Abstract
Melatonin is a neurohormone secreted from the pineal gland and has a wide‐ranging regulatory and neuroprotective role. It has been reported that melatonin level is disturbed in some neurological conditions such as stroke, Alzheimer's disease, and Parkinson's disease, which indicates its involvement in the pathophysiology of these diseases. Its properties qualify it to be a promising potential therapeutic neuroprotective agent, with no side effects, for some neurological disorders. This review discusses and localizes the effect of melatonin in the pathophysiology of some diseases.
Keywords: melatonin, ischemia, alzheimer, parkinson
1. INTRODUCTION
Melatonin is a neurohormone secreted from the pineal gland, which is situated at the center of the brain (Tan, Manchester, Sanchez‐Barcelo, Mediavilla, & Reiter, 2010). It has a wide range of regulatory and protective effects, such as synchronizing circadian rhythm, protecting against oxidative stress, regulating energy metabolism, modulating the immune system, and postponing the ageing process (Tan, Manchester, Terron, Flores, & Reiter, 2007; De Pedro, Martinez‐Alvarez, & Delgado, 2008; Caballero et al., 2009; Shieh, Wu, Cheng, & Cheng, J., 2009; Hardeland, 2010; Tan et al., 2010). Melatonin is also secreted from extra‐pineal sources, the highest melatonin release coming from the gut and the skin (Tan et al., 2007). Other extra‐pineal sources are the retina, the testes, the ovary, the placenta, glial cells, and lymphocytes (Tan et al., 2007). However, melatonin secreted from extra‐pineal sources has a small effect on the plasma melatonin circadian oscillation, as pinealectomy is found to disturb melatonin rhythm (Pelham, 1975). Extra‐pineal areas secrete melatonin, which remains within these tissues and acts mainly as an antioxidant agent (Tan et al., 2007).
Normally, melatonin is secreted during the first year of life, starting from a very low level before the age of three months and then increasing rhythmically to reach its peak at 1–3 years, followed by a gradual decrease until adulthood (Waldhauser, Ehrhart, & Forster, 1993). Diurnally, melatonin is found to be highly secreted between 03:00 and 04:00 (Claustrat & Leston, 2015). Melatonin circulating in the blood is distributed widely throughout the body, including in saliva, urine, cerebrospinal fluid, preovulatory follicles, semen, amniotic fluid and milk (Reiter, 1991). The melatonin level in the bloodstream indicates active pineal gland function (Reiter, 1991). Melatonin is lipophilic and hydrophilic in nature, and this amphiphilic property bestows the advantage of crossing the blood–brain barrier (Pardridge & Mietus, 1980).
2. BIOSYNTHESIS
Melatonin synthesis occurs in pinealocytes from tryptophan, which is converted to serotonin and finally into melatonin in a four‐step process (Tan et al., 2010). Tryptophan is naturally present in the bloodstream and its acute depletion has been shown to be capable of reducing the nocturnal melatonin level in humans (Zimmermann et al., 1993). As folate is essential in the methylation step of converting N‐acetylserotonin to melatonin, folate deficiency decreases melatonin release in rats (Fournier, Ploye, Cottet‐Emard, Brun, & Claustrat, 2002). Moreover, vitamin B6 (pyridoxine) plays an important role in tryptophan decarboxylation and increases the release of melatonin by the pineal gland in children but not in adults (Munoz‐Hoyos et al., 1996; Luboshitzky et al., 2002).
Two enzymes are essential for the conversion of serotonin into melatonin, namely serotonin‐N‐acetyltransferase (NAT; also known as arylalkylamine N‐acetyltransferase [AANAT] and hydroxyindole‐O‐methyltransferase [HIOMT]; [Tan, Manchester et al., 2010]). It has been found that the expression of mRNAs encoding these enzymes in a chicken's pineal gland is controlled by day/night circadian oscillators that reach their maximum level at night (Bernard et al., 1999). Norepinephrine (NE) has a key role in initiating melatonin synthesis, in which the binding of NE to adrenergic 1 receptors in pinealocytes stimulates cyclic AMP (cAMP) and therefore NAT formation (Tan et al., 2010).
![]()
3. METABOLISM
In the brain, melatonin is oxidized by formamidase and produces N1‐acetyl‐N2‐formyl‐5‐methoxykynuramine (AFMK; Hirata, Hayaishi, Tokuyama, & Seno, 1974; Cardinali, 1981). N1‐acetyl‐5‐methoxy‐kynuramine (AMK) is another well‐known melatonin metabolite. AFMK and AMK can be produced by enzymatic, free radical or ultraviolet radiation pathways (Hirata et al., 1974; Cardinali, 1981; Tan et al., 2001; Hardeland, Tan, & Reiter, 2009). Both melatonin metabolites are considered potent antioxidants with the capacity to scavenge free radicals and activate the free radical scavenging cascade (Tan et al., 2001; Hardeland et al., 2009).
In the liver, circulating melatonin is hydroxylated by cytochrome CYP1A2 into 6‐hydroxymelatonin, with wide variance in CYP1A2 activity between individuals (Gunes & Dahl, 2008). Different factors are shown to affect this activity, which in turn alters the melatonin level. For example, caffeine is shown to counteract CYP1A2 action, thus inhibiting melatonin metabolism in the liver and raising the melatonin level (Hartter et al., 2003; Hartter et al., 2006). Cigarette smoking is shown to activate CYP1A2 and depress the exogenous melatonin level (Ursing, von Bahr, Brismar, & Rojdmark, 2005). 6‐hydroxymelatonin is excreted in the urine in the form of sulphate and glucuronide (Isidorov & Nazaruk, 2017). The urinary level of 6‐hydroxymelatonin sulphate is reportedly associated with the plasma melatonin level (Arendt, Bojkowski, Franey, Wright, & Marks, 1985).
4. REGULATION OF MELATONIN SECRETION
Melatonin in mammals is released in a rhythmic oscillation pattern, which is regulated by suprachiasmatic nuclei (SCN) of the hypothalamus in animals and humans (Cohen & Albers, 1991; Moore, 1992; Edgar, Dement, & Fuller, 1993; Cardinali & Pevet, 1998). Melatonin release is controlled by the diurnal cycle, in which daylight supresses melatonin release by signals transmitted by the retino‐hypothalamic tract to the SCN. It has been found that an adequate intensity of artificial light in the room at night suppresses melatonin release in humans (Lewy, Wehr, Goodwin, Newsome, & Markey, 1980). The intensity of light is inversely proportional to the nocturnal plasma level of melatonin (Bojkowski et al., 1987). Exposure to a light intensity of about 2000 to 2500 lx between 02:00 and 04:00 significantly supresses the melatonin plasma level (Bojkowsk et al., 1987).
A neuronal connection is found between the SCN and the sympathetic system through the superior cervical ganglion, in which fibres projected from the ganglion directly synapse with the pineal gland and secrete norepinephrine to activate melatonin synthesis and release (Moller, 1992; Moller & Baeres, 2002). Therefore, blocking 1‐adrenergic receptors by atenolol suppresses melatonin release, as detected by urinary melatonin metabolites (Arendt et al., 1985).
5. SOURCES
A wide variety of dietary elements contain melatonin, including nuts, seeds, fruits, vegetables, and cereals (Manchester et al., 2000; Iriti, 2009; Iriti & Faoro, 2009; Paredes, Korkmaz, Manchester, Tan, & Reiter, 2009). A radioimmunoassay showed an increased level of plasma melatonin following an intake of food rich in melatonin (Hattori et al., 1995). It is known that the plasma level of melatonin is reflected in melatonin level in the body (Tan et al., 2010). The normal melatonin level in the blood varies diurnally within a range of a few pg/ml during the day to more than 50 pg/ml at night. However, the plasma melatonin level is much lower than in other areas such as the gut and bone marrow, both of which are considered extra‐pineal sources of melatonin. Thus, the level of melatonin in plasma does not reflect its concentration in other areas of the body (Huether, 1993; Tan et al., 1999).
6. MELATONIN IN THE CENTRAL NERVOUS SYSTEM (CNS)
AANAT and HIOMT are two crucial enzymes in the melatonin synthesis pathway. AANAT is important in converting serotonin into N‐acetylserotonin, while HIOMT converts N‐acetylserotonin into melatonin (Hirata et al., 1974). The HIOMT enzyme plays a physiological role in regulating melatonin peak release at night (Ribelayga, Pevet, & Simonneaux, 2000).
![]()
Previously, it was believed that the pineal source of melatonin is the origin of the level of melatonin in the blood and CNS. However, recent data show that there are other CNS sources of melatonin. Reverse transcription polymerase chain reaction (RT‐PCR) identified mRNA encoding AANAT and HIOMT in a wide range of rat brain areas (Stefulj et al., 2001). This reflects the possible endogenous melatonin synthesis and release from different brain regions. AANAT and HIOMT enzymes are found in astrocytes, which release melatonin in cell cultures of the rat cortex and glioma C6 cell line (Liu et al., 2007).
Both melatonin metabolites, AFMK, and AMK are present in the brain. AFMK was first discovered in a rat brain in 1974 by Hirata et al. and since then it is believed to be the main melatonin metabolite in the brain (Hirata et al., 1974; Tan et al., 2007).
The AFMK concentration in cases of meningitis is found to be several times higher than in normal healthy cerebrospinal fluid (CSF; Silva, Ximenes, Livramento, Catalani, & Campa, 2005). Given that every unit of melatonin produces a single AFMK, a high concentration of CSF AFMK in meningitis cases exceeds the pineal gland's capacity to produce melatonin, which in turn suggests another source of melatonin secretion to the CSF. The synthesis and release of melatonin are considered to be a stress‐mediated mechanism in which melatonin is a potent neuroprotector and antioxidant (Carloni et al., 2008; Carretero et al., 2009; Manda, Ueno, & Anzai, 2009). Stress‐mediated melatonin release has been demonstrated in the case of acute pancreatitis in rats and severe traumatic brain in humans (Jaworek et al., 2003; Seifman et al., 2008).
7. THE PINEAL GLAND AND MELATONIN IN THE CSF
The direct connection of the pineal recess to the third ventricle was first recognized in 1969 (Sheridan, Reiter, & Jacobs, 1969; Sheridan & Reiter, 1970). Melatonin concentration in sheep was found to reach its highest level in the third ventricle near the pineal recess, the concentration gradually decreasing in the CSF with increased distance from the third ventricle (Tricoire, Locatelli, Chemineau, & Malpaux, 2002). The high‐performance liquid chromatography (HPLC) technique used in humans showed a high level of melatonin in the third ventricles and lower levels in the lateral and fourth ventricles (Longatti et al., 2007). However, HPLC can measure the level of free melatonin only, thus missing a high percentage of melatonin in bound form (Rizzo et al., 2002). Surgical sealing of the pineal recess leads to a drop in the melatonin concentration in the third ventricle (Tricoire et al., 2002). This result is consistent with the direct release of pineal melatonin to third ventricle through the pineal recess. All of these data have opened the field for more research to investigate whether the CNS level of melatonin is of pineal origin only. What is the role of melatonin secreted from the glial cells? Is it activated during stress only or is there a regulatory system controlling its synthesis and release?
8. DISTRIBUTION OF MELATONIN RECEPTORS IN THE NERVOUS SYSTEM
Melatonin binds to two types of G‐protein‐coupled receptors, namely MT1 and MT2 (Dubocovich & Markowska, 2005; Benitez‐King, 2006; Ng, Leong, Liang, & Paxinos, 2017). MT1 is distributed in a wide area of the nervous system, including the hippocampus, the caudate putamen, the suprachiasmatic nucleus (SCN), the paraventricular nucleus, the periventricular nucleus, the supraoptic nucleus, the Meynert nucleus, the nucleus accumbens, the substantia nigra tuberomammillary nucleus, the mammillary bodies, and the retina (Dubocovich & Markowska, 2005; Wu et al., 2006; Ng et al., 2017). On the other hand, MT2 is mainly expressed in the hippocampus, the SCN and the retina (Dubocovich & Markowska 2005; Ng et al., 2017). Both receptors are expressed by neurons and glial cells of the cerebral and cerebellar cortex, thalamus, and pineal gland (Brunner et al., 2006; Wu et al., 2006; Ng et al., 2017). Interestingly, it has been found that expression of the mRNA encoding MT1 receptor is affected by the day/night cycle, and that there is a relation between plasma melatonin level and MR1 mRNA expression (Masana, Benloucif, & Dubocovich, 2000).
