Abstract
In the thymus, medullary thymic epithelial cells (mTEC) determine the fate of newly selected CD4+ and CD8+ single positive (SP) thymocytes. For example, mTEC expression of Aire controls intrathymic self‐antigen availability for negative selection. Interestingly, alterations in both Foxp3+ Regulatory T‐cells (T‐Reg) and conventional SP thymocytes in Aire−/− mice suggest additional, yet poorly understood, roles for Aire during intrathymic T‐cell development. To examine this, we analysed thymocytes from Aire −/− mice using Rag2GFP and Foxp3 expression, and a recently described CD69/MHCI subset definition of post‐selection CD4+ conventional thymocytes. We show that while Aire is dispensable for de novo generation of conventional αβT‐cells, it plays a key role in controlling the intrathymic T‐Reg pool. Surprisingly, a decline in intrathymic T‐Reg in Aire−/− mice maps to a reduction in mature recirculating Rag2GFP− T‐Reg that express CCR6 and re‐enter the thymus from the periphery. Furthermore, we show mTEC expression of the CCR6 ligand CCL20 is reduced in Aire−/− mice, and that CCR6 is required for T‐Reg recirculation back to the thymus. Collectively, our study re‐defines requirements for late stage intrathymic αβT‐cell development, and demonstrates that Aire controls a CCR6‐CCL20 axis that determines the developmental makeup of the intrathymic T‐Reg pool.
Keywords: Thymic epithelium, Thymic selection, Thymocyte, Thymopoiesis, Thymus
Introduction
In the thymus, distinct stromal cell types support the generation of functionally competent αβT‐cells. For example, cortical thymic epithelial cells (cTEC) are specialised for positive selection, and form ‘thymic nurse cell’ complexes 1, 2 with DP thymocytes to enable secondary TCRα rearrangements and enhance opportunities for further maturation 3. Complementary to cortex specialisation are unique features of the thymus medulla that control later stages of thymocyte development. While dendritic cells in the medulla mediate negative selection 4, medullary thymic epithelial cells (mTEC) express MHCII and CD80/CD86 5, 6, Aire 7, 8, 9, 10, 11, and CCL21 12, 13 to control the selection, differentiation and migration of newly generated CD4+ and CD8+ single positive (SP) thymocytes prior to thymic exit. Thus, cTEC/mTEC specialisation is an important feature of intrathymic microenvironments that guide step‐wise T‐cell development.
In order to mediate negative selection, mTEC express Aire and Fezf2 for the intrathymic expression of self‐antigens that eliminate autoreactive thymocytes by apoptosis 14, 15, 16, 17, 18. Significantly, while mTEC also support lineage divergence in CD4+ thymocytes to create Foxp3+ Regulatory T‐cells (T‐Reg) 19, 20, their role in this process is not fully understood. For example, while the intrathymic generation of naturally diverse polyclonal Foxp3+ T‐Reg is dependent upon Aire in the neonate 21, the presence of peripheral recirculating T‐Reg in the adult thymus 22, 23, 24, 25, 26 makes it difficult to accurately quantitate de novo T‐Reg development at this later stage of life. Thus, the impact of Aire on the adult intrathymic T‐Reg pool is unclear. In addition, the medulla has also been linked to stages in the post‐selection development of conventional SP thymocytes 27, 28. Importantly however, while CCR4, CCR7, CCR9 19, 29, 30 and CD69, 6C10, Qa2 31 define CD4+ SP thymocyte heterogeneity, recent work 28 has raised doubts about the suitability of some of these markers (e.g. Qa2) to identify specific developmental stages. Interestingly, the same study described ‘Semi‐Mature’ (SM, CD69+MHCIlo), ‘Mature 1’ (M1, CD69+MHCI+) and ‘Mature 2’ (M2, CD69−MHCI+) subsets that represent an accurate developmental sequence of CD4+ SP thymocytes, with functional maturation residing within M1 and M2 cells 28, 32. However, while these studies define SP thymocyte heterogeneity, the role of the thymus medulla in controlling transit through these stages is not clear. For example, while the reduction in late‐stage conventional CD4+ thymocytes in adult Aire−/− mice suggests a role for mTEC and Aire in post‐selection conventional thymocyte differentiation 31, 33, 34, other studies showed that mTEC are not required for conventional CD4+ thymocyte development 19. Consequently, the role of mTEC and Aire in late stage CD4+ αβT‐cell development is poorly understood.
Given these uncertainties, we have studied mechanisms controlling the developmental progression of CD4+ thymocytes, and examined the impact of the thymus medulla on this process. By analysing Rag2GFP expression in intrathymic Foxp3+ T‐Reg and SM/M1/M2 CD4+ SP conventional thymocyte subsets in adult Aire−/− mice, we show that Aire is dispensable for de novo conventional and Foxp3+ T‐Reg development. In contrast, we find that Aire exerts a surprising influence on the intrathymic T‐Reg pool, by controlling recirculation of peripheral CCR6+ T‐Reg back to the thymus. Moreover, we show this involves Aire‐mediated regulation of the chemokine CCL20 in mTEC, and identify a novel CCR6‐CCL20 axis for T‐Reg thymus recirculation. Collectively, our study redefines the requirements of post‐selection intrathymic T‐cell development, and describes a new role for Aire in controlling migration of T‐Reg between peripheral tissues and the thymus.
Results
Aire controls developmental heterogeneity in the intrathymic Foxp3+ T‐Reg pool
The thymus medulla contains Foxp3+ T‐Reg that branch off from the programme of conventional thymocyte development during the CD4+ stage 19, 35, 36, and mTEC play an essential role in the generation of Foxp3+ T‐Reg and their precursors 19, 20. Consistent with this, Aire influences Foxp3+ T‐Reg generation in neonatal mice 21. However, as the naturally diverse T‐Reg pool in the adult thymus consists of both newly produced cells and recirculating T‐Reg that have re‐entered from the periphery 22, 23, 24, the requirement for Aire during adult intrathymic T‐Reg development is not clear. To address this directly, we crossed Aire−/− mice with Rag2GFP mice, providing an accurate means to distinguish newly produced (GFP+) and recirculating (GFP−) cells in the thymus 22, 23, and examined the developmental makeup of thymic T‐Reg. The gating strategy used to define intrathymic Foxp3+ T‐Reg development is shown in Supporting Information Fig. 1. Interestingly, while total T‐Reg numbers showed a significant reduction in Aire−/−Rag2GFP mice (Fig. 1A), the numbers of CD25+Foxp3− and CD25−Foxp3+ thymocytes that represent T‐Reg progenitors 35, 36 were unchanged (Fig. 1A). Moreover, when we analysed GFP expression in both T‐Reg and T‐Reg progenitor populations from thymuses of WTRag2GFP and Aire−/−Rag2GFP mice, we saw no differences in the absolute numbers of GFP+ thymocytes representing de novo generated cells (Fig. 1B and C). Thus, newly generated Rag2GFP+ T‐Reg and T‐Reg progenitors develop effectively in the absence of Aire, indicating that the reduction in total T‐Reg in Aire−/− mice cannot be explained by a role for Aire in intrathymic T‐Reg development. Surprisingly, further analysis of Aire−/−Rag2GFP mice revealed a significant decrease in the number of intrathymic T‐Reg that lacked GFP expression (Fig. 1D), indicating that a selective decline in Rag2GFP− T‐Reg provides an explanation for the reduction in overall intrathymic T‐Reg numbers in Aire−/− mice. As these cells have been shown previously to represent thymus‐recirculating cells 22, 23, this suggests that Aire influences the thymus re‐entry of mature T‐Reg from the periphery.
