPC who have failed conventional RT might not be applicable to patients with NPC treated in the modern era, including those in the current series because the majority of patients herein were treated in the IMRT era.
High doses from previous irradiation to the critical organs adjacent to disease foci always limit the doses of reirradiation that are required for disease control. Despite the prevailing use of IMRT for patients with NPC, reirradiation with IMRT to the postnasal space is limited by doses delivered to the brainstem, temporal lobes, optic nerve, and chiasm, as well as the nasopharyngeal mucosa. Therefore, it is important to limit the reirradiation dose to the disease foci only, without overdosing the normal surrounding tissues.
Heavy charged particles such as carbon ion have a finite range and a distant Bragg peak, and have been applied successfully in the treatment of malignancies of the skull base such as chordoma, chondrosarcoma, and meningioma. Early data also have suggested that the use of proton, neon, or helium for the management of patients with locally advanced NPC or the reirradiation of patients with LR‐NPC is feasible and potentially effective.13, 14, 25 In an early study of 16 patients with LR‐NPC who were treated with proton beam RT, Lin et al discovered that a local control rate of 60% can be achieved if a sufficient dose (>60 GyE at 1.8 GyE or 2.0 GyE/daily fraction) can be delivered. With a cumulative dose of 133.5 Gy (range, 110‐148 Gy) for both initial and proton beam reirradiation, only 2 of the 9 surviving patients (22.2%) developed grade 3 or 4 late toxicity (osteonecrosis and mucosal necrosis), and no patient experienced a neurological deficit.13 Neon or helium also were used successfully in the management of patients with LR‐NPC. In a series of 11 patients with NPC who failed initial high‐dose RT, retreatment at the Lawrence Berkeley National Laboratory using helium or neon to a median dose of 50 GyE (range, 31.8‐62.3 GyE) achieved a local control rate of 45% and a median survival of 42 months. No fatal complications were observed.14 It is important to mention that the salvage treatment for all patients included in these 2 small series had no support from modern imaging technologies such as MRI or PET‐CT for diagnosing and defining the recurrent disease foci and ruling out distant metastasis. In fact, some of the patients who received heavy ion RT at the Lawrence Berkeley National Laboratory had distant metastasis at the time of salvage treatment. Under such suboptimal circumstances, we consider the outcome after reirradiation with proton, neon, or helium particles acceptable.
Previous experience has demonstrated that more effective dose distributions in terms of vital tissue sparing can be achieved with IMCT compared with IMRT in patients with head and neck cancer.12 However, to the best of our knowledge, CIRT has never been used in the treatment of patients with LR‐NPC, and there is no literature to date suggesting the proper dose of CIRT to be used for reirradiation in patients with head and neck cancer recurrences. CIRT has a higher linear energy transfer compared with photon or proton RT, and the estimated relative biologic effectiveness of CIRT is 2 to 5 times that of photon RT depending on the irradiated tissues and cells.15, 16 Therefore, it is reasonable to postulate that CIRT is more effective for disease control for patients with photon‐resistant NPC recurrences after previous conventional RT. Nevertheless, to the best of our knowledge, the proper dose of CIRT for patients with NPC who failed initial definitive RT is unknown. Although a dose of ≥60 Gy with photon RT traditionally was suggested for improved control of recurrent SCC of the head and neck area,26, 27 such a dose scheme may or may not be applicable for CIRT.
The initial findings of the current study suggested that reirradiation with IMCT at a dose of 50 to 66 GyE (at 2.0‐3 GyE/daily fraction) is feasible and potentially effective for controlling locoregionally recurrent foci of NPC. With a median follow‐up of 15.4 months, all patients except for 2 were alive, and only 1 patient had died of NPC or a treatment‐related cause. Acute and late adverse events of grade ≥2 were infrequent. These outcomes are highly comparable to those among head and neck cancer patients with base‐of‐skull recurrences who are treated with similar technology and at a similar facility at the Heidelberg Ion Therapy Center. In a recently published series of 18 patients with recurrent skull base diseases of various diagnoses who underwent reirradiation with IMCT, the median dose of 51 GyE (over 17 daily fractions, 7 days/week) was found to be well tolerated.28 Grade 1 or 2 early or late toxicities only were observed in 5 patients. No patient developed grade 3 or 4 early or late toxicities after a median follow‐up of 2 years. Furthermore, in our previously published data regarding reirradiation with IMRT, only 40% of patients had T3/4 lesions, which is substantially less than the rate of 73% reported in the current series.10 However, the 1‐year OS rate of approximately 98% is far superior after IMCT, whereas the PFS rates were similar between the 2 technologies. We attribute such a phenomenon to the superb toxicity profile of IMCT.
