Abstract
Objectives
Synthetic biphasic calcium phosphate (BCP) with a hydroxyapatite/ß‐tricalcium phosphate (HA/ß‐TCP) ratio of 60/40 (BCP60/40) is successfully used as alternative for autologous bone in patients undergoing maxillary sinus floor elevation (MSFE) for dental implant placement. A high percentage of HA in BCP60/40 may hamper efficient scaffold remodeling. Osteogenesis and neovascularization are pivotal in effective bone regeneration. We aimed to investigate whether differences exist in osteogenic and/or vasculogenic potential of BCP60/40 and BCP20/80 in patients undergoing MSFE.
Materials and methods
Twenty patients undergoing MSFE were treated with BCP60/40 (n = 10) or BCP20/80 (n = 10). Bone and graft volumes were determined by micro‐computed tomography and histomorphometrical analysis of biopsies of the augmented region. Osteoid volumes, number of osteoclasts, and blood vessels were determined by histomorphometrical analysis. The biopsies were taken 6.5 months (26 weeks) postoperatively prior to dental implant placement.
Results
Bone and osteoid volumes were 9.7% and 0.8% higher at the most cranial side of the BCP20/80 biopsies compared to the BCP60/40 biopsies. Graft volumes, number of osteoclasts, and blood vessels were similar in both groups.
Conclusions
BCP20/80 showed enhanced osteogenic potential in patients undergoing MSFE compared to BCP60/40, due to either a faster bone remodeling rate or an earlier start of bone formation in BCP20/80‐treated patients, suggesting that a higher TCP content positively contributes to the bone remodeling rate. Therefore, BCP20/80 might perform better, at least in the short term, as a scaffold for bone augmentation in the MSFE model than BCP60/40 as more bone is formed, and more osteoid is deposited at the cranial side in BCP20/80‐treated patients compared to BCP60/40‐treated patients. However, catch‐up of BCP60/40 in the long term cannot be ruled out.
Keywords: bone regeneration, bone substitutes, clinical research, clinical trials, guided tissue regeneration, morphometric analysis, sinus floor elevation
1. INTRODUCTION
Maxillary sinus floor elevation (MSFE) is a frequently performed surgical procedure to restore insufficient jaw bone height in the posterior maxilla allowing dental implant placement (Wallace & Froum, 2003; Zijderveld, van den Bergh, Cune, ten Bruggenkate & van Waas, 1997). Autologous bone is the golden standard for clinical bone augmentation in MSFE (Zijderveld, Zerbo, van den Bergh, Schulten & ten Bruggenkate, 2005), because it has osteoconductive as well as osteoinductive properties, contains osteogenic cells, and does not evoke immunogenic responses. Drawbacks of using autologous bone are, for example, limited availability of bone grafts and morbidity at the donor site (Klijn, Meijer, Bronkhorst & Jansen, 2010). An alternative to the golden standard is biphasic calcium phosphate (BCP) containing hydroxyapatite (HA) and ß‐tricalcium phosphate (ß‐TCP). BCP is used as bone substitute material for dental and orthopedic applications. The chemical composition of BCP resembles the inorganic part of the natural bone matrix (Frenken et al., 2010). HA is rigid, brittle, and hardly resorbed after application in MSFE, while ß‐TCP degrades faster and has a different resorption pattern (Fujita, Yokoyama, Kawasaki & Kohgo, 2003). Furthermore, mesenchymal stromal cells on ß‐TCP form more bone than mesenchymal stromal cells on HA (Prins, Fernandes, et al., 2016; Yuan et al., 2010), which can be caused by differences in spatial distribution of bone‐forming cells on HA and ß‐TCP (Prins, Fernandes, et al., 2016). For efficient scaffold remodeling, there should be a proper balance between the resorption time of the scaffold and the timing of new bone formation. BCP with a HA/ß‐TCP ratio of 60/40 (BCP60/40) represents the slowest resorbing variant of BCP currently used in the clinic, while a bone substitute with 100% ß‐TCP has the shortest resorption time and may lose its scaffolding properties too early. Several studies have evaluated and compared bone substitutes with different HA/ß‐TCP ratios against autologous bone or each other (Hallman & Thor, 2008; Kunert‐Keil, Scholz, Gedrange & Gredes, 2015; Miron et al., 2016; Schmitt et al., 2013; Tadjoedin, de Lange, Bronckers, Lyaruu & Burger, 2003; Zijderveld et al., 2005).
Up to now, two animal studies have been published comparing BCP60/40, BCP with a HA/ß‐TCP ratio of 20/80 (BCP20/80), and other bone substitutes with autologous bone (Jensen, Bornstein, Dard, Bosshardt & Buser, 2009; Yang et al., 2013). One comparative study on BCP with different HA/ß‐TCP ratios in mandibular bone defects in minipigs showed that BCP20/80 results in similar bone formation as autologous bone after 52 weeks (Jensen et al., 2009). This study also showed that BCP60/40 results in similar bone formation as deproteinized bovine bone mineral (Jensen et al., 2009). Another comparative study on BCP with different HA/TCP ratios in a calvarial bone defect in rabbits demonstrated that BCP60/40 and BCP20/80 provided more newly formed bone and increased bone density compared to pure ß‐TCP after 8 weeks (Yang et al., 2013). The same study also showed that BCP60/40 and BCP20/80 exhibit similar bone healing and biodegradation patterns after this period (Yang et al., 2013). BCP60/40 is successfully used as alternative for autologous bone in patients undergoing MSFE for dental implant placement; however, the high percentage of HA in BCP60/40 may hamper efficient scaffold remodeling. Whether BCP20/80 is more desirable to use as an alternative for autologous bone in patients undergoing MSFE for dental implant placement compared to BCP60/40 is still unclear as to the best of our knowledge BCP20/80 has not been tested before in a clinical human model.
A novel bone substitute should offer a framework for regenerating and healing of the host bone as well as formation of blood vessels. Osteogenesis and angiogenesis are tightly coupled processes, and vascular development needs to be induced before osteogenesis can take place. A comparative study of HA, TCP, and BCP60/40 in subcutaneous tissue under the thin skin muscle of the subscapular region in rats for up to 30 days showed that BCP60/40 results in similar vascularization as the TCP group after 15 days, whereas the vascularization in the BCP60/40 group is comparable as the HA group at the end of the study (Ghanaati et al., 2012). Whether BCP20/80 enhances vascularization compared to BCP60/40 is still unclear.
As implantation of BCP with different HA/TCP ratios in patients undergoing MSFE for dental implant placement may influence the in vivo tissue reaction, we aimed to investigate whether differences exist in osteogenic and/or angiogenic potential of BCP60/40 or BCP20/80 in patients undergoing MSFE. We hypothesized that the use of BCP20/80 in human MSFE results in an increased bone volume and/or improved bone structure and/or enhanced vascularization compared to the widely used BCP60/40. In this study, we report the first comparison of BCP60/40 and BCP20/80 for osteogenic and vasculogenic potential in patients undergoing MSFE.
2. MATERIAL AND METHODS
2.1. Biphasic calcium phosphate scaffolds
Two types of calcium phosphate scaffolds were used: (i) Straumann® BoneCeramic 60/40 (Institut Straumann AG, Basel, Switzerland), a porous BCP scaffold composed of 60% HA and 40% ß‐TCP (BCP60/40), and (ii) Straumann® BoneCeramic 20/80 (Institut Straumann AG, Basel, Switzerland), a porous BCP scaffold composed of 20% HA and 80% ß‐TCP (BCP20/80). To properly compare material composition without the risk of topographical influences overruling the compositional effects due to differences in manufacturing techniques, the two BCPs were produced by the same company and had similar particle size (500–1000 μm), microporosity (2%), interconnected pores (100–500 μm), and porosity (90%). BCP20/80 had a crystal size of 1.0–6.0 μm and a specific surface area of 9.5 × 10−3 m2/g, while BCP60/40 had a crystal size of 0.6–6.0 μm and a specific surface area of 6.9 × 10−3 m2/g. Characteristics and surface morphology have been described earlier (van Esterik, Zandieh‐Doulabi, Kleverlaan & Klein‐Nulend, 2016).