Figure 1.

Selective reduction in Rag2GFP− Foxp3+ thymic T‐Reg in Aire−/− mice. Flow cytometric analysis of CD4+CD8−TCRβ+CD25+Foxp3+, CD4+CD8−TCRβ+CD25+Foxp3− and CD4+CD8−TCRβ+CD25−Foxp3+ thymocytes from both WTRag2GFP (white) and Aire−/−Rag2GFP (black) mice. Gating strategy is shown in Supporting Information Fig. 1. Bar graphs in panel (A) show total numbers of each population, and panel (B) shows representative examples of Rag2GFP expression within each population, gray histograms indicated fluorescence levels on GFP− thymocytes. Panel (C) shows numbers of Rag2GFP+ T‐Reg, CD25+Foxp3− and CD25−Foxp3+ cells in WTRag2GFP (white) and Aire−/−Rag2GFP (black) mice. Panel (D) shows numbers of Rag2GFP− T‐Reg, CD25+Foxp3− and CD25−Foxp3+ cells in WTRag2GFP (white) and Aire−/−Rag2GFP (black) mice. Error bars indicate SEM, using an unpaired Student's two‐tailed t‐test: *p < 0.05; ***p < 0.001. Data are pooled from at least three separate experiments, with at least 6 mice of each strain in total.
Aire controls recirculation of peripheral T‐Reg back to the thymus
To directly examine the requirement for Aire in thymus T‐Reg recirculation, we performed thymus transplantation experiments to visualise and quantitate peripheral T‐Reg homing to the thymus. Thus, freshly isolated WT embryonic day 18 (E18) thymus lobes from BoyJ mice, which contain a cohort of CD45.1+ congenically marked thymocytes, were transplanted under the kidney capsule of CD45.2+ WT or CD45.2+ Aire−/− adult mice. In this way, grafted thymuses generate a single wave of T‐cell development, and so allow direct analysis of thymus recirculation through the identification of graft‐derived CD45.1+ T‐cells within the host thymus 22. After 5 weeks, host thymus and spleen tissues were harvested from WT and Aire−/− hosts, and CD45.1 expression was used to identify graft‐derived cells alongside analysis of CD4, CD8, TCRβ and Foxp3 by flow cytometry. When we analysed host thymuses from WT and Aire−/− mice, we saw striking differences in the frequency of graft‐derived T‐cells. Thus, the numbers of both total donor T‐cells and Foxp3+ T‐Reg, were significantly reduced in the Aire−/− host thymus (Fig. 2A). Moreover, while in the WT host thymus, graft‐derived T‐Reg:T‐conv were present at a ratio of 3:1, in the thymus of Aire−/− hosts we saw a markedly skewed ratio of 1:1, indicating reduced graft‐derived T‐Reg entry to the Aire−/− thymus (Fig. 2A). Importantly, in the spleens of both WT and Aire−/− mice, the numbers of total graft‐derived CD45.1+ donor cells and CD45.1+Foxp3+ T‐Reg, were comparable (Fig. 2B). Moreover, splenic ratios of T‐Reg:T‐conv CD45.1+ graft‐derived T‐cells were comparable in both WT and Aire−/− mice (Fig. 2B). Thus, WT thymus‐graft derived T‐Reg are represented equally in the spleen of WT and Aire−/− hosts, indicating that limitations in their availability cannot explain the reduction in thymus‐recirculating cells in Aire−/− mice. Rather, these findings indicate that absence of Aire limits the entry of peripheral T‐Reg to thymus.
Figure 2.
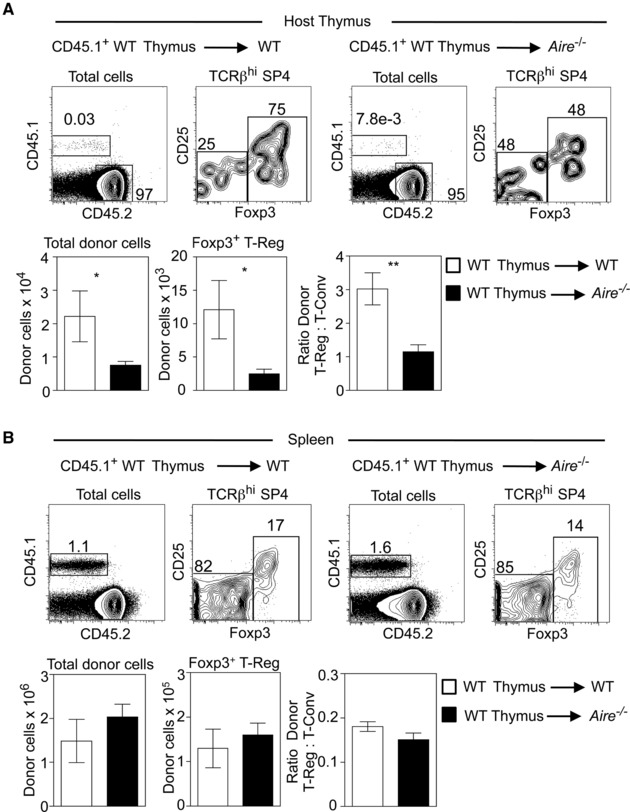
Aire regulates the thymus recirculation of peripheral Foxp3+ T‐Reg. panel (A) shows flow cytometric analysis of the host thymus of either CD45.2+ WT or Aire−/− mice, previously grafted with an embryonic CD45.1+ WT thymus. Upper panels show representative examples of the detection of graft‐derived CD45.1+CD45.2− thymic cells in the host mouse thymus, and detection of CD25+Foxp3+ T‐Reg within these cells. Lower panels show absolute cell numbers of graft‐derived donor cells, and the ratio of donor T‐Reg:T‐conv present in the thymus of WT (white bars) and Aire−/− (black bars) mice. Panel (B) shows identical analysis of splenocytes from WT (white bars) and Aire−/− (black bars) mice transplanted with a CD45.1+ WT embryonic thymus. Error bars indicate SEM, using an unpaired Student's two‐tailed t‐test: *p < 0.05; **p < 0.01. Data are representative of at least two separate experiments, with at least 5 mice of each strain in total.