9. THE BRAIN IS AN ORGAN THAT IS SENSITIVE TO ENERGY DISTURBANCE
Although the brain makes up only 2% of the human body weight, it consumes around 20% of the body's oxygen. This high level of oxygen consumption can initiate a harmful process known as oxidative stress. Oxidative stress is the appearance of reactive oxygen species (ROS) in a way that exceeds the capacity of the antioxidant effect (Gupta Y., Gupta, & Kohli, 2003). ROS are unstable molecules with one or more unpaired electrons in their outer layer (Guetens, De Boeck, Highley, van Oosterom, & De Bruijn, 2002); for example, superoxide (O2 −), hydroxyl (OH−), peroxyl (RO2 −), alkoxyl (RO−) radicals or covalent molecules as H2O2 (Caimi et al., 2004). These molecules are harmful and destroy DNA, proteins, and the cell membrane (Gupta et al., 2003).
A high level of fat in the brain distributed in the cell membranes and myelin sheath makes it more prone to targeted damage by ROS. A lower level of antioxidant enzymes compared to other body areas generates an imbalance between ROS production and the opposing effect of antioxidants (Skaper et al., 1999). ROS damage extensively affecting the brain function leaves the blood–brain barrier leaky, disturbing mitochondrial respiration and changing the tubulin arrangement (Gupta et al., 2003). Studies showed that ROS enhances a release of excitatory neurotransmitter as glutamate to the extracellular space, which acts on different types of receptors, mainly NMDA receptors, and triggers an anoxic depolarization (Gilman, Bonner, & Pellmar, 1993). Moreover, ROS alters gene expression, mediates apoptotic cascade, and eventually decreases neuronal viability (Gilgun‐Sherki, Rosenbaum, Melamed, & Offen, 2002).
10. NEUROLOGICAL CONDITIONS AND MELATONIN
10.1. Melatonin and ischaemia
Ischaemic stroke is the second main cause of death and the leading cause of disability worldwide (Flynn, MacWalter, & Doney, 2008; Mathers, Boerma, & Ma Fat, 2009). There are two types of stroke, ischaemic stroke, which represents 85% of all strokes, and high‐mortality haemorrhagic stroke, which accounts for 15% of all strokes (Flynn et al., 2008). A complex cascade of cellular injury events is set in motion during ischaemia, consisting of excitotoxicity, ROS production, and inflammation.
Neurons are very active excitable cells that need a high metabolic rate to maintain their energy‐dependent activities (Hossmann, 1994). Thus, any restriction in the cerebral blood flow, as in the case of ischaemic stroke, is considered a serious situation for neurons, as they need a steady and consistent supply of oxygen and glucose. In the case of oxygen glucose deprivation, cell viability is affected by different mechanisms, starting from energy deprivation, which affects one of the main ATP‐dependent pumps, Na+/K+‐ATPase, causing its failure and reversing its function (efflux of K+ and influx of Na+; Stys, 1998). A high level of intracellular Na+ [Na+]I initiates anoxic depolarization, activates voltage gated calcium channels (VGCC) and reverses Na+‐Ca2+ exchange (Stys, 1998). Consequently, Ca2+ ions move inside the cells and activate a Ca2+ mediated injury process (Stys, 1998). This is consistent with the findings of Muller and Ballanyi, in which ischaemia initiated anoxic depolarization coincident with a huge increase in intracellular Ca2+ [Ca2+]I (Muller & Ballanyi, 2003). The level of glutamate, a major excitatory neurotransmitter, during ischaemia is increased extracellularly through inverse of its uptake and the release from presynaptic neurons (Obrenovitch, 1996; Rossi, Oshima, & Attwell, 2000). A prolonged high level of glutamate binds to NMDA and non‐NMDA receptors and initiates an excitotoxicity cell injury (Rothman & Olney, 1986; Arundine & Tymianski, 2003). NMDA receptors mediate Ca2+ influx and amplify Ca2+ overload, while AMPA receptors allow Na+ entry, leading to cell swelling and brain oedema (Dirnagl, Iadecola, & Moskowitz, 1999; Lipton, 1999).
Excitotoxicity is followed by an oxidative stress neurotoxic effect. High [Ca2+]I overload activates multiple oxidative enzymes (e.g., phospholipases, cyclooxygenases, NO synthase, and proteolytic enzymes). These enzymes accelerate the formation of free radicals, which in turn mediates a series of cellular injury events such as lipid peroxidation, DNA damage, mitochondrial injury, and blood–brain barrier (BBB) breakdown, which mediates brain oedema (Dirnagl et al., 1999; Lipton, 1999, Rodrigo, Fernandez, Serrano, Peinado, & Martinez, 2005).
Excitotoxicity, a high [Ca2+]I level and oxidative stress are followed by inflammation. The inflammation stage is started by the activation of different factors such as NFκB, hypoxia‐mediated factor 1, and STAT3, which are responsible for the production of inflammatory cytokines (TNF‐α, IL‐1β), enzymes (iNOS, COX‐2), and adhesion molecules (ICAM‐1, selectins) and for increasing the number of activated phagocytes (Dirnagl et al., 1999; Iadecola & Alexander, 2001).
These stages of ischaemic injury start from the first minutes of ischaemic insult and persist for several days, in which the ischaemic damage started at the core of the injury spreads out to the penumbra, a hypoperfused and functionally disturbed, but viable tissue (Dirnagl et al., 1999).
Clinically, in acute ischaemic stroke, thrombolysis or thrombectomy are established to remove the obstruction in the blood vessels and thereby regain cerebral circulation in the affected area. However, reperfusion will play a role in increasing the production of oxygen free radicals and therefore exacerbate oxidative stress and inflammatory injuries (Chen et al., 2011). Therefore, combining neuroprotective strategies with thrombosis or thrombolysis provides an effective way of treating stroke patients with better outcomes. The main objective is to save the penumbra from cell death.
Melatonin is considered one of the most potent antioxidant, playing an important protective role in ischaemic injury (see Figure 1; Watson, Diamandis, Gonzales‐Portillo, Reyes, & Borlongan, 2016; Wu et al., 2017). In middle cerebral artery occlusion (MCAO), a model of acute ischaemia in rats, receiving pineal gland transplantation improves the motor outcome and decreases the infarct size through the secretion of melatonin (Borlongan et al., 2003). Exogenous melatonin administration (4 mg/kg) significantly improves motor outcome and decreases infarct size by 40% in pinealectomized rats subjected to MCAO (Kilic, Ozdemir, Bolay, Kelestimur, & Dalkara, 1999). Moreover, it has been found that melatonin injections protect against oxidative brain injury in cases of subarachnoid haemorrhage (SAH) in rat models (Martinez‐Cruz, Espinar, Pozo, Osuna, & Guerrero, 2002; Ersahin et al., 2009; Wu et al., 2017).
Figure 1.
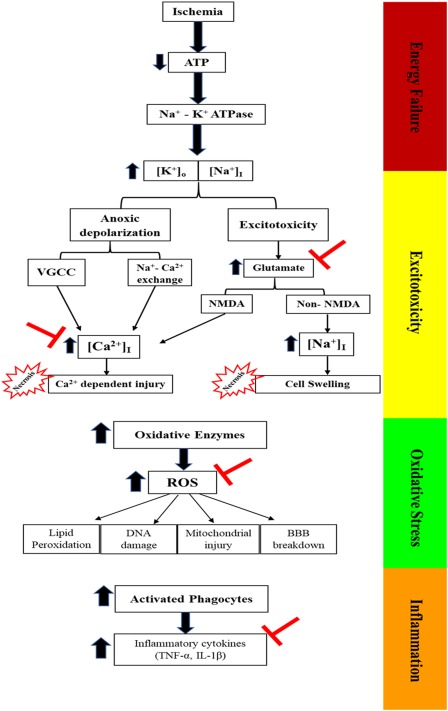
Ischaemia pathophysiology at four stages: energy failure, excitotoxicity, oxidative stress and inflammation. ( ) Represents the site of melatonin action
) Represents the site of melatonin action
Melatonin has a role in maintaining Ca2+ homeostasis and preventing any impairment. Moreover, it has been shown to decrease the extracellular level of glutamate in hippocampal sections by reversing its release in an oxygen glucose deprivation (OGD) model of rat ischaemia (Patino et al., 2016). Melatonin prevents an acid‐induced increase in [Ca2+]I levels (Bhattacharya, Pandey, Paul, & Patnaik, 2014). In addition, it diminishes a glutamate‐dependent increase in [Ca2+]I level by decreasing parvalbumin and hippocalcin, calcium‐buffering proteins in the rat's cerebral cortex (Koh, 2012).
A fluorescence live‐animal imaging system has shown that melatonin reduces free radical generation by acting on the MT2 receptor after a transient middle cerebral artery ischaemia in mice (Chern et al., 2012). Moreover, melatonin reportedly reduces Nox2 and Nox4 expression, thus decreasing the oxidative stress damage that is seen in ischaemic/reperfusion injury in rats (Li et al., 2014). Melatonin administration 60 minutes after MCAO has been shown to play a role as an antioxidant in reducing nitrite and malondialdehyde (MDA) levels and improving motor behavioural outcomes and brain oedema (Bhattacharya et al., 2014).
A study conducted on 45 human newborns diagnosed by hypoxic‐ischaemic encephalopathy showed that melatonin combined with hypothermia decreases plasma‐free radicals (nitric oxide [NO]), seizure attacks, and white matter insults and finally improves neurological outcomes (Aly et al., 2015). Another 10 asphyxiated human newborns treated with 8 doses of 10 mg melatonin demonstrated a better antioxidant effect, reducing serum malondialdehyde and nitrite/nitrate levels, therefore improving their survival outcomes (Fulia et al., 2001).
Melatonin administration decreases macrophage brain infiltration in transient focal cerebral ischaemia and MCAO of rats, which in turn prevents excess secretion of inflammatory cytokines and subsequent inflammatory injury (Chen et al., 2006; Lee et al., 2007). RT‐PCR demonstrated that melatonin administration significantly reduced the expression of interleukin‐1 beta (IL‐1β) and tumour necrosis factor alpha (TNF‐α) in ageing MCAO rats (Paredes et al., 2015). Messenger RNA expression of Bcl‐2‐associated death promoter (BAD), Bcl‐2‐associated X protein (BAX), glial fibrillary acidic protein (GFAP), B‐cell lymphoma 2 (Bcl‐2), and sirtuin 1 has been measured by reverse‐polymerase chain reaction. This also preserves the integrity of BBB and significantly diminishes its dysfunction through different mechanisms such as NO, ROS, and conserves tight junctions (Chen et al., 2006; Grossetete, Phelps, Arko, Yonas, & Rosenberg, 2009; Song et al., 2014; Moretti et al., 2015; Alluri et al., 2016). All these results suggest that melatonin can be of important therapeutic value in preventing BBB leakage and brain oedema.
10.2. Melatonin and Alzheimer's disease (AD)
Alzheimer's disease is an age‐related neurodegenerative disease marked by toxic protein aggregation inside and outside the neurons. Extracellular β‐amyloid (Aβ) and intracellular neurofibrillary tangles (NFTs) are the hallmark of Alzheimer's disease (Ittner & Gotz, 2011). These abnormal proteins are found to be accumulated in memory‐related areas in the brain, such as the neocortex and hippocampus, leading to progressive decline in cognitive function (He, Dong, & Huang, 2010). The accumulation of toxic proteins mediates oxidative stress, synaptic dysfunction and neuronal loss (Sultana & Butterfield, 2010; Jeong, 2017). The aetiology of the disease is still not clear; however, several factors are found to contribute to the disease, such as genetic factors, sex, lipid metabolism, ageing, diet, and metal ion toxicity (Sultana & Butterfield, 2010; Leszek, Sochocka, & Gasiorowski, 2012; Mustapic et al., 2012). The most common genes attributed to Alzheimer's disease are the amyloid precursor protein (APP), apolipoprotein E (ApoE), and presenilins 1 (PS1) and 2 (PS2; Price & Sisodia, 1998; Shimohama, 2000; Thomas, Thomas C, Radcliffe, & Itsiopoulos, 2015; Dominguez & Barbagallo, 2016; Loffler, Flunkert, Temmel, & Hutter‐Paier, 2016; Canerina‐Amaro et al., 2017; Dong, Gim, Yeo, & Kim, 2017; Li et al., 2017).