2.2. Patient selection
The number of patients assessed for sinus floor elevation on request of the referring dentist was 26. Six patients were excluded, four patients due to insufficient native alveolar residual bone height and two patients due to heavy smoking. Twenty patients, who were partially edentulous in the posterior maxilla and required dental implants for prosthetic rehabilitation, were included consecutively in a defined period between June 2008 and August 2011, and therefore, a sample size calculation was not considered. All patients had an adequate alveolar residual bone height at the posterior maxilla of at least 3.1 mm (Table 1). Therefore, a preoperative panoramic radiograph was made and carefully examined for contour lines of the maxillary sinus floor and to determine the residual bone height at the planned dental implant positions. The average age of the patients was 53 years, 14 years (mean, SD). Four females (54 years, 21 years; mean, SD) and six males (54, 10) undergoing MSFE were treated with BCP60/40, and seven females (46, 10) and three males (66, 9) undergoing MSFE were treated with BCP20/80 (Table 1). One female had natural remaining dentition, and three females and six males treated with BCP60/40 had partial remaining dentition (Table 1). In patients treated with BCP20/80, two females had natural remaining dentition, and five females and three males had partial remaining dentition (Table 1). The dental restorative treatments in the BCP60/40 group were six single crowns, three blocked crowns, and one bridge (Table 1). In the BCP20/80 group were the dental restorative treatments seven single crowns, two blocked crowns, and one bridge (Table 1). The patients included in this study had a healthy periodontium and were either nonsmokers or moderate smokers smoking less than 10 cigarettes per day. Patients who required horizontal bone augmentation, as well as patients with specific conditions—such as systemic diseases, drug abuse, heavy smokers, other semi‐invasive dental treatments, and/or pregnancy—were excluded from participation in this study. All procedures were performed by one oral surgeon either in the Alrijne Hospital in Leiderdorp or in the VU University Medical Center, Amsterdam, the Netherlands.
Table 1.
Patient data
| Scaffold | Gender (♀/♂) | Age (years) | Residual bone height( )(mm) | Dental implant position | Remaining dentition | Restorative treatment |
|---|---|---|---|---|---|---|
| BCP60/40 | ♀ | 24 | 5.5 | 16 | natural | single crown |
| BCP60/40 | ♀ | 68 | 3.7 | 25 | partial | single crown |
| BCP60/40 | ♀ | 58 | 5.4 | 26 | partial | single crown |
| BCP60/40 | ♀ | 67 | 7.1 | 16 | partial | bridge |
| BCP60/40 | ♂ | 57 | 3.1 | 16 | partial | blocked crowns |
| BCP60/40 | ♂ | 59 | 9.4 | 26 | partial | blocked crowns |
| BCP60/40 | ♂ | 45 | 6.8 | 26 | partial | single crown |
| BCP60/40 | ♂ | 55 | 6.7 | 15 | partial | single crown |
| BCP60/40 | ♂ | 39 | 5.5 | 16 | partial | single crown |
| BCP60/40 | ♂ | 66 | 12.3 | 15 | partial | blocked crowns |
| BCP20/80 | ♀ | 46 | 5.3 | 26 | partial | single crown |
| BCP20/80 | ♀ | 51 | 5.7 | 26 | partial | blocked crowns |
| BCP20/80 | ♀ | 47 | 3.2 | 26 | partial | blocked crowns |
| BCP20/80 | ♀ | 41 | 4.2 | 26 | partial | single crown |
| BCP20/80 | ♀ | 66 | 5.4 | 16 | natural | single crown |
| BCP20/80 | ♀ | 30 | 6.7 | 16 | natural | single crown |
| BCP20/80 | ♀ | 42 | 6.0 | 16 | partial | single crown |
| BCP20/80 | ♂ | 55 | 6.2 | 14 | partial | single crown |
| BCP20/80 | ♂ | 71 | 5.3 | 25 | partial | single crown |
| BCP20/80 | ♂ | 71 | 5.7 | 17 | partial | bridge |
Gender, age, residual bone height, dental implant position, remaining dentition, and restorative treatment in patients undergoing maxillary sinus floor elevation procedure treated with BCP60/40 or BCP20/80. BCP60/40, biphasic calcium phosphate with a hydroxyapatite/ß‐tricalcium phosphate ration of 60/40; BCP20/80, biphasic calcium phosphate with a hydroxyapatite/ß‐tricalcium phosphate ration of 20/80.
2.3. Maxillary sinus floor elevation
All 20 patients underwent a unilateral MSFE procedure as previously described (Tatum, 1986). Briefly, a preoperative clinical photograph was taken (Figure 1a), and a lateral bony window was prepared and turned inward and upward, the generated cavity within the maxillary sinus was filled with either BCP60/40 or BCP20/80, and the wound was closed with Gore‐Tex sutures (W.L. Gore and Associates, Newark, DE, USA), which were removed after 10–14 days. All patients received antibiotic prophylaxis, consisting of 500 mg amoxicillin, 4 times daily starting 1 day preoperatively and continuing 7 days postoperatively. After a healing period of 5 months post‐MSFE, prior to dental implant placement, a panoramic radiograph was made to determine the increase in vertical bone plus bone substitute height at the planned dental implant positions (Figure 1b).
Figure 1.
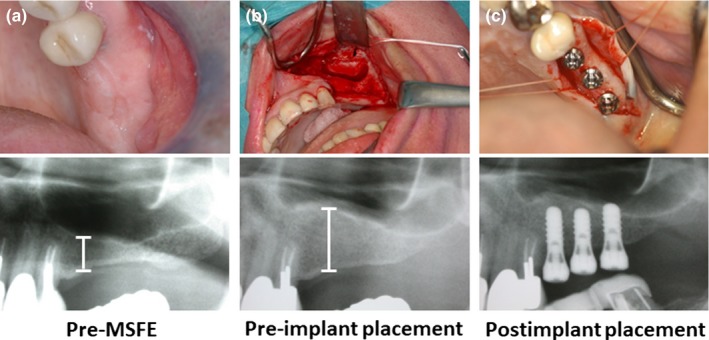
Clinical photographs of a maxillary sinus floor elevation procedure via a lateral approach allowing dental implant placement, and their corresponding radiographs to evaluate maxillary sinus and alveolar bone height. (a) Preoperative photograph of the maxillary sinus of the patient, (b) maxillary sinus filled with graft material, (c) dental implant placement after 6.5 months (26 weeks)
2.4. Dental implant surgery
Six and a half months, 0.9 months (mean, SD) after the MSFE procedure, dental implant surgery was performed under local anesthesia. A crestal incision was made with mesial and distal buccal vertical release incisions. A full‐thickness mucoperiosteal flap was raised to expose the underlying alveolar ridge, which was inspected for sufficient bone volume for the intended dental implant placement. Then, implant bed preparations were made and biopsies were collected with a hollow Straumann® trephine drill (Institute Straumann AG) with an outer diameter of 3.5 mm and an inner diameter of 2.5 mm, and using sterile saline for copious irrigation. Straumann® dental implants (Institute Straumann AG) with a diameter of 4.1 mm, a length of 10 or 12 mm, and a sand‐blasted, large‐grit, acid‐etched surface were placed in the augmented maxillary sinus. Dental implants were placed in a single‐stage surgical procedure, mounted with healing abutments (radiograph in Figure 1c), and sutured with Gore‐Tex sutures. Immediately after dental implant placement, another panoramic radiograph was made to check dental implant placement. The panoramic radiographs taken pre‐MSFE, as well as before dental implant placement, were used for morphometric measurements to determine the increase in vertical bone plus bone substitute height at the planned implant positions, using digital software. Calculations were performed with the use of a conversion factor (1.25 x) that adjusted for magnification of the panoramic radiograph. Sutures were removed after 10–14 days. Patients were instructed to avoid loading of the dental implants during the postimplant surgical osseointegration time. After a 3‐month osseointegration period, the superstructures were manufactured and placed. The follow‐up time for BCP60/40 was ≥ 59 months and for BCP20/80 was ≥ 58 months.