Aire controls a CCL20‐CCR6 axis for thymus T‐Reg recirculation
Within the intrathymic T‐Reg pool, recirculating T‐Reg can be distinguished from newly generated T‐Reg by their differential expression of chemokine receptors. Thus, while newly produced Rag2GFP+ T‐Reg contain CCR6−CCR7+ cells, recirculating Rag2GFP− cells are CCR6+CCR7− 22. As CCR6 is expressed by peripherally‐derived T‐Reg within the intrathymic pool, we next examined the involvement of this chemokine receptor in thymic recirculation. Initially, we transplanted lymphoid CD45.2+ embryonic thymus lobes from either WT or Ccr6−/− mice into congenically marked CD45.1+ WT mice. After 5 weeks, thymus and spleen from host mice was harvested, and the frequency and phenotype of graft‐derived CD45.2+ T‐cells was determined by flow cytometry. In the spleen, donor‐derived Foxp3+ T‐Reg numbers from Ccr6−/− thymus grafts were only slightly lower than those from WT grafts (Fig. 3A). However, when we analysed the number of graft‐derived cells in the host thymus, we saw a far greater reduction in Ccr6−/− graft‐derived T‐Reg compared to WT graft‐derived cells (Fig. 3B).
Figure 3.
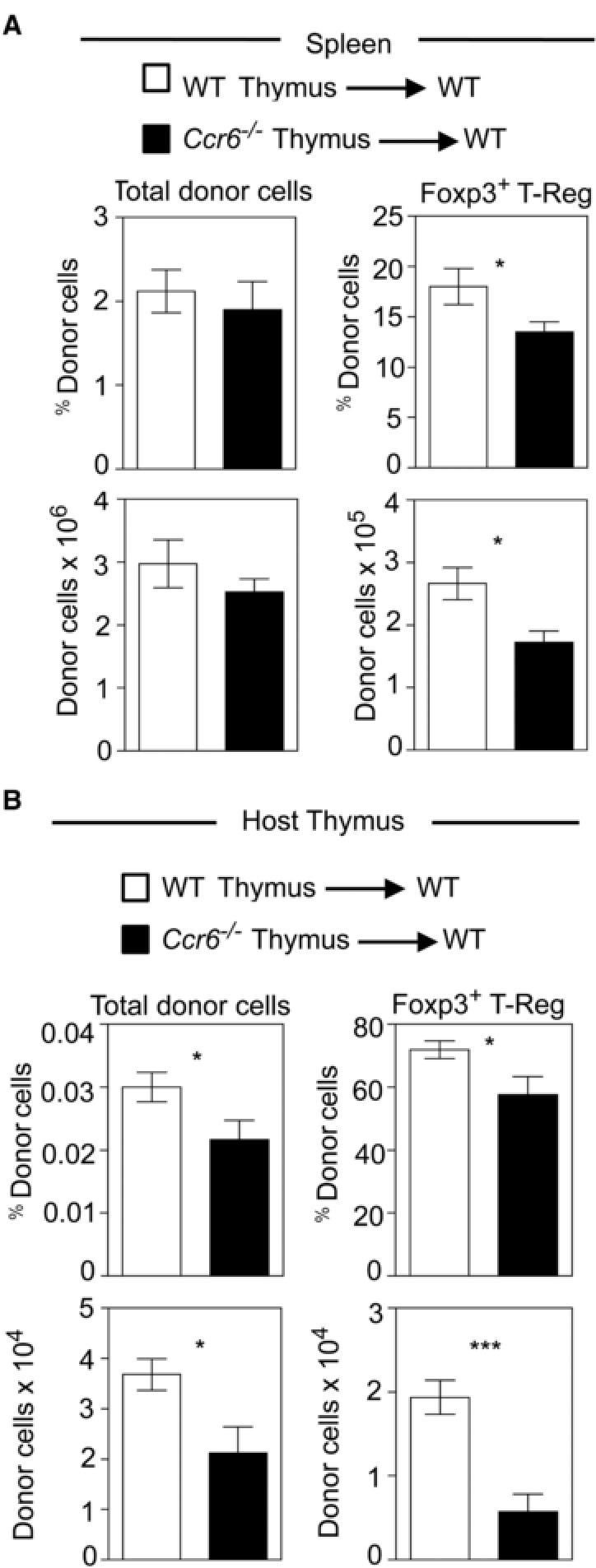
CCR6 controls the thymus homing of peripheral Foxp3+ T‐Reg. panel (A) shows flow cytometry analysis of splenocytes obtained from CD45.1+ WT mice previously grafted with either CD45.2+ WT (white bars) or Ccr6−/− (black bars) embryonic thymus lobes. Graphs show the absolute numbers of indicated donor cell populations. Panel (B) shows similar analysis of graft‐derived donor cells in the host thymus harvested from the same mice. Error bars indicate SEM using an unpaired Student's two‐tailed t‐test: * p < 0.05; *** p < 0.001. Data are representative of at least two separate experiments, with at least 5 mice of each strain in total.
As the above findings suggest that entry of peripheral Ccr6−/− T‐Reg into the host thymus is impaired, and to further examine the role of CCR6 in thymus T‐Reg recirculation, we next generated Ccr6−/−Rag2GFP mice and analysed developmental heterogeneity within the intrathymic T‐Reg pool (Fig. 4A and B). Importantly, by discriminating between recirculating GFP− cells and newly produced GFP+ cells, we saw a significant reduction in both the percentage and number of Rag2GFP− T‐Reg in the thymus of Ccr6−/−Rag2GFP mice (Fig. 4C). Thus, by both thymus transplantation and Rag2GFP expression analysis, these findings suggest that CCR6 is involved in the migration of peripheral T‐Reg back to the thymus. Finally, we next examined how CCR6 may be linked to the requirement for Aire in this process. As Aire has been shown to influence expression of intrathymic chemokines 33, 37, 38, 39, we analysed expression of the CCR6 ligand, CCL20, in purified TEC populations isolated from WT and Aire−/− mice. Interestingly, we found that Ccl20 mRNA was selectively expressed by mTEChi (Fig. 4D), the subset that contains Aire‐expressing cells. Moreover, direct comparison of mTEChi from WT and Aire−/− mice showed a striking reduction of Ccl20 mRNA in the latter (Fig. 4D). Thus, mTEChi express CCL20, which is reduced in the absence of Aire. Taken together with the expression of CCR6 by recirculating T‐Reg, and the diminished thymus recirculation of Ccr6−/− T‐Reg, our data suggest that Aire regulates a CCL20‐CCR6 axis required for peripheral T‐Reg homing to the thymus.
Figure 4.