High Aβ production is considered the primary cause of neuropathology in AD (Hardy & Selkoe, 2002). Senile plaques consist of Aβ peptides that are made up of around 40–43 amino acids (Soto, Branes, Alvarez, & Inestrosa, 1994; Soto, Castano, Frangione, & Inestrosa, 1995; Selkoe, 1998). Amyloid protein precursor (APP) undergoes proteolytic cleavage by β‐secretase at the C‐terminal and γ‐secretase at the N‐terminal to produce Aβ peptide (Nunan & Small, 2000). On the other hand, α‐secretase splits APP at the middle region of the Aβ sequence and does not produce Aβ (Vardy, Catto, & Hooper, 2005). Different gene mutations are suggested to play a role in Aβ formation, such as PS1 and PS2 (Bruni, 1998; Liu, Zhou, van Heerikhuize, Hofman, & Swaab, 1999; Seiffert et al., 2000; Lleo, Berezovska, Growdon, & Hyman, 2004). These gene mutations mediate soluble Aβ production. However, aggregation and deposition of soluble Aβ peptides produces a toxic fibrillary Aβ (Yankner, 1996; Alvarez et al., 1998; Soto et al., 1998). Two common types of Aβ are found in brain, Aβ40 and Aβ42, which differ in the number of amino acids (40 and 42, respectively) and are similar in their hydrophobic property (He et al., 2010). This property mediates Aβ fibrils to aggregate in a β‐sheet structure known as amyloid plaques (He et al., 2010). A cascade of Aβ plaque formation was presented by Louise C. Serpell, in which the cascade started to form APP, soluble Aβ, Aβ oligomers, Aβ protofilament, Aβ fibrils, and Aβ plaques (Serpell, 2000). Aβ fibrils are found to be condensed in the AD brain, and several studies reported its neurotoxic effects, including synaptic dysfunction, neuronal death, and hence, dementia (Lorenzo & Yankner, 1994; Younkin, 1995; Forloni, 1996; Selkoe, 1999; Iwata et al., 2000; Puglielli, Tanzi, & Kovacs, 2003).
Oxidative stress is involved in the aetiology and the subsequent neurodegenerative pathology of AD (Baldeiras et al., 2008; Greilberger et al., 2008; Padurariu et al., 2010; Ferreiro et al., 2012). A high level of free radicals in AD is mediated by several causes, such as Aβ deposition, mitochondrial dysfunction, inflammation, and activated microglia (Padurariu, et al., 2013). Aβ plaques are considered one of the main causes of oxidative stress in AD, with a two‐way effect, in which oxidative stress mediates lysosomal production of Aβ and Aβ itself induces lysosomal membrane dysfunction and finally cell death (Zheng, Roberg, Jerhammar, Marcusson & Terman, 2006).
The antiamyloidogenic effect of melatonin in AD has been reported in various studies (Figure 2; Shukla, Govitrapong, Boontem, Reiter, & Satayavivad 2017). It has been shown that melatonin inhibits the formation of soluble APP in vitro, which in turn could prevent Aβ production (Lahiri, 1999), consistent with the decrease in APP mRNA level after melatonin administration in P12 cells, but not in human neuroblastoma cells (Song & Lahiri, 1997). Moreover, it has been reported that melatonin can interfere with Aβ fibril production in vitro through interacting with A 40 and A 42 (Pappolla et al., 1998; Pappolla et al., 2000; Poeggeler et al., 2001).
Figure 2.
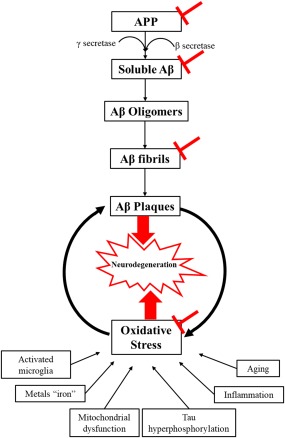
Alzheimer's pathophysiology with melatonin site of action ( )
)
It has been found that long‐term application of melatonin (for around two months) reduced immunoreactive Aβ deposition in the hippocampus and cortex by 43% and 37%, respectively in a transgenic rat model of Alzheimer's (Olcese et al., 2009). Melatonin administration during the active stage of disease progression reduced amyloid deposition in the hippocampus (β1–42 and β1–40) and frontal cortex (β1–42), decreased degenerative changes in the hippocampus, prevented mitochondrial dysfunction, and delayed anxiety and cognitive impairment in a sporadic rat model of Alzheimer's (Rudnitskaya, Muraleva et al., 2015). Chronic melatonin application after intracerebroventricular Aβ1–42‐injection demonstrated diminished tau hyperphosphorylation and Aβ mediated memory deficits, thus avoiding neurodegeneration in mice hippocampus (Ali & Kim, 2015).
Melatonin is also reported to be a potent antioxidant in AD (Figure 2; Shukla et al., 2017). Several studies have found that melatonin reduces Aβ mediated oxidative stress and lipid peroxidase (Daniels, van Rensburg, van Zyl, & Taljaard, 1998; Srinivasan et al., 2005; Shukla et al., 2017). Melatonin has been shown to regulate the level of mRNA encoding in some antioxidant enzymes (SOD‐1, glutathione peroxidase, and catalase) to its normal level in the cortex of transgenic AD mice (Olcese et al., 2009). It reduced the production of ROS by inhibiting the formation of NADPH oxidase, the main source of oxidative stress in AD, in β‐stimulated microglial culture (Zhou, Zhang, Zhao, & Wei, 2008). The oxidative stress end products are abundantly found in AD brains. Lipid peroxidation, DNA damage and oxidative altered protein metabolites are routinely identified in postmortem AD brains (Subbarao, Richardson, & Ang, 1990; Mecocci, MacGarvey, & Beal, 1994; Smith, Sayre, Monnier, & Perry, 1995; Markesbery, 1997). Moreover, neuroinflammation and the activated microglia worsen oxidative stress by enhancing NO generation, which mediates neuronal degeneration, especially in proximal death (Weldon et al., 1998).
It has been found that long administration of oral melatonin enhances hippocampal synaptic growth and preserves the neuronal and glial structure in a sporadic rat model with Alzheimer's (Stefanova et al., 2015). In addition, melatonin reportedly improves spatial memory, reduces synaptic dysfunction, and decreases astrogliosis in the rat hippocampus after intracerebroventricular injections of soluble Aβ1–42 (Zhang, Wang, Ren, Hu, & Bi, 2016). Further research demonstrated that melatonin diminishes apoptotic mediators in a senescence‐accelerated mice (SAMP8) model of Alzheimer's (Gutierrez‐Cuesta et al., 2008).
Clinically, multiple studies reported a decrease in melatonin level in AD patients compared to healthy people (Skene et al., 1990; Uchida, Okamoto, Ohara, & Morita, 1996; Liu et al., 1999; Mishima et al., 1999; Ohashi et al., 1999). The melatonin level in postmortem ventricular CSF is adversely related to Braak and modified Braak staging in the human cortex (Zhou, Liu, Kamphorst, Hofman, & Swaab, 2003). Thus, the melatonin level is considered as a marker for the progression of AD neuropathology. In Braak staging I and II “pre‐clinical” stages of Alzheimer's disease, melatonin circadian oscillation is lost owing to noradrenergic dysfunction and monoamine oxidase generation (Wu et al., 2003). A retrospective study showed that melatonin significantly improved Beck depression inventory scores (BDI), mini‐mental state examination results (MMSE), the cognitive subscale of the Alzheimer's disease assessment scale (ADAS‐Cog) and sleep quality in mildly cognitively impaired patients (Cardinali, Vigo, Olivar, Vidal, Furio, & Brusco, 2012). However, more studies are needed to investigate the mechanism and the therapeutic potentials of melatonin in AD patients.
Melatonin is known as anti‐β amyloid aggregation, antioxidant and anti‐inflammatory and therefore prevents synaptic dysfunction, neuronal loss and cognitive impairment.
10.3. Melatonin and Parkinson's disease (PD)
Several million people suffer from PD worldwide (Elbaz & Moisan, 2008; Wirdefeldt, Adami, Cole, Trichopoulos, & Mandel, 2011). Several risk factors are positively associated with PD incidence, including genetic factors, age, exposure to lead and manganese, and consumption of dairy products; while coffee and tea are inversely associated with PD (Elbaz & Moisan, 2008; Wirdefeldt et al., 2011; Hughes et al., 2017). A lot of controversies have been raised in the way of association of cigarette smoking with PD (Ma, Liu, Neumann, & Gao, 2017).
PD is characterized by dopaminergic neuronal loss in the substantia nigra pars compacta (SNc), leading to depletion in striatal dopamine, which in turn affects smooth, coordinated motor movements, mediating the appearance of rigidity, tremor, bradykinesia, and postural instability (Zhang et al., 1999; Tansey, McCoy, & Frank‐Cannon, 2007; Maguire‐Zeiss & Federoff, 2010). Non‐motor symptoms have also been reported in PD patients, including impulse control disorders (ICDs) and neuropsychiatric, autonomic, sleep, and sensory dysfunction (Weintraub, Comella, & Horn, 2008; Garcia‐Ruiz, Chaudhuri, & Martinez‐Martin, 2014). The pathological hallmark of PD is dopaminergic neuronal death, which may affect 60% of total dopaminergic neurons, thus affecting the connections with other neurons (Zigmond MJ). The histological hallmark of PD is the distribution of Lewy bodies and α‐synuclein protein aggregation on neurons (Spillantini, Crowther, Jakes, Hasegawa, & Goedert, 1998; Shults, 2006). Aggregation of Lewy bodies compromises mitochondrial dynamics, which mediates ROS release and cell death (Pozo Devoto, & Falzone, 2017).
Several types of PD animal models have been established to mediate dopamine neuronal death and augment the generation of sensory and motor deficit, which in turn expose PD symptoms, such as tremor, rigidity and akinesia (Schober, 2004; Terzioglu & Galter, 2008). Two mechanisms of PD animal model induction are commonly used, namely nigrostriatal injection of 6‐hydroxydopamine (6‐OHDA) and cerebral injections of neurotoxins such as MPTP (Schober, 2004; Terzioglu, & Galter, 2008). Transgenic models of PD have also been established, but some studies reflect some concerns about transgenic models and neurotoxic induced models, which should be taken into consideration (Terzioglu & Galter, 2008).
Evidence has been accumulating that age‐associated PD is combined with oxidative stress (Olanow 1992, Padurariu, Ciobica et al., 2013). It has been reported that melatonin injections interfere with lipid peroxidation in the hippocampus and striatum and that they inhibit neuronal death in the nigrostriatal area in an MPTP‐induced PD model (Acuna‐Castroviejo, Coto‐Montes et al., 1997, Antolin, Mayo et al., 2002). Moreover, melatonin elevates the level of antioxidant enzymes, such as catalase and superoxide dismutase, in the nigrostriatal pathway in a 6‐OHDA‐induced PD animal model (Saravanan, Sindhu, & Mohanakumar, 2007). Another study has elicited similar results of the neuroprotective effect of melatonin in the mouse nigrostriatum of a 6‐OHDA induced PD animal model through inhibiting OH generation and preventing glutathione (GSH) reduction (Thomas & Mohanakumar, 2004). Moreover, melatonin clearly counteracts the reduction in mitochondrial oxidative phosphorylation enzyme (complex I) in the substantia nigra of a 6‐OHDA‐induced PD animal model (Dabbeni‐Sala, Di Santo, Franceschini, Skaper, & Giusti, 2001). In a maneb‐ and paraquat‐induced PD mice model, melatonin improves locomotor activity by reducing the rise in nigrostriatal dopaminergic degeneration and lipid peroxidation (Singhal, Srivastava, Patel, Jain, & Singh, 2011). By downregulating the oxidative stress effects, melatonin is shown to be a potent antioxidant, which can improve the prognosis in PD (Figure 3).
Figure 3.
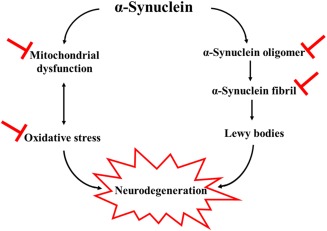
Parkinson's disease pathophysiology with melatonin site of action ( )
)
Toxic α‐synuclein is formed by α‐synuclein oligomerization, fibrillation, and finally by producing Lewy bodies, which mediates neurodegeneration cell death (Outeiro, Putcha et al., 2008). Melatonin has been shown to work as an anti‐assembly and to play a role in interfering with α‐synuclein toxic oligomer and α‐synuclein fibril, therefore reduceing α‐synuclein induced cytotoxicity (Ono, Mochizuki et al., 2012). This is consistent with the Western blot finding, which reported that melatonin inhibits arsenite‐induced apoptosis by reducing the accumulation of α‐synuclein in the rat brain (Lin, Fang, Chao, & Yang, 2007; Figure 3).