The results of univariate analyses indicated that both BED (<74.1 GyE vs ≥74.1 GyE) and fraction size (3 GyE vs <3 GyE) were potential significant prognostic factors for PFS. With limited severe adverse events observed at all CIRT dose levels, it is reasonable to observe an improved outcome with a higher BED and fraction dose. However, patients who received lower total and fraction doses were those who were treated soon after the initiation of the clinical practice at SPHIC. Although target volume delineation had been consistent for all patients, less experience at the beginning of the learning curve might well be a potential confounding factor for less optimal PFS.
The outcomes after IMCT for patients with locally recurrent skull base tumors were exciting, but we are unable to explain the underlying reason for such a low incidence of moderate or severe toxicities. A recently published translational research article studied the effects of ultra‐high dose rate RT in normal and cancer tissues, and discovered that RT delivered in <500 milliseconds could spare normal lung tissue whereas the efficacy against cancer tissue remained unchanged.29 It is possible that the ultra‐high scanning speed of 100 milliseconds per slice of our IONTRIS particle therapy system could play a role in limiting the acute adverse events in this group of patients. Nevertheless, this topic will be one of the foci of radiobiology research at SPHIC. The addition of concurrent chemotherapy to definitive RT was found to significantly improve local control and OS in patients with locally advanced NPC.30 Concurrent chemotherapy, when used with salvage IMRT, also can improve such clinical outcomes based on the long‐term results of a randomized phase 2 trial.31 To our knowledge, the question of whether the addition of concurrent chemotherapy to salvage IMCT can improve disease control further in patients with LR‐NPC after they fail definitive RT is unknown. This is the subject of one of the dose escalating trials that currently is ongoing at SPHIC.17
The use of Epstein‐Barr virus (EBV) DNA as a prognosticator and to guide treatment has been a focus of investigation for patients with NPC. In a pivotal study published by Lin et al,32 a higher pretreatment plasma EBV (pEBV) DNA concentration was found to be a significant predictor of worse outcome. In addition, patients with persistently detectable pEBV DNA after RT were found to have worse OS and recurrence‐free survival rates compared with those without detectable pEBV DNA. The same institute confirmed that pEBV DNA load is a significant independent predictor of treatment outcomes in patients with locally advanced NPC after long‐term follow‐up.33 Such findings are important to guide additional treatments for patients with a high probability of persistent or recurrent disease. More aggressive combinations can be provided to patients with high pretreatment pEBV DNA, and adjuvant therapy could be recommended for those with detectable pEBV DNA after treatment. Unfortunately, to the best of our knowledge, to date pEBV DNA has not been investigated and reported for patients with LR‐NPC. Because the 1‐year PFS rate was 86.6% in the current study cohort of patients with LR‐NPC after CIRT salvage therapy, the accurate identification of patients who may develop locoregional or distant failure could provide more precise guidance for additional treatment to improve prognoses further. At SPHIC, the peripheral blood of patients with NPC has been preserved based on an IRB‐approved study. pEBV DNA will be investigated for its predictive value for prognosis in patients with LR‐NPC who are treated with CIRT.
Conclusions
The use of IMCT for salvage treatment among patients with LR‐NPC appears to be safe and effective. With a median follow‐up of 15.4 months, the 1‐year OS, DSS, PFS, LRFS, RRFS, and DMFS rates were 98.1%, 98.1%, 82.2%, 86.6%, 97.9%, and 96.2%, respectively. No acute SAEs of any type were observed. Although 1 patient experienced grade 5 treatment‐induced mucosal necrosis and massive hemorrhage, the probability of a treatment‐induced late SAE remained infrequent compared with conventional IMRT. However, long‐term follow‐up is needed to determine the optimal dose and fractionation of IMCT for patients with LR‐NPC, as well as the long‐term outcome and late toxicities.