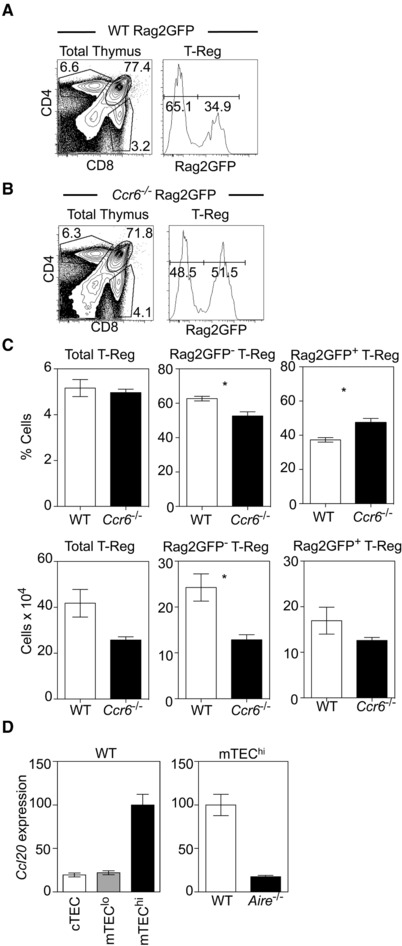
Recirculating T‐Reg are reduced in the thymus of Ccr6 −/−Rag2GFP mice. Flow cytometry analysis of CD4, CD8 expression in WTRag2GFP (Panel A) and Ccr6−/−Rag2GFP (Panel B) thymocytes, together with Rag2GFP levels on CD25+Foxp3+ SP4 thymic T‐Reg. Panel (C) shows percentages (upper panels) and absolute numbers (lower panels) of Rag2GFP+ and Rag2GFP− thymic T‐Reg in WTRag2GFP (white bars) and Ccr6−/−Rag2GFP (black bars) mice. Error bars indicate SEM using an unpaired Student's two‐tailed t‐test: * p < 0.05. Data are pooled from at least three separate experiments, with at least 6 mice of each strain in total. Panel (D) shows qPCR analysis of Ccl20 mRNA levels in indicated TEC populations isolated from WT mice, and in mTEChi isolated from either WT or Aire−/− mice. Fold levels represent the mean +/‐SEM of replicate reactions. Data is representative of at least 2 independent experiments, where cDNA samples were prepared from sorted cells from at least 3 mice.
Aire is not required for conventional CD4+ thymocyte development
In addition to housing conventional Foxp3+ T‐Reg, the thymus medulla contains conventional CD4+ and CD8+ thymocytes that consist of a series of subsets that are phenotypically and developmentally distinct 19, 27, 28. While the mechanisms controlling conventional post‐selection thymocyte development are not fully understood, a reduction in Qa2+CD4+ thymocytes in Aire −/− mice suggests a role for Aire in this process 31. However, as recent studies have raised doubts about the suitability of some previously used markers as indicators of maturation status 28, we adopted a method involving Rag2GFP, MHCI and CD69 expression that robustly identifies a developmental programme for CD4+ thymocytes 28. To directly assess the role of Aire in conventional thymocyte development, we analysed Aire−/−Rag2GFP mice in relation to semi‐mature (SM, CD69+MHCIlo), mature 1 (M1, CD69+MHCIhi) and mature 2 (M2, CD69+MHCIhi) CD4+ thymocyte subsets. In all cases, conventional CD4+ thymocytes were identified as CD4+8−TCRβ+Rag2GFP+CD25− cells (Fig. 5A and B). As shown previously 31, analysis of CD69 and Qa2 expression showed a marked reduction in Qa2hiCD69− cells in Aire−/−Rag2GFP mice, reported to represent the most mature CD4+ cells (Fig. 5A and B). In contrast, SM, M1 and M2 CD4+ subsets defined by MHCI, CD69 and Rag2GFP were readily detectable in both WTRag2GFP and Aire−/−Rag2GFP mice, which also showed a comparable progressive reduction in Rag2GFP levels (Fig. 5C and D). Moreover, while the percentage of SM cells in Aire−/−Rag2GFP mice showed a slight increase, no significant differences in the absolute numbers of SM, M1 and M2 CD4+ cells from WTRag2GFP and Aire−/− Rag2GFP mice were seen (Fig. 5D). Thus, our analysis of Aire−/− mice using Rag2GFP as a reporter of developmental status in association with SM/M1/M2 subset detection indicates that Aire is not required for conventional CD4+ thymocyte development. Moreover, that an unperturbed programme of maturation in Aire−/− mice is still accompanied by alterations in Qa2+ thymocyte frequency confirms the limited suitability of this marker to examine post‐selection thymocyte differentiation 28.
Figure 5.
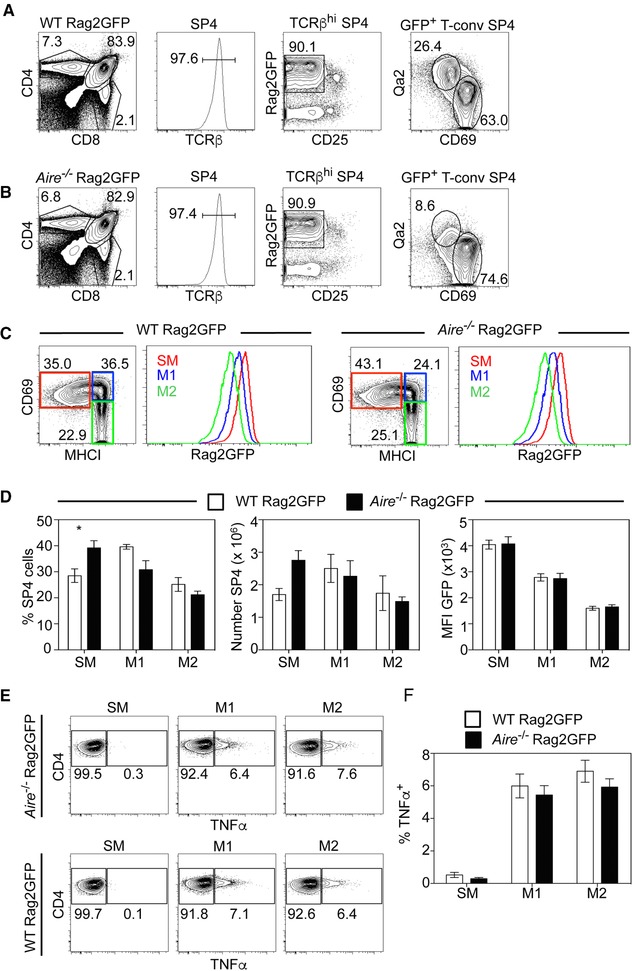
Aire is dispensable for post‐selection maturation and cytokine licencing in conventional thymocytes. Flow cytometric detection of CD4+CD8−TCRβ+CD25−Rag2GFP+ conventional CD4+ SP thymocytes in WTRag2GFP (A) and Aire−/−Rag2GFP (B) mice. Analysis of Qa2/CD69 expression in these cells is also shown. Panel (C) shows conventional CD4+ SP thymocytes from WTRag2GFP and Aire−/−Rag2GFP mice, analysed for expression of CD69 and MHCI to detect: SM (CD69+MHCIlow), M1 (CD69+MHCI+) and M2 (CD69−MHCI+) subsets. Histograms show the Rag2GFP levels in the indicated populations. Panel (D) shows proportions, numbers and Rag2GFP levels of populations identified in (C). Panels (E) and (F) show analysis of TNFα production in SM, M1 and M2 subsets of conventional CD4+ SP thymocytes from WTRag2GFP (white bars) and Aire−/−Rag2GFP (black bars) mice. Error bars indicate SEM, and a one‐way ANOVA was used, * p< 0.05. Data are pooled from at least three separate experiments, with at least 6 mice of each strain in total.