In humans, RT‐PCR of postmortem PD brains found reduced expression of MT1 and MT2 receptors in the amygdala and substantia nigra (Adi et al., 2010). Moreover, it has been reported that melatonin rhythm amplitude and 24‐hour plasma level are significantly reduced in PD patients compared to controls (Videnovic, Noble et al., 2014). These results reflect the involvement of the melatoninergic system in the pathophysiology of PD in humans. However, clinically, evidence has been accumulating that antagonizing the effect of a melatonin receptor by light therapy improves the motor outcome in PD patients (Paus, Schmitz‐Hubsch et al., 2007; Willis & Turner, 2007; Rutten, Vriend, van den Heuvel, Smit, Berendse, & van der Werf, 2012), consistent with some animal model studies (Willis, 2008; Cardinali et al., 2012). Further studies using external melatonin instead of light therapy are needed to better understand the role of melatonin in motor functions.
11. CONCLUSION
This review highlights the potential neuroprotective effect of melatonin in ischaemia, Alzheimer's disease, and Parkinson's disease. It is not only a widely known antioxidant, but also an anti‐excitotoxicity, anti‐ inflammatory, and anti‐misfolding molecule. Moreover, its ability to cross the blood–brain barrier and its short life with no significant side effects make melatonin a promising neuroprotector.
CONFLICT OF INTEREST STATEMENT
The author has no conflicts of interest to declare.
Alghamdi BS. The neuroprotective role of melatonin in neurological disorders. J Neuro Res. 2018;96:1136–1149. https://doi.org/10.1002/jnr.24220
Significance Stroke, Alzheimer's disease, and Parkinson's disease are known to be devastating neurological conditions. Therapeutic interventions in reducing their risk of progression and further neuronal damage would be of great value. Melatonin, a neurohormone secreted from the pineal gland, is found to have neuroprotective potential in these neurological conditions.
REFERENCES
- Acuna‐Castroviejo, D. , Coto‐Montes, A. , Gaia Monti, M. , Ortiz, G. G. , & Reiter, R. J. (1997). Melatonin is protective against MPTP‐induced striatal and hippocampal lesions. Life Sciences, 60(2),Pl23–29. [DOI] [PubMed] [Google Scholar]
- Adi, N. , Mash, D. C. , Ali, Y. , Singer, C. , Shehadeh, L. , & Papapetropoulos, S. (2010). Melatonin MT1 and MT2 receptor expression in Parkinson's disease. Medical Science Monitor, 16(2), Br61–67. [PubMed] [Google Scholar]
- Ali, T. , & Kim, M. O. (2015). Melatonin ameliorates amyloid beta‐induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3beta pathway in the mouse hippocampus. Journal of Pineal Research, 59(1), 47–59. https://doi.org/10.1111/jpi.12238 [DOI] [PubMed] [Google Scholar]
- Alluri, H. , Wilson, R. L. , Anasooya Shaji, C. , Wiggins‐Dohlvik, K. , Patel, S. , Liu, Y. , … Tharakan, B. (2016). Melatonin Preserves Blood‐Brain Barrier Integrity and Permeability via Matrix Metalloproteinase‐9 Inhibition. PLoS One, 11(5), e0154427 https://doi.org/10.1371/journal.pone.0154427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez, A. , Alarcon, R. , Opazo, C. , Campos, E. O. , Munoz, F. J. , Calderon, F. H. , … Inestrosa, N. C. (1998). Stable complexes involving acetylcholinesterase and amyloid‐beta peptide change the biochemical properties of the enzyme and increase the neurotoxicity of Alzheimer's fibrils. Journal of Neuroscience, 18(9), 3213–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly, H. , Elmahdy, H. , El‐Dib, M. , Rowisha, M. , Awny, M. , El‐Gohary, T. , … El‐Mashad, A. R. (2015). Melatonin use for neuroprotection in perinatal asphyxia: a randomized controlled pilot study. Journal of Perinatology, 35(3), 186–191. https://doi.org/10.1038/jp.2014.186 [DOI] [PubMed] [Google Scholar]
- Antolin, I. , Mayo, J. C. , Sainz, R. M. , del Brio Mde, L. , Herrera, F. , Martin, V. , & Rodriguez, C. (2002). Protective effect of melatonin in a chronic experimental model of Parkinson's disease. Brain Research, 943(2), 163–173. [DOI] [PubMed] [Google Scholar]
- Arendt, J. , Bojkowski, C. , Franey, C. , Wright, J. , & Marks, V. (1985). Immunoassay of 6‐hydroxymelatonin sulfate in human plasma and urine: abolition of the urinary 24‐hour rhythm with atenolol. The Journal of Clinical Endocrinology & Metabolism, 60(6), 1166–1173. https://doi.org/10.1210/jcem-60-6-1166 [DOI] [PubMed] [Google Scholar]
- Arundine, M. , & Tymianski, M. (2003). Molecular mechanisms of calcium‐dependent neurodegeneration in excitotoxicity. Cell Calcium, 34(4–5), 325–337. [DOI] [PubMed] [Google Scholar]
- Baldeiras, I. , Santana, I. , Proenca, M. T. , Garrucho, M. H. , Pascoal, R. , Rodrigues, A. , … Oliveira, C. R. (2008). Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer's disease. Journal of Alzheimer's Disease, 15(1), 117–128. [DOI] [PubMed] [Google Scholar]
- Benitez‐King, G. (2006). Melatonin as a cytoskeletal modulator: implications for cell physiology and disease. Journal of Pineal Research, 40(1), 1–9. https://doi.org/10.1111/j.1600-079X.2005.00282.x [DOI] [PubMed] [Google Scholar]
- Bernard, M. , Guerlotte, J. , Greve, P. , Grechez‐Cassiau, A. , Iuvone, M. P. , Zatz, M. , … Voisin, P. (1999). Melatonin synthesis pathway: circadian regulation of the genes encoding the key enzymes in the chicken pineal gland and retina. Reproduction Nutrition Development, 39(3), 325–334. [DOI] [PubMed] [Google Scholar]
- Bhattacharya, P. , Pandey, A. K. , Paul, S. , & Patnaik, R. (2014). Melatonin renders neuroprotection by protein kinase C mediated aquaporin‐4 inhibition in animal model of focal cerebral ischemia. Life Sciences, 100(2), 97–109. https://doi.org/10.1016/j.lfs.2014.01.085 [DOI] [PubMed] [Google Scholar]
- Bojkowski, C. J. , Aldhous, M. E. , English, J. , Franey, C. , Poulton, A. L. , Skene, D. J. , & Arendt, J. (1987). Suppression of nocturnal plasma melatonin and 6‐sulphatoxymelatonin by bright and dim light in man. Hormone and Metabolic Research, 19(9), 437–440. https://doi.org/10.1055/s-2007-1011846 [DOI] [PubMed] [Google Scholar]
- Borlongan, C. V. , Sumaya, I. , Moss, D. , Kumazaki, M. , Sakurai, T. , Hida, H. , & Nishino, H. (2003). Melatonin‐secreting pineal gland: a novel tissue source for neural transplantation therapy in stroke. Cell Transplant, 12(3), 225–234. [DOI] [PubMed] [Google Scholar]
- Bruni, A. C. (1998). Cloning of a gene bearing missense mutations in early onset familial Alzheimer's disease: a Calabrian study. Functional Neurology, 13(3), 257–261. [PubMed] [Google Scholar]
- Brunner, P. , Sozer‐Topcular, N. , Jockers, R. , Ravid, R. , Angeloni, D. , Fraschini, F. , … Savaskan, E. (2006). Pineal and cortical melatonin receptors MT1 and MT2 are decreased in Alzheimer's disease. European Journal of Histochemistry, 50(4), 311–316. [PubMed] [Google Scholar]
- Caballero, B. , Vega‐Naredo, I. , Sierra, V. , Huidobro‐Fernandez, C. , Soria‐Valles, C. , De Gonzalo‐Calvo, D. , … Coto‐Montes, A. (2009). Melatonin alters cell death processes in response to age‐related oxidative stress in the brain of senescence‐accelerated mice. Journal of Pineal Research, 46(1), 106–114. https://doi.org/10.1111/j.1600-079X.2008.00637.x [DOI] [PubMed] [Google Scholar]
- Caimi, G. , Carollo, C. , & Lo Presti, R. (2004). Chronic renal failure: oxidative stress, endothelial dysfunction and wine. Clinical Nephrology, 62(5), 331–335. [DOI] [PubMed] [Google Scholar]
- Canerina‐Amaro, A. , Hernandez‐Abad, L. G. , Ferrer, I. , Quinto‐Alemany, D. , Mesa‐Herrera, F. , Ferri, C. , … Marin, R. (2017). Lipid raft ER signalosome malfunctions in menopause and Alzheimer's disease. Frontiers in Bioscience, 9, 111–126. [DOI] [PubMed] [Google Scholar]
- Cardinali, D. P. (1981). Melatonin.l A mammalian pineal hormone. Endocrine Reviews, 2(3), 327–346. https://doi.org/10.1210/edrv-2-3-327 [DOI] [PubMed] [Google Scholar]
- Cardinali, D. P. , & Pevet, P. (1998). Basic aspects of melatonin action. Sleep Medicine Reviews, 2(3), 175–190. [DOI] [PubMed] [Google Scholar]
- Cardinali, D. P. , Vigo, D. E. , Olivar, N. , Vidal, M. F. , Furio, A. M. , & Brusco, L. I. (2012). Therapeutic application of melatonin in mild cognitive impairment. American Journal of Neurodegenerative Disease, 1(3), 280–291. [PMC free article] [PubMed] [Google Scholar]
- Carloni, S. , Perrone, S. , Buonocore, G. , Longini, M. , Proietti, F. , & Balduini, W. (2008). Melatonin protects from the long‐term consequences of a neonatal hypoxic‐ischemic brain injury in rats. Journal of Pineal Research, 44(2), 157–164. https://doi.org/10.1111/j.1600-079X.2007.00503.x [DOI] [PubMed] [Google Scholar]
- Carretero, M. , Escames, G. , Lopez, L. C. , Venegas, C. , Dayoub, J. C. , Garcia, L. , & Acuna‐Castroviejo, D. (2009). Long‐term melatonin administration protects brain mitochondria from aging. Journal of Pineal Research, 47(2), 192–200. https://doi.org/10.1111/j.1600-079X.2009.00700.x [DOI] [PubMed] [Google Scholar]
- Chen, H. , Yoshioka, H. , Kim, G. S. , Jung, J. E. , Okami, N. , Sakata, H. , … Chan, P. H. (2011). Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxidants & Redox Signaling, 14(8), 1505–1517. https://doi.org/10.1089/ars.2010.3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. Y. , Chen, T. Y. , Lee, M. Y. , Chen, S. T. , Hsu, Y. S. , Kuo, Y. L. , … Lee, E. J. (2006). Melatonin decreases neurovascular oxidative/nitrosative damage and protects against early increases in the blood‐brain barrier permeability after transient focal cerebral ischemia in mice. Journal of Pineal Research, 41(2), 175–182. https://doi.org/10.1111/j.1600-079X.2006.00351.x [DOI] [PubMed] [Google Scholar]
- Chern, C. M. , Liao, J. F. , Wang, Y. H. , & Shen, Y. C. (2012). Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic‐stroke mice. Free Radical Biology & Medicine, 52(9), 1634–1647. https://doi.org/10.1016/j.freeradbiomed.2012.01.030 [DOI] [PubMed] [Google Scholar]
- Claustrat, B. , & Leston, J. (2015). Melatonin: Physiological effects in humans. Neurochirurgie, 61(2–3), 77–84. https://doi.org/10.1016/j.neuchi.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Cohen, R. A. , & Albers, H. E. (1991). Disruption of human circadian and cognitive regulation following a discrete hypothalamic lesion: a case study. Neurology, 41(5), 726–729. [DOI] [PubMed] [Google Scholar]
- Dabbeni‐Sala, F. , Di Santo, S. , Franceschini, D. , Skaper, S. D. , & Giusti, P. (2001). Melatonin protects against 6‐OHDA‐induced neurotoxicity in rats: a role for mitochondrial complex I activity. Faseb Journal, 15(1), 164–170. https://doi.org/10.1096/fj.00-0129com [DOI] [PubMed] [Google Scholar]
- Daniels, W. M. , van Rensburg, S. J. , van Zyl, J. M. , & Taljaard, J. J. (1998). Melatonin prevents beta‐amyloid‐induced lipid peroxidation. Journal of Pineal Research, 24(2), 78–82. [DOI] [PubMed] [Google Scholar]