FUNDING SUPPORT
Supported by grants from the National Key Research and Development Program of China (project no. 2017YFC0108603), Joint Breakthrough Project for New Frontier Technologies of the Shanghai Hospital Development Center (project no. SHDC 12015118), and Science and Technology Commission of Shanghai Municipality (project no. 15411950102 and 15411950106).
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
AUTHOR CONTRIBUTIONS
Jiyi Hu: Conceptualization, data curation, formal analysis, investigation, methodology, resources, validation, visualization, writing‐original draft, and writing‐review and editing. Cihang Bao: Data curation, formal analysis, investigation, methodology, resources, validation, writing‐original draft, and writing‐review and editing. Jing Gao: Data curation, resources, investigation, and validation. Xiyin Guan: Data curation, resources, investigation, and validation. Weixu Hu: Data curation, resources, and investigation. Jing Yang: Data curation, resources, and investigation. Chaosu Hu: Resources. Lin Kong: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, resources, supervision, validation, writing‐original draft, and writing‐review and editing. Jiade J. Lu: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing‐original draft, and writing‐review and editing
The first 2 authors contributed equally to this article.
Contributor Information
Lin Kong, Email: lin.kong@sphic.org.cn.
Jiade J. Lu, Email: jiade.lu@sphic.org.cn.
REFERENCES
- 1. Wang TJC, Riaz N, Cheng SK, Lu JJ, Lee NY. Intensity‐modulated radiation therapy for nasopharyngeal carcinoma: a review. J Radiat Oncol. 2012;1:129‐146. [Google Scholar]
- 2. Sze H, Blanchard P, Ng WT, Pignon JP, Lee AW. Chemotherapy for nasopharyngeal carcinoma‐current recommendation and controversies. Hematol Oncol Clin North Am. 2015;29:1107‐1122. [DOI] [PubMed] [Google Scholar]
- 3. Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33:3356‐3364. [DOI] [PubMed] [Google Scholar]
- 4. Lin JC. Adjuvant chemotherapy in advanced nasopharyngeal carcinoma based on plasma EBV load. J Radiat Oncol. 2012;1:117‐127. [Google Scholar]
- 5. Suarez C, Rodrigo JP, Rinaldo A, Langendijk JA, Shaha AR, Ferlito A. Current treatment options for recurrent nasopharyngeal cancer. Eur Arch Otorhinolaryngol. 2010;267:1811‐1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siddiqui F, Raben D, Lu JJ, et al. Emerging applications of stereotactic body radiation therapy for head and neck cancer. Expert Rev Anticancer Ther. 2011;11:1429‐1436. [DOI] [PubMed] [Google Scholar]
- 7. Qiu S, Lin S, Tham IW, Pan J, Lu J, Lu JJ. Intensity‐modulated radiation therapy in the salvage of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2012;83:676‐683. [DOI] [PubMed] [Google Scholar]
- 8. Hua YJ, Han F, Lu LX, et al. Long‐term treatment outcome of recurrent nasopharyngeal carcinoma treated with salvage intensity modulated radiotherapy. Eur J Cancer. 2012;48:3422‐3428. [DOI] [PubMed] [Google Scholar]
- 9. Han F, Zhao C, Huang SM, et al. Long‐term outcomes and prognostic factors of re‐irradiation for locally recurrent nasopharyngeal carcinoma using intensity‐modulated radiotherapy. Clin Oncol (R Coll Radiol). 2012;24:569‐576. [DOI] [PubMed] [Google Scholar]
- 10. Kong L, Wang L, Shen C, et al. Salvage intensity‐modulated radiation therapy (IMRT) for locally recurrent nasopharyngeal cancer after definitive IMRT: A novel scenario of the modern era. Sci Rep. 2016;6:32883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Slater JM, Miller DW, Archambeau JO. Development of a hospital‐based proton beam treatment center. Int J Radiat Oncol Biol Phys. 1988;14:761‐775. [DOI] [PubMed] [Google Scholar]
- 12. Amirul Islam M, Yanagi T, Mizoe JE, Mizuno H, Tsujii H. Comparative study of dose distribution between carbon ion radiotherapy and photon radiotherapy for head and neck tumor. Radiat Med. 2008;26:415‐421. [DOI] [PubMed] [Google Scholar]