2.5. Biopsy analysis
The bone biopsies taken during dental implant surgery with a hollow Straumann® trephine drill were fixated in 4% phosphate‐buffered formaldehyde solution (Klinipath BV, Duiven, the Netherlands) for at least 24 hr, removed from the drill, transferred to 70% ethanol, and stored until use for micro‐CT analysis and histomorphometrical analysis, as described below. For each patient, one biopsy was selected in the middle of the grafted area to exclude the effect of surrounding bone containing mechanically loaded dental elements and bone near the nasal wall of the maxillary sinus.
2.6. Micro‐CT analysis
Three‐dimensional biopsy reconstructions of biopsies kept in 70% ethanol were obtained with a high‐resolution micro‐CT system PX5‐925EA (μCT 40; Scanco Medical AG, Bassersdorf, Switzerland). To this end, we fixed biopsies in synthetic foam, placed vertically in a custom‐made polyetherimide holder, and scanned them at an isotropic voxel size of 10 μm, source voltage of 70 kV, and current of 113 μA. Gray values, depending on radiolucency of the scanned material, were converted into corresponding values of degree of mineralization by the analysis software (Scanco Medical AG). The distinction between newly formed bone and graft material was made using the highest value of the degree of mineralization in the preexisting sinus floor bone as threshold value. A distinction could be made between the original nongrafted native bone of the residual maxillary sinus floor and the graft material, because the mineralization degree of the graft material was significantly higher than that of bone. The degree of mineralization was expressed in mg HA/cm3. A low threshold between 650 and 1300 mg HA/cm3 was defined to distinguish bone tissue from connective tissue and bone marrow, and a high threshold between 1300 and 2500 mg HA/cm3 was defined to distinguish graft material from bone tissue. Using this simple thresholding resulted initially in the detection of a thin layer of nonexisting “bone” covering the graft material throughout the grafted area of the sinus. Therefore, a new and so‐called onion‐peeling algorithm (evaluation program no. 12; Scanco Medical AG) was used to discriminate between the newly formed bone deposited on the graft material itself (Schulten et al., 2013). This method peels off voxels from the thin layer of “bone” (as measured with the simple thresholding method) and removes this layer when calcium phosphate is detected within a predefined extent of space. The digital images of the scanned biopsies were analyzed manually, starting from the caudal side of the biopsy and continuing toward the cranial side (Figure 2). Regions of interest (ROIs) of 1 mm2 were defined, and to obtain more insight into where the bone was formed within the grafted area, ROIs were numbered in a consecutive sequence starting from the residual sinus floor up to the most cranial part of the biopsy. The transition zone (tz) indicates the first ROI where substantial graft material (> 1% graft volume per total volume) was observed when analyzing manually the biopsy from the caudal to cranial side. Native bone (nb) is located caudally from the tz. Depending on the length of the biopsy, we divided the ROIs after the tz in region I, region II, and region III. The number of ROIs in region I was 2; in region II, it ranged from 0 to 3; and in region III, it ranged from 0 to 2 (Figure 2c). These ROIs were analyzed separately by micro‐CT and histomorphometry (Figure 2). For nb, tz, and each ROI, bone volume over total tissue volume (BV/TV) and graft volume over total tissue volume (GV/TV) were calculated and compared between the BCP60/40 group and BCP20/80 group.
Figure 2.
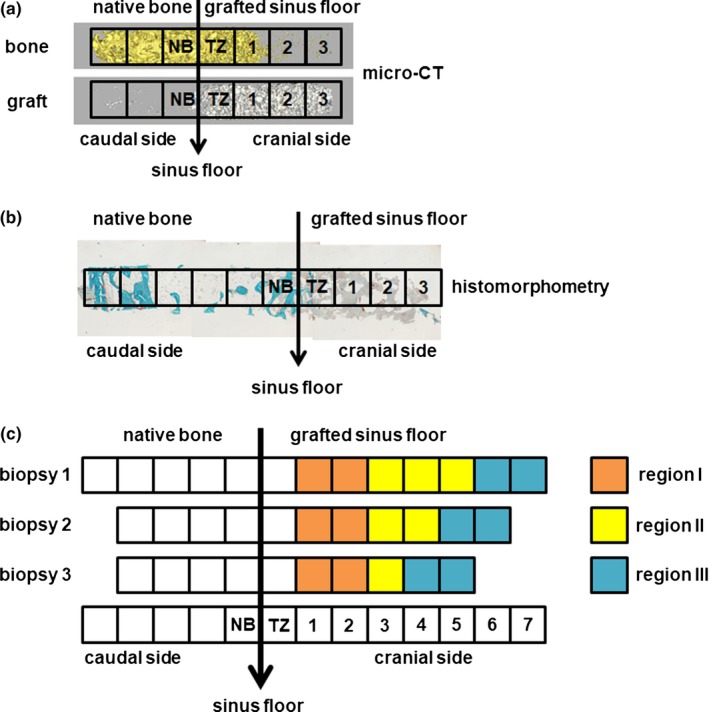
Methods of micro‐computed tomography and histomorphometrical analyses of bone biopsies. Bone biopsies are removed from the hollow burr and analyzed using (a) micro‐computed tomography and (b) histomorphometry. (c) Schematic diagram for micro‐computed tomography and histomorphometrical analyses. Biopsies were analyzed separately, starting from the caudal side of the biopsy and continuing toward the cranial side. Regions of interest (ROIs) of 1 mm2 were defined, and to obtain more insight into where the bone was formed within the grafted area, ROIs were numbered in a consecutive sequence starting from the sinus floor up to the most cranial part of the biopsy. The transition zone (tz) indicates the first ROI where substantial graft material (> 1% graft volume per total volume) was observed when analyzing the biopsy from the caudal to cranial side. Native bone (nb) is located before the tz (ROI 1). Depending on the length of the biopsy, we divided the ROIs after the tz in region I, region II, and region III. The number of ROIs in region I was 1 to 2; in region II, it ranged from 0 to 3, and in region III, it ranged from 0 to 2. For nb, tz, and each ROI, bone volume over total tissue volume (BV/TV) and graft volume over total tissue volume (GV/TV) were calculated and compared between the BCP60/40 group and BCP20/80 group
2.7. Histology and histomorphometrical analysis
After micro‐CT scanning and dehydration in ascending alcohol series, the bone specimens were embedded without prior decalcification in low‐temperature polymerizing methyl methacrylate (MMA; Merck Schuchardt OHG, Hohenbrunn, Germany, catalogue number 8.00590.2500). Longitudinal sections of 5 μm thickness were prepared with a Jung K microtome (Reichert Jung, Heidelberg, Germany). Midsagittal histological sections of each biopsy were stained with Goldner's trichrome to distinguish mineralized bone tissue (green) and unmineralized osteoid (red) (Plenk, 1989). The histological sections were also manually divided into ROIs of 1 mm2 for histomorphometrical analysis. The identity of the histological sections was blinded during analysis to avoid evaluation bias. Depending on the length of the biopsy, the number of ROIs ranged from 1 to 10. For each separate area of interest, we manually performed the histomorphometrical measurements with a computer using an electronic stage table and a Leica DC 200 digital camera. The computer software used was Leica QWin© (Leica Microsystems Image Solutions, Rijswijk, the Netherlands). Digital images of the sections were acquired at 100 × magnification. A manually placed demarcation line was indicated between the “native bone” and the regenerated “grafted sinus floor.” Consecutive ROIs of 1 mm2 were manually defined and numbered throughout the whole biopsy as described under “Micro‐CT analysis” (Figure 2). For nb, tz, and each ROI, bone ingrowth (in graft area; tz is not included), bone volume over total tissue volume (BV/TV), graft volume over total tissue volume (GV/TV), and osteoid volume over total tissue volume (OV/TV) were calculated as previously described (Parfitt et al., 1987) and compared between the BCP60/40 group and BCP20/80 group.