- De Pedro, N. , Martinez‐Alvarez, R. M. , & Delgado, M. J. (2008). Melatonin reduces body weight in goldfish (Carassius auratus): effects on metabolic resources and some feeding regulators. Journal of Pineal Research, 45(1), 32–39. https://doi.org/10.1111/j.1600-079X.2007.00553.x [DOI] [PubMed] [Google Scholar]
- Dirnagl, U. , Iadecola, C. , & Moskowitz, M. A. (1999). Pathobiology of ischaemic stroke: an integrated view. Trends in Neuroscience, 22(9), 391–397. [DOI] [PubMed] [Google Scholar]
- Dominguez, L. J. , & Barbagallo, M. (2016). Dietary Approaches and Supplements in the Prevention of Cognitive Decline and Alzheimer's Disease. Current Pharmaceutical Design, 22(6), 688–700. [DOI] [PubMed] [Google Scholar]
- Dong, H. K. , Gim, J. A. , Yeo, S. H. , & Kim, H. S. (2017). Integrated late onset Alzheimer's disease (LOAD) susceptibility genes: Cholesterol metabolism and trafficking perspectives. Gene, 597, 10–16. https://doi.org/10.1016/j.gene.2016.10.022 [DOI] [PubMed] [Google Scholar]
- Dubocovich, M. L. , & Markowska, M. (2005). Functional MT1 and MT2 melatonin receptors in mammals. Endocrine, 27(2), 101–110. https://doi.org/10.1385/endo:27:2:101 [DOI] [PubMed] [Google Scholar]
- Edgar, D. M. , Dement, W. C. , & Fuller, C. A. (1993). Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep‐wake regulation. Journal of Neuroscience, 13(3), 1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz, A. , & Moisan, F. (2008). Update in the epidemiology of Parkinson's disease. Current Opinion in Neurology, 21(4), 454–460. https://doi.org/10.1097/WCO.0b013e3283050461 [DOI] [PubMed] [Google Scholar]
- Ersahin, M. , Toklu, H. Z. , Cetinel, S. , Yuksel, M. , Yegen, B. C. , & Sener, G. (2009). Melatonin reduces experimental subarachnoid hemorrhage‐induced oxidative brain damage and neurological symptoms. Journal of Pineal Research, 46(3), 324–332. https://doi.org/10.1111/j.1600-079X.2009.00664.x [DOI] [PubMed] [Google Scholar]
- Ferreiro, E. , Baldeiras, I. , Ferreira, I. L. , Costa, R. O. , Rego, A. C. , Pereira, C. F. , & Oliveira, C. R. (2012). Mitochondrial‐ and endoplasmic reticulum‐associated oxidative stress in Alzheimer's disease: from pathogenesis to biomarkers. International Journal of Cell Biology, 2012, 735206. https://doi.org/10.1155/2012/735206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn, R. W. , MacWalter, R. S. , & Doney, A. S. (2008). The cost of cerebral ischaemia. Neuropharmacology, 55(3), 250–256. https://doi.org/10.1016/j.neuropharm.2008.05.031 [DOI] [PubMed] [Google Scholar]
- Forloni, G. (1996). Neurotoxicity of beta‐amyloid and prion peptides. Current Opinion in Neurology, 9(6), 492–500. [DOI] [PubMed] [Google Scholar]
- Fournier, I. , Ploye, F. , Cottet‐Emard, J. M. , Brun, J. , & Claustrat, B. (2002). Folate deficiency alters melatonin secretion in rats. Journal of Nutrition, 132(9), 2781–2784. [DOI] [PubMed] [Google Scholar]
- Fulia, F. , Gitto, E. , Cuzzocrea, S. , Reiter, R. J. , Dugo, L. , Gitto, P. , … Barberi, I. (2001). Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: reduction by melatonin. Journal of Pineal Research, 31(4), 343–349. [DOI] [PubMed] [Google Scholar]
- Garcia‐Ruiz, P. J. , Chaudhuri, K. R. , & Martinez‐Martin, P. (2014). Non‐motor symptoms of Parkinson's disease A review…from the past. Journal of the Neurological Sciences, 338(1–2), 30–33. https://doi.org/10.1016/j.jns.2014.01.002 [DOI] [PubMed] [Google Scholar]
- Gilgun‐Sherki, Y. , Rosenbaum, Z. , Melamed, E. , & Offen, D. (2002). Antioxidant therapy in acute central nervous system injury: current state. Pharmacological Reviews, 54(2), 271–284. [DOI] [PubMed] [Google Scholar]
- Gilman, S. C. , Bonner, M. J. , & Pellmar, T. C. (1993). Effect of oxidative stress on excitatory amino acid release by cerebral cortical synaptosomes. Free Radical Biology & Medicine, 15(6), 671–675. [DOI] [PubMed] [Google Scholar]
- Greilberger, J. , Koidl, C. , Greilberger, M. , Lamprecht, M. , Schroecksnadel, K. , Leblhuber, F. , … Oettl, K. (2008). Malondialdehyde, carbonyl proteins and albumin‐disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer's disease. Free Radical Research, 42(7), 633–638. https://doi.org/10.1080/10715760802255764 [DOI] [PubMed] [Google Scholar]
- Grossetete, M. , Phelps, J. , Arko, L. , Yonas, H. , & Rosenberg, G. A. (2009). Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery, 65(4), 702–708. https://doi.org/10.1227/01.neu.0000351768.11363.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guetens, G. , De Boeck, G. , Highley, M. , van Oosterom, A. T. , & de Bruijn, E. A. (2002). Oxidative DNA damage: biological significance and methods of analysis. Critical Reviews in Clinical Laboratory Sciences, 39(4–5), 331–457. [DOI] [PubMed] [Google Scholar]
- Gunes, A. , & Dahl, M. L. (2008). Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics, 9(5), 625–637. https://doi.org/10.2217/14622416.9.5.625 [DOI] [PubMed] [Google Scholar]
- Gupta, Y. K. , Gupta, M. , & Kohli, K. (2003). Neuroprotective role of melatonin in oxidative stress vulnerable brain. Indian Journal of Physiology and Pharmacology, 47(4), 373–386. [PubMed] [Google Scholar]
- Gutierrez‐Cuesta, J. , Tajes, M. , Jimenez, A. , Coto‐Montes, A. , Camins, A. , & Pallas, M. (2008). Evaluation of potential pro‐survival pathways regulated by melatonin in a murine senescence model. Journal of Pineal Research, 45(4), 497–505. https://doi.org/10.1111/j.1600-079X.2008.00626.x [DOI] [PubMed] [Google Scholar]
- Hardeland, R. (2010). Melatonin metabolism in the central nervous system. Current Neuropharmacology, 8(3), 168–181. https://doi.org/10.2174/157015910792246244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeland, R. , Tan, D. X. , & Reiter, R. J. (2009). Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. Journal of Pineal Research, 47(2), 109–126. https://doi.org/10.1111/j.1600-079X.2009.00701.x [DOI] [PubMed] [Google Scholar]
- Hardy, J. , & Selkoe, D. J. (2002). The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science, 297(5580), 353–356. https://doi.org/10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- Hartter, S. , Korhonen, T. , Lundgren, S. , Rane, A. , Tolonen, A. , Turpeinen, M. , & Laine, K. (2006). Effect of caffeine intake 12 or 24 hours prior to melatonin intake and CYP1A2*1F polymorphism on CYP1A2 phenotyping by melatonin. Basic Clinical Pharmacology Toxicology, 99(4), 300–304. https://doi.org/10.1111/j.1742-7843.2006.pto_491.x [DOI] [PubMed] [Google Scholar]
- Hartter, S. , Nordmark, A. , Rose, D. M. , Bertilsson, L. , Tybring, G. , & Laine, K. (2003). Effects of caffeine intake on the pharmacokinetics of melatonin, a probe drug for CYP1A2 activity. British Journal of Clinical Pharmacology, 56(6), 679–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori, A. , Migitaka, H. , Iigo, M. , Itoh, M. , Yamamoto, K. , Ohtani‐Kaneko, R. , … Reiter, R. J. (1995). Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochemistry and Molecular Biology International, 35(3), 627–634. [PubMed] [Google Scholar]
- He, H. , Dong, W. , & Huang, F. (2010). Anti‐amyloidogenic and anti‐apoptotic role of melatonin in Alzheimer disease. Current Neuropharmacology, 8(3), 211–217. https://doi.org/10.2174/157015910792246137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata, F. , Hayaishi, O. , Tokuyama, T. , & Seno, S. (1974). In vitro and in vivo formation of two new metabolites of melatonin. The Journal of Biological Chemistry, 249(4), 1311–1313. [PubMed] [Google Scholar]
- Hossmann, K. A. (1994). Viability thresholds and the penumbra of focal ischemia. Annals of Neurology, 36(4), 557–565. https://doi.org/10.1002/ana.410360404 [DOI] [PubMed] [Google Scholar]
- Huether, G. (1993). The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia, 49(8), 665–670. [DOI] [PubMed] [Google Scholar]
- Hughes, K. C. , Gao, X. , Kim, I. Y. , Wang, M. , Weisskopf, M. G. , Schwarzschild, M. A. , & Ascherio, A. (2017). Intake of dairy foods and risk of Parkinson disease. Neurology, 89(1), 46–52. https://doi.org/10.1212/wnl.0000000000004057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola, C. , & Alexander, M. (2001). Cerebral ischemia and inflammation. Current Opinion in Neurology, 14(1), 89–94. [DOI] [PubMed] [Google Scholar]
- Iriti, M. (2009). Melatonin in grape, not just a myth, maybe a panacea. Journal of Pineal Research, 46(3), 353 https://doi.org/10.1111/j.1600-079X.2008.00616.x [DOI] [PubMed] [Google Scholar]
- Iriti, M. , & Faoro, F. (2009). Bioactivity of grape chemicals for human health. Natural Product Communications, 4(5), 611–634. [PubMed] [Google Scholar]
- Isidorov, V. A. , & Nazaruk, J. (2017). Gas chromatographic‐mass spectrometric determination of glycosides without prior hydrolysis. Journal of Chromatography A, 1521, 161–166. https://doi.org/10.1016/j.chroma.2017.09.033 [DOI] [PubMed] [Google Scholar]
- Ittner, L. M. , & Gotz, J. (2011). Amyloid‐beta and tau–a toxic pas de deux in Alzheimer's disease. Nature Reviews Neuroscience, 12(2), 65–72. https://doi.org/10.1038/nrn2967 [DOI] [PubMed] [Google Scholar]
- Iwata, N. , Tsubuki, S. , Takaki, Y. , Watanabe, K. , Sekiguchi, M. , Hosoki, E. , … Saido, T. C. (2000). Identification of the major Abeta1–42‐degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nature Medicine, 6(2), 143–150. https://doi.org/10.1038/72237 [DOI] [PubMed] [Google Scholar]
- Jaworek, J. , Leja‐Szpak, A. , Bonior, J. , Nawrot, K. , Tomaszewska, R. , Stachura, J. , … Konturek, S. J. (2003). Protective effect of melatonin and its precursor L‐tryptophan on acute pancreatitis induced by caerulein overstimulation or ischemia/reperfusion. Journal of Pineal Research, 34(1), 40–52. [DOI] [PubMed] [Google Scholar]
- Jeong, S. (2017). Molecular and Cellular Basis of Neurodegeneration in Alzheimer's Disease. Molecules and Cells, 40(9), 613–620. https://doi.org/10.14348/molcells.2017.0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic, E. , Ozdemir, Y. G. , Bolay, H. , Kelestimur, H. , & Dalkara, T. (1999). Pinealectomy aggravates and melatonin administration attenuates brain damage in focal ischemia. Journal of Cerebral Blood Flow & Metabolism, 19(5), 511–516. https://doi.org/10.1097/00004647-199905000-00005 [DOI] [PubMed] [Google Scholar]
- Koh, P. O. (2012). Melatonin regulates the calcium‐buffering proteins, parvalbumin and hippocalcin, in ischemic brain injury. Journal of Pineal Research, 53(4), 358–365. https://doi.org/10.1111/j.1600-079X.2012.01005.x [DOI] [PubMed] [Google Scholar]
- Lahiri, D. K. (1999). Melatonin affects the metabolism of the beta‐amyloid precursor protein in different cell types. Journal of Pineal Research, 26(3), 137–146. [DOI] [PubMed] [Google Scholar]
- Lee, M. Y. , Kuan, Y. H. , Chen, H. Y. , Chen, T. Y. , Chen, S. T. , Huang, C. C. , … Lee, E. J. (2007). Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. Journal of Pineal Research, 42(3), 297–309. https://doi.org/10.1111/j.1600-079X.2007.00420.x [DOI] [PubMed] [Google Scholar]