In addition to phenotypic changes, CD4+ thymocytes undergo functional maturation as they progress through post‐selection stages of development 27, 28, 32, 40. This includes the ability to produce the cytokine TNFα in response to TCR stimulation, a process termed cytokine licencing 28. To examine whether Aire plays a role in the functional maturation of CD4+ thymocytes, we next assessed the cytokine licencing capabilities of SM, M1 and M2 subsets from WT and Aire −/− mice. Thymocytes were stimulated with anti‐CD3/anti‐CD28 and TNFα production was analysed following cell permeabilisation 28. Interestingly, CD4+ thymocytes from both WTRag2GFP and Aire−/−Rag2GFP mice were comparable in their TNFα production (Fig. 5E and F). Thus, consistent with their immature status, stimulation of the least mature SM CD4+ cells from both WTRag2GFP and Aire−/−Rag2GFP mice failed to induce TNFα expression. However, more mature M1 and M2 subsets from both WTRag2GFP and Aire−/−Rag2GFP mice generated TNFα‐producing cells at similar frequencies (Fig. 5E and F). Thus, the ability of CD4+ thymocytes to acquire cytokine licencing properties, and the developmental timing of this process, occurs normally in Aire−/− mice. Taken together with our phenotypic analysis of post‐selection maturation stages, these findings demonstrate that Aire is not required for completion of intrathymic CD4+ thymocyte development.
Discussion
The thymus medulla houses both conventional and Foxp3+ T‐Reg generated during T‐cell development 41, 42. As a consequence, medullary functions are diverse in order to influence multiple events within distinct αβT‐cell lineages. The importance of the medulla is well studied, which is due at least in part to mTEC expression of Aire, and its control of intrathymic availability of self‐antigens for negative selection 11, 15, 16. Interestingly, Aire has been implicated in other aspects of medulla function, including post‐selection development of conventional CD4+ thymocytes and Foxp3+ T‐Reg development 21, 31, 33. However, understanding these additional roles for Aire has been limited by uncertainty in identifying post‐selection stages in T‐cell development 28, and the presence of recirculating peripheral T‐Reg in the adult thymus, that hinder accurate analysis of de novo T‐Reg selection 22, 23, 24, 25. Here, we used a variety of approaches to examine mechanisms regulating CD4+ conventional and Foxp3+ T‐Reg in the thymus. For conventional thymocytes, we adopted an approach in which step‐wise progression through three SM, M1 and M2 CD4+ stages was examined alongside de novo Rag2GFP+ thymocyte maturation and the timing of acquisition of functional competency 28. By performing this analysis in Aire−/− mice, we show that SM/M1/M2 post‐selection phenotypic progression, and acquisition of TNFα cytokine licencing, both occur independently of Aire. Thus, Aire is not a key regulator of post‐selection thymocyte differentiation. This conclusion differs from earlier studies where a reduction in Qa2hiCD69− CD4+ thymocytes in Aire−/− mice (also shown here) was taken as evidence for a role for Aire in post‐selection thymocyte maturation 31. Importantly, as Qa2 expression has recently been shown to indicate type 1 interferon signalling in thymocytes rather than their maturation status 28, its suitability as a marker to identify stages in post‐thymic differentiation is uncertain. Indeed, our finding that post‐selection thymocyte maturation is regulated by Aire‐independent mechanisms is compatible with studies showing that mTEC, major expressers of Aire in the thymus, are not required for the generation of mature CD4+ thymocytes 19. As such, our data suggest the primary role of Aire in conventional adult αβT‐cell development is tolerance induction, and not post‐selection differentiation.
Foxp3+ T‐Reg require the medulla, and mTEC in particular, for their development 19. Here, using Rag2GFP expression and thymus transplantation approaches, we reveal an unexpected impact of Aire on the developmental makeup of the intrathymic T‐Reg pool in adult mice, and show it controls the frequency of mature T‐Reg entering the thymus from the periphery. Thus, a reduction in T‐Reg recirculation, not de novo production, may explain the smaller T‐Reg pool size in the thymus of adult Aire−/− mice. In addition, it has also been shown that T‐Reg can be retained within the thymus for longer periods compared to conventional thymocytes 43. While these findings emphasise the developmental heterogeneity that exists within T‐Reg in the thymus, it is currently unclear whether Aire impacts the intrathymic T‐Reg pool by influencing both intrathymic retention and re‐entry from peripheral tissues. Importantly, these findings on the role of Aire in T‐Reg recirculation may still be consistent with studies demonstrating a requirement for Aire in TCR‐transgenic T‐Reg development in adult mice 20, 44, 45, 46. Thus, while we show naturally diverse intrathymic T‐Reg are produced in comparable numbers in WT and Aire−/− mice, they may differ in the αβTCR repertoires they express. This would be consistent with Aire controlling the development of those cells selected by Aire‐dependent TRAs. In addition, by demonstrating Aire‐dependent expression of CCL20 for entry of CCR6+ peripheral T‐Reg, we provide new insight into Aire's ability to exert influence on thymus populations by affecting intrathymic chemokine availability. Thus, while Aire controls XCL1‐mediated thymic DC positioning 37 and CCR4/CCR7 ligand‐mediated thymocyte migration 33, 38, we now show that Aire also controls CCL20 for T‐Reg migration between the thymus and periphery (Fig. 6). That CCR6 is expressed by recirculating cells within intrathymic T‐Reg 22 is consistent with this, and further highlights Aire's impact on T‐Reg extends beyond any influence on their intrathymic production. It is interesting to note that while thymic T‐Reg recirculation is reduced in both Aire−/− and Ccr6−/− mice, some peripherally‐derived thymic T‐Reg are still detectable. Whether this reflects additional roles for other Aire‐dependent and –independent chemokines is unclear.
Figure 6.
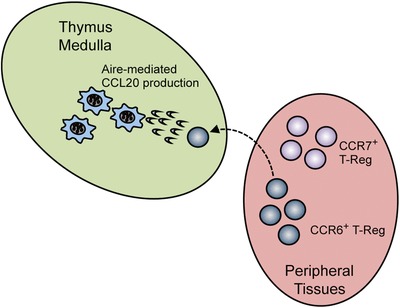
Aire controls A CCR6‐CCL20 axis for Foxp3+ T‐Reg recirculation to the thymus. In the model shown, mTEC produce the chemokine CCL20 in an Aire‐dependent manner. This enables mature CCR6+ T‐Reg to recirculate back to the thymus from peripheral tissues. Newly produced T‐Reg within peripheral tissues are CCR7+CCR6−, and so do not undergo CCL20‐mediated thymus recirculation.