Tartrate‐resistant acid phosphatase (TRAcP) staining was used to visualize bone resorbing multinuclear cells (osteoclasts) within the biopsy sections. These sections were selected adjacent to biopsy sections that were stained with Goldner's trichrome method. TRAcP staining was performed according to a standardized protocol (van de Wijngaert & Burger, 1986). The number of TRAcP osteoclasts in each section was manually measured at 200 × magnification with the same computer software and microscope used for quantification of bone ingrowth, BV/TV, GV/TV, and OV/TV in the Goldner trichrome‐stained sections. Each tissue section used for TRAcP staining of osteoclasts was manually divided into consecutive optical areas of 1 mm2 and numbered throughout the whole biopsy as described under “Micro‐CT analysis” (Figure 2), overlapping with the optical areas in the Goldner's trichrome‐stained sections as closely as possible. Within each area all red‐colored multinuclear cells were identified as TRAcP‐positive osteoclasts, and for each section, the total number of TRAcP‐positive osteoclasts was manually calculated and compared between the BCP60/40 group and BCP20/80 group.
The number of blood vessels was used to determine the vasculogenic potential within the biopsy sections. Blood vessels were manually counted at 200 × and/or 400 × magnification with the computer software and microscope used for quantification of BV and OV in the Goldner's trichrome‐stained sections. The size of the blood vessels was automatically calculated as the total blood vessel area in μm2. Depending on the size, blood vessels were divided into small (0–400 μm2), medium (401–1200 μm2), or large (>1200 μm2) and compared between the BCP60/40 group and BCP20/80 group.
2.8. Statistical analysis
Data were obtained from 20 patients. Data are presented as mean, SD (median, interquartile range). Differences in osteogenic and/or vasculogenic potential in patients undergoing MSFE treated with BCP60/40 or BCP20/80 were tested with Mann–Whitney U test. A Mann–Whitney U test was used because of a small sample size, and no assumptions were made about the probability distributions of the test parameters. The equality of means was tested in two independent groups of patients using a two‐tailed statistical test as the direction of the results was unknown, and to check both possibilities in order to prevent making mistakes to state that one type of BCP was better than the other type. Therefore, differences were considered significant at p < .025. Statistical analysis was performed using IBM® SPSS® Statistics version 21 software package (SPSS Inc., Chicago, IL, USA) and GraphPad Prism® 5.0 (GraphPad Software Inc., La Jolla, USA).
2.9. Study approval
The use of calcium phosphate ceramics (alloplastic bone substitutes) for MSFE is an accepted grafting method (Katsuyama & Jensen, 2011). As the study involved CE‐marked devices (calcium phosphates) being used for their intended purpose, to be used as carrier material for bone augmentation in MSFE procedures, no specific regulatory approval from a medical ethical committee was required. The study was performed in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all patients before the study‐related procedures were undertaken.
3. RESULTS
3.1. Clinical evaluation and implant survival
All 20 patients with a healthy periodontium showed no adverse events/complications during and after the MSFE procedure. The augmentation height increased after the MSFE procedure, and the increase was similar in the BCP60/40 group (6.1 mm, 1.7 mm (6.3 mm, 1.6 mm) (mean, SD (median, interquartile range))) and the BCP20/80 group (6.5 mm, 1.6 mm (6.5 mm, 2.7 mm)). Wound healing went uneventful, and no implants were lost after implantation.
3.2. Micro‐CT analysis
Micro‐CT analysis was performed to determine bone volume over total tissue volume (BV/TV; Figure 3a) and graft volume over total tissue volume (GV/TV; Figure 3b). Bone volume decreased from the sinus floor toward the cranial side of the biopsies and increased at the most cranial side. The percentages of BV/TV in the grafted area were similar for all regions in the BCP60/40 group and BCP20/80 group (Figure 3a). In region l, BV/TV in the BCP60/40 group was 12.5%, 8.9% (13.2%, 11.4%) (mean, SD (median, interquartile range)) and in the BCP20/80 group 20.4%, 16.3% (19.1%, 29.0%). The percentage of BV/TV in region ll was 1.7%, 3.5% (0.4%, 0.9%) (mean, SD (median, interquartile range)) for the BCP60/40 group and 2.5%, 3.4% (2.0%, 2.0%) for the BCP20/80 group. In region lll, BV/TV in the BCP60/40 group was 8.1%, 12.0% (1.8%, 11.5%) (mean, SD (median, interquartile range)) and 14.2%, 11.6% (13.5%, 14.0%) in the BCP20/80 group. No differences were observed in percentage of graft volume between both BCP groups (Figure 3b). GV/TV remained constant throughout the more cranial side of the biopsies from both BCP groups (BCP60/40; region l: 12.6%, 4.9% (12.6%, 7.3%) (mean, SD (median, interquartile range)), region ll: 11.6%, 7.5% (14.6%, 11.2%), region lll: 12.1%, 7.6% (15.2%, 7.8%), and BCP20/80; region l: 13.4%, 7.8% (15.9%, 12.3%), region ll: 14.1%, 3.9% (13.6%, 5.3%), and region lll: 11.4%, 5.5% (14.0%, 5.9%); Figure 3b).
Figure 3.
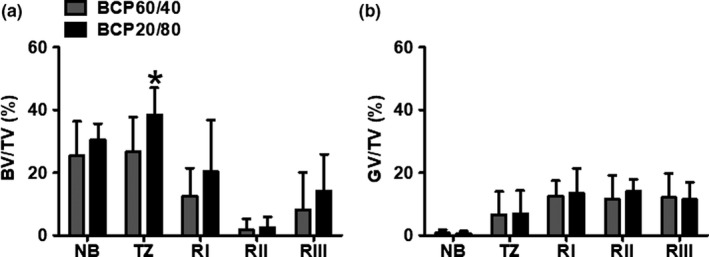
Micro‐computed tomography analysis of biopsies taken after maxillary sinus floor elevation with BCP60/40 or BCP20/80. (a) Bone volume over total tissue volume (BV/TV) and (b) graft volume over total tissue volume (GV/TV) as assessed by micro‐computed tomography analysis retrieved after maxillary sinus floor elevation with BCP60/40 or BCP20/80. BV/TV and GV/TV were assessed for native bone, transition zone, region l, region ll, and region lll. Values are mean, SD (n = 7–10). *Significantly different from BCP60/40, p < .025. BCP60/40, biphasic calcium phosphate with a hydroxyapatite/ß‐tricalcium phosphate ratio of 60/40; BCP20/80, biphasic calcium phosphate with a hydroxyapatite/ß‐tricalcium phosphate ratio of 20/80; BV, bone volume; TV, total volume; GV, graft volume; nb, native bone; tz, transition zone; Rl, region l; Rll, region ll; Rlll, region lll
3.3. Histology and histomorphometrical analysis
Histomorphometrical analysis was performed to determine bone ingrowth (in graft area, tz is not included), bone volume over total tissue volume (BV/TV), graft volume over total tissue volume (GV/TV), osteoid volume over total tissue volume (OV/TV), the number of TRAcP‐positive stained osteoclasts, and the number of blood vessels. The bone ingrowth from the native bone toward the cranial side of the biopsy was similar in both BCP groups (BCP60/40: 1.5 mm, 1.2 mm (1.0 mm, 0.8 mm) (mean, SD (median, interquartile range)); BCP20/80: 1.8 mm, 1.8 mm (1.0 mm, 1.8 mm); Figure 4a). This indicates that the rate of bone ingrowth was 0.2 mm per month for the BCP60/40 group and 0.3 mm per month for the BCP20/80 group.