- Leszek, J. , Sochocka, M. , & Gasiorowski, K. (2012). Vascular factors and epigenetic modifications in the pathogenesis of Alzheimer's disease. Journal of the Neurological Sciences, 323(1–2), 25–32. https://doi.org/10.1016/j.jns.2012.09.010 [DOI] [PubMed] [Google Scholar]
- Lewy, A. J. , Wehr, T. A. , Goodwin, F. K. , Newsome, D. A. , & Markey, S. P. (1980). Light suppresses melatonin secretion in humans. Science, 210(4475), 1267–1269. [DOI] [PubMed] [Google Scholar]
- Li, H. , Lv, C. , Yang, C. , Wei, D. , Chen, K. , Li, S. , & Zhang, Z. (2017). SORL1 rs1699102 polymorphism modulates age‐related cognitive decline and gray matter volume reduction in non‐demented individuals. European Journal of Neurology, 24(1), 187–194. https://doi.org/10.1111/ene.13182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Wang, Y. , Feng, D. , Liu, Y. , Xu, M. , Gao, A. , … Chen, G. (2014). Alterations in the time course of expression of the Nox family in the brain in a rat experimental cerebral ischemia and reperfusion model: effects of melatonin. Journal of Pineal Research, 57(1), 110–119. https://doi.org/10.1111/jpi.12148 [DOI] [PubMed] [Google Scholar]
- Lin, A. M. , Fang, S. F. , Chao, P. L. , & Yang, C. H. (2007). Melatonin attenuates arsenite‐induced apoptosis in rat brain: involvement of mitochondrial and endoplasmic reticulum pathways and aggregation of alpha‐synuclein. Journal of Pineal Research, 43(2), 163–171. https://doi.org/10.1111/j.1600-079X.2007.00456.x [DOI] [PubMed] [Google Scholar]
- Lipton, P. (1999). Ischemic cell death in brain neurons. Physiological Reviews, 79(4), 1431–1568. [DOI] [PubMed] [Google Scholar]
- Liu, R. Y. , Zhou, J. N. , van Heerikhuize, J. , Hofman, M. A. , & Swaab, D. F. (1999). Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer's disease, and apolipoprotein E‐epsilon4/4 genotype. The Journal of Clinical Endocrinology & Metabolism, 84(1), 323–327. https://doi.org/10.1210/jcem.84.1.5394 [DOI] [PubMed] [Google Scholar]
- Liu, Y. J. , Zhuang, J. , Zhu, H. Y. , Shen, Y. X. , Tan, Z. L. , & Zhou, J. N. (2007). Cultured rat cortical astrocytes synthesize melatonin: absence of a diurnal rhythm. Journal of Pineal Research, 43(3), 232–238. https://doi.org/10.1111/j.1600-079X.2007.00466.x [DOI] [PubMed] [Google Scholar]
- Lleo, A. , Berezovska, O. , Growdon, J. H. , & Hyman, B. T. (2004). Clinical, pathological, and biochemical spectrum of Alzheimer disease associated with PS‐1 mutations. The American Journal of Geriatric Psychiatry, 12(2), 146–156. [DOI] [PubMed] [Google Scholar]
- Loffler, T. , Flunkert, S. , Temmel, M. , & Hutter‐Paier, B. (2016). Decreased Plasma Abeta in Hyperlipidemic APPSL Transgenic Mice Is Associated with BBB Dysfunction. Frontiers in Neuroscience, 10, 232 https://doi.org/10.3389/fnins.2016.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longatti, P. , Perin, A. , Rizzo, V. , Comai, S. , Giusti, P. , & Costa, C. V. (2007). Ventricular cerebrospinal fluid melatonin concentrations investigated with an endoscopic technique. Journal of Pineal Research, 42(2), 113–118. https://doi.org/10.1111/j.1600-079X.2006.00391.x [DOI] [PubMed] [Google Scholar]
- Lorenzo, A. , & Yankner, B. A. (1994). Beta‐amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proceedings of the National Academy of Sciences of the United States of America, 91(25), 12243–12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luboshitzky, R. , Ophir, U. , Nave, R. , Epstein, R. , Shen‐Orr, Z. , & Herer, P. (2002). The effect of pyridoxine administration on melatonin secretion in normal men. Neuroendocrinology Letters, 23(3), 213–217. [PubMed] [Google Scholar]
- Ma, C. , Liu, Y. , Neumann, S. , & Gao, X. (2017). Nicotine from cigarette smoking and diet and Parkinson disease: a review. Translational Neurodegeneration, 6, 18 https://doi.org/10.1186/s40035-017-0090-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire‐Zeiss, K. A. , & Federoff, H. J. (2010). Future directions for immune modulation in neurodegenerative disorders: focus on Parkinson's disease. Journal of Neural Transmission, 117(8), 1019–1025. https://doi.org/10.1007/s00702-010-0431-6 [DOI] [PubMed] [Google Scholar]
- Manchester, L. C. , Tan, D. X. , Reiter, R. J. , Park, W. , Monis, K. , & Qi, W. (2000). High levels of melatonin in the seeds of edible plants: possible function in germ tissue protection. Life Sciences, 67(25), 3023–3029. [DOI] [PubMed] [Google Scholar]
- Manda, K. , Ueno, M. , & Anzai, K. (2009). Cranial irradiation‐induced inhibition of neurogenesis in hippocampal dentate gyrus of adult mice: attenuation by melatonin pretreatment. Journal of Pineal Research, 46(1), 71–78. https://doi.org/10.1111/j.1600-079X.2008.00632.x [DOI] [PubMed] [Google Scholar]
- Markesbery, W. R. (1997). Oxidative stress hypothesis in Alzheimer's disease. Free Radical Biology & Medicine, 23(1), 134–147. [DOI] [PubMed] [Google Scholar]
- Martinez‐Cruz, F. , Espinar, A. , Pozo, D. , Osuna, C. , & Guerrero, J. M. (2002). Melatonin prevents focal rat cerebellum injury as assessed by induction of heat shock protein (HO‐1) following subarachnoid injections of lysed blood. Neuroscience Letters, 331(3), 208–210. [DOI] [PubMed] [Google Scholar]
- Masana, M. I. , Benloucif, S. , & Dubocovich, M. L. (2000). Circadian rhythm of mt1 melatonin receptor expression in the suprachiasmatic nucleus of the C3H/HeN mouse. Journal of Pineal Research, 28(3), 185–192. [DOI] [PubMed] [Google Scholar]
- Mathers, C. D. , Boerma, T. , & Ma Fat, D. (2009). Global and regional causes of death. British Medical Bulletin, 92, 7–32. https://doi.org/10.1093/bmb/ldp028 [DOI] [PubMed] [Google Scholar]
- Mecocci, P. , MacGarvey, U. , & Beal, M. F. (1994). Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. Annals of Neurology, 36(5), 747–751. https://doi.org/10.1002/ana.410360510 [DOI] [PubMed] [Google Scholar]
- Mishima, K. , Tozawa, T. , Satoh, K. , Matsumoto, Y. , Hishikawa, Y. , & Okawa, M. (1999). Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer's type with disturbed sleep‐waking. Biological Psychiatry, 45(4), 417–421. [DOI] [PubMed] [Google Scholar]
- Moller, M. (1992). Fine structure of the pinealopetal innervation of the mammalian pineal gland. Microscopy Research and Technique, 21(3), 188–204. https://doi.org/10.1002/jemt.1070210303 [DOI] [PubMed] [Google Scholar]
- Moller, M. , & Baeres, F. M. (2002). The anatomy and innervation of the mammalian pineal gland. Cell and Tissue Research, 309(1), 139–150. https://doi.org/10.1007/s00441-002-0580-5 [DOI] [PubMed] [Google Scholar]
- Moore, R. Y. (1992). The fourth C.U. Ariens Kappers lecture. The organization of the human circadian timing system. Progress in Brain Research, 93, 99–115; discussion 115–117. [PubMed] [Google Scholar]
- Moretti, R. , Zanin, A. , Pansiot, J. , Spiri, D. , Manganozzi, L. , Kratzer, I. , … Titomanlio, L. (2015). Melatonin reduces excitotoxic blood‐brain barrier breakdown in neonatal rats. Neuroscience, 311, 382–397. https://doi.org/10.1016/j.neuroscience.2015.10.044 [DOI] [PubMed] [Google Scholar]
- Muller, M. , & Ballanyi, K. (2003). Dynamic recording of cell death in the in vitro dorsal vagal nucleus of rats in response to metabolic arrest. Journal of Neurophysiology, 89(1), 551–561. https://doi.org/10.1152/jn.00559.2002 [DOI] [PubMed] [Google Scholar]
- Munoz‐Hoyos, A. , Amoros‐Rodriguez, I. , Molina‐Carballo, A. , Uberos‐Fernandez, J. , & Acuna‐Castroviejo, D. (1996). Pineal response after pyridoxine test in children. Journal of Neural Transmission, 103(7), 833–842. https://doi.org/10.1007/bf01273361 [DOI] [PubMed] [Google Scholar]
- Mustapic, M. , Popovic Hadzija, M. , Pavlovic, M. , Pavkovic, P. , Presecki, P. , Mrazovac, D. , … Muck‐Seler, D. (2012). Alzheimer's disease and type 2 diabetes: the association study of polymorphisms in tumor necrosis factor‐alpha and apolipoprotein E genes. Metabolic Brain Disease, 27(4), 507–512. https://doi.org/10.1007/s11011-012-9310-1 [DOI] [PubMed] [Google Scholar]
- Ng, K. Y. , Leong, M. K. , Liang, H. , & Paxinos, G. (2017). Melatonin receptors: distribution in mammalian brain and their respective putative functions. Brain Structure and Function. https://doi.org/10.1007/s00429-017-1439-6 [DOI] [PubMed] [Google Scholar]
- Nunan, J. , & Small, D. H. (2000). Regulation of APP cleavage by alpha‐, beta‐ and gamma‐secretases. FEBS Letters, 483(1), 6–10. [DOI] [PubMed] [Google Scholar]
- Obrenovitch, T. P. (1996). Origins of glutamate release in ischaemia. Acta Neurochirurgica Supplement, 66, 50–55. [DOI] [PubMed] [Google Scholar]
- Ohashi, Y. , Okamoto, N. , Uchida, K. , Iyo, M. , Mori, N. , & Morita, Y. (1999). Daily rhythm of serum melatonin levels and effect of light exposure in patients with dementia of the Alzheimer's type. Biological Psychiatry, 45(12), 1646–1652. [DOI] [PubMed] [Google Scholar]
- Olanow, C. W. (1992). An introduction to the free radical hypothesis in Parkinson's disease. Annals of Neurology, 32 Suppl, S2–9. [DOI] [PubMed] [Google Scholar]
- Olcese, J. M. , Cao, C. , Mori, T. , Mamcarz, M. B. , Maxwell, A. , Runfeldt, M. J. , … Arendash, G. W. (2009). Protection against cognitive deficits and markers of neurodegeneration by long‐term oral administration of melatonin in a transgenic model of Alzheimer disease. Journal of Pineal Research, 47(1), 82–96. https://doi.org/10.1111/j.1600-079X.2009.00692.x [DOI] [PubMed] [Google Scholar]
- Ono, K. , Mochizuki, H. , Ikeda, T. , Nihira, T. , Takasaki, J. , Teplow, D. B. , & Yamada, M. (2012). Effect of melatonin on alpha‐synuclein self‐assembly and cytotoxicity. Neurobiology of Aging, 33(9), 2172–2185. https://doi.org/10.1016/j.neurobiolaging.2011.10.015 [DOI] [PubMed] [Google Scholar]
- Outeiro, T. F. , Putcha, P. , Tetzlaff, J. E. , Spoelgen, R. , Koker, M. , Carvalho, F. , … McLean, P. J. (2008). Formation of toxic oligomeric alpha‐synuclein species in living cells. PLoS One, 3(4), e1867 https://doi.org/10.1371/journal.pone.0001867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padurariu, M. , Ciobica, A. , Hritcu, L. , Stoica, B. , Bild, W. , & Stefanescu, C. (2010). Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer's disease. Neuroscience Letters, 469(1), 6–10. https://doi.org/10.1016/j.neulet.2009.11.033 [DOI] [PubMed] [Google Scholar]
- Padurariu, M. , Ciobica, A. , Lefter, R. , Serban, I. L. , Stefanescu, C. , & Chirita, R. (2013). The oxidative stress hypothesis in Alzheimer's disease. Psychiatria Danubina, 25(4), 401–409. [PubMed] [Google Scholar]
- Pappolla, M. , Bozner, P. , Soto, C. , Shao, H. , Robakis, N. K. , Zagorski, M. , … Ghiso, J. (1998). Inhibition of Alzheimer beta‐fibrillogenesis by melatonin. The Journal of Biological Chemistry, 273(13), 7185–7188. [DOI] [PubMed] [Google Scholar]
- Pappolla, M. A. , Chyan, Y. J. , Poeggeler, B. , Frangione, B. , Wilson, G. , Ghiso, J. , & Reiter, R. J. (2000). An assessment of the antioxidant and the antiamyloidogenic properties of melatonin: implications for Alzheimer's disease. Journal of Neural Transmission, 107(2), 203–231. https://doi.org/10.1007/s007020050018 [DOI] [PubMed] [Google Scholar]