The functional relevance of T‐Reg recirculation to the thymus is not fully understood. However, recent studies have shown that it may control de novo T‐Reg development by creating intrathymic competition for IL‐2 23, 24. However, despite reduced recirculating T‐Reg in Aire−/− mice, we saw no changes in the numbers of de novo produced T‐Reg precursors or more mature Foxp3+ T‐Reg. Whether the reduced peripheral T‐Reg numbers in the thymus of Aire−/− mice are still able to effectively compete for IL‐2 and limit new T‐Reg production is not clear. Interestingly, CCL20 directs CCR6+ T‐Reg to peripheral inflammatory sites 47, and its expression by mTEC for the attraction of CCR6+ T‐Reg correlates with conditions within medullary thymic microenvironments that support constitutive MHC class II expression and elevated co‐stimulatory molecules on thymic APC 48. Whether recirculating T‐Reg influence the thymic medullary microenvironments they reside within is currently not known.
In conclusion, by analysing conventional and Foxp3+ thymocytes and separating intrathymic production from thymic recirculation, we have examined the requirement for Aire in specialisation of the thymus medulla during αβT‐cell development. Collectively, our findings support models in which the major role of Aire+ medullary microenvironments in adult thymus is the control of tolerance induction and not thymocyte differentiation. In contrast, by revealing an Aire‐mediated mechanism that controls the entry of peripherally derived T‐Reg to the thymus, we demonstrate the ability of Aire to regulate communication between sites of T‐cell production and function.
Materials and methods
Mice
Wildtype (WT) C57BL/6, Rag2GFP 49, Aire−/− 50 and Ccr6 −/− mice (obtained from The Jackson Laboratory, stock number 005793 51 on a C57BL/6 background were used at 8–12 weeks of age. All mice were housed at the Biomedical Services Unit at The University of Birmingham, and all procedures were performed with permission for the UK Home Office. Rag2GFP mice were interbred with Aire−/− and Ccr6−/− mice to generate Aire −/−Rag2GFP and Ccr6 −/−Rag2GFP mice respectively. Embryos from WT CD45.1+ BoyJ and C57BL/6 CD45.2+ mice were generated from timed matings, with the day of vaginal plug detection designated as day 0.
Thymocyte cytokine licencing
TNFα production in freshly isolated thymocytes was performed as described 28. Briefly, 5 × 106 thymocytes were cultured with plate‐bound anti‐CD3 (10 ug/ml, clone 145‐2C11, eBioscience) and anti‐CD28 (20 ug/ml, clone 37.51, eBioscience) for 6 hours in GolgiPlug (BD Bioscience). Cells were stained with antibodies described below to detect SM, M1 and M2 CD4+ cells, fixed using BD Cytofix/Cytoperm (BD Bioscience) for 30 min on ice, and TNFα production was detected by flow cytometry using an anti‐TNFα antibody (MP6‐XT22, BD Bioscience).
Antibodies, immunoconjugates and flow cytometry
Thymus and spleen were mechanically teased with glass slides to acquire single cell suspensions and cells were stained with antibodies to the following: CD45.1 (A20, eBioscience), CD45.2 (104, eBioscience), CD4 (RM4‐5, Biolegend), CD8 (53‐6.7, Biolegend), CD25 (PC61.5, Biolegend), CD44 (IM7, eBioscience), Qa2 (695H1‐9‐9, Biolegend), TCRβ (H57‐597, eBioscience), CD69 (H1.2F3, eBioscience), and H‐2Kb (AF6‐88.5, Biolegend). Reagents were conjugated to Pacific Blue, Brilliant Violet (BV) 421, BV510, BV711, PE, PE‐Cy7, PerCP–eFluor 710, allophycocyanin– eFluor 780 and Alexa Fluor 700. Streptavidin PE‐Cy7 (eBioscience) was used to detect biotinylated antibodies. A Foxp3 fixation kit (eBioscience) was used in conjunction with anti‐Foxp3 (FJK‐16s, eBioscience) or the BD Cytofix/Cytoperm Kit (BD Biosciences) to preserve the GFP signal. Data were acquired using a BD LSR Fortessa and FACSDiva 2.6 software and analysed using FloJo software (Tree Star).
Preparation and purification of thymic epithelial cells
Thymus tissue was enzymatically digested with Collagenase/Dispase (2.5 mg/ml; Roche) and DNase 1 (40 mg/ml; Roche). After digestion, CD45+ thymocytes were depleted using anti‐CD45 microbeads and LS columns (Miltenyi Biotec). Antibodies against the following were used to sort TEC populations: CD45 (30‐F11, eBioscience), EpCAM1 (G8.8, eBioscience), I‐Ab (AF6‐120.1, BD Bioscience), Ly51 (6C3, Biolegend), CD80 (16‐10A1, Biolegend). Reagents were conjugated to Pacific Blue, BV 421, BV605, PE, PerCP–eFluor 710, allophycocyanin–eFluor 780. Streptavidin PE‐Cy7 was used to detect biotinylated antibodies. Sorting was performed using a FACS Aria Fusion 1 sorter (BD) with a purity typically >98%.
qPCR
The following FACS‐sorted TEC populations were analysed by qPCR: cTEC: CD45−EpCAM1+Ly51+; mTEClo: CD45−EpCAM1+Ly51−MHCIIloCD80lo; mTEChi: CD45−EpCAM1+Ly51−MHCIIhiCD80hi. mRNA isolation, cDNA synthesis and qPCR were performed exactly as described 29. Primer sequences are as follows:
Thymus transplantation
Freshly isolated embryonic day (E) 18 thymus lobes were used as thymus transplants. To assess the role of Aire in thymus recirculation, thymus lobes from WT CD45.1+ mice were transplanted under the kidney capsule 52 of congenically marked CD45.2 WT or Aire−/− mice. To assess the role of CCR6, CD45.2+ WT or Ccr6−/− thymus lobes were transplanted into WT CD45.1+ adult mice. After 5 weeks, host thymus and spleen tissues were harvested and graft derived T‐cell subsets identified by expression of CD45.1 or CD45.2, as appropriate.
Conflict of interests
The authors declare no commercial or financial conflict of interest.
Abbreviations
- cTEC
cortical thymic epithelial cells
- DP
Double Positive
- mTEC
medullary thymic epithelial cells
- SP
Single Positive
Supporting information
Peer review correspondence
Supporting Information Figure 1
Acknowledgements
We thank Dr Andrea Bacon and BMSU staff for expert animal husbandry, Dr David Withers for Ccr6−/− mice, and Dr Matthew MacKenzie for cell sorting. This work was supported by an MRC Programme Grant to GA, a BBSRC project grant to WEJ, and a Wellcome Trust Seed Award to AJW. JEC, SB, NIM, AJW performed experiments and analysed data, GA and WEJ designed experiments, JEC and GA designed the study and wrote the manuscript.