Figure 4.
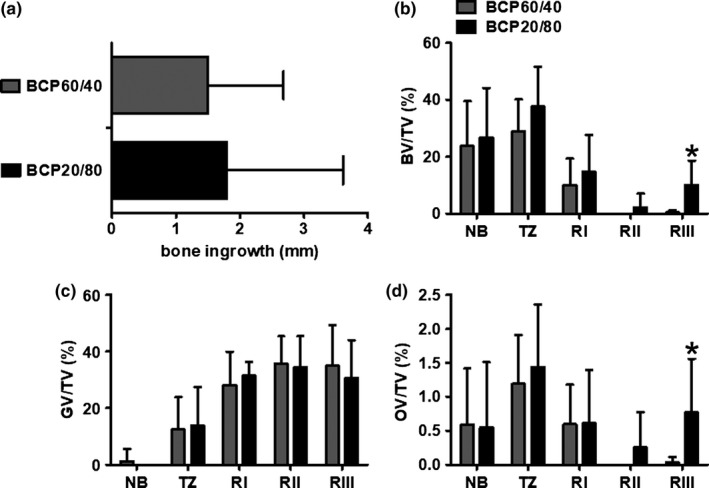
Histomorphometrical analysis of biopsies taken after maxillary sinus floor elevation with BCP60/40 or BCP20/80. (a) Bone ingrowth in mm was determined in biopsies taken after maxillary sinus floor with BCP60/40 or BCP20/80 from the maxillary sinus floor toward the cranial side of the biopsies. (b) Bone volume over total tissue volume (BV/TV), (c) graft volume over total tissue volume (GV/TV), and (d) osteoid volume over total tissue volume (OV/TV) as assessed by histomorphometrical analysis retrieved after maxillary sinus floor elevation with BCP60/40 or BCP20/80. BV/TV, GV/TV, and OV/TV were assessed for native bone, transition zone, region l, region ll, and region lll. Values are mean, SD (n = 6–10). *Significantly different from BCP60/40, p < .025. BCP60/40, biphasic calcium phosphate with a hydroxyapatite/ß‐tricalcium phosphate ratio of 60/40; BCP20/80, biphasic calcium phosphate with a hydroxyapatite/ß‐tricalcium phosphate ratio of 20/80; BV, bone volume; TV, total volume; GV, graft volume; OV, osteoid volume; nb, native bone; tz, transition zone; Rl, region l; Rll, region ll; Rlll, region lll
Histomorphometrical analysis revealed that bone volume decreased from the sinus floor toward the cranial side of the biopsies and increased again at the most cranial side. The percentage of BV/TV in the grafted area was similar in the BCP60/40 group and BCP20/80 group for region I (BCP60/40: 10.0%, 9.4% (10%, 11.1%) (mean, SD (median, interquartile range)), BCP20/80: 14.7%, 13.0% (16.8%, 18.9%)) and for region II (BCP60/40: 0%, 0% (0%, 0%); BCP20/80: 2.2%, 4.8% (0%, 1.5%); Figure 4b). At the most cranial side of the biopsy, region lll, 9.7% more bone formation (p = .022) was found in the BCP20/80 group (10.1%, 8.4% (14.0%, 14.4%) (mean, SD (median, interquartile range))) than in the BCP60/40 group (0.4%, 0.7% (0%, 0.4%); Figure 4b). No differences were observed in percentage of graft volume between BCP60/40 and BCP20/80 groups. GV/TV remained constant throughout the more cranial side of the biopsies from both BCP groups for region I (BCP60/40: 28.1%, 11.8% (27.3%. 14.2%) (mean, SD (median, interquartile range)); BCP20/80: 31.5%, 4.8% (30.3%, 4.9%)), region ll (BCP60/40: 35.7%, 9.7% (31.7%, 11.8%); BCP20/80: 34.4%, 11.0% (34.0%, 11.2%)), and region lll (BCP60/40: 35.0%, 14.4% (33.6%, 17.1%); BCP20/80: 30.7%, 13.3% (28.3%, 13.8%); Figure 4c). Osteoid volume showed a similar trend as bone volume measurements (Figure 4b,d). The osteoid volume decreased from the sinus floor toward the cranial side of the biopsies and increased at the most cranial side. The mean and median OV/TV were 0.8% and 0.4% higher (p = .014 and p = .021) in region lll in the BCP20/80 group (0.8%, 0.8% (0.4%, 0.9%) (mean, SD (median, interquartile range))) than in the BCP60/40 group (0%, 0.1% (0%, 0%); Figure 4d).
Clusters of TRAcP‐positive osteoclasts were found at the site of newly formed bone as well as native bone (Figure 5a). The number of TRAcP‐positive osteoclasts from sinus floor toward the cranial side of the biopsies was similar in the BCP60/40 group and BCP20/80 group (Figure 5b). In region l, the number of TRAcP‐positive osteoclasts in the BCP60/40 group was 6.2, 8.4 (1.3, 11.3) (mean, SD (median, interquartile range)) and in the BCP20/80 group 1.2, 1.7 (0.3, 1.9). The number of TRAcP‐positive osteoclasts in region ll was 5.7, 6.6 (3.0, 7.3) for the BCP60/40 group and 1.8, 2.5 (0, 3.5) for the BCP20/80 group. In region lll, the number of TRAcP‐positive osteoclasts in the BCP60/40 group was 4.3, 6.2 (1.0, 6.0), and 1.2, 2.2 (0, 1.1) in the BCP20/80 group.
Figure 5.
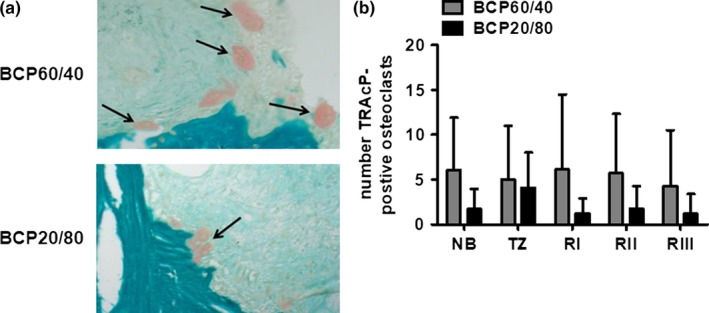
The number of TRAcP‐positive osteoclasts in biopsies taken after maxillary sinus floor elevation with BCP60/40 or BCP20/80. (a) To visualize and calculate the number of osteoclasts within the biopsies, consecutive sections were tartrate‐resistant acid phosphatase (TRAcP)‐stained (red) in biopsies taken after maxillary sinus floor elevation with BCP60/40 or BCP20/80. (b) The number of TRAcP‐positive osteoclasts was assessed for native bone, transition zone, region l, region ll, and region lll. Values are mean, SD (n = 7–10). BCP60/40, BCP with a hydroxyapatite/ß‐tricalcium phosphate of 60/40; BCP20/80, BCP with a hydroxyapatite/ß‐tricalcium phosphate ratio of 20/80; TRAcP, tartrate‐resistant acid phosphatase. Black arrows: TRAcP‐positive osteoclasts. Magnification: 200 ×
The number of blood vessels was counted to study blood vessel formation in biopsies taken after maxillary sinus floor elevation with BCP60/40 or BCP20/80. Depending on the size, blood vessels were defined as small (0–400 μm2), medium (401–1200 μm2), or large (>1200 μm2) blood vessel. No differences in number of small, medium, or large blood vessels could be observed in the BCP20/80 group compared to the BCP60/40 group (Table 2).