- Pardridge, W. M. , & Mietus, L. J. (1980). Transport of albumin‐bound melatonin through the blood‐brain barrier. Journal of Neurochemistry, 34(6), 1761–1763. [DOI] [PubMed] [Google Scholar]
- Paredes, S. D. , Korkmaz, A. , Manchester, L. C. , Tan, D. X. , & Reiter, R. J. (2009). Phytomelatonin: a review. Journal of Experimental Botany, 60(1), 57–69. https://doi.org/10.1093/jxb/ern284 [DOI] [PubMed] [Google Scholar]
- Paredes, S. D. , Rancan, L. , Kireev, R. , Gonzalez, A. , Louzao, P. , Gonzalez, P. , … Tresguerres, J. A. (2015). Melatonin counteracts at a transcriptional level the inflammatory and apoptotic response secondary to ischemic brain injury induced by middle cerebral artery blockade in aging rats. Bioresearch Open Access, 4(1), 407–416. https://doi.org/10.1089/biores.2015.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino, P. , Parada, E. , Farre‐Alins, V. , Molz, S. , Cacabelos, R. , Marco‐Contelles, J. , … Egea, J. (2016). Melatonin protects against oxygen and glucose deprivation by decreasing extracellular glutamate and Nox‐derived ROS in rat hippocampal slices. Neurotoxicology, 57, 61–68. https://doi.org/10.1016/j.neuro.2016.09.002 [DOI] [PubMed] [Google Scholar]
- Paus, S. , Schmitz‐Hubsch, T. , Wullner, U. , Vogel, A. , Klockgether, T. , & Abele, M. (2007). Bright light therapy in Parkinson's disease: a pilot study. Movement Disorders, 22(10), 1495–1498. https://doi.org/10.1002/mds.21542 [DOI] [PubMed] [Google Scholar]
- Pelham, R. W. (1975). A serum melatonin rhythm in chickens and its abolition by pinealectomy. Endocrinology, 96(2), 543–546. https://doi.org/10.1210/endo-96-2-543 [DOI] [PubMed] [Google Scholar]
- Poeggeler, B. , Miravalle, L. , Zagorski, M. G. , Wisniewski, T. , Chyan, Y. J. , Zhang, Y. , … Pappolla, M. A. (2001). Melatonin reverses the profibrillogenic activity of apolipoprotein E4 on the Alzheimer amyloid Abeta peptide. Biochemistry, 40(49), 14995–15001. [DOI] [PubMed] [Google Scholar]
- Pozo Devoto, V. M. , & Falzone, T. L. (2017). Mitochondrial dynamics in Parkinson's disease: a role for alpha‐synuclein? Disease Models & Mechanisms, 10(9), 1075–1087. https://doi.org/10.1242/dmm.026294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, D. L. , & Sisodia, S. S. (1998). Mutant genes in familial Alzheimer's disease and transgenic models. Annual Review of Neuroscience, 21, 479–505. https://doi.org/10.1146/annurev.neuro.21.1.479 [DOI] [PubMed] [Google Scholar]
- Puglielli, L. , Tanzi, R. E. , & Kovacs, D. M. (2003). Alzheimer's disease: the cholesterol connection. Nature Neuroscience, 6(4), 345–351. https://doi.org/10.1038/nn0403-345 [DOI] [PubMed] [Google Scholar]
- Reiter, R. J. (1991). Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocrine Reviews, 12(2), 151–180. https://doi.org/10.1210/edrv-12-2-151 [DOI] [PubMed] [Google Scholar]
- Ribelayga, C. , Pevet, P. , & Simonneaux, V. (2000). HIOMT drives the photoperiodic changes in the amplitude of the melatonin peak of the Siberian hamster. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 278(5), R1339–1345. [DOI] [PubMed] [Google Scholar]
- Rizzo, V. , Porta, C. , Moroni, M. , Scoglio, E. , & Moratti, R. (2002). Determination of free and total (free plus protein‐bound) melatonin in plasma and cerebrospinal fluid by high‐performance liquid chromatography with fluorescence detection. Journal of Chromatography B, 774(1), 17–24. [DOI] [PubMed] [Google Scholar]
- Rodrigo, J. , Fernandez, A. P. , Serrano, J. , Peinado, M. A. , & Martinez, A. (2005). The role of free radicals in cerebral hypoxia and ischemia. Free Radical Biology & Medicine, 39(1), 26–50. https://doi.org/10.1016/j.freeradbiomed.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Rossi, D. J. , Oshima, T. , & Attwell, D. (2000). Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature, 403(6767), 316–321. https://doi.org/10.1038/35002090 [DOI] [PubMed] [Google Scholar]
- Rothman, S. M. , & Olney, J. W. (1986). Glutamate and the pathophysiology of hypoxic–ischemic brain damage. Annals of Neurology, 19(2), 105–111. https://doi.org/10.1002/ana.410190202 [DOI] [PubMed] [Google Scholar]
- Rudnitskaya, E. A. , Muraleva, N. A. , Maksimova, K. Y. , Kiseleva, E. , Kolosova, N. G. , & Stefanova, N. A. (2015). Melatonin Attenuates Memory Impairment, Amyloid‐beta Accumulation, and Neurodegeneration in a Rat Model of Sporadic Alzheimer's Disease. Journal of Alzheimer's Disease, 47(1), 103–116. https://doi.org/10.3233/jad-150161 [DOI] [PubMed] [Google Scholar]
- Rutten, S. , Vriend, C. , van den Heuvel, O. A. , Smit, J. H. , Berendse, H. W. , & van der Werf, Y. D. (2012). Bright light therapy in Parkinson's disease: an overview of the background and evidence. Parkinsons Disease, 2012, 767105. https://doi.org/10.1155/2012/767105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan, K. S. , Sindhu, K. M. , & Mohanakumar, K. P. (2007). Melatonin protects against rotenone‐induced oxidative stress in a hemiparkinsonian rat model. Journal of Pineal Research, 42(3), 247–253. https://doi.org/10.1111/j.1600-079X.2006.00412.x [DOI] [PubMed] [Google Scholar]
- Schober, A. (2004). Classic toxin‐induced animal models of Parkinson's disease: 6‐OHDA and MPTP. Cell and Tissue Research, 318(1), 215–224. https://doi.org/10.1007/s00441-004-0938-y [DOI] [PubMed] [Google Scholar]
- Seiffert, D. , Bradley, J. D. , Rominger, C. M. , Rominger, D. H. , Yang, F. , Meredith, J. E., Jr. , … Zaczek, R. (2000). Presenilin‐1 and −2 are molecular targets for gamma‐secretase inhibitors. The Journal of Biological Chemistry, 275(44), 34086–34091. https://doi.org/10.1074/jbc.M005430200 [DOI] [PubMed] [Google Scholar]
- Seifman, M. A. , Adamides, A. A. , Nguyen, P. N. , Vallance, S. A. , Cooper, D. J. , Kossmann, T. , … Morganti‐Kossmann, M. C. (2008). Endogenous melatonin increases in cerebrospinal fluid of patients after severe traumatic brain injury and correlates with oxidative stress and metabolic disarray. Journal of Cerebral Blood Flow & Metabolism, 28(4), 684–696. https://doi.org/10.1038/sj.jcbfm.9600603 [DOI] [PubMed] [Google Scholar]
- Selkoe, D. J. (1998). The cell biology of beta‐amyloid precursor protein and presenilin in Alzheimer's disease. Trends in Cell Biology, 8(11), 447–453. [DOI] [PubMed] [Google Scholar]
- Selkoe, D. J. (1999). Translating cell biology into therapeutic advances in Alzheimer's disease. Nature, 399(6738 Suppl), A23–31. [DOI] [PubMed] [Google Scholar]
- Serpell, L. C. (2000). Alzheimer's amyloid fibrils: structure and assembly. Biochimica et Biophysica Acta, 1502(1), 16–30. [DOI] [PubMed] [Google Scholar]
- Sheridan, M. N. , & Reiter, R. J. (1970). Observations on the pineal system in the hamster. II. Fine structure of the deep pineal. Journal of Morphology, 131(2), 163–177. https://doi.org/10.1002/jmor.1051310204 [DOI] [PubMed] [Google Scholar]
- Sheridan, M. N. , Reiter, R. J. , & Jacobs, J. J. (1969). An interesting anatomical relationship between the hamster pineal gland and the ventricular system of the brain. Journal of Endocrinology, 45(1), 131–132. [DOI] [PubMed] [Google Scholar]
- Shieh, J. M. , Wu, H. T. , Cheng, K. C. , & Cheng, J. T. (2009). Melatonin ameliorates high fat diet‐induced diabetes and stimulates glycogen synthesis via a PKCzeta‐Akt‐GSK3beta pathway in hepatic cells. Journal of Pineal Research, 47(4), 339–344. https://doi.org/10.1111/j.1600-079X.2009.00720.x [DOI] [PubMed] [Google Scholar]
- Shimohama, S. (2000). Apoptosis in Alzheimer's disease–an update. Apoptosis, 5(1), 9–16. [DOI] [PubMed] [Google Scholar]
- Shukla, M. , Govitrapong, P. , Boontem, P. , Reiter, R. J. , & Satayavivad, J. (2017). Mechanisms of Melatonin in Alleviating Alzheimer's Disease. Current Neuropharmacology, 15(7), 1010–1031. https://doi.org/10.2174/1570159x15666170313123454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shults, C. W. (2006). Lewy bodies. Proceedings of the National Academy of Sciences of the United States of America, 103(6), 1661–1668. https://doi.org/10.1073/pnas.0509567103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, S. O. , Ximenes, V. F. , Livramento, J. A. , Catalani, L. H. , & Campa, A. (2005). High concentrations of the melatonin metabolite, N1‐acetyl‐N2‐formyl‐5‐methoxykynuramine, in cerebrospinal fluid of patients with meningitis: a possible immunomodulatory mechanism. Journal of Pineal Research, 39(3), 302–306. https://doi.org/10.1111/j.1600-079X.2005.00247.x [DOI] [PubMed] [Google Scholar]
- Singhal, N. K. , Srivastava, G. , Patel, D. K. , Jain, S. K. , & Singh, M. P. (2011). Melatonin or silymarin reduces maneb‐ and paraquat‐induced Parkinson's disease phenotype in the mouse. Journal of Pineal Research, 50(2), 97–109. https://doi.org/10.1111/j.1600-079X.2010.00819.x [DOI] [PubMed] [Google Scholar]
- Skaper, S. D. , Floreani, M. , Ceccon, M. , Facci, L. , & Giusti, P. (1999). Excitotoxicity, oxidative stress, and the neuroprotective potential of melatonin. Annals of the New York Academy of Sciences, 890, 107–118. [DOI] [PubMed] [Google Scholar]
- Skene, D. J. , Vivien‐Roels, B. , Sparks, D. L. , Hunsaker, J. C. , Pevet, P. , Ravid, D. , & Swaab, D. F. (1990). Daily variation in the concentration of melatonin and 5‐methoxytryptophol in the human pineal gland: effect of age and Alzheimer's disease. Brain Research, 528(1), 170–174. [DOI] [PubMed] [Google Scholar]
- Smith, M. A. , Sayre, L. M. , Monnier, V. M. , & Perry, G. (1995). Radical AGEing in Alzheimer's disease. Trends in Neuroscience, 18(4), 172–176. [DOI] [PubMed] [Google Scholar]
- Song, J. , Kang, S. M. , Lee, W. T. , Park, K. A. , Lee, K. M. , & Lee, J. E. (2014). The beneficial effect of melatonin in brain endothelial cells against oxygen‐glucose deprivation followed by reperfusion‐induced injury. Oxidative Medicine and Cellular Longevity, 2014, 639531 https://doi.org/10.1155/2014/639531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W. , & Lahiri, D. K. (1997). Melatonin alters the metabolism of the beta‐amyloid precursor protein in the neuroendocrine cell line PC12. Journal of Molecular Neuroscience, 9(2), 75–92. https://doi.org/10.1007/bf02736852 [DOI] [PubMed] [Google Scholar]
- Soto, C. , Branes, M. C. , Alvarez, J. , & Inestrosa, N. C. (1994). Structural determinants of the Alzheimer's amyloid beta‐peptide. Journal of Neurochemistry, 63(4), 1191–1198. [DOI] [PubMed] [Google Scholar]
- Soto, C. , Castano, E. M. , Frangione, B. , & Inestrosa, N. C. (1995). The alpha‐helical to beta‐strand transition in the amino‐terminal fragment of the amyloid beta‐peptide modulates amyloid formation. The Journal of Biological Chemistry, 270(7), 3063–3067. [DOI] [PubMed] [Google Scholar]
- Soto, C. , Sigurdsson, E. M. , Morelli, L. , Kumar, R. A. , Castano, E. M. , & Frangione, B. (1998). Beta‐sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: implications for Alzheimer's therapy. Nature Medicine, 4(7), 822–826. [DOI] [PubMed] [Google Scholar]