Current address: Jennifer E. Cowan, T Cell Biology and Development Section, Laboratory of Genome Integrity, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
References
- 1. Wekerle, H. and Ketelsen, U. P. , Thymic nurse cells–Ia‐bearing epithelium involved in T‐lymphocyte differentiation? Nature 1980. 283: 402–404. [DOI] [PubMed] [Google Scholar]
- 2. Kyewski, B. A. and Kaplan, H. S. , Lymphoepithelial interactions in the mouse thymus: phenotypic and kinetic studies on thymic nurse cells. J. Immunol. 1982. 128: 2287–2294. [PubMed] [Google Scholar]
- 3. Nakagawa, Y. , Ohigashi, I. , Nitta, T. , Sakata, M. , Tanaka, K. , Murata, S. , Kanagawa, O. et al, Thymic nurse cells provide microenvironment for secondary T cell receptor alpha rearrangement in cortical thymocytes. Proc. Natl. Acad. Sci. USA 2012. 109: 20572–20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cosway, E. J. , Lucas, B. , James, K. D. , Parnell, S. M. , Carvalho‐Gaspar, M. , White, A. J. , Tumanov, A. V. et al, Redefining thymus medulla specialization for central tolerance. J. Exp. Med. 2017. 214: 3183–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gray, D. H. , Seach, N. , Ueno, T. , Milton, M. K. , Liston, A. , Lew, A. M. , Goodnow, C. C. et al, Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood 2006. 108: 3777–3785. [DOI] [PubMed] [Google Scholar]
- 6. Gabler, J. , Arnold, J. and Kyewski, B. , Promiscuous gene expression and the developmental dynamics of medullary thymic epithelial cells. Eur. J. Immunol. 2007. 37: 3363–3372. [DOI] [PubMed] [Google Scholar]
- 7. Rossi, S. W. , Kim, M. Y. , Leibbrandt, A. , Parnell, S. M. , Jenkinson, W. E. , Glanville, S. H. , McConnell, F. M. et al, RANK signals from CD4(+)3(‐) inducer cells regulate development of Aire‐expressing epithelial cells in the thymic medulla. J. Exp. Med. 2007. 204: 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chuprin, A. , Avin, A. , Goldfarb, Y. , Herzig, Y. , Levi, B. , Jacob, A. , Sela, A. et al, The deacetylase Sirt1 is an essential regulator of Aire‐mediated induction of central immunological tolerance. Nat. Immunol. 2015. 16: 737–745. [DOI] [PubMed] [Google Scholar]
- 9. Kyewski, B. and Peterson, P. , Aire, master of many trades. Cell 2010. 140: 24–26. [DOI] [PubMed] [Google Scholar]
- 10. Laan, M. and Peterson, P. , The many faces of aire in central tolerance. Front Immunol. 2013. 4: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson, M. S. , Venanzi, E. S. , Klein, L. , Chen, Z. , Berzins, S. P. , Turley, S. J. , von Boehmer, H. et al, Projection of an immunological self shadow within the thymus by the aire protein. Science 2002. 298: 1395–1401. [DOI] [PubMed] [Google Scholar]
- 12. Lkhagvasuren, E. , Sakata, M. , Ohigashi, I. and Takahama, Y. , Lymphotoxin beta receptor regulates the development of CCL21‐expressing subset of postnatal medullary thymic epithelial cells. J. Immunol. 2013. 190: 5110–5117. [DOI] [PubMed] [Google Scholar]
- 13. Kozai, M. , Kubo, Y. , Katakai, T. , Kondo, H. , Kiyonari, H. , Schaeuble, K. , Luther, S. A. et al, Essential role of CCL21 in establishment of central self‐tolerance in T cells. J. Exp. Med. 2017. 214: 1925–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takaba, H. , Morishita, Y. , Tomofuji, Y. , Danks, L. , Nitta, T. , Komatsu, N. , Kodama, T. et al, Fezf2 Orchestrates a Thymic Program of Self‐Antigen Expression for Immune Tolerance. Cell 2015. 163: 975–987. [DOI] [PubMed] [Google Scholar]
- 15. Sansom, S. N. , Shikama‐Dorn, N. , Zhanybekova, S. , Nusspaumer, G. , Macaulay, I. C. , Deadman, M. E. , Heger, A. et al, Population and single‐cell genomics reveal the Aire dependency, relief from Polycomb silencing, and distribution of self‐antigen expression in thymic epithelia. Genome Res. 2014. 24: 1918–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abramson, J. and Goldfarb, Y. , AIRE: From promiscuous molecular partnerships to promiscuous gene expression. Eur. J. Immunol. 2015. [DOI] [PubMed] [Google Scholar]
- 17. Derbinski, J. , Gabler, J. , Brors, B. , Tierling, S. , Jonnakuty, S. , Hergenhahn, M. , Peltonen, L. et al, Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J. Exp. Med. 2005. 202: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liston, A. , Lesage, S. , Wilson, J. , Peltonen, L. and Goodnow, C. C. , Aire regulates negative selection of organ‐specific T cells. Nat. Immunol. 2003. 4: 350–354. [DOI] [PubMed] [Google Scholar]
- 19. Cowan, J. E. , Parnell, S. M. , Nakamura, K. , Caamano, J. H. , Lane, P. J. , Jenkinson, E. J. , Jenkinson, W. E. et al, The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. J. Exp. Med. 2013. 210: 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aschenbrenner, K. , D'Cruz, L. M. , Vollmann, E. H. , Hinterberger, M. , Emmerich, J. , Swee, L. K. , Rolink, A. et al, Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat. Immunol. 2007. 8: 351–358. [DOI] [PubMed] [Google Scholar]
- 21. Yang, S. , Fujikado, N. , Kolodin, D. , Benoist, C. and Mathis, D. , Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self‐tolerance. Science 2015. 348: 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cowan, J. E. , McCarthy, N. I. and Anderson, G. , CCR7 Controls Thymus Recirculation, but Not Production and Emigration, of Foxp3(+) T Cells. Cell Rep. 2016. 14: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thiault, N. , Darrigues, J. , Adoue, V. , Gros, M. , Binet, B. , Perals, C. , Leobon, B. et al, Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat. Immunol. 2015. 16: 628–634. [DOI] [PubMed] [Google Scholar]
- 24. Weist, B. M. , Kurd, N. , Boussier, J. , Chan, S. W. and Robey, E. A. , Thymic regulatory T cell niche size is dictated by limiting IL‐2 from antigen‐bearing dendritic cells and feedback competition. Nat. Immunol. 2015. 16: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bosco, N. , Agenes, F. , Rolink, A. G. and Ceredig, R. , Peripheral T cell lymphopenia and concomitant enrichment in naturally arising regulatory T cells: the case of the pre‐Talpha gene‐deleted mouse. J. Immunol. 2006. 177: 5014–5023. [DOI] [PubMed] [Google Scholar]