Table 2.
Histomorphometrical analysis of number of blood vessels (%) in biopsies taken after MSFE with BCP60/40 or BCP20/80
| Size blv | Region I | Region ll | Region III | |
|---|---|---|---|---|
| BCP60/40 | small | 33 | 31 | 32 |
| medium | 33 | 22 | 23 | |
| large | 35 | 47 | 45 | |
| BCP20/80 | small | 40 | 28 | 15 |
| medium | 21 | 20 | 62 | |
| large | 40 | 52 | 23 |
Values are mean (n = 6–7). BCP60/40, biphasic calcium phosphate with a hydroxyapatite/ß‐tricalcium phosphate ration of 60/40; BCP20/80, biphasic calcium phosphate with a hydroxyapatite/ß‐tricalcium phosphate ration of 20/80; blv, blood vessel.
4. DISCUSSION
This study aimed to investigate whether differences exist in osteogenic and/or vasculogenic potential of BCP60/40 and BCP20/80 in patients undergoing MSFE. We found that (i) the bone ingrowth (in mm) from the native bone toward the cranial side of the biopsy was similar in both BCP groups; (ii) BV/TV was 9.7% higher in the BCP20/80 group than the BCP60/40 group at the most cranial side of the biopsy (histomorphometry); (iii) GV/TV was similar in both BCP groups in all regions; (iv) OV/TV was 0.8% higher in the BCP20/80 group than in the BC60/40 group at the most cranial side of the biopsy (histomorphometry); (v) the number of TRAcP‐positive osteoclasts was similar in both BCP groups; and (vi) no differences in number of small, medium, or large blood vessels could be observed in both BCP groups. Therefore, our results showed 9.7% more bone formation and 0.8% osteoid deposition at the most cranial side of the biopsy in patients who underwent MSFE with BCP20/80 than patients with BCP60/40, demonstrating that BCP20/80 might perform better as a scaffold for bone augmentation in the MSFE model than BCP60/40.
In this study, BCP60/40 and BCP20/80 were manufactured by the same company to exclude possible differences as a result of manufacturing. BCP60/40 and BCP20/80 showed formation of new bone when placed in the maxillary sinus. In a bilateral sinus elevation procedure, the bone ingrowth speed in the BCP60/40 group was between 0.5 and 1.0 mm per month (de Lange et al., 2014), while the rate of bone ingrowth from the native bone toward the cranial side of the biopsy in this study was lower for both BCP groups. The difference in bone ingrowth speed might be explained by the different method used for numbering of the regions of interest or by the time point of dental implant placement. In the present study, dental implants were placed 6.5 months (26 weeks) after the MSFE procedure, while in our previously published bilateral sinus elevation procedure study, the dental implants were placed 3 to 8 months after the MSFE procedure (de Lange et al., 2014). HA is rigid, brittle, and hardly resorbed, while ß‐TCP degrades faster and has a different resorption pattern (Fujita et al., 2003). The lower the HA/ß‐TCP ratio, the more the dissolution of BCP (LeGeros, 1993). Based on the lower HA/ß‐TCP ratio in BCP20/80, a higher resorption rate in BCP20/80‐grafted biopsies than in BCP60/40‐grafted biopsies was expected, but this observation was not observed. As we only analyzed biopsies after a short term (up to 6 months), a higher resorption rate in BCP20/80‐grafted biopsies may be observed in the long term (> 6 months). A higher resorption rate in BCP20/80‐grafted biopsies would allow more space for new bone ingrowth as well as blood vessel formation in the BCP20/80 group. The micro‐CT and histomorphometrical analyses showed similar bone formation from the native bone toward the cranial side in the biopsies of both BCPs; but although micro‐CT analysis showed a similar trend, only histomorphometrical analysis resulted in significant more bone formation in the BCP20/80 group at the most cranial side of the biopsy. The same pattern as found for bone formation was observed for osteoid deposition in the grafted areas. Active bone formation at the cranial side of the biopsies has also been observed in our clinical trial, where patients undergoing bilateral MSFE were treated with ß‐TCP or BCP60/40 with and without freshly isolated autologous stromal vascular fraction (SVF) of adipose tissue containing multipotent cells (Prins, Schulten, ten Bruggenkate, Klein‐Nulend & Helder, 2016). In that study, more active bone formation was observed at the cranial side of the biopsies with SVF than without SVF, which might be due to transdifferentiation of adipose stem cells within the SVF toward bone‐forming cells or caused by increased adipose stem cell paracrine recruitment of progenitor cells from the lateral bony window capping the bone reconstruction compartment and/or the human Schneiderian membrane of the maxillary sinus. It has recently been shown that this membrane, which is lifted during MSFE to insert the graft material, contains a cell population with potential for osteogenic differentiation (Berbéri et al., 2016). As in our study we did not include multipotent cells on the scaffolds, the observed high bone volumes in the most cranial side of the biopsies may be due to bone ingrowth from the cranial side from this membrane or lifted lateral bony window. The noted divergence between the levels of bone ingrowth between both BCP types, with the HA/ß‐TCP ratio being the only parameter being different, suggests that a higher TCP content may positively contribute to the bone ingrowth rate. This is in line with a previous study showing that TCP has a relatively late‐occurring remodeling phase, supports cell ingrowth, and promotes osteogenic differentiation of osteoprogenitor cells (Zerbo, Bronckers, de Lange & Burger, 2005). Moreover, the findings in our study support the suggestion to keep the lateral bony window of the maxillary sinus in situ (Prins, Schulten, et al., 2016). Comparison of our data with other studies using a variety of biomaterials is rather difficult as a large heterogeneity exists in bone formation among several studies using bovine bone, autologous bone, HA, TCP, BCP, and calcium sulfate (Corbella, Taschieri, Weinstein & Del Fabbro, 2016).
The results from the micro‐CT and histomorphometrical analyses showed a similar trend in remaining graft volumes in both BCP groups, but a lower percentage of graft volume was observed by micro‐CT analysis than our histomorphometrical analysis. A similar discrepancy was found in our previous studies (Prins, Schulten, et al., 2016; Schulten et al., 2013) and can be explained by the fact that micro‐CT analysis comprises three‐dimensional measurements, while histomorphometrical analysis comprises two‐dimensional measurements. During histological preparation, graft material is dissolved, which gives the false impression that the granules are solid while being porous. Despite the differences in data obtained by micro‐CT and histomorphometrical analyses, they seem to complement each other; micro‐CT analysis is based on differences between structures and mineralized compounds, while histomorphometrical analysis provides information on, for example cells, and soft tissue organization and structure.
We found similar numbers of TRAcP‐positive osteoclasts laying against bone matrix in the BCP60/40 group and the BCP20/80 group in all regions, but lower numbers of osteoclasts were observed in the BCP20/80 group than the BCP60/40 group for the grafted area. The micro‐CT and histomorphometrical analyses showed more bone formation and subsequent bone remodeling in the BCP20/80 group, thus explaining the lower number of osteoclasts we found at the time point of our analysis. HA is degraded by osteoclastic resorption, and more TRAcP‐positive cells are present on HA disks than on β‐TCP disks (Lee et al., 2014). Therefore, the lower percentage of HA in BCP20/80 compared to BCP60/40 may explain the lower number of osteoclasts we observed in the BCP20/80 group.