- 13. Lin R, Slater JD, Yonemoto LT, et al. Nasopharyngeal carcinoma: repeat treatment with conformal proton therapy‐dose‐volume histogram analysis. Radiology. 1999;213:489‐494. [DOI] [PubMed] [Google Scholar]
- 14. Feehan PE, Castro JR, Phillips TL, et al. Recurrent locally advanced nasopharyngeal carcinoma treated with heavy charged particle irradiation. Int J Radiat Oncol Biol Phys. 1992;23:881‐884. [DOI] [PubMed] [Google Scholar]
- 15. Elsasser T, Kramer M, Scholz M. Accuracy of the local effect model for the prediction of biologic effects of carbon ion beams in vitro and in vivo. Int J Radiat Oncol Biol Phys. 2008;71:866‐872. [DOI] [PubMed] [Google Scholar]
- 16. Jones B. A simpler energy transfer efficiency model to predict relative biological effect for protons and heavier ions. Front Oncol. 2015;5:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kong L, Gao J, Hu J, et al. Phase I/II trial evaluating concurrent carbon‐ion radiotherapy plus chemotherapy for salvage treatment of locally recurrent nasopharyngeal carcinoma. Chin J Cancer. 2016;35:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kong L, Hu J, Guan X, et al. Phase I/II trial evaluating carbon ion radiotherapy for salvaging treatment of locally recurrent nasopharyngeal carcinoma. J Cancer. 2016;7:774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nieder C, Milas L, Ang KK. Tissue tolerance to reirradiation. Semin Radiat Oncol. 2000;10:200‐209. [DOI] [PubMed] [Google Scholar]
- 20. Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. [DOI] [PubMed] [Google Scholar]
- 21. Koto M. Skull Base and Upper Cervical Spine Tumors. In: Tsujii H, Kamada T, Shirai T, Noda K, Tsuji H, Karasawa K, eds. Carbon‐Ion Radiotherapy. Tokyo: Springer; 2014. [Google Scholar]
- 22. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 23. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341‐1346. [DOI] [PubMed] [Google Scholar]
- 24. Kong L, Lu JJ. Reirradiation of locally recurrent nasopharyngeal cancer: history, advances, and promises for the future. Chin Clin Oncol. 2016;5:26. [DOI] [PubMed] [Google Scholar]
- 25. Holliday EB, Frank SJ. Proton therapy for nasopharyngeal carcinoma. Chin Clin Oncol. 2016;5:25. [DOI] [PubMed] [Google Scholar]
- 26. Wang CC. Re‐irradiation of recurrent nasopharyngeal carcinoma–treatment techniques and results. Int J Radiat Oncol Biol Phys. 1987;13:953‐956. [DOI] [PubMed] [Google Scholar]
- 27. Lee AW, Foo W, Law SC, et al. Reirradiation for recurrent nasopharyngeal carcinoma: factors affecting the therapeutic ratio and ways for improvement. Int J Radiat Oncol Biol Phys. 1997;38:43‐52. [DOI] [PubMed] [Google Scholar]
- 28. Combs SE, Kalbe A, Nikoghosyan A, et al. Carbon ion radiotherapy performed as re‐irradiation using active beam delivery in patients with tumors of the brain, skull base and sacral region. Radiother Oncol. 2011;98:63‐67. [DOI] [PubMed] [Google Scholar]
- 29. Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose‐rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6:245ra293. [DOI] [PubMed] [Google Scholar]
- 30. Tan WL, Tan EH, Lim DW, et al. Advances in systemic treatment for nasopharyngeal carcinoma. Chin Clin Oncol. 2016;5:21. [DOI] [PubMed] [Google Scholar]
- 31. Guan Y, Liu S, Wang HY, et al. Long‐term outcomes of a phase II randomized controlled trial comparing intensity‐modulated radiotherapy with or without weekly cisplatin for the treatment of locally recurrent nasopharyngeal carcinoma. Chin J Cancer. 2016;35:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin JC, Wang WY, Chen KY, et al. Quantification of plasma Epstein‐Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350:2461‐2470. [DOI] [PubMed] [Google Scholar]
- 33. Wang WY, Twu CW, Chen HH, et al. Long‐term survival analysis of nasopharyngeal carcinoma by plasma Epstein‐Barr virus DNA levels. Cancer. 2013;119:963‐970. [DOI] [PubMed] [Google Scholar]