- Spillantini, M. G. , Crowther, R. A. , Jakes, R. , Hasegawa, M. , & Goedert, M. (1998). alpha‐Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proceedings of the National Academy of Sciences of the United States of America, 95(11), 6469‐6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan, V. , Pandi‐Perumal, S. R. , Maestroni, G. J. , Esquifino, A. I. , Hardeland, R. , & Cardinali, D. P. (2005). Role of melatonin in neurodegenerative diseases. Neurotoxicity Research, 7(4), 293–318. [DOI] [PubMed] [Google Scholar]
- Stefanova, N. A. , Maksimova, K. Y. , Kiseleva, E. , Rudnitskaya, E. A. , Muraleva, N. A. , & Kolosova, N. G. (2015). Melatonin attenuates impairments of structural hippocampal neuroplasticity in OXYS rats during active progression of Alzheimer's disease‐like pathology. Journal of Pineal Research, 59(2), 163–177. https://doi.org/10.1111/jpi.12248 [DOI] [PubMed] [Google Scholar]
- Stefulj, J. , Hortner, M. , Ghosh, M. , Schauenstein, K. , Rinner, I. , Wolfler, A. , … Liebmann, P. M. (2001). Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. Journal of Pineal Research, 30(4), 243–247. [DOI] [PubMed] [Google Scholar]
- Stys, P. K. (1998). Anoxic and ischemic injury of myelinated axons in CNS white matter: from mechanistic concepts to therapeutics. Journal of Cerebral Blood Flow & Metabolism, 18(1), 2–25. https://doi.org/10.1097/00004647-199801000-00002 [DOI] [PubMed] [Google Scholar]
- Subbarao, K. V. , Richardson, J. S. , & Ang, L. C. (1990). Autopsy samples of Alzheimer's cortex show increased peroxidation in vitro. Journal of Neurochemistry, 55(1), 342–345. [DOI] [PubMed] [Google Scholar]
- Sultana, R. , & Butterfield, D. A. (2010). Role of oxidative stress in the progression of Alzheimer's disease. Journal of Alzheimer's Disease, 19(1), 341–353. https://doi.org/10.3233/jad-2010-1222 [DOI] [PubMed] [Google Scholar]
- Tan, D. X. , Hardeland, R. , Manchester, L. C. , Paredes, S. D. , Korkmaz, A. , Sainz, R. M. , … Reiter, R. J. (2010). The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biological Reviews of the Cambridge Philosophical Society, 85(3), 607–623. https://doi.org/10.1111/j.1469-185X.2009.00118.x [DOI] [PubMed] [Google Scholar]
- Tan, D. X. , Manchester, L. C. , Burkhardt, S. , Sainz, R. M. , Mayo, J. C. , Kohen, R. , … Reiter, R. J. (2001). N1‐acetyl‐N2‐formyl‐5‐methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. Faseb Journal, 15(12), 2294–2296. https://doi.org/10.1096/fj.01-0309fje [DOI] [PubMed] [Google Scholar]
- Tan, D. X. , Manchester, L. C. , Reiter, R. J. , Qi, W. B. , Zhang, M. , Weintraub, S. T. , … Mayo, J. C. (1999). Identification of highly elevated levels of melatonin in bone marrow: its origin and significance. Biochimica et Biophysica Acta, 1472(1–2), 206–214. [DOI] [PubMed] [Google Scholar]
- Tan, D. X. , Manchester, L. C. , Sanchez‐Barcelo, E. , Mediavilla, M. D. , & Reiter, R. J. (2010). Significance of high levels of endogenous melatonin in Mammalian cerebrospinal fluid and in the central nervous system. Current Neuropharmacology, 8(3), 162–167. https://doi.org/10.2174/157015910792246182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, D. X. , Manchester, L. C. , Terron, M. P. , Flores, L. J. , & Reiter, R. J. (2007). One molecule, many derivatives: a never‐ending interaction of melatonin with reactive oxygen and nitrogen species? Journal of Pineal Research, 42(1), 28–42. https://doi.org/10.1111/j.1600-079X.2006.00407.x [DOI] [PubMed] [Google Scholar]
- Tansey, M. G. , McCoy, M. K. , & Frank‐Cannon, T. C. (2007). Neuroinflammatory mechanisms in Parkinson's disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Experimental Neurology, 208(1), 1–25. https://doi.org/10.1016/j.expneurol.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzioglu, M. , & Galter, D. (2008). Parkinson's disease: genetic versus toxin‐induced rodent models. Febs Journal, 275(7), 1384–1391. https://doi.org/10.1111/j.1742-4658.2008.06302.x [DOI] [PubMed] [Google Scholar]
- Thomas, B. , & Mohanakumar, K. P. (2004). Melatonin protects against oxidative stress caused by 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine in the mouse nigrostriatum. Journal of Pineal Research, 36(1), 25–32. [DOI] [PubMed] [Google Scholar]
- Thomas, J. , Thomas, C. J. , Radcliffe, J. , & Itsiopoulos, C. (2015). Omega‐3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer's Disease. Biomedical Research International, 2015, 172801. https://doi.org/10.1155/2015/172801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire, H. , Locatelli, A. , Chemineau, P. , & Malpaux, B. (2002). Melatonin enters the cerebrospinal fluid through the pineal recess. Endocrinology, 143(1), 84–90. https://doi.org/10.1210/endo.143.1.8585 [DOI] [PubMed] [Google Scholar]
- Uchida, K. , Okamoto, N. , Ohara, K. , & Morita, Y. (1996). Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Research, 717(1–2), 154–159. [DOI] [PubMed] [Google Scholar]
- Ursing, C. , von Bahr, C. , Brismar, K. , & Rojdmark, S. (2005). Influence of cigarette smoking on melatonin levels in man. European Journal of Clinical Pharmacology, 61(3), 197–201. https://doi.org/10.1007/s00228-005-0908-7 [DOI] [PubMed] [Google Scholar]
- Vardy, E. R. , Catto, A. J. , & Hooper, N. M. (2005). Proteolytic mechanisms in amyloid‐beta metabolism: therapeutic implications for Alzheimer's disease. Trends in Molecular Medicine, 11(10), 464–472. https://doi.org/10.1016/j.molmed.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Videnovic, A. , Noble, C. , Reid, K. J. , Peng, J. , Turek, F. W. , Marconi, A. , … Zee, P. C. (2014). Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol, 71(4), 463–469. https://doi.org/10.1001/jamaneurol.2013.6239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhauser, F. , Ehrhart, B. , & Forster, E. (1993). Clinical aspects of the melatonin action: impact of development, aging, and puberty, involvement of melatonin in psychiatric disease and importance of neuroimmunoendocrine interactions. Experientia, 49(8), 671–681. [DOI] [PubMed] [Google Scholar]
- Watson, N. , Diamandis, T. , Gonzales‐Portillo, C. , Reyes, S. , & Borlongan, C. V. (2016). Melatonin as an Antioxidant for Stroke Neuroprotection. Cell Transplant, 25(5), 883–891. https://doi.org/10.3727/096368915x689749 [DOI] [PubMed] [Google Scholar]
- Weintraub, D. , Comella, C. L. , & Horn, S. (2008). Parkinson's disease–Part 1: Pathophysiology, symptoms, burden, diagnosis, and assessment. The American Journal of Managed Care, 14(2 Suppl), S40–48. [PubMed] [Google Scholar]
- Weldon, D. T. , Rogers, S. D. , Ghilardi, J. R. , Finke, M. P. , Cleary, J. P. , O'Hare, E. , … Mantyh, P. W. (1998). Fibrillar beta‐amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. Journal of Neuroscience, 18(6), 2161–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, G. L. (2008). Intraocular microinjections repair experimental Parkinson's disease. Brain Research, 1217, 119–131. https://doi.org/10.1016/j.brainres.2008.03.083 [DOI] [PubMed] [Google Scholar]
- Willis, G. L. , & Turner, E. J. (2007). Primary and secondary features of Parkinson's disease improve with strategic exposure to bright light: a case series study. Chronobiology International, 24(3), 521–537. https://doi.org/10.1080/07420520701420717 [DOI] [PubMed] [Google Scholar]
- Wirdefeldt, K. , Adami, H. O. , Cole, P. , Trichopoulos, D. , & Mandel, J. (2011). Epidemiology and etiology of Parkinson's disease: a review of the evidence. European Journal of Epidemiology, 26 Suppl 1, S1–58. https://doi.org/10.1007/s10654-011-9581-6 [DOI] [PubMed] [Google Scholar]
- Wu, H. J. , Wu, C. , Niu, H. J. , Wang, K. , Mo, L. J. , Shao, A. W. , … Wang, Y. R. (2017). Neuroprotective Mechanisms of Melatonin in Hemorrhagic Stroke. Cellular and Molecular Neurobiology, 37(7), 1173–1185. https://doi.org/10.1007/s10571-017-0461-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. H. , Feenstra, M. G. , Zhou, J. N. , Liu, R. Y. , Torano, J. S. , Van Kan, H. J. , … Swaab, D. F. (2003). Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages. The Journal of Clinical Endocrinology & Metabolism, 88(12), 5898–5906. https://doi.org/10.1210/jc.2003-030833 [DOI] [PubMed] [Google Scholar]
- Wu, Y. H. , Zhou, J. N. , Balesar, R. , Unmehopa, U. , Bao, A. , Jockers, R. , … Swaab, D. F. (2006). Distribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: colocalization of MT1 with vasopressin, oxytocin, and corticotropin‐releasing hormone. Journal of Comparative Neurology, 499(6), 897–910. https://doi.org/10.1002/cne.21152 [DOI] [PubMed] [Google Scholar]
- Yankner, B. A. (1996). Mechanisms of neuronal degeneration in Alzheimer's disease. Neuron, 16(5), 921–932. [DOI] [PubMed] [Google Scholar]
- Younkin, S. G. (1995). Evidence that A beta 42 is the real culprit in Alzheimer's disease. Annals of Neurology, 37(3), 287–288. https://doi.org/10.1002/ana.410370303 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Perry, G. , Smith, M. A. , Robertson, D. , Olson, S. J. , Graham, D. G. , & Montine, T. J. (1999). Parkinson's disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. American Journal of Pathology, 154(5), 1423–1429. https://doi.org/10.1016/s0002-9440(10)65396-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Wang, P. , Ren, L. , Hu, C. , & Bi, J. (2016). Protective effect of melatonin on soluble Abeta1–42‐induced memory impairment, astrogliosis, and synaptic dysfunction via the Musashi1/Notch1/Hes1 signaling pathway in the rat hippocampus. Alzheimer's Research & Therapy, 8(1), 40 https://doi.org/10.1186/s13195-016-0206-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, L. , Roberg, K. , Jerhammar, F. , Marcusson, J. , & Terman, A. (2006). Oxidative stress induces intralysosomal accumulation of Alzheimer amyloid beta‐protein in cultured neuroblastoma cells. Annals of the New York Academy of Sciences, 1067, 248–251. https://doi.org/10.1196/annals.1354.032 [DOI] [PubMed] [Google Scholar]
- Zhou, J. , Zhang, S. , Zhao, X. , & Wei, T. (2008). Melatonin impairs NADPH oxidase assembly and decreases superoxide anion production in microglia exposed to amyloid‐beta1–42. Journal of Pineal Research, 45(2), 157–165. https://doi.org/10.1111/j.1600-079X.2008.00570.x [DOI] [PubMed] [Google Scholar]
- Zhou, J. N. , Liu, R. Y. , Kamphorst, W. , Hofman, M. A. , & Swaab, D. F. (2003). Early neuropathological Alzheimer's changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. Journal of Pineal Research, 35(2), 125–130. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, B. R. Neuropsychopharmacology: The Fifth Generation of Progress.
- Zimmermann, R. C. , McDougle, C. J. , Schumacher, M. , Olcese, J. , Mason, J. W. , Heninger, G. R. , & Price, L. H. (1993). Effects of acute tryptophan depletion on nocturnal melatonin secretion in humans. The Journal of Clinical Endocrinology & Metabolism, 76(5), 1160–1164. https://doi.org/10.1210/jcem.76.5.8496306 [DOI] [PubMed] [Google Scholar]