Bone is highly vascularized, and vascular development needs to be induced prior to osteogenesis. Both BCP60/40 and BCP20/80 in patients undergoing MSFE apparently had sufficient and comparable angiogenic potential to form new bone. In contrast, in our clinical trial where 6/10 patients underwent bilateral MSFE and were treated with either ß‐TCP or BCP60/40 with and without freshly isolated autologous SVF of adipose tissue, bone formation could be linked to increased blood vessel formation (Prins, Schulten, et al., 2016; Farré‐Guasch et al., 2013. The SVF consists of a heterogeneous mixture of cells including endothelial cells and lineage‐committed progenitor cells (Zuk et al., 2001, 2002). This suggests that freshly isolated autologous SVF of adipose tissue may trigger increased blood vessel formation, which will allow nutrient diffusion and waste removal crucial for cell recruitment, survival, proliferation, differentiation, and tissue remodeling. It seems that freshly isolated autologous SVF of adipose tissue increases nutrient delivery, enabling more cell penetration toward the interior of the grafted area, and thus faster tissue remodeling compared to our current study without cells. SVF fits in a so‐called one‐step surgical procedure (Farré‐Guasch et al., 2013). Our results suggest that SVF‐seeded BCP20/80 might perform better than SVF‐seeded BCP60/40 in the MSFE model during a one‐step surgical procedure. Further preclinical in vivo studies are needed before clinical implantation can be considered.
A limitation of the present study is that we compared two BCPs using biopsies from unilateral sinus floor elevation in different patients. As the anatomical structure of the maxillary sinus varies between patients, bilateral sinus floor elevation models would be more appropriate to compare two BCPs. Another limitation of the present study is that we only analyzed biopsies after a short term, while BCP60/40 may have a beneficial long‐term outcome due to a lower resorption rate. In future studies, BCP60/40 and BCP20/80 should be compared in bilateral sinus floor elevation models to eliminate the variation in anatomical structure of the sinus between patients after a longer healing period.
In summary, the use of BCP20/80 in human MSFE resulted in a 9.7% higher bone and 0.8% osteoid volume at the cranial side of the biopsies than BCP60/40, but no differences in vascularization could be observed. Therefore, we conclude that BCP20/80 showed enhanced osteogenic potential in patients undergoing MSFE compared to BCP60/40, due to a faster bone remodeling rate, or an earlier start of bone remodeling in BCP20/80‐treated patients, suggesting that a higher TCP content positively contributes to the bone remodeling rate. This demonstrates that BCP20/80 might perform better as a scaffold for bone augmentation in the MSFE model than BCP60/40 in the short term as more bone is formed, and more osteoid is deposited at the cranial side in BCP20/80‐treated patients compared to BCP60/40‐treated patients.
Supporting information
ACKNOWLEDGEMENTS
The work of Fransisca A.S. van Esterik was supported by a grant from the University of Amsterdam for the stimulation of the research priority area Oral Regenerative Medicine. The authors thank Marion A. van Duin for histological processing and histomorphometrical analysis, Henk‐Jan Prins for data gathering, in particular histomorphometry, data interpretation, and manuscript correction, Leo J. van Ruijven for micro‐computed tomography assistance, Janice R. Overman for histomorphometrical and micro‐computed tomography analysis, and the Institut Straumann AG for providing Straumann® BoneCeramic 20/80 and the trephine drills.
Helder MN, van Esterik FAS, Kwehandjaja MD, ten Bruggenkate CM, Klein‐Nulend J, Schulten EAJM. Evaluation of a new biphasic calcium phosphate for maxillary sinus floor elevation: Micro‐CT and histomorphometrical analyses. Clin Oral Impl Res. 2018;29:488–498. https://doi.org/10.1111/clr.13146
REFERENCES
- Berbéri, A. , Al‐Nemer, F. , Hamade, E. , Noujeim, Z. , Badran, B. , & Zibara, K. (2016). Mesenchymal stem cells with osteogenic potential in human maxillary sinus membrane: An in vitro study. Clinical Oral Investigations, 21(5), 1599–1609. [DOI] [PubMed] [Google Scholar]
- Corbella, S. , Taschieri, S. , Weinstein, R. , & Del Fabbro, M. (2016). Histomorphometric outcomes after lateral sinus floor elevation procedure: A systematic review of the literature and meta‐analysis. Clinical Oral Implants Research, 27, 1106–1122. https://doi.org/10.1111/clr.12702 [DOI] [PubMed] [Google Scholar]
- De Lange, G. L. , Overman, J. R. , Farré‐Guasch, E. , Korstjens, C. M. , Hartman, B. , Langenbach, G. E. J. , … Klein‐Nulend, J. (2014). A histomorphometric and micro‐computed tomography study of bone regeneration in the maxillary sinus comparing biphasic calcium phosphate and deproteinized cancellous bovine bone in a human split‐mouth model. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, 117, 8–22. https://doi.org/10.1016/j.oooo.2013.08.008 [DOI] [PubMed] [Google Scholar]
- Farré‐Guasch, E. , Prins, H. J. , Overman, J. R. , ten Bruggenkate, C. M. , Schulten, E. A. J. M. , Helder, M. N. , & Klein‐Nulend, J. (2013). Human maxillary sinus floor elevation as a model for bone regeneration enabling the application of one‐step surgical procedures. Tissue Engineering Part B: Reviews, 19, 69–82. https://doi.org/10.1089/ten.teb.2012.0404 [DOI] [PubMed] [Google Scholar]
- Frenken, J. W. F. H. , Bouwman, W. F. , Bravenboer, N. , Zijderveld, S. A. , Schulten, E. A. J. M. , & ten Bruggenkate, C. M. (2010). The use of Straumann® Bone Ceramic in a maxillary sinus floor elevation procedure: A clinical, radiological, histological and histomorphometric evaluation with a 6‐month healing period. Clinical Oral Implants Research, 21, 201–208. https://doi.org/10.1111/j.1600-0501.2009.01821.x [DOI] [PubMed] [Google Scholar]
- Fujita, R. , Yokoyama, A. , Kawasaki, T. , & Kohgo, T. (2003). Bone augmentation osteogenesis using hydroxyapatite and ß‐tricalcium phosphate blocks. Journal of Oral and Maxillofacial Surgery, 9, 1045–1053. https://doi.org/10.1016/S0278-2391(03)00317-3 [DOI] [PubMed] [Google Scholar]
- Ghanaati, S. , Barbeck, M. , Detsch, R. , Deisinger, U. , Hilbig, U. , Rausch, V. , … Kirkpatrick, C. J. (2012). The chemical composition of synthetic bone substitutes influences tissue reactions in vivo: Histological and histomorphometrical analysis of the cellular inflammatory response to hydroxyapatite, beta‐tricalcium phosphate and biphasic calcium phosphate ceramics. Biomedical Materials, 7, 015005 https://doi.org/10.1088/1748-6041/7/1/015005 [DOI] [PubMed] [Google Scholar]
- Hallman, M. , & Thor, A. (2008). Bone substitutes and growth factors as an alternative/complement to autogenous bone for grafting in implant dentistry. Periodontology 2000, 47, 172–192. https://doi.org/10.1111/j.1600-0757.2008.00251.x [DOI] [PubMed] [Google Scholar]
- Jensen, S. S. , Bornstein, M. M. , Dard, M. , Bosshardt, D. D. , & Buser, D. (2009). Comparative study of biphasic calcium phosphates with different HA/TCP ratios in mandibular bone defects. A long‐term histomorphometric study in minipigs. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 90, 171–181. [DOI] [PubMed] [Google Scholar]