- 26. Hale, J. S. and Fink, P. J. , Back to the thymus: peripheral T cells come home. Immunol. Cell Biol. 2009. 87: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Webb, L. V. , Ley, S. C. and Seddon, B. , TNF activation of NF‐kappaB is essential for development of single‐positive thymocytes. J. Exp. Med. 2016. 213: 1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xing, Y. , Wang, X. , Jameson, S. C. and Hogquist, K. A. , Late stages of T cell maturation in the thymus involve NF‐kappaB and tonic type I interferon signaling. Nat. Immunol. 2016. 17: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cowan, J. E. , McCarthy, N. I. , Parnell, S. M. , White, A. J. , Bacon, A. , Serge, A. , Irla, M. et al, Differential requirement for CCR4 and CCR7 during the development of innate and adaptive alphabetaT cells in the adult thymus. J. Immunol. 2014. 193: 1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu, Z. , Lancaster, J. N. , Sasiponganan, C. and Ehrlich, L. I. , CCR4 promotes medullary entry and thymocyte‐dendritic cell interactions required for central tolerance. J. Exp. Med. 2015. 212: 1947–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li, J. , Li, Y. , Yao, J. Y. , Jin, R. , Zhu, M. Z. , Qian, X. P. , Zhang, J. et al, Developmental pathway of CD4+CD8− medullary thymocytes during mouse ontogeny and its defect in Aire−/− mice. Proc. Natl. Acad. Sci. USA 2007. 104: 18175–18180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ki, S. , Thyagarajan, H. M. , Hu, Z. , Lancaster, J. N. and Ehrlich, L. I. R. , EBI2 contributes to the induction of thymic central tolerance in mice by promoting rapid motility of medullary thymocytes. Eur. J. Immunol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin, R. , Aili, A. , Wang, Y. , Wu, J. , Sun, X. , Zhang, Y. and Ge, Q. , Critical role of SP thymocyte motility in regulation of thymic output in neonatal Aire‐/‐ mice. Oncotarget 2017. 8: 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jin, R. , Teng, F. , Xu, X. , Yao, Y. , Zhang, S. , Sun, X. , Zhang, Y. et al, Redox balance of mouse medullary CD4 single‐positive thymocytes. Immunol. Cell Biol. 2013. 91: 634–641. [DOI] [PubMed] [Google Scholar]
- 35. Lio, C. W. and Hsieh, C. S. , A two‐step process for thymic regulatory T cell development. Immunity 2008. 28: 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tai, X. , Erman, B. , Alag, A. , Mu, J. , Kimura, M. , Katz, G. , Guinter, T. et al, Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity 2013. 38: 1116–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lei, Y. , Ripen, A. M. , Ishimaru, N. , Ohigashi, I. , Nagasawa, T. , Jeker, L. T. , Bosl, M. R. et al, Aire‐dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J. Exp. Med. 2011. 208: 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laan, M. , Kisand, K. , Kont, V. , Moll, K. , Tserel, L. , Scott, H. S. and Peterson, P. , Autoimmune regulator deficiency results in decreased expression of CCR4 and CCR7 ligands and in delayed migration of CD4+ thymocytes. J. Immunol. 2009. 183: 7682–7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hubert, F. X. , Kinkel, S. A. , Davey, G. M. , Phipson, B. , Mueller, S. N. , Liston, A. , Proietto, A. I. et al, Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood 2011. 118: 2462–2472. [DOI] [PubMed] [Google Scholar]
- 40. Kishimoto, H. and Sprent, J. , Negative selection in the thymus includes semimature T cells. J. Exp. Med. 1997. 185: 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cowan, J. E. , Jenkinson, W. E. and Anderson, G. , Thymus medulla fosters generation of natural Treg cells, invariant gammadelta T cells, and invariant NKT cells: what we learn from intrathymic migration. Eur. J. Immunol. 2015. 45: 652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weinreich, M. A. and Hogquist, K. A. , Thymic emigration: when and how T cells leave home. J. Immunol. 2008. 181: 2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang, E. , Zou, T. , Leichner, T. M. , Zhang, S. L. and Kambayashi, T. , Both retention and recirculation contribute to long‐lived regulatory T‐cell accumulation in the thymus. Eur. J. Immunol. 2014. 44: 2712–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perry, J. S. , Lio, C. W. , Kau, A. L. , Nutsch, K. , Yang, Z. , Gordon, J. I. , Murphy, K. M. et al, Distinct contributions of Aire and antigen‐presenting‐cell subsets to the generation of self‐tolerance in the thymus. Immunity 2014. 41: 414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Malchow, S. , Leventhal, D. S. , Nishi, S. , Fischer, B. I. , Shen, L. , Paner, G. P. , Amit, A. S. et al, Aire‐dependent thymic development of tumor‐associated regulatory T cells. Science 2013. 339: 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Malchow, S. , Leventhal, D. S. , Lee, V. , Nishi, S. , Socci, N. D. and Savage, P. A. , Aire Enforces Immune Tolerance by Directing Autoreactive T Cells into the Regulatory T Cell Lineage. Immunity 2016. 44: 1102–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamazaki, T. , Yang, X. O. , Chung, Y. , Fukunaga, A. , Nurieva, R. , Pappu, B. , Martin‐Orozco, N. et al, CCR6 regulates the migration of inflammatory and regulatory T cells. J. Immunol. 2008. 181: 8391–8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kroger, C. J. , Wang, B. and Tisch, R. , Temporal increase in thymocyte negative selection parallels enhanced thymic SIRPalpha+ DC function. Eur. J. Immunol. 2016. 46: 2352–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yu, W. , Nagaoka, H. , Jankovic, M. , Misulovin, Z. , Suh, H. , Rolink, A. , Melchers, F. et al, Continued RAG expression in late stages of B cell development and no apparent re‐induction after immunization. Nature 1999. 400: 682–687. [DOI] [PubMed] [Google Scholar]
- 50. Ramsey, C. , Winqvist, O. , Puhakka, L. , Halonen, M. , Moro, A. , Kampe, O. , Eskelin, P. et al, Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum. Mol. Genet. 2002. 11: 397–409. [DOI] [PubMed] [Google Scholar]
- 51. Paradis, T. J. , Cole, S. H. , Nelson, R. T. and Gladue, R. P. , Essential role of CCR6 in directing activated T cells to the skin during contact hypersensitivity. J. Invest. Dermatol. 2008. 128: 628–633. [DOI] [PubMed] [Google Scholar]
- 52. White, A. J. , Jenkinson, W. E. , Cowan, J. E. , Parnell, S. M. , Bacon, A. , Jones, N. D. , Jenkinson, E. J. et al, An essential role for medullary thymic epithelial cells during the intrathymic development of invariant NKT cells. J. Immunol. 2014. 192: 2659–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peer review correspondence
Supporting Information Figure 1