- Katsuyama, H. , & Jensen, S. S. (2011). ITI treatment guide, sinus floor elevation procedures, 5. Berlin, Germany: Quintessence Publishing Co Ltd. [Google Scholar]
- Klijn, R. J. , Meijer, G. J. , Bronkhorst, E. M. , & Jansen, J. A. (2010). Sinus floor augmentation surgery using autologous bone grafts from various donor sites: A meta‐analysis of the total bone volume. Tissue Engineering Part B: Reviews, 16, 295–303. https://doi.org/10.1089/ten.teb.2009.0558 [DOI] [PubMed] [Google Scholar]
- Kunert‐Keil, C. , Scholz, F. , Gedrange, T. , & Gredes, T. (2015). Comparative study of biphasic calcium phosphate with beta‐tricalcium phosphate in rat cranial defects – A molecular biological and histological study. Annals of Anatomy, 199, 79–84. https://doi.org/10.1016/j.aanat.2013.12.001 [DOI] [PubMed] [Google Scholar]
- Lee, K. S. , Hwang, C. J. , Kim, J. H. , Lee, H. S. , Ryu, H. S. , Chang, J. S. , & Lee, D. H. (2014). Study of difference in biodegradation mechanism on the surface of hydroxyapatite and ß‐tricalcium phosphate. Science of Advanced Materials, 6, 2238–2243. https://doi.org/10.1166/sam.2014.2073 [Google Scholar]
- LeGeros, R. Z. (1993). Biodegradation and bioresorption of calcium phosphate bioceramics. Clinical Materials, 14, 65–68. https://doi.org/10.1016/0267-6605(93)90049-D [DOI] [PubMed] [Google Scholar]
- Miron, R. J. , Zhang, Q. , Sculean, A. , Buser, D. , Pippenger, B. E. , Dard, M. , … Zhang, Y. (2016). Osteoinductive potential of 4 commonly employed bone grafts. Clinical Oral Investigations, 20(8), 2259–2265. https://doi.org/10.1007/s00784-016-1724-4 [DOI] [PubMed] [Google Scholar]
- Parfitt, A. M. , Drezner, M. K. , Gloreux, F. H. , Kanis, J. A. , Malluche, H. , Meunier, P. J. , … Recker, R. R. (1987). Bone histomorphometry: Standardization of nomenclature, symbols, and units: Reports of the asbmr histomorphometry nomenclature committee. Journal of Bone and Mineral Research, 2, 595–610. [DOI] [PubMed] [Google Scholar]
- Plenk, H. Jr (1989).Bone tissue and teeth In Böck P. (Ed.), Romeis microscopic technique, (17th edn, pp. 527–566). Munich, Germany: Urban & Schwarzenberg. [Google Scholar]
- Prins, H. J. , Fernandes, H. , Rozemuller, H. , van Blitterswijk, C. , de Boer, J. , & Martens, A. C. M. (2016). Spatial distribution and survival of human and goat mesenchymal stromal cells on hydroxyapatite and ß‐tricalcium phosphate. Journal of Tissue Engineering and Regenerative Medicine, 10, 233–244. https://doi.org/10.1002/term.1681 [DOI] [PubMed] [Google Scholar]
- Prins, H. J. , Schulten, E. A. J. M. , ten Bruggenkate, C. M. , Klein‐Nulend, J. , & Helder, M. N. (2016). Bone regeneration using the freshly isolated autologous stromal vascular fraction of adipose tissue in combination with calcium phosphate ceramics. Stem Cells Translational Medicine, 5, 1362–1374. https://doi.org/10.5966/sctm.2015-0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt, C. M. , Doering, H. , Schmidt, T. , Lutz, R. , Neukam, F. W. , & Schlegel, K. A. (2013). Histological results after maxillary sinus augmentation with Straumann® BoneCeramic, Bio‐Oss®, Puros®, and autologous bone. A randomized controlled clinical trial. Clinical Oral Implants Research, 24, 576–585. https://doi.org/10.1111/j.1600-0501.2012.02431.x [DOI] [PubMed] [Google Scholar]
- Schulten, E. A. J. M. , Prins, H. J. , Overman, J. R. , Helder, M. N. , ten Bruggenkate, C. M. , & Klein‐Nulend, J. (2013). A novel approach revealing the effect of a collagenous membrane on osteoconduction in maxillary sinus floor elevation with ß‐tricalcium phosphate. European Cells & Materials, 25, 215–228. https://doi.org/10.22203/eCM [DOI] [PubMed] [Google Scholar]
- Tadjoedin, E. S. , de Lange, G. L. , Bronckers, A. L. J. J. , Lyaruu, D. M. , & Burger, E. H. (2003). Deproteinized cancellous bovine bone (Bio‐Oss®) as bone substitute for sinus floor elevation. A retrospective, histomorphometrical study of five cases. Journal of Clinical Periodontology, 30, 261–270. https://doi.org/10.1034/j.1600-051X.2003.01099.x [DOI] [PubMed] [Google Scholar]
- Tatum, H. Jr (1986). Maxillary and sinus implant reconstructions. Dental Clinics of North America, 30, 207–229. [PubMed] [Google Scholar]
- Van de Wijngaert, F. P. , & Burger, E. H. (1986). Demonstration of tartrate‐resistant acid phosphatase in un‐decalcified, glycomethyacrylate‐embedded mouse bone: A possible marker for (pre)osteoclast identification. Journal of Histochemistry & Cytochemistry, 34, 1317–1323. https://doi.org/10.1177/34.10.3745910 [DOI] [PubMed] [Google Scholar]
- Van Esterik, F. A. S. , Zandieh‐Doulabi, B. , Kleverlaan, C. J. , & Klein‐Nulend, J. (2016). Enhanced osteogenic and vasculogenic differentiation potential of human adipose stem cells on biphasic calcium phosphate scaffolds in fibrin gels. Stem Cells International, 2016, 1934720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, S. S. , & Froum, S. J. (2003). Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Annals of Periodontology, 8, 328–343. https://doi.org/10.1902/annals.2003.8.1.328 [DOI] [PubMed] [Google Scholar]
- Yang, C. , Unursaikhan, O. , Lee, J. S. , Jung, U. W. , Kim, C. S. , & Choi, S. H. (2013). Osteoconductivity and biodegradation of synthetic bone substitutes with different tricalcium phosphate contents in rabbits. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 102, 80–88. [DOI] [PubMed] [Google Scholar]
- Yuan, H. , Fernandes, H. , Habibovic, P. , de Boer, J. , Barradas, A. M. C. , de Ruiter, A. , … de Bruijn, J. D. (2010). Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proceedings of the National Academy of Sciences of the United States of America, 107, 13614–13619. https://doi.org/10.1073/pnas.1003600107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo, I. R. , Bronckers, A. L. J. J. , de Lange, G. , & Burger, E. H. (2005). Localisation of osteogenic and osteoclastic cells in porous ß‐tricalcium phosphate particles used for human maxillary sinus floor elevation. Biomaterials, 26, 1445–1451. https://doi.org/10.1016/j.biomaterials.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Zijderveld, S. A. , van den Bergh, J. P. A. , Cune, M. S. , ten Bruggenkate, C. M. , & van Waas, M. A. (1997). Pre‐implantation surgery of the atrophied maxilla. A review of the literature. Nederlands Tijdschrift Voor Tandheelkunde, 104, 259–261. [PubMed] [Google Scholar]
- Zijderveld, S. A. , Zerbo, I. R. , van den Bergh, J. P. A. , Schulten, E. A. J. M. , & ten Bruggenkate, C. M. (2005). Maxillary sinus floor augmentation using a ß‐tricalcium phosphate (Cerasorb) alone compared to autogenous bone grafts. International Journal of Oral & Maxillofacial Implants, 20, 432–440. [PubMed] [Google Scholar]
- Zuk, P. A. , Zhu, M. , Ashjian, P. , de Ugarte, D. A. , Huang, J. I. , Mizuno, H. , … Hedrick, M. H. (2002). Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell, 13, 4279–4295. https://doi.org/10.1091/mbc.E02-02-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk, P. A. , Zhu, M. , Mizuno, H. , Huang, J. , Futrell, J. W. , Katz, A. J. , … Hedrick, M. H. (2001). Multilineage cells from human adipose tissue: Implications for cell‐based therapies. Tissue Engineering, 7, 211–228. https://doi.org/10.1089/107632701300062859 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


